Effects of Proanthocyanidins on Growth Performance, Intestinal Inflammation and Barrier Function, and Bile Acid Metabolism-Related Genes in Weaned Piglets Challenged with Lipopolysaccharide
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals, Diets, and Experimental Design
2.3. Sample Collection
2.4. Piglet Growth Performance and Fecal Scores
2.5. Histomorphological Examination and Periodic Acid–Schiff (PAS) Staining of the Jejunum and Ileum Tissues
2.6. Serum Immune Indices and Immune Function
2.7. Gene Expression
2.8. Data Processing and Statistics Analysis
3. Results
3.1. Growth Performance
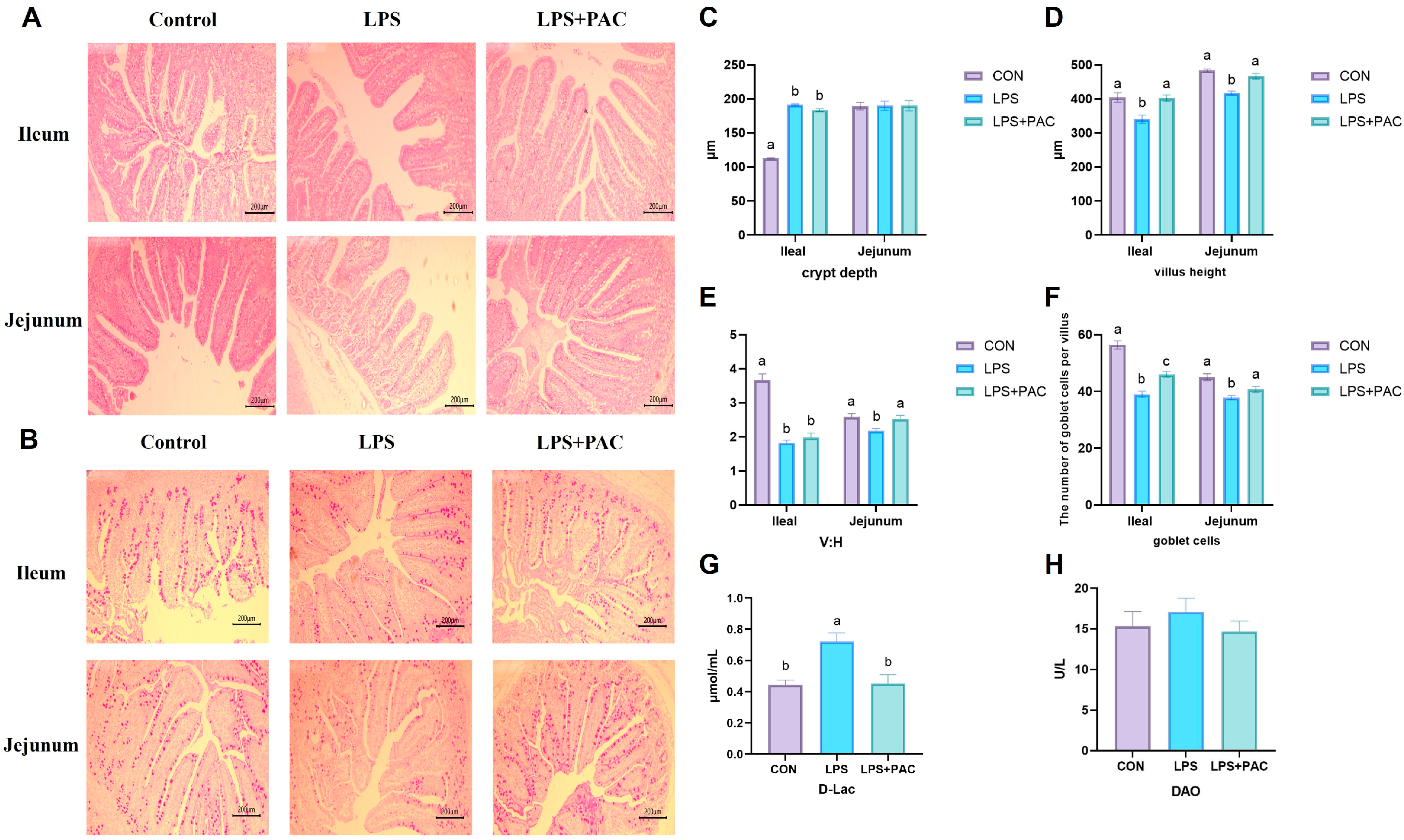
3.2. Intestinal Morphology
3.3. Intestinal Permeability
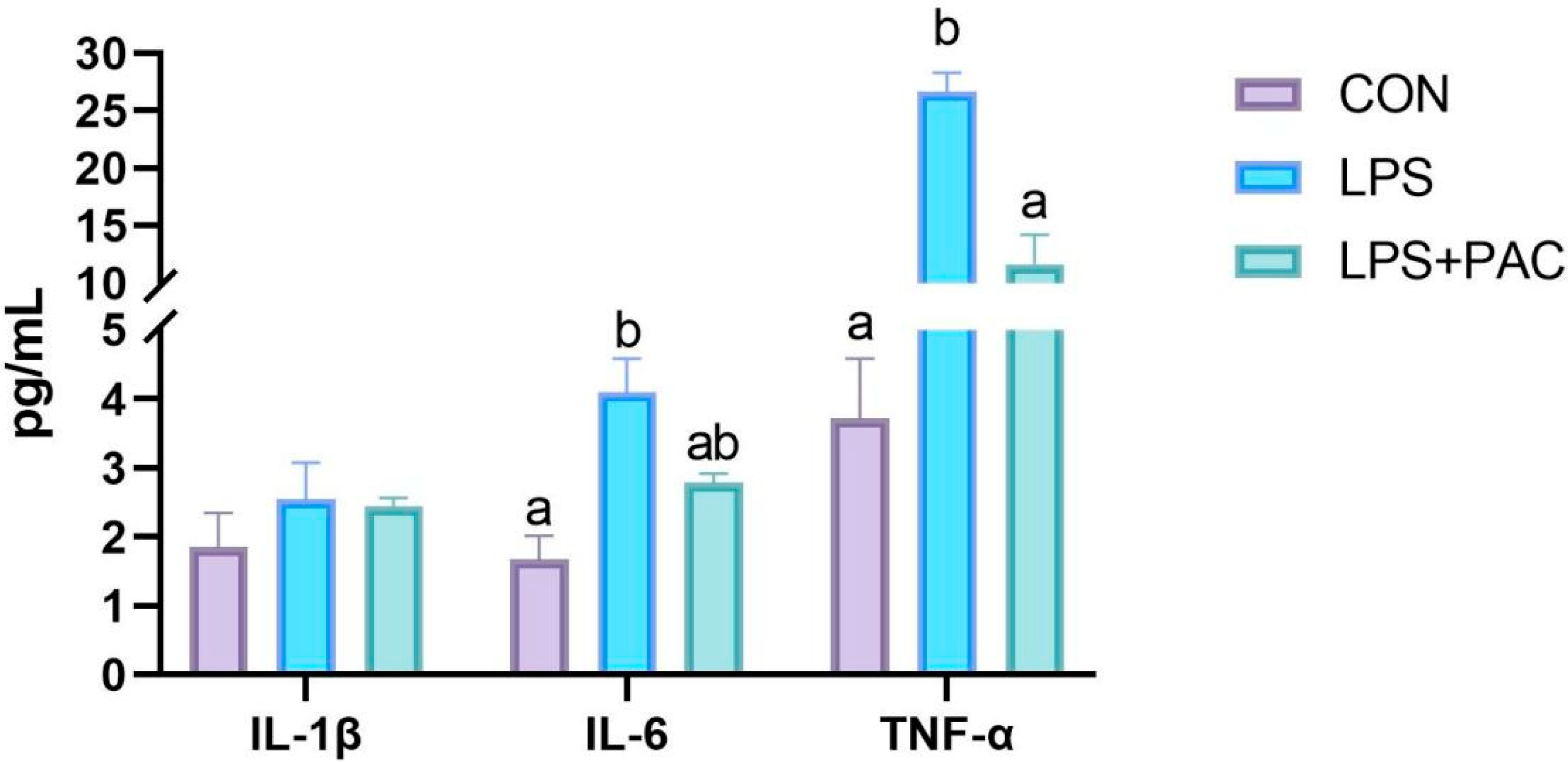
3.4. Cytokine Content
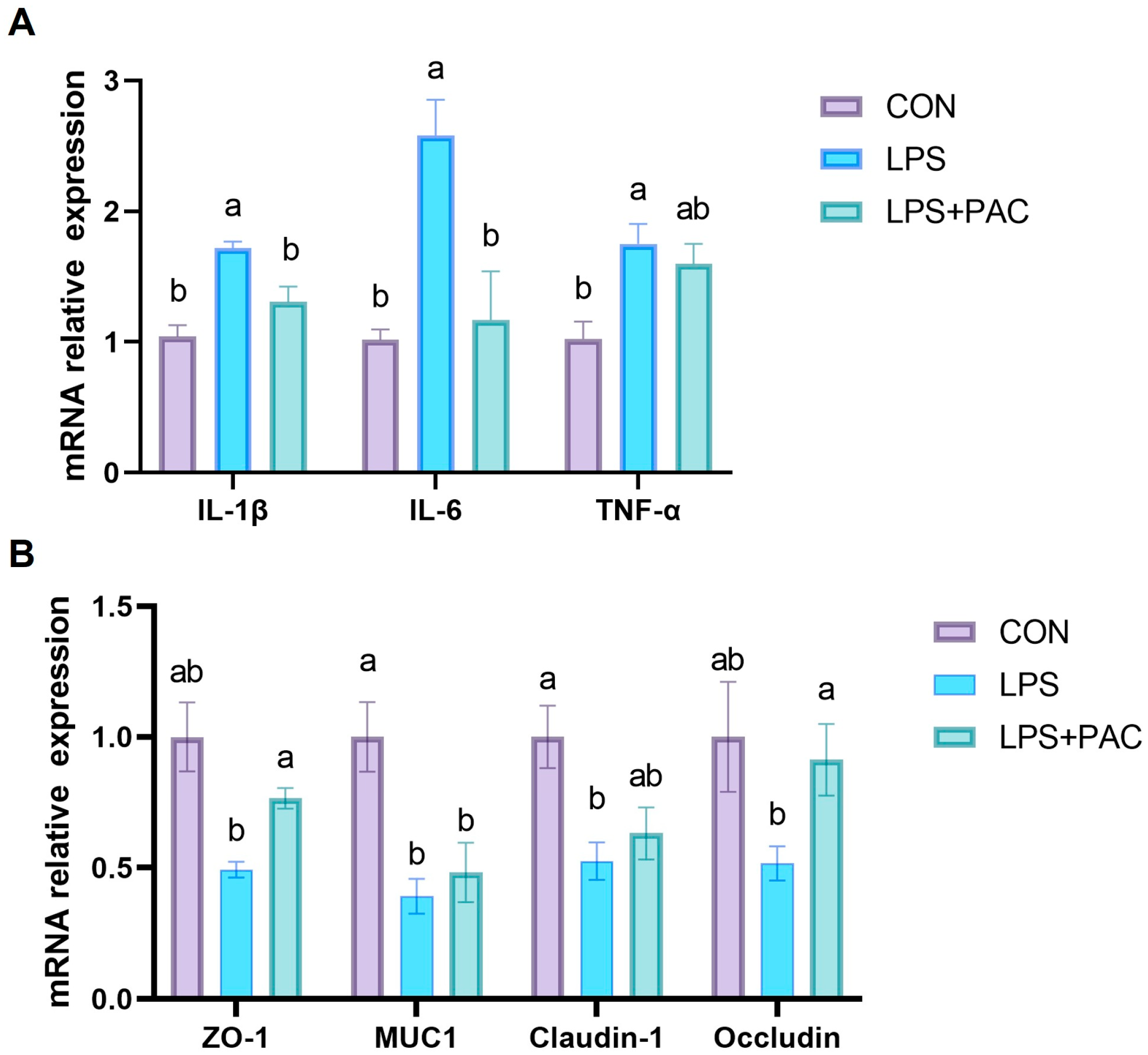
3.5. Gene Expression of Ileum Inflammatory Factors and Tight Junction Proteins
3.6. Hematological and Biochemical Indices
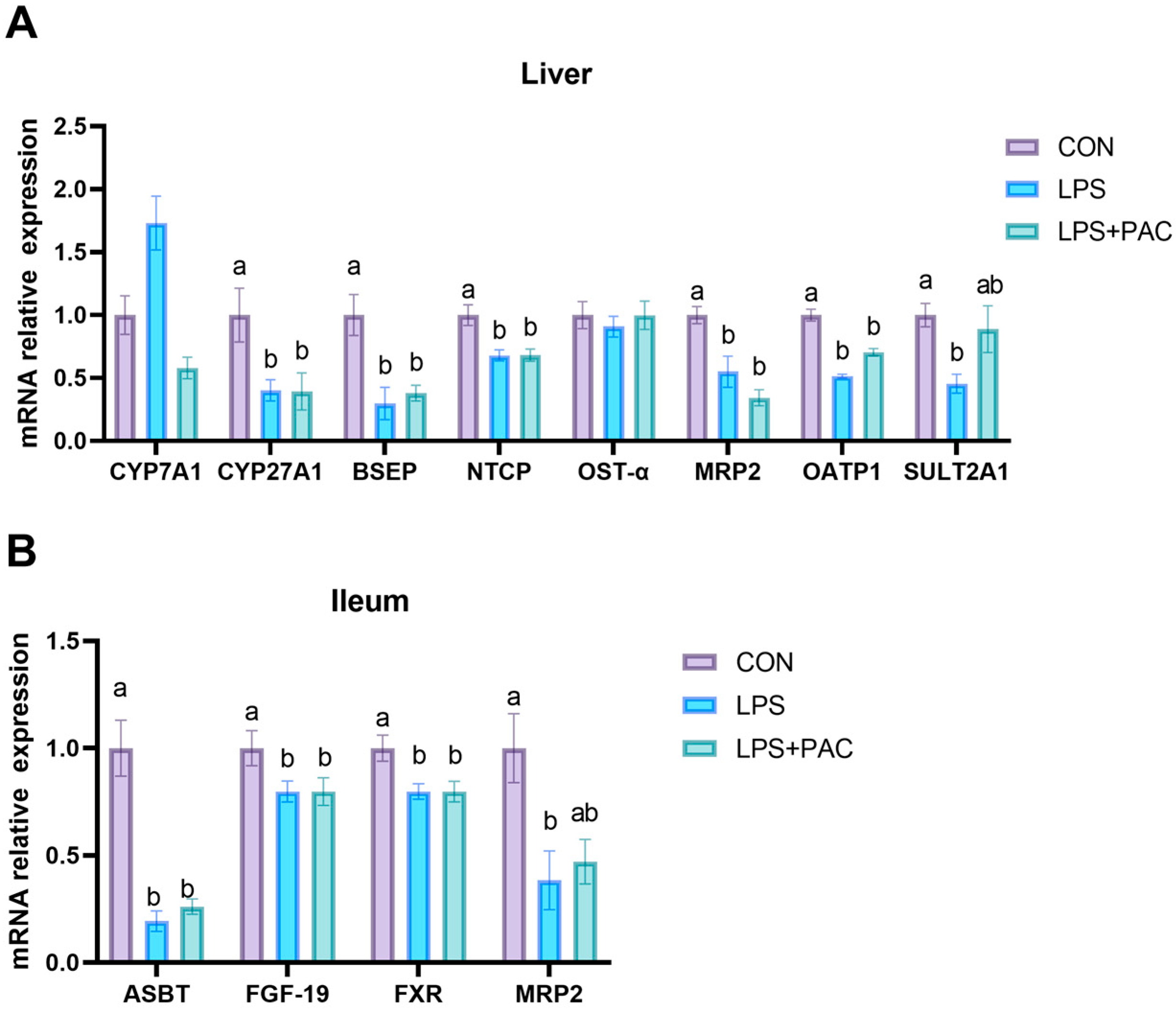
3.7. Gene Expression of Liver and Ileum of Bile Acid Synthesis and Transport-Related Proteins
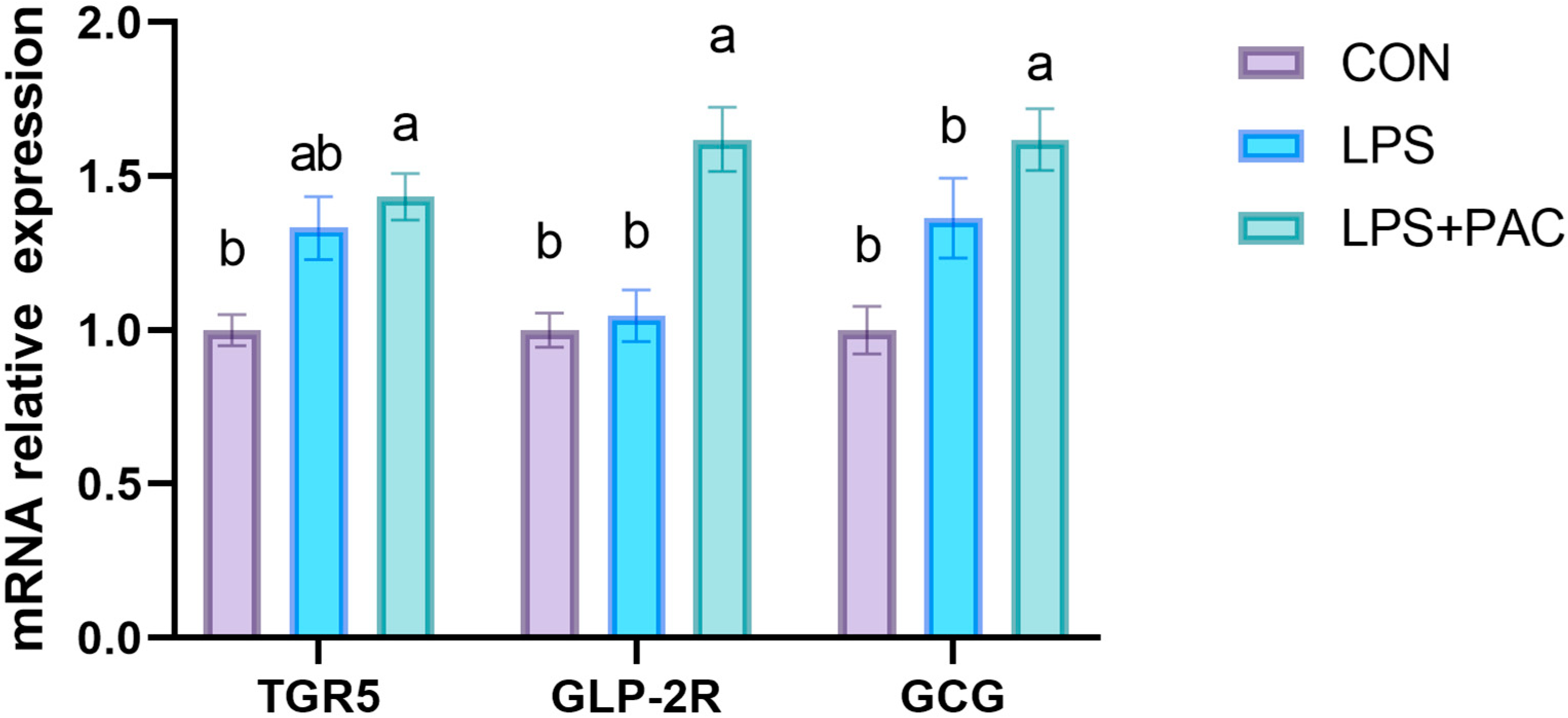
3.8. Gene Expression of GLP-2 Related Genes in Ileum of Piglets
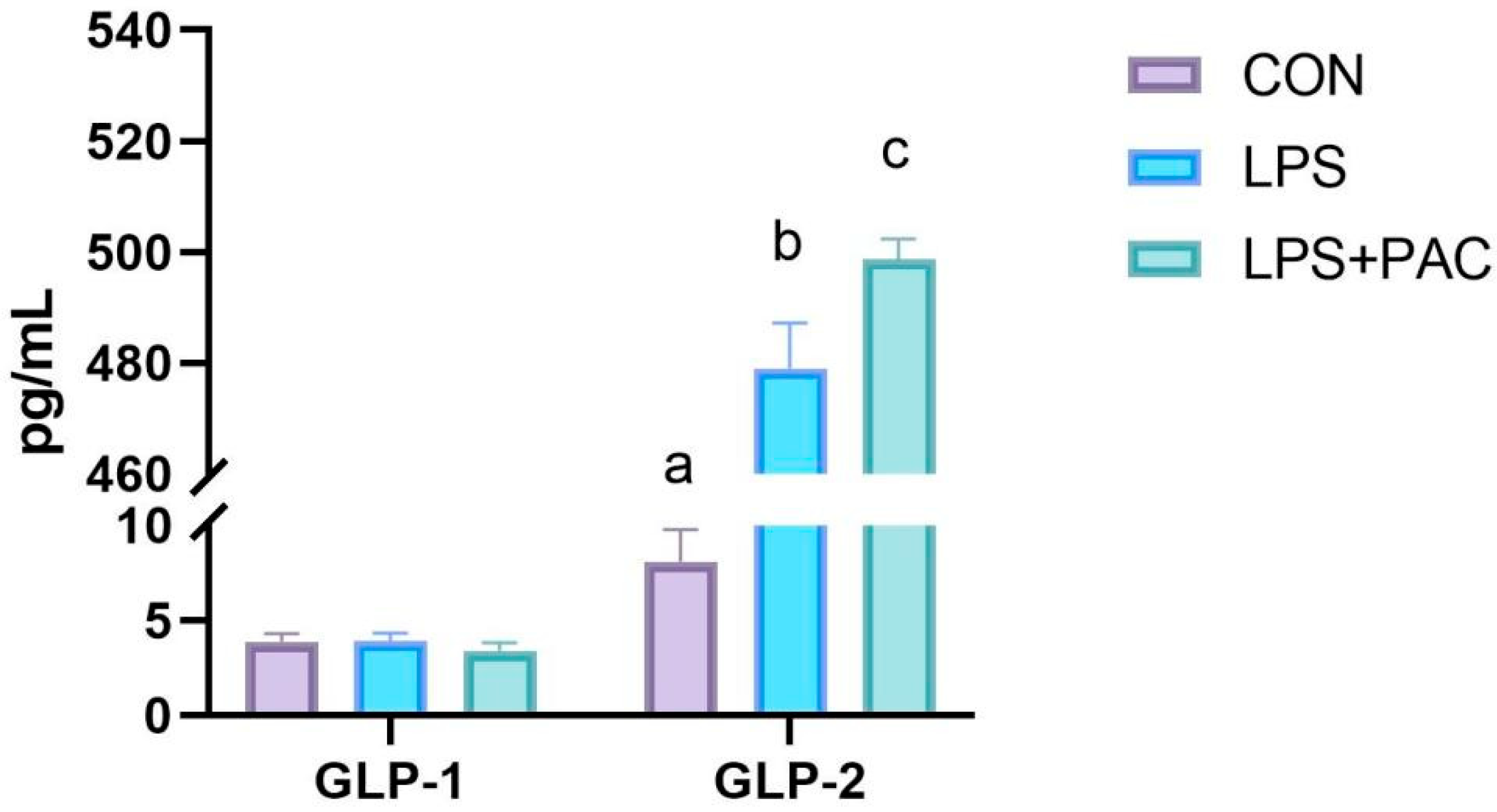
3.9. The Content of Serum GLP-1 and GLP-2 of Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning Stress and Intestinal Health of Piglets: A Review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Wijtten, P.J.A.; Meulen, J.v.d.; Verstegen, M.W.A. Intestinal Barrier Function and Absorption in Pigs after Weaning: A Review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef]
- Wensley, M.R.; Potter, M.L.; Tokach, M.D.; Woodworth, J.C.; Goodband, R.D.; DeRouchey, J.M.; Gebhardt, J.T.; Menegat, M.B.; Allerson, M.W. Effects of Mat Feeding on the Growth Performance, Removal, and Mortality of Pigs after Weaning. J. Anim. Sci. 2022, 100, skac344. [Google Scholar] [CrossRef]
- Lin, S.; Yang, X.; Yuan, P.; Yang, J.; Wang, P.; Zhong, H.; Zhang, X.; Che, L.; Feng, B.; Li, J.; et al. Undernutrition Shapes the Gut Microbiota and Bile Acid Profile in Association with Altered Gut-Liver Fxr Signaling in Weaning Pigs. J. Agric. Food Chem. 2019, 67, 3691–3701. [Google Scholar] [CrossRef]
