Simple Summary
Fish swimming is crucial to their survival and reproduction, and is usually influenced by species, morphology, habitat environment and physiological condition. Understanding the changes in fish swimming ability and identifying the driving factors behind these changes are of pressing importance. Our findings showed that body length parameters had a minimal effect on the induced swimming speed, but played a positive role in critical and burst swimming ability. Weight parameters were more highly correlated with swimming ability than body length parameters. Fish preferring the lotic environment exhibited higher Ucrit and Uburst, and fish with a streamlined morphology had smaller Uind. The burst swimming speed of fish during the spawning period was slower than that during the non-spawning period. Generally, the swimming ability was mainly influenced by the species identity, followed by environmental conditions and morphological factors. The findings of this study are of great significance to the conservation of rare and endemic fish in mountainous rivers, and could provide a theoretical basis and data support for the design of river restoration measures.
Abstract
Swimming is crucial to fish survival and reproduction, and is usually influenced by species, morphology, habitat environment and physiological condition. Understanding the changes in fish swimming ability and identifying the driving factors behind these changes is vital. In this study, seven endemic fish species from the Qingshui River, an important tributary of the upper Pearl River in Southwest China, were used to explore the effects of biological and environmental factors on fish swimming ability. The results indicated that the body length parameters had a minimal effect on the induced swimming speed (Uind) but played a positive role in critical and burst swimming ability (Ucrit and Uburst). Weight (W) and W/SL (the ratio of weight to standard body length) correlated more highly with swimming ability than body length parameters. Fish preferring the lotic environment exhibited higher Ucrit and Uburst, and fish with a streamlined morphology had a smaller Uind. The Uind, Uind/SL, Uind/W and Uind/(W/SL) of Discogobio yunnanensis (Regan, 1907) and Pseudocrossocheilus tridentis (Cui & Chu, 1986) were significantly higher during the spawning period. Acrossocheilus yunnanensis (Regan, 1904) performed better during the spawning period in Ucrit and Ucrit/SL. The Uburst of fish during the spawning period was smaller than that during the non-spawning period. Generally, the species difference had the greatest contribution to the swimming ability difference, followed by environmental conditions and fish morphology.
1. Introduction
Fish swimming is essential for their survival and reproduction, and is related to the achievement of behaviors such as schooling, migration, reproduction, foraging and predator avoidance [1,2]. The strength of fish’s swimming ability directly influences the sustainability of fish populations and is of great ecological importance [3]. Fish’s anaerobic exercise relies on the size of their white muscle mass and aerobic exercise is supported by red muscle tissue [4,5,6], leading to the introduction of three important metrics: induced swimming speed (Uind), critical swimming speed (Ucrit) and burst swimming speed (Uburst) [7]. These swimming metrics help to distinguish fish swimming speeds intuitively, and can be used to evaluate the fish migratory movements.
Differences in habitat environment, morphology and life periods have led to the evolution of fish with different swimming performances [8]. Over a long evolutionary history, different fish species have developed corresponding swimming abilities adapted to their living environment. For example, fish living in the lotic environment showed a preference for high flow velocities, while those in the lentic environment were weaker swimmers [9]. Different morphologies shape the different swimming capabilities of fish. The spindle-shaped body makes Cottus bairdii (Girard, 1850) and Rhinichthys cataractae (Valenciennes, 1842) good swimmers for long-distance migration [10,11], while lateral flattened fish were found to be more susceptible to the disturbance caused by water currents than spindle-shape ones [12]. Reproduction is a critical period in the life cycle of fish when the energy requirement of fish is at its highest [13]. During reproduction, potamodromous freshwater fish allocate energy to gamete production, potentially reducing energy for locomotion, as predicted by the trade-offs from biological evolution theory [14]. Swimming performance varies by species and life periods [15,16]. Due to spawning, fish usually optimize their allocation of energy by subduing the locomotor ability to compensate for reproduction. However, the differences in fish swimming ability between spawning and non-spawning periods has received less attention.
