Diagnosis and Treatment of an Ununited Anconeal Process in a California Sea Lion (Zalophus californianus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Description
2.2. Anesthesia and Diagnostics
2.2.1. First Anesthesia for Radiology
2.2.2. Second Anesthesia for Surgery
2.3. Elbow Surgery
Surgical Procedure
2.4. Necropsy
2.4.1. Post-Surgery Anesthesia for Critical Care
2.4.2. Standard Necropsy
2.5. Histology
2.6. Three-Dimensional Visualization
3. Results
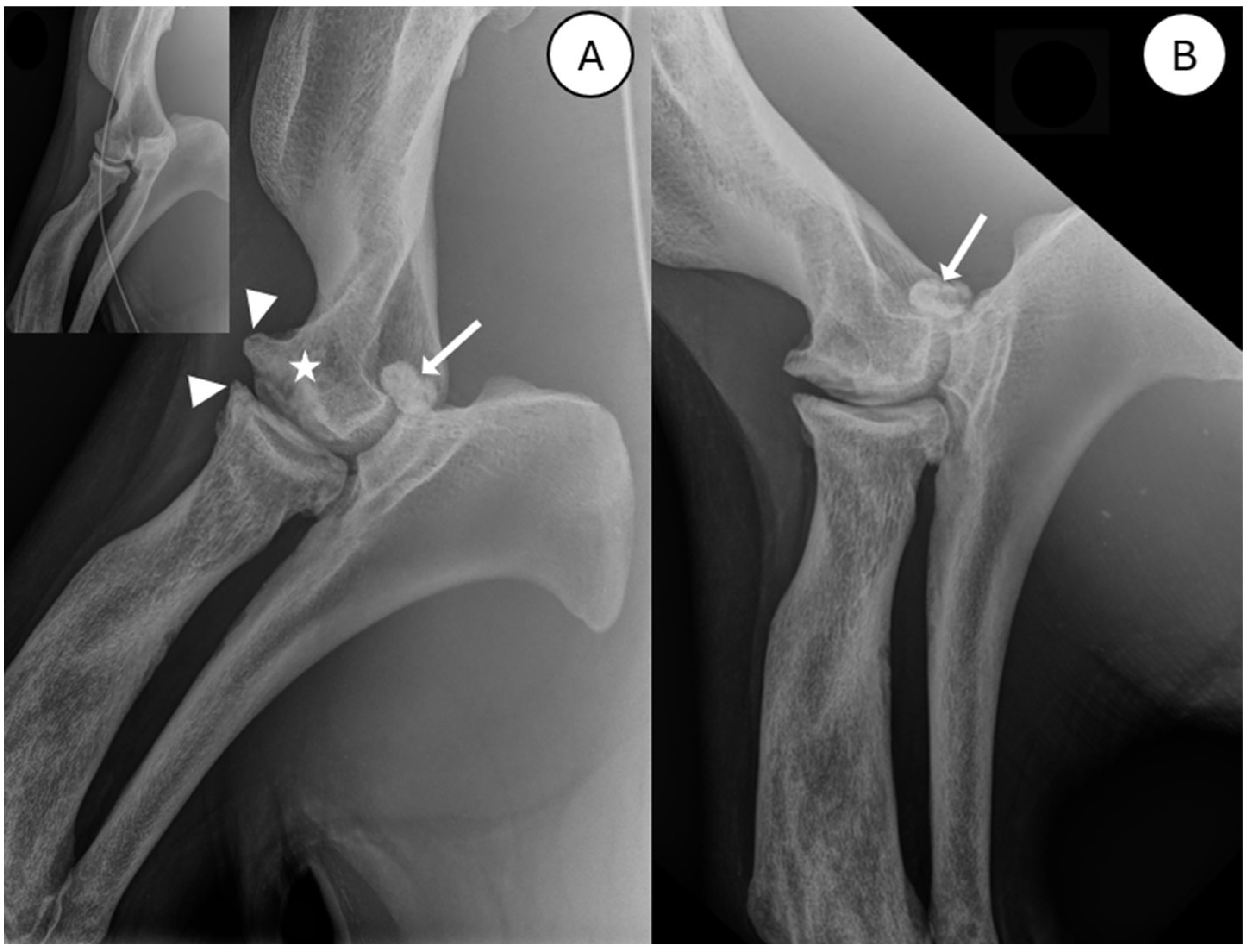
3.1. Radiography
3.2. Surgical and Post-Surgical Data
3.2.1. Arthrocentesis
3.2.2. Post-Surgical Evolution
3.2.3. Histology of Intra-Articular Fragment
3.3. Necropsy, Histology, and 3D Visualization
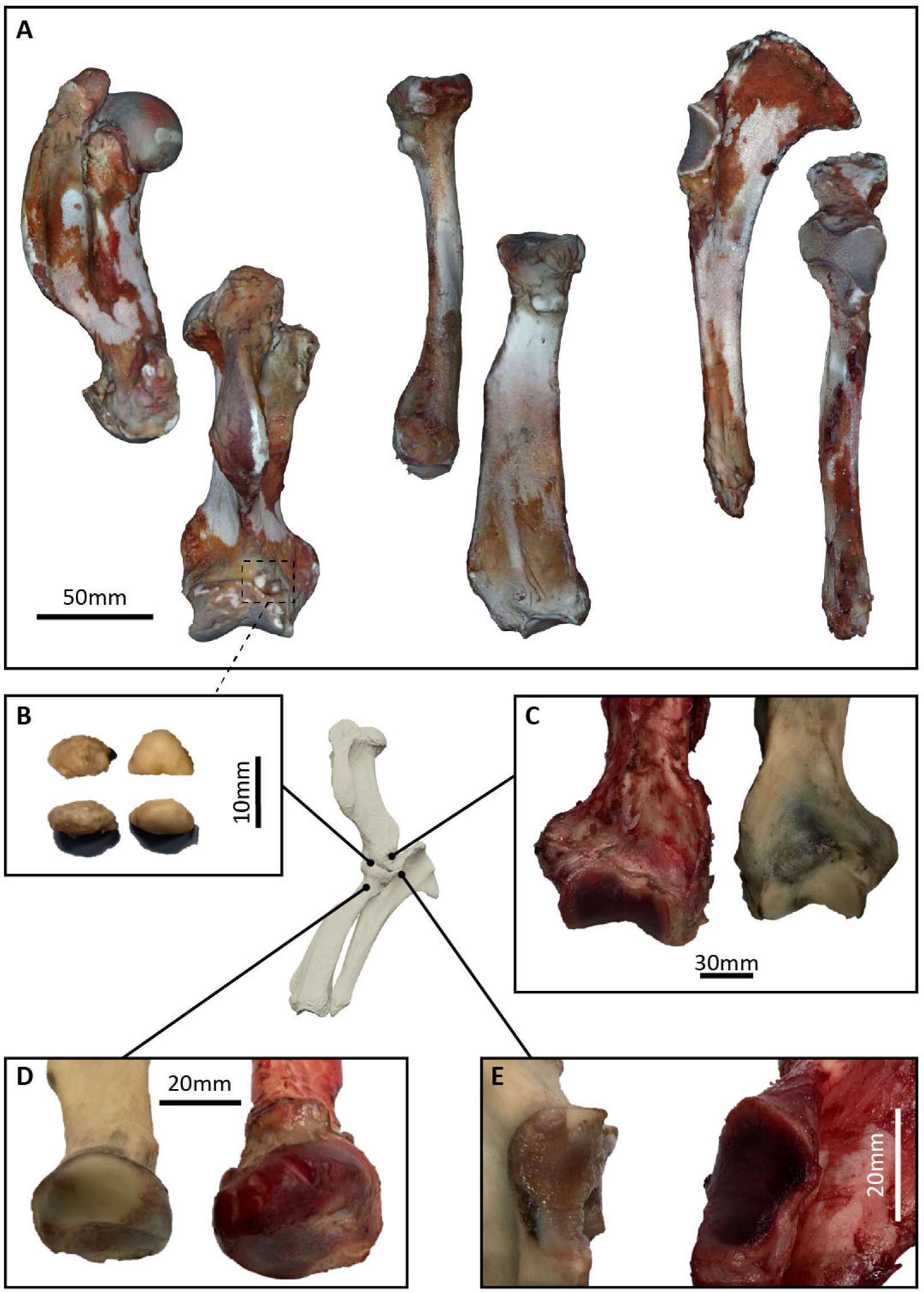
3.3.1. Necropsy
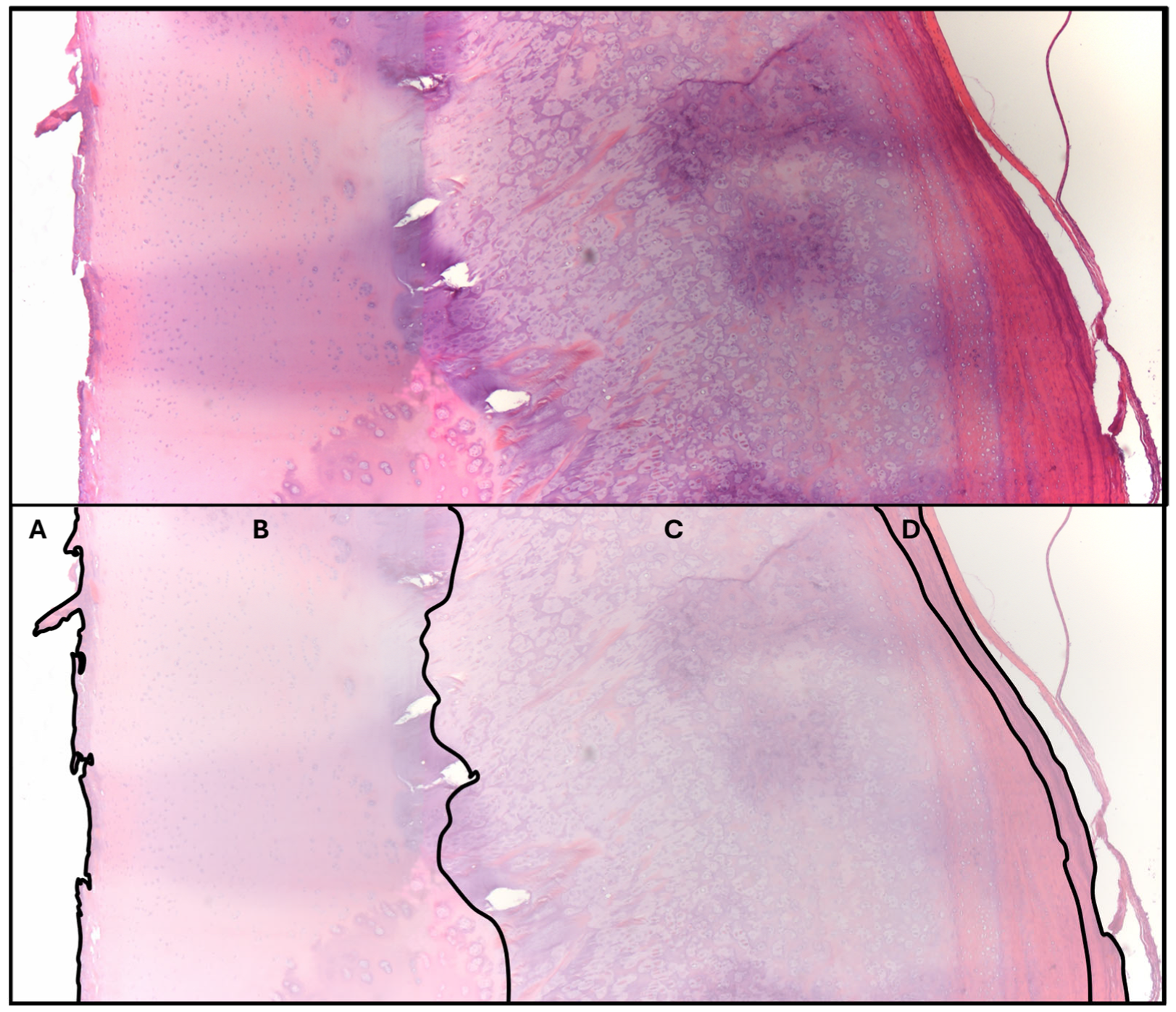
3.3.2. Histopathologic Data