- Hu, C.H.; Xiao, K.; Luan, Z.S.; Song, J. Early Weaning Increases Intestinal Permeability, Alters Expression of Cytokine and Tight Junction Proteins, and Activates Mitogen-Activated Protein Kinases in Pigs. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, Y.; Shen, S.; Tao, W.; Zhang, Y.; Ye, X.; Chen, S.; Pan, H. Analysis of Proanthocyanidins in Plant Materials Using Hydrophilic Interaction Hplc-Qtof-Ms. Molecules 2022, 27, 2684. [Google Scholar] [CrossRef]
- Han, X.; Zhou, Q.; Gao, Z.; Xu, G.B.; Chen, H.; Chitrakar, B.; Sun, Y.; Zhao, W.; Lin, X.; Zhou, K.; et al. Characterization of Procyanidin Extracts from Hawthorn (Crataegus pinnatifida) in Human Colorectal Adenocarcinoma Cell Line Caco-2, Simulated Digestion, and Fermentation Identified Unique and Novel Prebiotic Properties. Food Res. Int. 2023, 165, 112393. [Google Scholar] [CrossRef]
- Fang, L.; Li, M.; Zhao, L.; Han, S.; Li, Y.; Xiong, B.; Jiang, L. Dietary Grape Seed Procyanidins Suppressed Weaning Stress by Improving Antioxidant Enzyme Activity and Mrna Expression in Weanling Piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1178–1185. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, Y.; Yu, B.; Luo, Y.; Huang, Z.; Mao, X.; Yu, J.; Zheng, P.; Yan, H.; Li, Y.; et al. Functional Characterization and Toxicological Study of Proanthocyanidins in Weaned Pigs. Toxins 2023, 15, 558. [Google Scholar] [CrossRef]
- Merlen, G.; Kahale, N.; Ursic-Bedoya, J.; Bidault-Jourdainne, V.; Simerabet, H.; Doignon, I.; Tanfin, Z.; Garcin, I.; Péan, N.; Gautherot, J.; et al. Tgr5-Dependent Hepatoprotection through the Regulation of Biliary Epithelium Barrier Function. Gut 2020, 69, 146–157. [Google Scholar] [CrossRef]
- Lin, S.; Stoll, B.; Robinson, J.; Pastor, J.J.; Marini, J.C.; Ipharraguerre, I.R.; Hartmann, B.; Holst, J.J.; Cruz, S.; Lau, P.; et al. Differential Action of Tgr5 Agonists on Glp-2 Secretion and Promotion of Intestinal Adaptation in a Piglet Short Bowel Model. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 316, G641–G652. [Google Scholar] [CrossRef]
- Ginés, I.; Gil-Cardoso, K.; Terra, X.; Blay, M.; Pérez-Vendrell, A.M.; Pinent, M.; Ardévol, A. Grape Seed Proanthocyanidins Target the Enteroendocrine System in Cafeteria-Diet-Fed Rats. Mol. Nutr. Food Res. 2019, 63, e1800912. [Google Scholar] [CrossRef]
- Fiorucci, S.; Mencarelli, A.; Palladino, G.; Cipriani, S. Bile-Acid-Activated Receptors: Targeting Tgr5 and Farnesoid-X-Receptor in Lipid and Glucose Disorders. Trends Pharmacol. Sci. 2009, 30, 570–580. [Google Scholar] [CrossRef]