Currently, the construction and operation of massive hydropower projects all over the world have blocked fish migration in rivers [17,18,19,20], further affecting the survival of fish. These hydropower projects have also significantly altered the hydrodynamic characteristics of the rivers, leading to changes in the flow field suitable for fish spawning [21]. The disappearance of some shoals, deep pools and rapids simplifies the complex river system, and what is worse, the environment for fish reproduction may also be destroyed [22]. In recent years, fish passage facilities have constantly emerged to restore the deteriorated connectivity of rivers [23]. The successful design of fish passage facilities not only depended on the hydrodynamic characteristics, but was also closely related to the biological characteristics of the target fish species which were in need of migration. Fish swimming performance is the key metric to guarantee passing efficiency. For example, 52% of Hybognathus amarus passed over the dual vertical-slot fishway in Grand River with a less turbulent flow of 78 cm/s, while the passage rate decreased to 8% at a faster and more turbulent flow of 87 cm/s, which exceeded the Ucrit of Hybognathus amarus [24].
There are abundant fish resources and a wide range of endemic fish species in Southwest China [25,26]. However, most of the current studies on fish swimming ability are species-specific, and have difficulty in helping to comprehensively understand the key factors driving fish swimming ability. The hydropower resources in China are mainly concentrated in the Southwest region [27]. Under the impetus of the western development and West-to-East Power Transmission policies enacted recently, the construction of hydropower projects in major basins of the Southwest has been accelerated to an unprecedented degree [27,28], causing the ecological destruction of the river ecosystem to be increasingly prominent. Relevant environmental impact assessments (EIAs) for hydropower projects require the deployment of fish passages, fish ramps, or fish lifts to protect fish genetic integrity [29,30]. Fish swimming speeds are vital metrics to evaluate the operational efficiency of fish passage facilities and exhibit distinct preferences to water depth, water velocity and substrates [31]. Fish with a streamlined morphology usually inhabit high-velocity environments over gravel substrates, while fish with a dorsoventrally flattened morphology always occupy deeper positions with lower velocities and silt–sand substrates [32,33]. However, relevant studies on fish swimming performance were insufficient to support the increasingly strict requirements of river connectivity restoration. It is particularly urgent at this stage to reveal the patterns in the variation of fish swimming ability and explore the driving factors behind these changes. This study hypothesizes that the biological characteristics of fish and environmental conditions mainly impact fish swimming performance. Seven endemic fish species from the Qingshui River, an important tributary of the upper Pearl River in Southwest China, were taken as control species to explore the effects of biological characteristics and environmental factors on fish swimming ability. The findings of this study are vital to the conservation of rare and endemic fish in mountainous rivers, and could provide a theoretical basis and data support for the design of river restoration measures.
2. Materials and Methods
2.1. Study Area and Experimental Fish
The Qingshui River is an important tributary of the upper Pearl River in Southwest China (Wenshan City, Yunnan Province), with a total length of 134 km. A large variation in the water level leads to complex habitats, cultivating plenty of endemic fish species. Among the endemic fish in the Qingshui River basin, Acrossocheilus yunnanensis (Regan, 1904), Abbottina rivularis (Basilewsky, 1855) and Onychostoma elongatum (Pellegrin & Chevey, 1934) have a short-distance migration requirement during their spawning period. The Qingshui River basin is endowed with abundant water energy storage and large water-level differences. Several hydropower projects have been developed in the Qingshui River basin, adversely affecting the short-distance migration of fish and blocking the exchange of fish genes upstream and downstream of dams.