3.3.3. Three-Dimensional Visualization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baltzer, W. Rehabilitation of companion animals following orthopaedic surgery. New Zealand Veter. J. 2020, 68, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Duerr, F.; Lambrechts, N.; Duncan, C.; Gibbs, C.P.; West, A.; Rishniw, M.; Elam, L. What to Teach in Small Animal Veterinary Orthopedics: A Survey of Practicing Veterinarians to Inform Curriculum Development. J. Veter. Med. Educ. 2023, 50, 677–684. [Google Scholar] [CrossRef] [PubMed]
- González, M.S.; Carrasco, D.C. Advances in Exotic Animal Osteosynthesis. Veter. Clin. N. Am. Exot. Anim. Pr. 2019, 22, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.P.; Isaza, R.; Aczm, D. Concepts and issues with interspecies scaling in zoological pharmacology. J. Zoo Wildl. Med. 2008, 39, 517–526. [Google Scholar] [CrossRef]
- Higgins, J.L.; Hendrickson, D.A. Surgical procedures in pinniped and cetacean species. J. Zoo Wildl. Med. 2013, 44, 817–836. [Google Scholar] [CrossRef]
- Wilson, G.J.; Blyde, D.; Forsayeth, A.M.; Pearce, E. Tooth Resorption in an Australian Sea Lion. J. Veter. Dent. 2014, 31, 106–107. [Google Scholar] [CrossRef]
- Hespel, A.-M.; Bernard, F.; Davies, N.J.; Huuskonen, V.; Skelly, C.; David, F. Surgical repair of a tibial fracture in a two-week-old grey seal (Halichoerus grypus). Veter. Comp. Orthop. Traumatol. 2013, 26, 82–87. [Google Scholar] [CrossRef]
- Lucas, R.J.; Barnett, J.; Riley, P. Treatment of lesions of osteomyelitis in the hind flippers of six grey seals (Halkchoerus grypus). November 1999. Available online: https://pubmed.ncbi.nlm.nih.gov/10609572/ (accessed on 21 June 2025).
- Malabia, A.; Lacave, G.; Ria, J.; Marquez, M. Open reduction surgery of an elbow luxation in a California sea lion (Zalophus californianus). Proc. Int. Assoc. Aquat. Anim. Med. 2011, 42, 179. [Google Scholar]
- Morgan, J.P.; Wind, A.; Davidson, A.P. Hereditary Bone and Joint Diseases in the Dog: Osteochondroses, Hip Dysplasia, Elbow Dysplasia; Schlütersche: Hannover, Germany, 2000. [Google Scholar]
- Vezzoni, A.; Benjamino, K. Canine Elbow Dysplasia. Veter. Clin. N. Am. Small Anim. Pr. 2021, 51, 439–474. [Google Scholar] [CrossRef]
- Sjöström, L. Ununited anconeal process in the dog. Veter. Clin. N. Am. Small Anim. Pr. 1998, 28, 75–86. [Google Scholar] [CrossRef]
- Krotchek, U.; Böttcher, P. Surgical Diseases of the Elbow, Veterinary Surgery: Small Animal. In TA-TT-, Second Edi., St. Louis, Missouri SE—2 Volumes (xxii, 2379, I–86 Pages): Illustrations (Some Color); Elsevier: Amsterdam, The Netherlands, 2018; Available online: https://worldcat.org/title/958796229 (accessed on 15 September 2024).