- Katsuma, S.; Hirasawa, A.; Tsujimoto, G. Bile Acids Promote Glucagon-Like Peptide-1 Secretion through Tgr5 in a Murine Enteroendocrine Cell Line Stc-1. Biochem. Biophys. Res. Commun. 2005, 329, 386–390. [Google Scholar] [CrossRef]
- Drucker, D.J.; Li, K.K.; Hadjiyanni, I. Glucagon-Like Peptide-2 Reduces Intestinal Permeability but Does Not Modify the Onset of Type 1 Diabetes in the Nonobese Diabetic Mouse. Endocrinology 2009, 150, 592–599. [Google Scholar] [CrossRef]
- Moore, B.A.; Peffer, N.; Pirone, A.; Bassiri, A.; Sague, S.; Palmer, J.M.; Johnson, D.L.; Nesspor, T.; Kliwinski, C.; Hornby, P.J. Glp-2 Receptor Agonism Ameliorates Inflammation and Gastrointestinal Stasis in Murine Postoperative Ileus. J. Pharmacol. Exp. Ther. 2010, 333, 574–583. [Google Scholar] [CrossRef]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan Oligosaccharide as Potential Therapy of Inflammatory Bowel Disease: Therapeutic Efficacy and Possible Mechanisms of Action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef]
- Han, M.; Song, P.; Huang, C.; Rezaei, A.; Farrar, S.; Brown, M.A.; Ma, X. Dietary Grape Seed Proanthocyanidins (Gsps) Improve Weaned Intestinal Microbiota and Mucosal Barrier Using a Piglet Model. Oncotarget 2016, 7, 80313–80326. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Tan, H.; Luo, J.; et al. Grape Seed Proanthocyanidins Improves Growth Performance, Antioxidative Capacity, and Intestinal Microbiota in Growing Pigs. Front. Microbiol. 2024, 15, 1501211. [Google Scholar] [CrossRef]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal Absorption and Antioxidant Activity of Grape Pomace Polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Gao, Y.Q.; Lippton, H.; Hyman, A.; Spitzer, J.J. The Roles of Nitric Oxide and Hydrogen Peroxide Production in Lipopolysaccharide-Induced Intestinal Damage. Shock 1994, 2, 185–191. [Google Scholar] [CrossRef]
- Wu, Q.J.; Wang, Y.Q.; Qi, Y.X. The Protective Effect of Procyanidin against Lps-Induced Acute Gut Injury by the Regulations of Oxidative State. SpringerPlus 2016, 5, 1645. [Google Scholar] [CrossRef]
- Cai, C.-H.; Li, W.; Chen, J.; Li, X.; Chen, S.-l. Diamine Oxidase as a Marker for Diagnosis of Superior Mesenteric Arterial Occlusion. Hepato-Gastroenterol. 2012, 59, 155–158. [Google Scholar]
