Genetic Erosion in Captive Alpine Musk Deer Highlights the Challenges of Conserving Endangered Species in Closed Populations
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Fecal Samples from Captive Alpine Musk Deer
2.2. Amplification of Mitochondrial Genes in Captive Alpine Musk Deer
2.2.1. DNA Extraction
2.2.2. PCR Amplification and Sequencing
2.2.3. Acquisition of Mitochondrial Data from Wild Alpine Musk Deer
2.3. Data Analysis
2.3.1. Phylogenetic Tree Construction
2.3.2. Haplotype Network Construction, Polymorphism Analysis
2.3.3. Genetic Differentiation Coefficient and Molecular Variance Analysis Methods
2.3.4. Richness Estimation
2.3.5. Sequence Concatenation
3. Results
3.1. D-Loop Gene Sequence and Haplotype
3.2. COI Sequence and Haplotype
3.3. Cytb Sequence and Haplotype
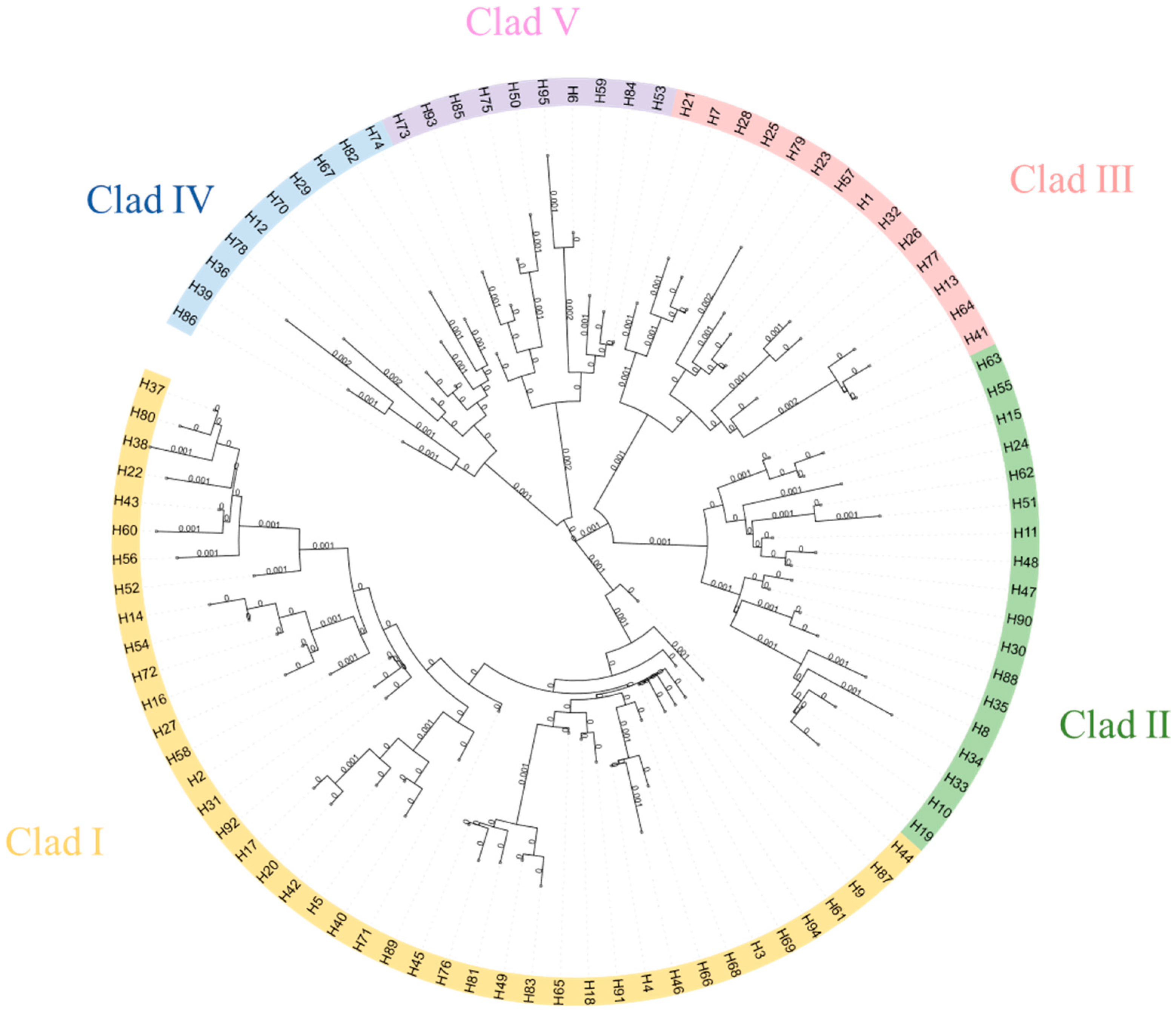
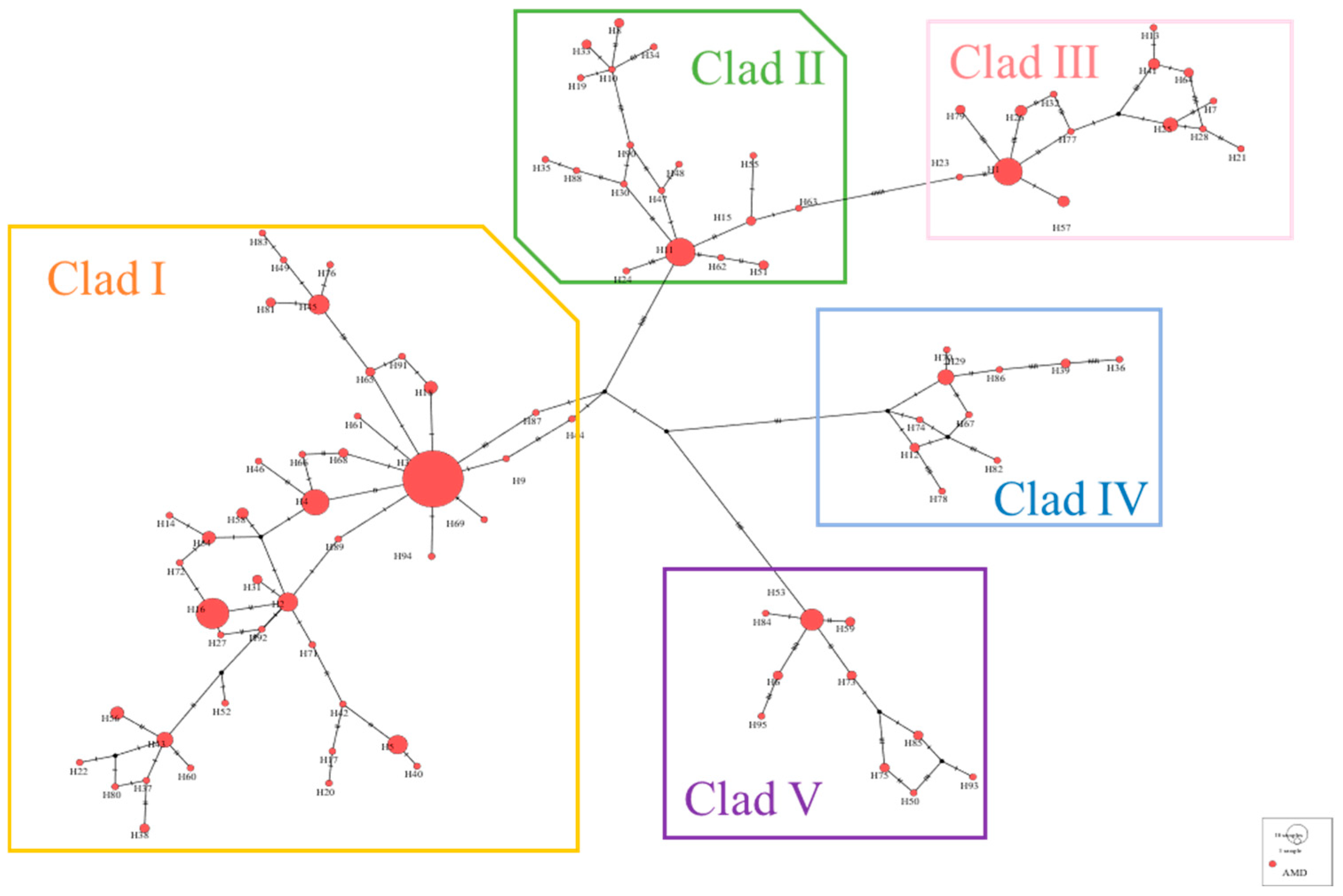
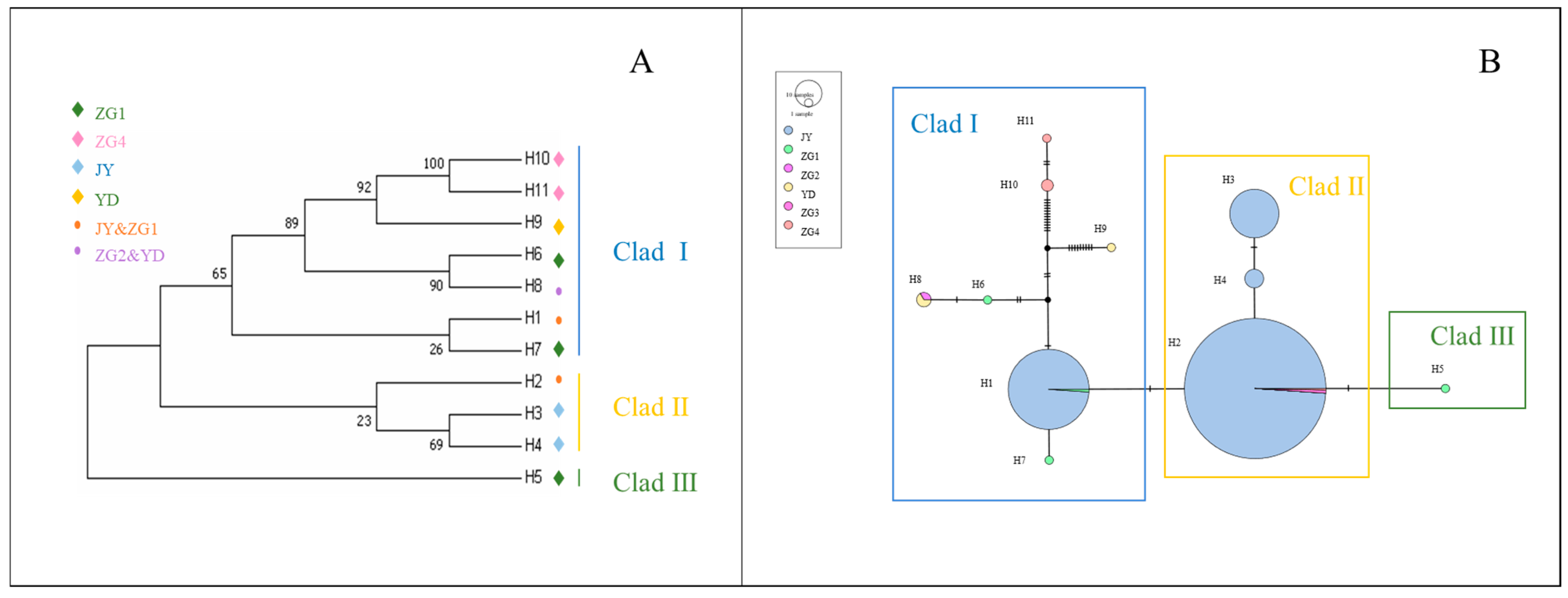
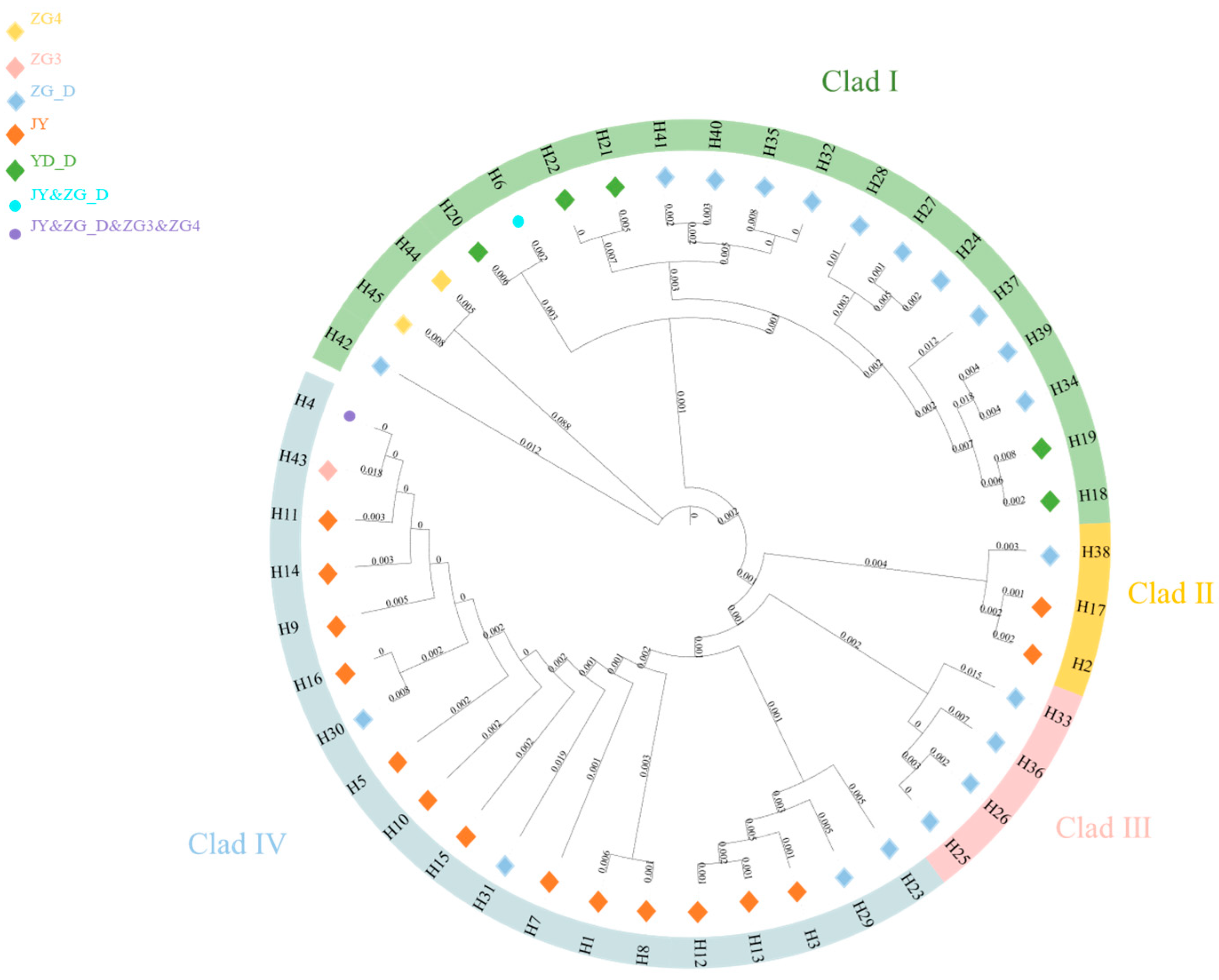
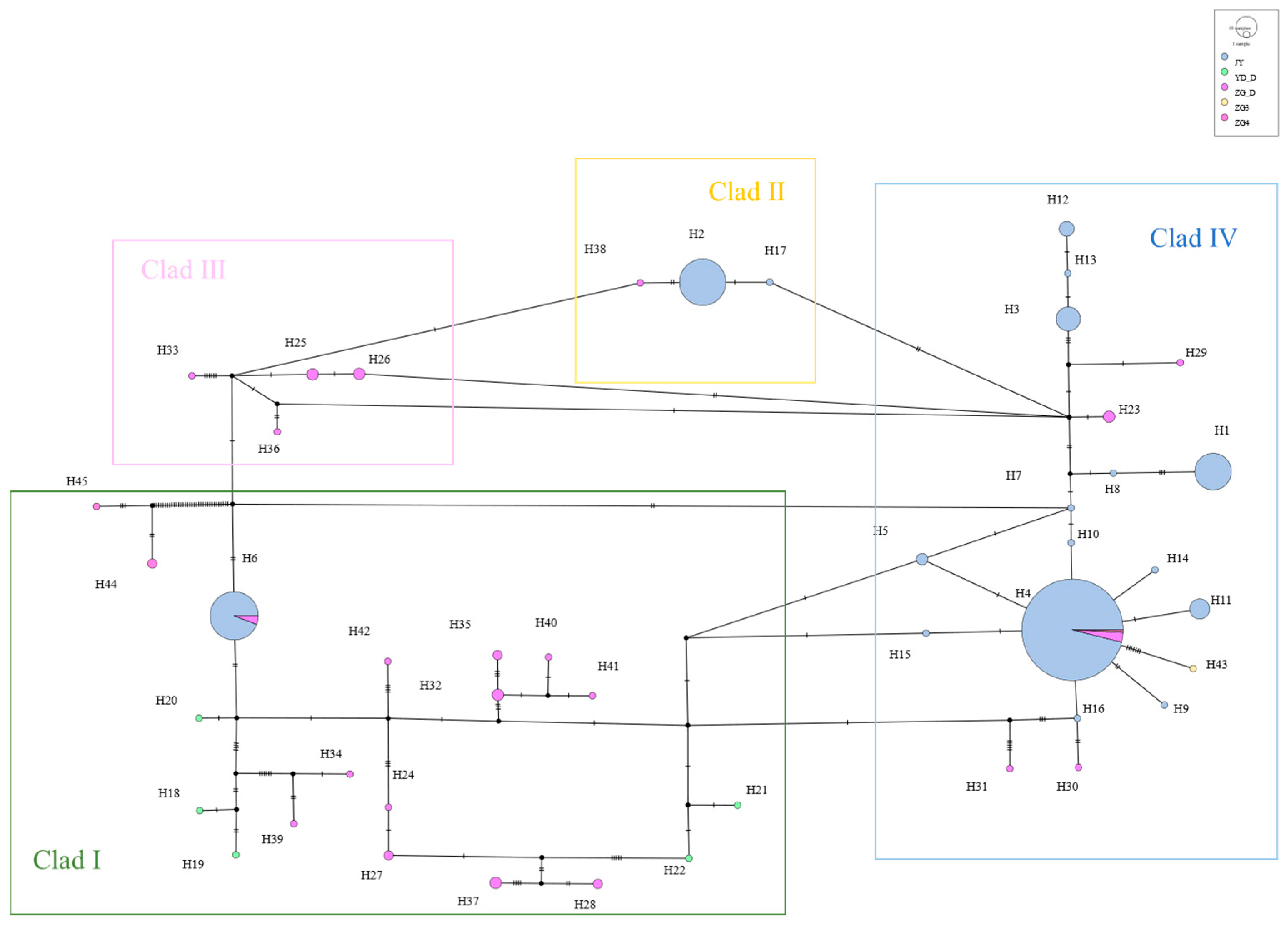
3.4. Phylogenetic and Network Relationship Analysis
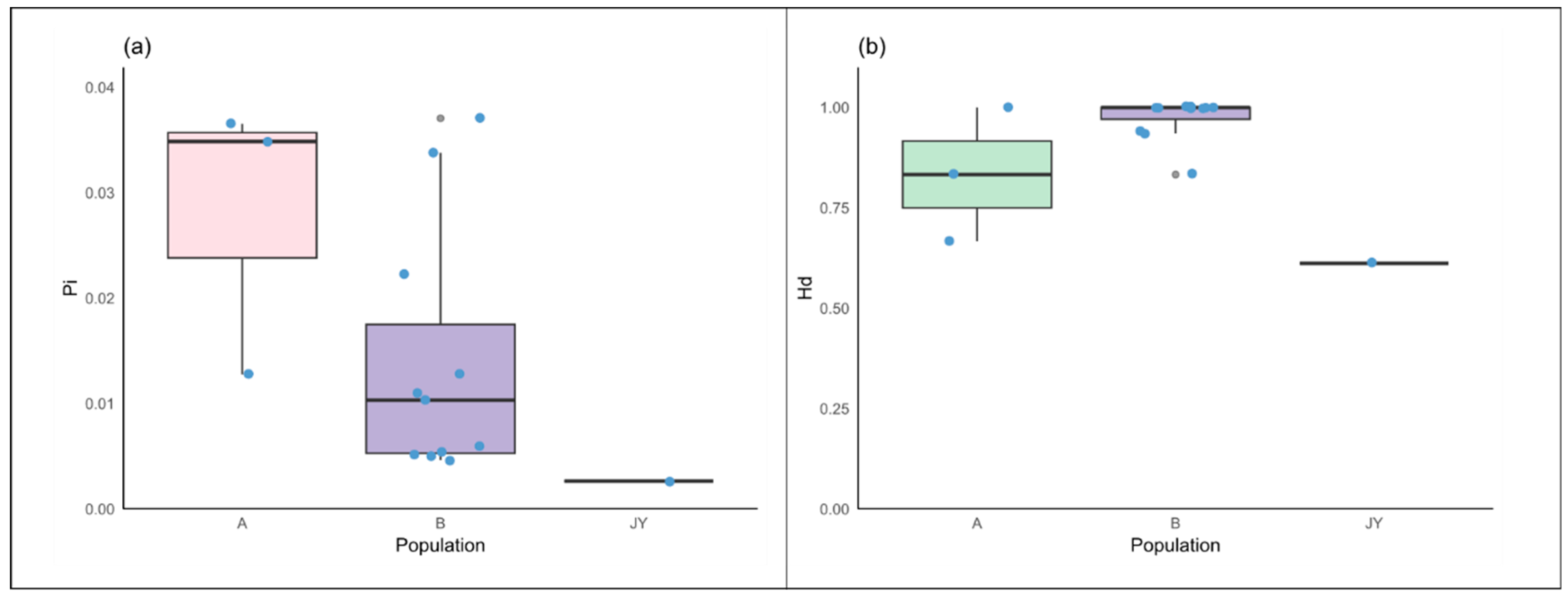
3.5. Comparison of Genetic Diversity Between Captive and Wild Alpine Musk Deer
3.5.1. Comparison of Genetic Diversity Based on Cytb