The spawning period of fish in the Qingshui River Basin lasts from March to July, and fish collections were conducted in April (the spawning period) and October (the non-spawning period) in this study. Seven endemic fish species (Pseudocrossocheilus tridentis (Cui & Chu, 1986), Acrossocheilus yunnanensis, Discogobio yunnanensis (Regan, 1907), Abbottina rivularis, Sinocyclocheilus grahami (Regan, 1904), Hemibarbus maculatus (Bleeker, 1871), Onychostoma elongatum) were obtained in October 2020 and April 2021, respectively, at three typical sections of the Qingshui River basin (site 1: E104.28°, N24.05°; site 2: E104.52°, N24.08°; site 3: E104.52°, N24.23°; Figure 1). Obtained fish were temporarily kept in keeping tanks with natural river water and enriched oxygen for 48 h to eliminate the stress reaction of the fish transportation [34]. The seven endemic fish species were selected based on their ecological significance in the Qingshui River basin, their short-distance migration requirements during spawning and their susceptibility to hydropower-induced habitat fragmentation, making them representative objects for studying swimming performance in this basin.
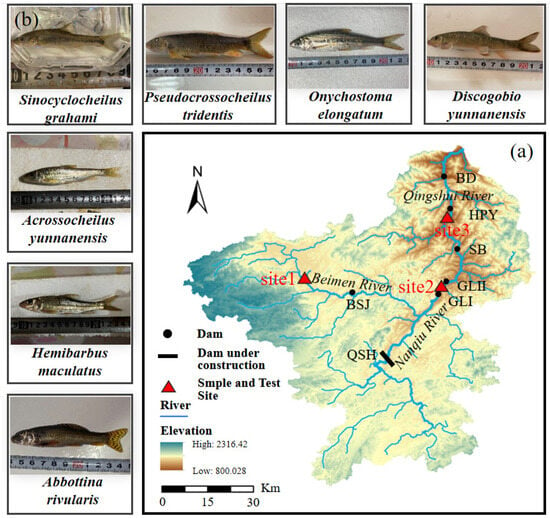
Figure 1.
The experimental fish and their sample sites in this study. (a) indicates the positional relationship between the Qingshui River basin and the test sites in this study, and (b) shows the seven experimental fish. Qingshuihe (QSH), Bisongjiu (BSJ), Gelei I (GLI), Gelei II (GLII), Shibie (SB), Houpayan (HPY) and Bada (BD) in this figure represent the different constructed hydropower plants in this area.
After the swimming speed test finished, fish morphology parameters, including fish standard body length (SL), fish fork length (FL), fish total length (TL) and fish weight (W), were measured using digital calipers (±0.1 mm) and an electronic scale (±0.1 g), following standardized protocols [35]. SL, FL and TL were measured from the tip of the snout to the posterior end of the vertebral column (caudal peduncle), the center of the caudal fin fork and the distal tip of the longest caudal fin ray, respectively. Environmental factors, including dissolved oxygen (DO) and the temperature (T) of the river water, were measured by the dissolved oxygen portable meter (YSI Pro20).
2.2. Fish Swimming Ability Test
Uind, Ucrit and Uburst are three important metrics frequently used to evaluate fish swimming ability. Uind refers to the minimum speed at which a fish can discern the flow direction of water. Ucrit is the maximum aerobic speed of a fish, which can be used to determine the maximum oxygen consumption capacity of a fish during the swimming process. Uburst is the transient and rapid swimming speed where fish are in a state of anaerobic respiration and lasts for less than 20 s [8,36].
The swimming ability test system (SY10800, Danish Loligo Company, Viborg, Denmark), with a volume of 30 L for the sealing area and a volume of 9 L for the test chamber, was adopted to carry out the fish swimming ability test. The motor drove the rotating paddle to generate the flow velocity from 5 to 180 cm/s. A linear relationship between the propeller rotation speed and the flow velocity was built and the desired flow velocity could be obtained by controlling the rotating frequency. The front rectifier grid was provided at the inlet of the test area to stabilize the flow pattern and ensure a uniformly distributed flow velocity (Figure 2). At the beginning of the swimming ability test, fish were acclimated in the test chamber for 20 min with a velocity of 5 cm/s to eliminate the stress of the transfer process [37,38]. The increasing flow velocity method was used to test Uind, Ucrit and Uburst in this study.