- Birchenough, G.M.; Johansson, M.E.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New Developments in Goblet Cell Mucus Secretion and Function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef]
- Xing, S.; Zhang, B.; Lin, M.; Zhou, P.; Li, J.; Zhang, L.; Gao, F.; Zhou, G. Effects of Alanyl-Glutamine Supplementation on the Small Intestinal Mucosa Barrier in Weaned Piglets. Asian-Australas. J. Anim. Sci. 2017, 30, 236–245. [Google Scholar] [CrossRef]
- Umeda, K.; Ikenouchi, J.; Katahira-Tayama, S.; Furuse, K.; Sasaki, H.; Nakayama, M.; Matsui, T.; Tsukita, S.; Furuse, M.; Tsukita, S. Zo-1 and Zo-2 Independently Determine Where Claudins Are Polymerized in Tight-Junction Strand Formation. Cell 2006, 126, 741–754. [Google Scholar] [CrossRef]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The Tight Junction Protein Zo-1 Establishes a Link between the Transmembrane Protein Occludin and the Actin Cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef]
- Aroche, R.; Gao, G.; Li, Y.; Zhang, Y.; Rodríguez, R.; Martínez, Y.; Li, X. Effect of Anacardium Occidentale Leaf Powder on Growth Performance, Diarrhea Incidence, Blood Biochemistry, and Intestinal Traits in Weaned Piglets. Animals 2024, 14, 3382. [Google Scholar] [CrossRef]
- Lou, Y.; Jiang, S.; Song, M.; Wang, H.; Han, M.; Tian, X.; Zhao, Y.; Gao, J.; Song, Y.; Ma, S.; et al. Epithelial Tipe1 Protein Guards against Colitis by Inhibiting Tnf-A-Mediated Inflammation. J. Immunol. 2023, 211, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, S.; Pathak, J.L. Pro-Inflammatory Cytokines and Osteocytes. Curr. Osteoporos. Rep. 2019, 17, 97–104. [Google Scholar] [CrossRef]
- Andersen-Civil, A.I.S.; Arora, P.; Williams, A.R. Regulation of Enteric Infection and Immunity by Dietary Proanthocyanidins. Front. Immunol. 2021, 12, 637603. [Google Scholar] [CrossRef]
- Hao, R.; Li, Q.; Zhao, J.; Li, H.; Wang, W.; Gao, J. Effects of Grape Seed Procyanidins on Growth Performance, Immune Function and Antioxidant Capacity in Weaned Piglets. Livest. Sci. 2015, 178, 237–242. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Y.; Bai, J.; Li, X.; Ma, L.; Man, S. Litchi Procyanidins Ameliorate Dss-Induced Colitis through Gut Microbiota-Dependent Regulation of Treg/Th17 Balance. J. Agric. Food Chem. 2024, 72, 24823–24832. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, W.; Liu, X.; Bai, J.; Jia, Q.; Shi, Q. Procyanidin A1 Alleviates Dss-Induced Ulcerative Colitis Via Regulating Ampk/Mtor/P70s6k-Mediated Autophagy. J. Physiol. Biochem. 2022, 78, 213–227. [Google Scholar] [CrossRef]