3.5.2. Comparison of Genetic Diversity Based on D-Loop
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellegren, H.; Galtier, N. Determinants of genetic diversity. Nat. Rev. Genet. 2016, 17, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Leigh, D.M.; Hendry, A.P.; Vazquez-Dominguez, E.; Friesen, V.L. Estimated six per cent loss of genetic variation in wild populations since the industrial revolution. Evol. Appl. 2019, 12, 1505–1512. [Google Scholar] [CrossRef]
- De Woody, J.A.; Harder, A.M.; Mathur, S.; Willoughby, J. The long-standing significance of genetic diversity in conservation. Mol. Ecol. 2021, 30, 4147–4154. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Rob, D.; Olive, A.R.; Howard, C.R. Conservation Genetics in the Age of Genomics; Columbia University Press: New York, NY, USA, 2009. [Google Scholar]
- Wang, Y.F.; Ma, S.M.; Liu, C.P.; Wang, M. The Development of Genetic Markers and Detection Techniques for Molecular Markers. J. Northwest A&F Univ. 2001, 29, 130–136. [Google Scholar]
- Ding, X.H. Phylogeographic Study of Blackfin Icefish Based on Mitochondrial and Nuclear Gene Molecular Markers. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2020. [Google Scholar]
- Yang, Z.H.; Xia, R.C.; Jiang, Z.W. Identification of Chinese sturgeon species based on mitochondrial DNA and nuclear DNA genetic markers. Chin. Judic. Apprais. 2024, 61–65. [Google Scholar] [CrossRef]
- Chu, J.N.; Xu, H.T.; He, Z.J. Comparative study on mitochondrial genomic genetic diversity between captive and wild populations of Northeast tigers in China. J. Wildl. Sci. 2024, 45, 231–241. [Google Scholar]
- Zhao, X.B.; Li, N.; Wu, C.X. Genetic Variation Analysis of the D-loop Region of Pig Mitochondrial DNA. Hereditas 1997, 19 (Suppl. S1), 23–26. [Google Scholar]
- Zhou, J.L.; Zhang, Y.P.; Huang, M.H. Analysis of Mitochondrial Cytochrome b Gene Sequences and Phylogeny of Viperinae Snakes. Curr. Zool. 2001, 47, 361–366. [Google Scholar]
- Su, B.; Wang, Y.X.; Wang, Q.S. Mitochondrial DNA Sequences lmply Anhui Musk Deera Valid Species in Genus Moschus. Zool. Res. 2001, 22, 169–173. [Google Scholar]
- Li, Y. Study of Genetic Diversity in the Cold-Resistant Frog Based on Mitochondrial Gene Cyt b and Nuclear Gene rag2. Ph.D. Thesis, Central South University of Forestry and Technology, Changsha, China, 2022. [Google Scholar]