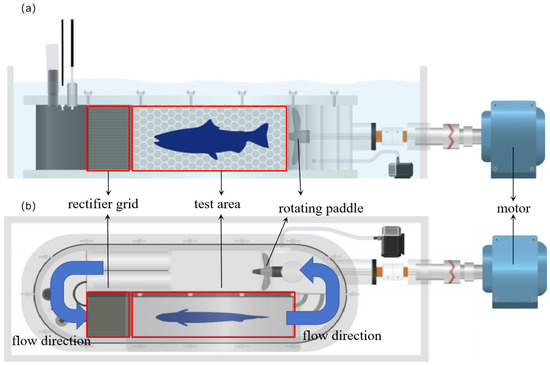
Figure 2.
The swimming ability test system. (a) and (b), respectively, represent the front view and the top view of the device.
The swimming speeds were tested after fish acclimation. In terms of the fish Uind test, the flow velocity was increased by 5 cm/s every 1 min from the acclimated velocity. The movement of the fish was observed until the fish exhibited rheotaxis and turned its direction to swim against the current. The flow velocity at this time was taken as the Uind of the experimental fish. The calculation formula for Uind is as follows [35]:
where is the velocity when the fish exhibit rheotaxis, is the flow velocity increment, is the time to start reversing course and is the interval of each increase. For the Uind test, 5 cm/s was taken as and 1 min was taken as .
For the Ucrit test, the flow velocity was increased by 5 cm/s every 20 min from the acclimated velocity. The movement of the fish was observed until the fish was exhausted and leaned against the downstream wire mesh for more than 2 min, when the flow velocity was taken as the Ucrit of the experimental fish. The calculation formula for Ucrit is as follows [39]:
where is the velocity when the fish is exhausted, is the flow velocity increment, is the time from the last increase to the fish exhaustion and is the interval of each increase. For the Ucrit test, 5 cm/s was taken as and 20 min was taken as .
Uburst has a similar test methodology to Ucrit, except that the interval of each increase is changed to 1 min. The calculation formula for Uburst is as follows [35]:
To eliminate the effect of body length and weight on the swimming ability, the absolute swimming metrics (Uind, Ucrit and Uburst) were divided by SL, W and W/SL, respectively, to obtain relative swimming metrics, including Uind/SL, Uind/W, Uind/(W/SL), Ucrit/SL, Ucrit/W, Ucrit/(W/SL), Uburst/SL, Uburst/W and Uburst/(W/SL).
2.3. Data Analysis
Bivariate correlation was used to test the relationship between the three swimming speeds and the morphology parameters of the experimental fish, with a significance level of p < 0.05. The linear or power regression was applied for swimming speeds and the morphology parameters where significant correlation existed. The mantel test is a non-parametric statistical method used to evaluate the correlation between two matrices. It was used to evaluate whether fish swimming speeds had correlations with the biological characteristics (species, SL, FL, TL, W and W/SL) and environmental factors (the life periods, sample sites, water temperature and DO). We used Rstudio to implement random forest (RF) coding to rank the importance of the factors affecting fish swimming speeds. The independent samples t test was used to analyze the difference in each morphological parameter and swimming speed between the two periods for each fish species. For datasets meeting normality assumptions, one-way ANOVA was employed to test interspecies differences in swimming abilities. When normality assumptions were violated, the non-parametric Kruskal–Wallis test was used. The normality of data distribution was verified using the Shapiro–Wilk test (p > 0.05). Post hoc multiple comparisons were conducted using the least significant difference (LSD) test for homogeneous variances (Levene’s test, p > 0.05) or Tamhane’s T2 test for heterogeneous variances.
2.4. Ethical Approval
The animal study proposal was approved by the Ethics Committee for Animal Experiments of Sichuan University (No. 2019062101). All experimental procedures were performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of the People’s Republic of China.