- Zhang, C.; Gan, Y.; Lv, J.-W.; Qin, M.-Q.; Hu, W.-R.; Liu, Z.-B.; Ma, L.; Song, B.-D.; Li, J.; Jiang, W.-Y.; et al. The Protective Effect of Obeticholic Acid on Lipopolysaccharide-Induced Disorder of Maternal Bile Acid Metabolism in Pregnant Mice. Int. Immunopharmacol. 2020, 83, 106442. [Google Scholar] [CrossRef]
- Downing, L.E.; Heidker, R.M.; Caiozzi, G.C.; Wong, B.S.; Rodriguez, K.; Del Rey, F.; Ricketts, M.L. A Grape Seed Procyanidin Extract Ameliorates Fructose-Induced Hypertriglyceridemia in Rats Via Enhanced Fecal Bile Acid and Cholesterol Excretion and Inhibition of Hepatic Lipogenesis. PLoS ONE 2015, 10, e0140267. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, T.; Zhao, A.; Ning, Z.; Kuang, J.; Wang, S.; You, Y.; Bao, Y.; Ma, X.; Yu, H.; et al. Hyocholic Acid Species as Novel Biomarkers for Metabolic Disorders. Nat. Commun. 2021, 12, 1487. [Google Scholar] [CrossRef]
- Jia, W.; Li, Y.; Cheung, K.C.P.; Zheng, X. Bile Acid Signaling in the Regulation of Whole Body Metabolic and Immunological Homeostasis. Sci. China-Life Sci. 2024, 67, 865–878. [Google Scholar] [CrossRef]
- Puddu, A.; Mach, F.; Nencioni, A.; Viviani, G.L.; Montecucco, F. An Emerging Role of Glucagon-Like Peptide-1 in Preventing Advanced-Glycation-End-Product-Mediated Damages in Diabetes. Mediat. Inflamm. 2013, 2013, 591056. [Google Scholar] [CrossRef]
- Brubaker, P.L. Glucagon-Like Peptide-2 and the Regulation Of intestinal Growth and Function. Compr. Physiol. 2018, 8, 1185–1210. [Google Scholar] [CrossRef]
- Baccari, M.C.; Vannucchi, M.G.; Idrizaj, E. Glucagon-Like Peptide-2 in the Control of Gastrointestinal Motility: Physiological Implications. Curr. Protein Pept. Sci. 2022, 23, 61–69. [Google Scholar] [CrossRef]
| Ingredients | Content % | Nutrition Level 3 | Content % |
|---|---|---|---|
| Corn | 28.85 | Digestible energy, MJ/kg | 14.83 |
| Expanded corn | 10.00 | Metabolizable energy, MJ/kg | 14.23 |
| Extruded soybean | 14.00 | Net energy, MJ/kg | 10.25 |
| Fermented soybean meal | 11.50 | Crude protein % | 21.11 |
| Soybean powder | 7.00 | Calcium Ca % | 0.90 |
| Fishmeal | 3.00 | Total phosphorus % | 0.63 |
| Whey powder | 15.00 | SID lysine % | 1.40 |