- Wang, N.X.; Feng, X.; Jiang, G.F.; Fang, N.; Xuan, W.J. Molecular phylogenetic analysis of five subfamilies of the Acrididae (Orthoptera: Acridoidea) based on the mitochondrial cytochrome b and cytochrome e oxidase subunit I gene sequences. Acta Entomol. Sin. 2008, 51, 1187–1195. [Google Scholar]
- Zhao, Y. Population Structure Study of the Indo-Pacific Flying Squid (Sthenoteuthis oualaniensis) Based on Molecular. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2022. [Google Scholar]
- Sheng, H.L.; Liu, Z.X. The Musk Deer in China; The Shanghai Scientific & Technical Publishers: Shanghai, China, 2007. [Google Scholar]
- Wu, J.Y.; Wang, W. The Musk Deer of China; The China Forestry Publishing House: Beijing, China, 2006. [Google Scholar]
- Wang, G.; Shen, L.Q.; Gao, H.X. Habitat characteristics of wild musk deer resting in winter in Xinglongshan Nature Reserve, Gansu Province. Chin. J. Zool. 2023, 58, 209–217. [Google Scholar]
- Zhang, Z.R. Current Status and Conservation Genetics of Musk Deer Population in Helan Mountain Area. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2023. [Google Scholar]
- Kang, F.G.; Sheng, Y.; Ma, L.T. The Secretion of Musk in Domesticated Musk Deer and Its Relationship with Population Dynamics and Age Structure in the Xinglong Mountain Nature Reserve. Acta Ecol. Sin. Sheng T’ai Hsueh Pao 2015, 35, 4993–4999. [Google Scholar]