3. Results
3.1. Fish Collection and Field Environmental Conditions
A total of 142 fish were collected during the non-spawning and spawning periods. Seven fish species were used to test Uind and Uburst, while Ucrit was tested for five fish species. The sample size for each swimming ability test ranged from 3 to 12 fish, and each fish was used only once (Table 1). Natural river water was used in keeping tanks, with a T of 20.3 °C and a DO of 6.5 mg/L during the non-spawning period, and a T of 20.4 °C and a DO of 7.12 mg/L during the spawning period. The swimming speeds of endemic fish in the Qingshui River basin were tested during the non-spawning period (October 2020) and the spawning period of the following year (April 2021). River water with 24 h of aeration, which had a DO over 7.0 mg/L, was used as the test water in this study. The temperature of the river water ranged from 17.2 to 22.4 °C during the non-spawning period and from 18.65 to 25.7 °C during the spawning period.

Table 1.
Sample sizes for swimming ability tests (Uind, Ucrit and Uburst) across seven fish species during spawning and non-spawning periods. Slashes (/) indicate that tests were not conducted.
3.2. Fish Morphological Characteristics
As shown in Figure 3, the SL, FL, TL and W of the seven experimental fish species differed significantly during the non-spawning period. The SL, FL, TL and W of Hemibarbus maculatus were significantly higher than those of other fish during the non-spawning period, with Discogobio yunnanensis and Abbottina rivularis being the lowest (one-way ANOVA for the non-spawning period: FSL = 11.671, pSL < 0.001; FFL = 10.450, pFL < 0.001; FTL = 10.737, pTL < 0.001; FW = 8.665, pW < 0.001). During the spawning period, Onychostoma elongatum had the largest morphological parameters among all species, while Discogobio yunnanensis and Abbottina rivularis had the smallest (one-way ANOVA for the spawning period: FSL = 4.963, pSL = 0.211 > 0.05; FFL = 3.880, pFL < 0.05; FTL = 5.970, pTL < 0.001; FW = 3.408, pW < 0.001). The morphological parameters of Discogobio yunnanensis and Onychostoma elongatum were significantly higher during the spawning period than those during the non-spawning period (independent sample T test for Discogobio yunnanensis: FSL = −2.71, pSL = 0.006, FFL = −2.13, pFL = 0.005, FTL = −2.84, pTL = 0.015, FW = -5.86, pW = 0.018; independent sample T test for Onychostoma elongatum: FSL = −2.69, pSL < 0.001, FFL = −2.48, pFL < 0.001, FTL = −2.65, pTL = 0.005, FW = −4.89, pW = 0.006), and the rest of fish species had no significant differences (Figure 4).
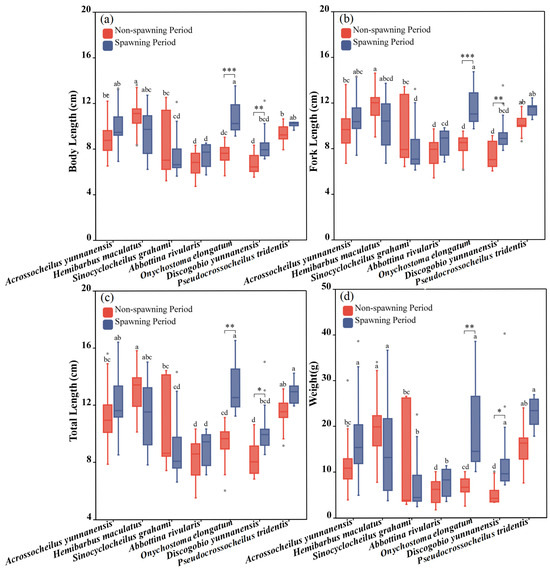
Figure 3.
Morphological characteristics of experimental fish during the non-spawning and spawning period. (a–d) represent differences of standard body length (SL), fork length (FL), total length (TL) and weight (W) between two periods and seven studied fish, respectively. The boxes in the figure illustrate the range of lower and upper quartiles. The whiskers indicate the 1.5 interquartile range. The median values are represented by solid lines, respectively. The gray dots outside the boxes represent data that are outliers. The asterisks indicate significant differences in morphological parameters between the two periods (* indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001). Letters above boxes represents the results of post-hoc multiple comparisons.