- Herron, M. Ununited anconeal process in the dog. Veter. Clin. N. Am. 1971, 1, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Frazho, J.K.; Graham, J.; Peck, J.N.; De Haan, J.J. Radiographic evaluation of the anconeal process in skeletally immature dogs. Veter. Surg. 2010, 39, 829–832. [Google Scholar] [CrossRef]
- Berta, A.; Churchill, M.; Boessenecker, R.W. The Origin and Evolutionary Biology of Pinnipeds: Seals, Sea Lions, and Walruses. Annu. Rev. Earth Planet. Sci. 2018, 46, 203–228. [Google Scholar] [CrossRef]
- Lavrijsen, I.; Heuven, H.; Meij, B.; Theyse, L.; Nap, R.; Leegwater, P.; Hazewinkel, H. Prevalence and co-occurrence of hip dysplasia and elbow dysplasia in Dutch pure-bred dogs. Prev. Veter- Med. 2014, 114, 114–122. [Google Scholar] [CrossRef]
- Hebel, M.; Panek, W.K.; Ruszkowski, J.J.; Nabzdyk, M.; Niedzielski, D.; Pituch, K.C.; Jackson, A.M.; Kiełbowicz, M.; Pomorska-Mól, M. Computed tomography findings in a cohort of 169 dogs with elbow dysplasia—A retrospective study. BMC Veter. Res. 2021, 17, 296. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Fehr, M.; Nolte, I. Co-existence of ununited anconeal process and fragmented medial coronoid process of the ulna in the dog. J. Small Anim. Pr. 2006, 47, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Horning, M.; Haulena, M.; Tuomi, P.A.; Mellish, J.-A.E. Intraperitoneal implantation of life-long telemetry transmitters in otariids. BMC Veter. Res. 2008, 4, 51. [Google Scholar] [CrossRef]
- Le Boeuf, B.J.; Riedman, M.; Keyes, R.S. White Shark Predation on Pinnipeds in California Coastal Waters. Fish. Bull. 1982, 80, 891–895. [Google Scholar]
- English, A.W.M. Structural correlates of forelimb function in fur seals and sea lions. J. Morphol. 1977, 151, 325–352. [Google Scholar] [CrossRef]
- Fitzpatrick, N.; Smith, T.J.; Evans, R.B.; Yeadon, R. Radiographic and arthroscopic findings in the elbow joints of 263 dogs with medial coronoid disease. Veter. Surg. 2009, 38, 213–223. [Google Scholar] [CrossRef]
- RRead, R.A.; Armstrong, S.J.; O’KEefe, J.D.; Eger, C.E. Fragmentation of the medial coronoid process of the ulna in dogs: A study of 109 cases. J. Small Anim. Pr. 1990, 31, 330–334. [Google Scholar] [CrossRef]