- Huang, W.; Wu, J.Y.; Shen, L.Q. The behavioral and physiological effects of manual fragrance extraction on captive musk deer. Chin. J. Zool. 2024, 59, 379–386. [Google Scholar]
- Wang, X.L.; Wu, J.Y.; Zhou, X. The relationship between aggression and fecal steroid hormone levels and musk secretion in captive musk deer. Acta Theriol. Sin. 2024, 44, 344–350. [Google Scholar]
- Huang, Y.D.; Wang, P.M.; Jiang, Y.W. Determination of physiological and biochemical normal values of musk deer. Chin. J. Zool. 2003, 38, 45–47. [Google Scholar]
- Zhang, Y.Z.; Xiang, J.; Zhang, J.D. Genetic Diversity of Macaca mulatta in the Western Regions of China Based on Cyt b Gene Sequences. Sichuan Anim. 2023, 42, 133–140. [Google Scholar]
- Xia, T.; Xu, H.; Li, D.; Xie, M.; Ni, Q.; Zhang, M.; Yao, Y. Complete mitochondrial genome and phylogenetic analysis of Alpine musk deer (Moschus chrysogaster). Mitochondrial DNA Part B 2019, 4, 2042–2043. [Google Scholar] [CrossRef]
- Piggott, M.P.; Taylor, A.C. Extensive evaluation of faecal preservation and DNA extraction methods in Australian native and introduced species. Aust. J. Zool. 2003, 51, 341–355. [Google Scholar] [CrossRef]