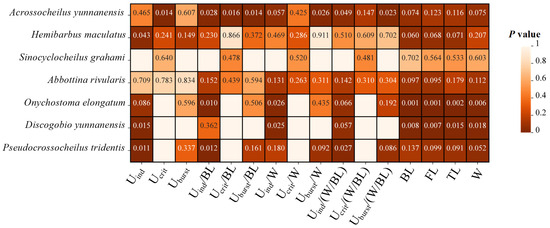
Figure 4.
Heat map describing the differences in the swimming ability (Uind, Uind/SL, Uind/W, Uind/(W/SL), Ucrit, Ucrit/SL, Ucrit/W, Ucrit/(W/SL), Uburst, Uburst/SL, Uburst/W, Uburst/(W/SL)) of seven fish species in the Qingshui River basin between the two periods. The color in cells ranging from dark to light with P values represents the significance level from high to low, while empty cells without values indicate that the data were not obtained.
3.3. Relationship Between Swimming Ability and Fish Morphology
The ranges of Uind, Ucrit and Uburst for experimental fish in the Qingshui River basin were 5–12.75 cm/s, 25.5–149 cm/s and 36.3–179.2 cm/s, respectively. Bivariate correlation shows that there was no significant correlation between Uind and SL (p = 0.3166, Figure 5a), while Ucrit was positively correlated with SL (p = 0.0002, Figure 5b). Although Uburst had a significant correlation with SL (p = 0.0227), there only existed a weak linear relationship between them (R = 0.074). Uind and Ucrit were negatively correlated with SL2 (Uind: p = 0.0001, Ucrit: p = 0.0204; Figure 5d,e), while Uburst was not found to be significantly related to SL2 (p = 0.216, Figure 5f). Compared to SL and SL2, fish swimming speeds showed better power function correlations with W and W/SL, where the R values were over 0.7 (Figure 5g–l).
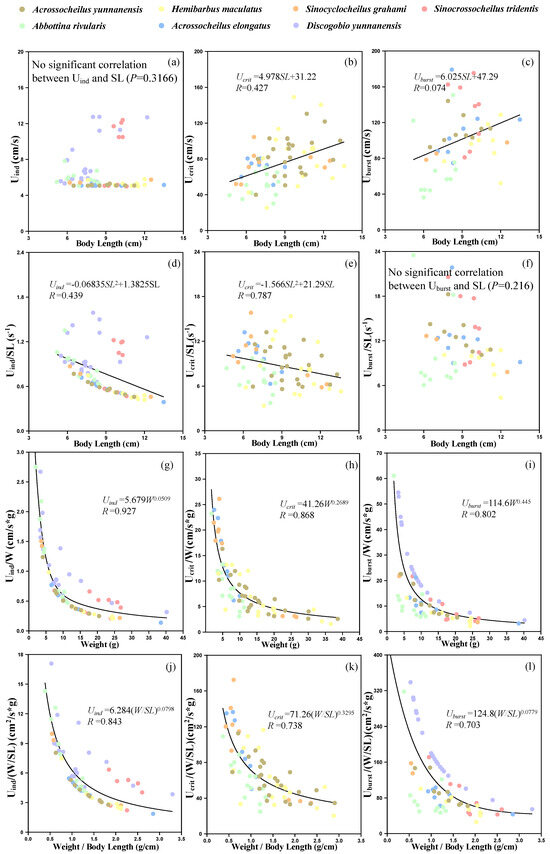
Figure 5.
Relationship between morphological parameters and swimming ability. (a–c) represents relationship between standard body length and swimming ability (Uind, Ucrit and Uburst), respectively. (d–f) represents relationship between standard body length and swimming ability (Uind/SL, Ucrit/SL and Uburst/SL), respectively. (g–i) represents relationship between weight and swimming ability (Uind/W, Ucrit/W and Uburst/W), respectively. (j–l) represents relationship between weight/standard body length and swimming ability (Uind/(W/SL), Ucrit/(W/SL) and Uburst/(W/SL)), respectively.