| Whey protein concentrate | 1.00 | SID methionine % | 0.40 |
| Soyabean oil | 1.00 | SID threonine % | 0.82 |
| Sucrose | 3.00 | SID tryptophan % | 0.23 |
| Calcium citrate | 1.85 | SID isoleucine % | 0.72 |
| Dicalcium phosphate | 0.60 | SID methionine + cystine % | 0.75 |
| Sodium chloride | 0.25 | ||
| L-lysine | 0.55 | ||
| DL-methionine | 0.15 | ||
| L-threonine | 0.20 | ||
| L-tryptophan | 0.05 | ||
| L- valine | 0.10 | ||
| Choline chloride | 0.20 | ||
| Vitamin premix 1 | 0.40 | ||
| Mineral premix 2 | 1.30 |
| Gene | Primer Sequence (5′-3′) | Product Size (bp) | |
|---|---|---|---|
| β-actin | FW | AGAGCAAGAGAGGCATCCTG | 111 |
| RW | CACGCAGCTCGTTGTAGAAG | ||
| IL-6 | FW | TGTC GAGGCTGTGCAGATTA | 102 |
| RW | GCATTTGTGGTGGGGTTAGG | ||
| IL-1β | FW | AGACCCCAAAAGATACCCAAAGAGG | 129 |
| RW | TCTGCTTGAGAGGTGCTGATGTAC | ||
| TNF-α | FW | GGCCCAAGGACTCAGATCAT | 105 |
| RW | GCATACCC ACTCTGCCATTG | ||
| ZO-1 | FW | TCCTGAGTTTGATAGTGGCGTTGAC | 148 |
| RW | CACGGTGTGACCATCCTCATCTTC | ||
| Occludin | FW | TTACTCCTACGCTGGTGACAACATTG | 93 |
| RW | TGGATCTGCCCGGTGCTCTG | ||
| Muc1 | FW | GTGCCGCTGCCCACAACCTG | 141 |
| RW | AGCCGGGTACCCCAGACCCA | ||
| Claudin-1 | FW | AGAAGATGCGGATGGCTGTC | 193 |
| RW | CCCAGAAGGCAGAGAGAAGC | ||
| BSEP | FW | GCCTGACCACGAGCATCT | 60 |
| RW | AGGTCAGTTTCCAACCCTGAT | ||
| ABST | FW | ACTTTCGGAAACCTAAGGGACT | 88 |
| RW | AAGAGCTTGCCCAGTGCAAAG | ||
| NTCP | FW | GTGGTACCCGAGAGCAACTT | 132 |
| RW | CTTTACGTGCCCCAGGAACT | ||
| CYP7A1 | FW | GAAAGAGAGACCACATCTCGG | 123 |
| RW | GAATGGTGTTGGCTTGCGAT | ||
| CYP27A1 | FW | GAACTCACTCTACGCCACCTTCC | 85 |
| RW | GGTATTCCAGCCATCCAGGTATCG | ||
| OSTα | FW | GGGTGAACTCTGAGATAGGAAC | 73 |
| RW | CTGGGTCTTTCCTTCTGGCT | ||
| MRP2 | FW | TGCAAGTACGGACCAGTGTC | 83 |
| RW | AACGGTGTACTGCTTCCTGG | ||
| OATP1A2 | FW | TGGCTGTGGTGGTCGTGATAAG | 82 |
| RW | TCCGATGGCAGCGTCTTTGG | ||
| SULT2A1 | FW | CCATGCGAGACAAGGAGAAC | 155 |
| RW | CATGACCTGGAAGGAGCTGT | ||
| FXR | FW | TTTGTGTCGTTTGCGGAGAG | 128 |
| RW | GTTGCCCCCATTTTTACACTTG | ||
| FGF-19 | FW | AAGATGCAAGGGCAGACTCA | 101 |
| RW | AGATGGTGTTTCTTGGACCAGT | ||
| TGR5 | FW | CCATGCACCCCTGTTGCT | 66 |
| RW | GGTGCTGTTGGGTGTCATCTT | ||
| GCG | FW | CTCGATAATCTTGCCACCCGA | 115 |
| RW | CTGGCAGGTGATGTTGTGAAC | ||
| GLP-2R | FW | CCCTGCTGTTTCTGGTTTCC | 195 |
| RW | GGCAGGGAACAGAAACGTTT | ||
| Items | Treatments | p-Value | ||