- Tang, J.; Li, J.J.; Wang, B.; Suo, L.J.; Li, F.R.; Liu, W.H.; Wang, Y.; Yan, X.R. Efficiency and Analysis of DNA Extraction from the Feces of Forest Musk Deer Using Two Methods. Acta Agric. Boreali-Occident. Sin. 2018, 27, 326–330. [Google Scholar]
- Cui, L.Y.; Liu, B.Y.; Li, H.M.; Zhu, Y.X.; Zhou, Y.H.; Su, C.; Tian, Y.P.; Xu, H.T.; Liu, D.; Li, X.P.; et al. A simple and effective method to enrich endogenous DNA from mammalian faeces. Mol. Ecol. Resour. 2024, 24, e13939. [Google Scholar] [CrossRef]
- Burland, T.G. DNASTAR’s Lasergene sequence analysis software. Bioinform. Methods Protoc. 1999, 132, 71–91. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Swindell, S.R.; Plasterer, T.N. SEQMAN: Contig assembly. Seq. Data Anal. Guideb. 1997, 70, 75–89. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Han, L. Study on the Genetic Diversity of Hainan Gibbons Population. Ph.D. Thesis, Guizhou Normal University, Guiyang, China, 2019. [Google Scholar]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.K. Estimates: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9.1.0. 2019. Available online: https://www.robertkcolwell.org/pages/1407-estimates (accessed on 21 May 2025).