- Biancani, B.; Field, C.L.; Dennison, S.; Pulver, R.; Tuttle, A.D. Hiatal hernia in a harbor seal (Phoca vitulina) pup. J. Zoo Wildl. Med. 2012, 43, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Gili, C.; Meijer, G.; Lacave, G. EAZA and EAAM Best Practice Guidelines for Otariidae and Phocidae EAZA Marine Mammal TAG TAG Chair: EAZA Best Practice Guidelines Disclaimer. 2018. Available online: https://strapi.eaza.net/uploads/EAZA_EAAM_Pinniped_Guidelines_approved_701197aefb.pdf (accessed on 27 January 2025). [CrossRef]
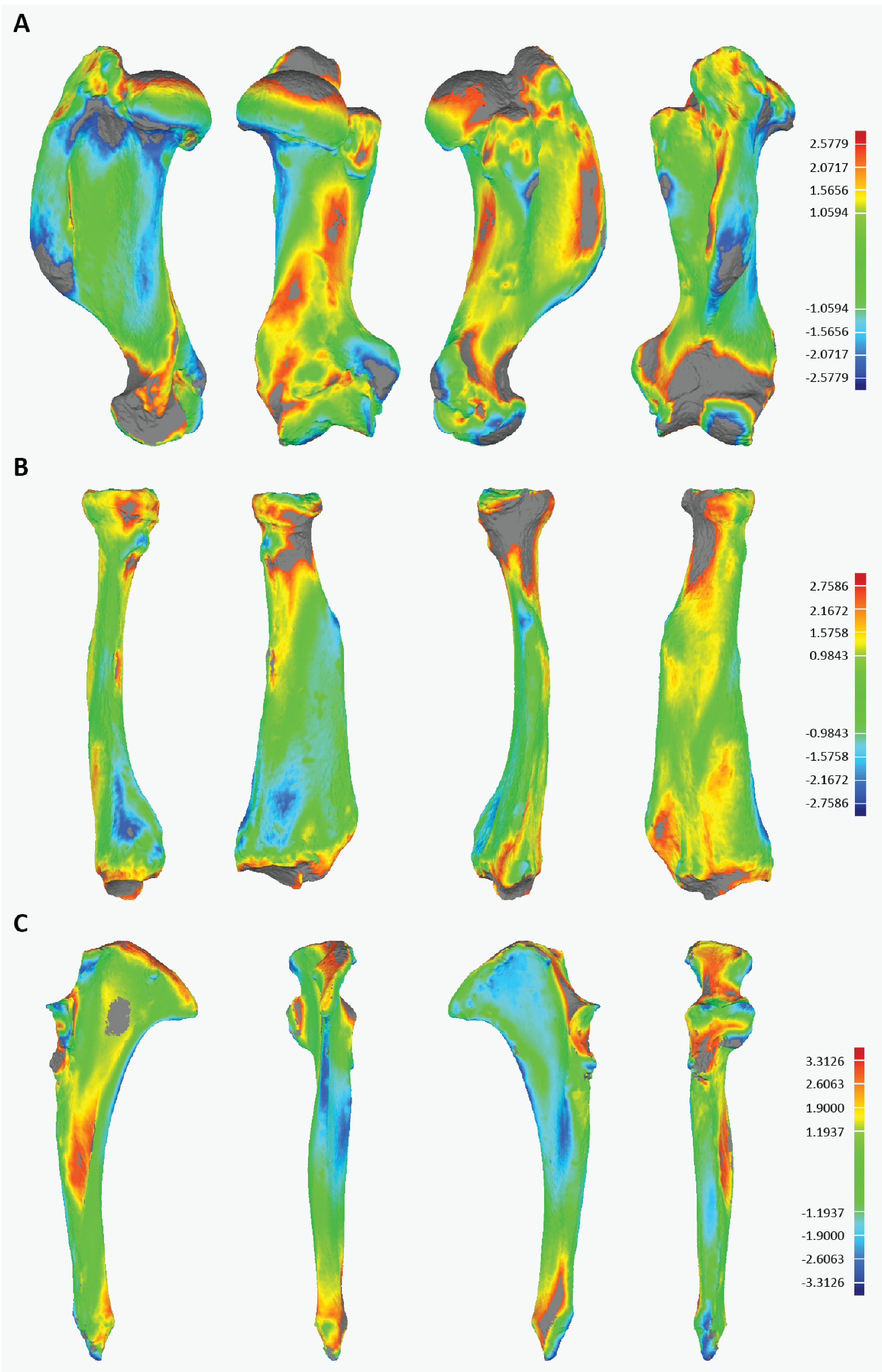
| Comparison | Maximum Deviation (mm) | Average Deviation (mm) | Standard Deviation (mm) |
|---|---|---|---|
| Humerus | |||
| RZSA healthy vs. RZSA pathological | 2.578 | 1.059 | 0.673 |
| RZSA healthy vs. USNM 200847 | 2.821 | 1.138 | 0.772 |
| Radius | |||
| RZSA healthy vs. RZSA pathological | 2.758 | 0.984 | 0.656 |
| RZSA healthy vs. USNM 21735 | 2.535 | 0.902 | 0.677 |
| Ulna | |||
| RZSA healthy vs. RZSA pathological | 3.313 | 1.194 | 0.822 |
| RZSA healthy vs. USNM 21735 | 3.078 | 0.879 | 0.737 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlake, A.; Van Mulders, L.; Dekkers, M.; Selini, A.; MacLaren, J.A.; Vercauteren, G.; Chiers, K.; Vercammen, F.; Spruyt, J. Diagnosis and Treatment of an Ununited Anconeal Process in a California Sea Lion (Zalophus californianus). Animals 2025, 15, 1865. https://doi.org/10.3390/ani15131865
Schlake A, Van Mulders L, Dekkers M, Selini A, MacLaren JA, Vercauteren G, Chiers K, Vercammen F, Spruyt J. Diagnosis and Treatment of an Ununited Anconeal Process in a California Sea Lion (Zalophus californianus). Animals. 2025; 15(13):1865. https://doi.org/10.3390/ani15131865
Chicago/Turabian StyleSchlake, Alexander, Laurens Van Mulders, Moniek Dekkers, Anastasia Selini, Jamie A. MacLaren, Griet Vercauteren, Koen Chiers, Francis Vercammen, and Jonas Spruyt. 2025. "Diagnosis and Treatment of an Ununited Anconeal Process in a California Sea Lion (Zalophus californianus)" Animals 15, no. 13: 1865. https://doi.org/10.3390/ani15131865
APA StyleSchlake, A., Van Mulders, L., Dekkers, M., Selini, A., MacLaren, J. A., Vercauteren, G., Chiers, K., Vercammen, F., & Spruyt, J. (2025). Diagnosis and Treatment of an Ununited Anconeal Process in a California Sea Lion (Zalophus californianus). Animals, 15(13), 1865. https://doi.org/10.3390/ani15131865