|---|---|---|---|---|
| CON | LPS | LPS + PAC | ||
| BW (kg) | ||||
| 1 d | 7.09 ± 0.15 | 7.12 ± 0.14 | 7.14 ± 0.15 | 0.941 |
| 14 d | 11.93 ± 0.29 | 11.47 ± 0.27 | 11.67 ± 0.46 | 0.815 |
| 21 d | 16.52 ± 0.30 | 14.28 ± 0.27 | 15.33 ± 0.46 | 0.247 |
| 1–14 d | ||||
| ADG (kg/d) | 0.35 ± 0.01 | 0.31 ± 0.01 | 0.35 ± 0.01 | 0.624 |
| ADFI (kg/d) | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.52 ± 0.02 | 0.954 |
| FCR | 1.49 ± 0.01 | 1.65 ± 0.03 | 1.53 ± 0.03 | 0.165 |
| Diarrhea index | 0.48 ± 0.03 | 0.52 ± 0.04 | 0.27 ± 0.03 | 0.761 |
| 15–21d | ||||
| ADG (kg/d) | 0.66 ± 0.02 a | 0.40 ± 0.02 c | 0.46 ± 0.02 b | 0.005 |
| ADFI (kg/d) | 0.97 ± 0.02 a | 0.73 ± 0.02 b | 0.81 ± 0.03 ab | 0.030 |
| FCR | 1.48 ± 0.01 b | 1.86 ± 0.03 a | 1.82 ± 0.04 a | 0.004 |
| Diarrhea index | 0.14 ± 0.03 b | 0.64 ± 0.05 a | 0.23 ± 0.05 ab | 0.015 |
| Items | Treatments | p-Value | ||
|---|---|---|---|---|
| CON | LPS | LPS + PAC | ||
| Hematological indices | ||||
| WBC (109/L) | 13.70 ± 0.38 | 14.42 ± 0.63 | 16.69 ± 0.82 | 0.43 |
| RBC (1012/L) | 6.48 ± 0.05 a | 6.03 ± 0.04 b | 6.03 ± 0.03 b | 0.02 |
| Hemoglobin (g/L) | 109.50 ± 0.52 a | 98.00 ± 0.66 b | 105.40 ± 0.95 b | <0.01 |
| Hematocrit (%) | 38.25 ± 0.22 a | 34.30 ± 0.25 b | 37.28 ± 0.27 a | <0.01 |
| MCV (fL) | 59.08 ± 0.41 ab | 56.93 ± 0.26 b | 61.86 ± 0.55 a | 0.02 |
| MCH (pg) | 16.95 ± 0.15 ab | 16.28 ± 0.09 b | 17.46 ± 0.15 a | 0.09 |
| MCHC (g/L) | 286.50 ± 0.77 | 285.67 ± 0.68 | 282.80 ± 0.94 | 0.46 |
| Platelets (109/L) | 693.00 ± 23.16 | 721.67 ± 9.98 | 834.60 ± 17.81 | 0.13 |
| RDW-SD (fL) | 37.93 ± 0.22 b | 39.10 ± 0.34 b | 45.00 ± 0.33 a | <0.01 |
| RDW-CV (%) | 19.20 ± 0.12 | 20.05 ± 0.17 | 20.80 ± 0.29 | 0.13 |
| PDW (fL) | 10.38 ± 0.06 | 11.75 ± 0.20 | 10.96 ± 0.17 | 0.16 |
| MPV (fL) | 9.58 ± 0.07 | 9.87 ± 0.08 | 9.56 ± 0.05 | 0.46 |
| p-LCR (%) | 22.25 ± 0.70 | 24.63 ± 0.81 | 21.76 ± 0.55 | 0.53 |
| Plateletcrit (%) | 0.69 ± 0.02 | 0.74 ± 0.01 | 0.80 ± 0.02 | 0.29 |
| NRBC (109/L) | 0.15 ± 0.02 | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.17 |
| Neutrophils (109/L) | 3.77 ± 0.21 | 4.89 ± 0.43 | 4.64 ± 0.42 | 0.13 |
| Lymphocytes (109/L) | 8.71 ± 0.25 | 8.50 ± 0.19 | 10.68 ± 0.57 | 0.21 |
| Monocytes (109/L) | 0.92 ± 0.01 | 0.83 ± 0.03 | 1.17 ± 0.14 | 0.45 |
| Eosinophils (109/L) | 0.20 ± 0.01 a | 0.14 ± 0.01 b | 0.12 ± 0.01 b | 0.05 |