- Li, Q. Species Accumulation Curves and Their Applications. Chin. J. Appl. Entomol. 2011, 48, 1882–1888. [Google Scholar]
- Xiang, C.Y.; Gao, F.L.; Jakovlić, I.; Lei, H.P.; Hu, Y.; Zhang, H.; Zou, H.; Wang, G.T.; Zhang, D. Using PhyloSuite for molecular phylogeny and tree-based analyses. IMeta 2023, 2, e87. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Giontella, A.; Cardinali, I.; Lancioni, H.; Giovannini, S.; Pieramati, C.; Silvestrelli, M.; Sarti, F.M. Mitochondrial DNA survey reveals the lack of accuracy in Maremmano horse studbook records. Animals 2020, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Crandall, K.A.; Templeton, A.R. Empirical tests of some predictions from coalescent theory with applications to intraspecific phylogeny reconstruction. Genetics 1993, 134, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Z.; Ma, B.; Li, L. Analysis of Genetic Diversity in the Populations of the Tibetan Yellow-Marbled Loach (Triplophysa yarlungzangboensis) Based on Mitochondrial DNA Cytochrome b Gene and Control Region Sequences. Chin. J. Zool. 2019, 54, 701–710. [Google Scholar]
- Bowen, W.; Bass, A.L.; Rocha, L.A.; Grant, W.S.; Robertson, D.R. Phylogeography of the trumpetfishes (Aulostomus): Ring species complex on a global scale. Evolution 2001, 55, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, G.; Gao, Y.; Yan, L.; Li, M.; Hu, D.; Zhang, D. mtDNA CR Evidence Indicates High Genetic Diversity of Captive Forest Musk Deer in Shaanxi Province, China. Animals 2023, 13, 2191. [Google Scholar] [CrossRef]
- Hai, L.Y.; Li, Y.X.; Lan, X.N.; Luo, Z.W.; Pei, C.; Hu, D.F. Insight into mtDNA diversity of wild forest musk deer (Moschus berezovskii) in Shanxi Province mountains. Sci. Rep. 2025, 15, 5523. [Google Scholar] [CrossRef]
- Peng, H.; Liu, S.; Zou, F.; Zeng, B.; Yue, B. Genetic diversity of captive forest musk deer (Moschus berezovskii) inferred from the mitochondrial DNA control region. Anim. Genet. 2009, 40, 65–72. [Google Scholar] [CrossRef]
- Fan, J.; Zheng, X.; Wang, H.; Qi, H.; Jiang, B.; Qiao, M.; Zhou, J.; Bu, S. Analysis of Genetic Diversity and Population Structure in Three Forest Musk Deer Captive Populations with Different Origins. G3 Genes Genomes Genet. 2019, 9, 1037–1044. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, B.F.; Chang, J.; Hu, X.L.; Li, C.; Xu, T.T.; Liu, S.Q.; Hu, D.F. Population genomics reveals moderate genetic differentiation between populations of endangered Forest Musk Deer located in Shaanxi and Sichuan. BMC Genom. 2022, 23, 668. [Google Scholar] [CrossRef]
- Fan, Z.X.; Li, W.J.; Jin, J.Z.; Cui, K.; Yan, C.C.; Peng, C.J.; Jian, Z.Y.; Bu, P.; Price, M.; Zhang, X.Y.; et al. The draft genome sequence of forest musk deer (Moschus berezovskii). GigaScience 2018, 7, giy038. [Google Scholar] [CrossRef]

| Locus | Primer Sequences (5′-3′) | Target Fragment (bp) |
|---|---|---|
| D-Loop | F: CCCTAAGACTCAAGGAAGAAGC R: TACCCCCACAGATTATGGGCC | 530 |
| Cytb | F: ATGATCAATATTCGAAAATCTCACCC R: TCTTCATTTTAGGAGATTATTTTCAATTT | 1140 |
| COI | F: TTGAAGCYGGAGCAGGAACAG R: AARTAGGCTCGTGTGTCRAC | 561 |
| Population | Sample Size (n) | Number of Variable Sites | Number of Haplotypes (H) | Nucleotide Diversity (Pi) | Haplotype Diversity (Hd) | Average Number of Nucleotide Differences (K) |
|---|---|---|---|---|---|---|
| ZG1 | 4 | 9 | 4 | 0.01278 | 1.000 | 4.500 |
| ZG4 | 4 | 83 | 3 | 0.03655 | 0.833 | 41.667 |
| YD | 3 | 17 | 2 | 0.03487 | 0.667 | 11.333 |
| Aba county * | 5 | 12 | 5 | 0.00464 | 1.000 | 4.800 |
| Muli county * | 5 | 13 | 5 | 0.00503 | 1.000 | 5.200 |
| Baiyu county * | 4 | 10 | 4 | 0.00516 | 1.000 | 5.333 |
| Yajiang county * | 4 | 21 | 3 | 0.01032 | 0.833 | 10.667 |
| Ganzi county * | 6 | 33 | 6 | 0.01103 | 1.000 | 11.400 |
| Luhuo county * | 16 | 92 | 12 | 0.03380 | 0.942 | 34.883 |
| Dege county * | 23 | 49 | 17 | 0.01278 | 0.935 | 13.217 |
| Shimian county * | 4 | 76 | 4 | 0.03707 | 1.000 | 38.333 |
| Zhangye city * | 3 | 23 | 2 | 0.02224 | 1.000 | 23.000 |
| Gongbujiangda county * | 4 | 12 | 4 | 0.00596 | 1.000 | 6.167 |
| Cangdu county * | 5 | 14 | 5 | 0.00542 | 1.000 | 5.600 |
| JY | 409 | 11 | 14 | 0.00264 | 0.612 | 2.602 |
| Population | JY | ZG1 | ZG2 | YD | ZG3 | ZG4 |
|---|---|---|---|---|---|---|
| JY | ||||||
| ZG1 | 0.42977 (+) | |||||
| ZG2 | 0.086429 (−) | 0.25000 (−) | ||||
| YD | 0.85017 (+) | 0.17207 (−) | −1.00000 (−) | |||
| ZG3 | −0.18146 (−) | 0.01370 (−) | 1.00000 (−) | 0.23982 (−) | ||
| ZG4 | 0.92278 (+) | 0.53398 (−) | 0.35714 (−) | 0.41640 (−) | 0.49415 (−) |
| Source of Variation | Degrees of Freedom | Sum of Squares | Variance Components | Percentage of Variation |
|---|---|---|---|---|
| Among populations | 5 | 54.381 | 1.91075 Va | 82.88 |
| Within populations | 417 | 164.541 | 0.39458 Vb | 17.12 |
| Total | 422 | 218.922 | 2.30534 | |
| Fixation Index | 0.82884 (++) |
| Population | Sample Size (n) | Number of Variable Sites | Number of Haplotypes (H) | Nucleotide Diversity (Pi) | Haplotype Diversity (Hd) | Average Number of Nucleotide Differences (K) |
|---|---|---|---|---|---|---|
| ZG_D | 43 | 60 | 26 | 0.02469 | 0.961 | 13.083 |
| ZG3 | 2 | 7 | 2 | 0.01799 | 1.000 | 7.000 |
| ZG4 | 4 | 44 | 3 | 0.05771 | 0.833 | 22.333 |
| YD_D | 5 | 16 | 5 | 0.02262 | 1.000 | 8.800 |
| JY | 386 | 23 | 19 | 0.01073 | 0.639 | 5.417 |
| Population | JY | YD-D | ZG-D | ZG3 | ZG4 |
|---|---|---|---|---|---|
| JY | |||||
| YD-D | 0.54239 (+) | ||||
| ZG-D | 0.20985 (+) | 0.15723 (+) | |||
| ZG3 | 0.25312 (−) | 0.42647 (−) | 0.22867 (+) | ||
| ZG4 | 0.82610 (+) | 0.57926 (+) | 0.66563 (+) | 0.46716 (+) |
| Source of Variation | Degrees of Freedom | Sum of Squares | Variance Components | Percentage of Variation |
|---|---|---|---|---|
| Among populations | 4 | 160.340 | 1.55429 Va | 39.64 |
| Within populations | 435 | 1029.633 | 2.36697 Vb | 60.36 |
| Total | 439 | 1189.973 | 3.92126 | |
| Fixation Index | 0.39638 (++) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Hai, L.; Luo, Z.; Lan, X.; Zhou, M.; Qin, X.; Hu, D. Genetic Erosion in Captive Alpine Musk Deer Highlights the Challenges of Conserving Endangered Species in Closed Populations. Animals 2025, 15, 1827. https://doi.org/10.3390/ani15131827
Jiang H, Hai L, Luo Z, Lan X, Zhou M, Qin X, Hu D. Genetic Erosion in Captive Alpine Musk Deer Highlights the Challenges of Conserving Endangered Species in Closed Populations. Animals. 2025; 15(13):1827. https://doi.org/10.3390/ani15131827
Chicago/Turabian StyleJiang, Han, Luyao Hai, Zhengwei Luo, Xianna Lan, Mi Zhou, Xinghu Qin, and Defu Hu. 2025. "Genetic Erosion in Captive Alpine Musk Deer Highlights the Challenges of Conserving Endangered Species in Closed Populations" Animals 15, no. 13: 1827. https://doi.org/10.3390/ani15131827
APA StyleJiang, H., Hai, L., Luo, Z., Lan, X., Zhou, M., Qin, X., & Hu, D. (2025). Genetic Erosion in Captive Alpine Musk Deer Highlights the Challenges of Conserving Endangered Species in Closed Populations. Animals, 15(13), 1827. https://doi.org/10.3390/ani15131827