| Basophils (109/L) | 0.10 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.35 |
| Neutrophils (%) | 27.22 ± 0.89 | 31.60 ± 1.50 | 27.66 ± 1.86 | 0.66 |
| Lymphocytes (%) | 63.62 ± 0.89 | 60.90 ± 1.34 | 64.64 ± 1.72 | 0.73 |
| Monocytes (%) | 6.97 ± 0.26 | 6.00 ± 0.22 | 6.54 ± 0.46 | 0.69 |
| Eosinophils (%) | 1.50 ± 0.08 a | 1.00 ± 0.07 ab | 0.74 ± 0.01 b | 0.03 |
| Basophils (%) | 0.70 ± 0.05 | 0.50 ± 0.07 | 0.42 ± 0.03 | 0.20 |
| Biochemical indices | ||||
| AST (IU/L) | 41.20 ± 4.43 a | 27.00 ± 2.42 b | 41.75 ± 3.90 a | 0.02 |
| ALT (IU/L) | 45.00 ± 3.65 | 41.50 ± 3.17 | 44.80 ± 4.65 | 0.76 |
| ALP (IU/L) | 425.67 ± 9.57 | 403.60 ± 5.72 | 399.60 ± 7.57 | 0.43 |
| GGT (IU/L) | 29.17 ± 1.34 | 31.00 ± 1.14 | 35.80 ± 1.60 | 0.37 |
| TBIL (μmol/L) | 1.02 ± 0.03 a | 1.10 ± 0.12 a | 0.72 ± 0.05 b | 0.02 |
| DBIL (μmol/L) | 0.37 ± 0.03 | 0.33 ± 0.05 | 0.24 ± 0.02 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, A.; Wang, Z.; Wang, S.; Zhao, W.; Chen, L.; Wang, D.; Li, Z.; Wang, Y.; Fang, Z.; Lin, S. Effects of Proanthocyanidins on Growth Performance, Intestinal Inflammation and Barrier Function, and Bile Acid Metabolism-Related Genes in Weaned Piglets Challenged with Lipopolysaccharide. Animals 2025, 15, 1826. https://doi.org/10.3390/ani15131826
Yu A, Wang Z, Wang S, Zhao W, Chen L, Wang D, Li Z, Wang Y, Fang Z, Lin S. Effects of Proanthocyanidins on Growth Performance, Intestinal Inflammation and Barrier Function, and Bile Acid Metabolism-Related Genes in Weaned Piglets Challenged with Lipopolysaccharide. Animals. 2025; 15(13):1826. https://doi.org/10.3390/ani15131826
Chicago/Turabian StyleYu, Aiying, Zhenjiang Wang, Sutian Wang, Weiguo Zhao, Lian Chen, Dan Wang, Zhiyi Li, Yuan Wang, Zhengfeng Fang, and Sen Lin. 2025. "Effects of Proanthocyanidins on Growth Performance, Intestinal Inflammation and Barrier Function, and Bile Acid Metabolism-Related Genes in Weaned Piglets Challenged with Lipopolysaccharide" Animals 15, no. 13: 1826. https://doi.org/10.3390/ani15131826
APA StyleYu, A., Wang, Z., Wang, S., Zhao, W., Chen, L., Wang, D., Li, Z., Wang, Y., Fang, Z., & Lin, S. (2025). Effects of Proanthocyanidins on Growth Performance, Intestinal Inflammation and Barrier Function, and Bile Acid Metabolism-Related Genes in Weaned Piglets Challenged with Lipopolysaccharide. Animals, 15(13), 1826. https://doi.org/10.3390/ani15131826