3.4. Comparison of Swimming Ability Considering Different Periods and Species
There were significant differences in Uind among the seven fish species during both the non-spawning and spawning periods (one-way ANOVA for the non-spawning period: F = 3.458, p = 0.038; one-way ANOVA for the spawning period: F = 44.002, p < 0.001; Figure 6a). Discogobio yunnanensis and Abbottina rivularis had the largest Uind during the non-spawning period, while the greatest Uind during the spawning period was recorded by Pseudocrossocheilus tridentis and Discogobio yunnanensis. For the same species, Pseudocrossocheilus tridentis (independent sample T test: F = −15.888, p = 0.011), Discogobio yunnanensis (independent sample T test: F = −2.879, p = 0.015) and Hemibarbus maculatus (independent sample T test: F = −3.103, p = 0.043) had significantly higher Uind during the spawning period than those during the non-spawning period, while the rest of the fish species had no significant differences (Figure 6a).
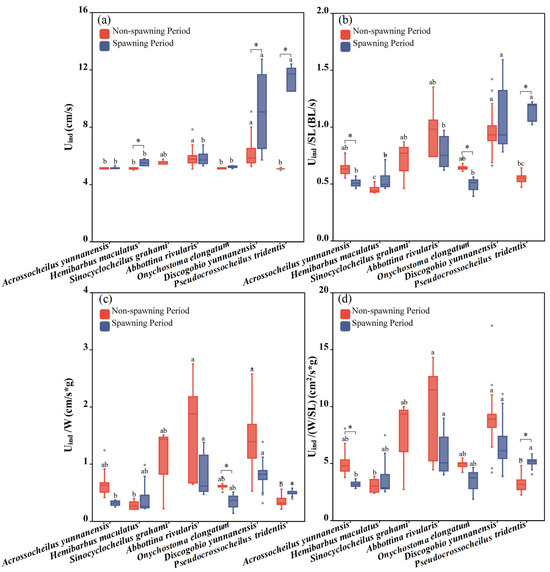
Figure 6.
Differences between the Uind, Uind/SL, Uind/W and Uind/(W/SL) of experimental fish across species and periods. (a–d) represent differences of Uind, Uind/SL, Uind/W and Uind/(W/SL) between two periods and seven studied fish, respectively. The boxes in the figure illustrate the range of lower and upper quartiles. The whiskers indicate the 1.5 interquartile range. The median values are represented by solid lines, respectively. The gray dots outside the boxes represent data that are outliers. * represents a statistically significant difference in Uind between the non-spawning and spawning periods (* indicates p < 0.05). Letters above boxes represents the results of post-hoc multiple comparisons.
Significant differences among fish species in Uind/SL, Uind/W and Uind/(W/SL) were found during both the non-spawning period and spawning period (one-way ANOVA for the non-spawning period: FUind/SL = 10.414, pUind/SL = 0.001, FUind/W = 7.672, pUind/W = 0.002, FUind/(W/SL) = 5.678, pUind/(W/SL) = 0.008; one-way ANOVA for the spawning period: FUind/SL = 33.775, pUind/SL < 0.001, FUind/W = 7.615, pUind/W = 0.004, F Uind/(W/SL) = 8.284, pUind/(W/SL) = 0.003; Figure 6a–c). The significances in Uind/W and Uind/(W/SL) had the same distribution among the seven fish species during both the non-spawning period and spawning period. During the non-spawning period, Discogobio yunnanensis showed a stronger performance in terms of Uind/SL, Uind/W and Uind/(W/SL), while Pseudocrossocheilus tridentis and Hemibarbus maculatus were weaker swimmers. During the spawning period, Discogobio yunnanensis and Pseudocrossocheilus tridentis had the strongest Uind/SL, Uind/W and Uind/(W/SL), and Abbottina rivularis performed weaker in Uind/SL while was the strongest in Uind/W and Uind/(W/SL).
For the same species, in terms of Uind/SL, Acrossocheilus yunnanensis (independent sample T test: F = 2.756, p = 0.028) and Onychostoma elongatum (independent sample T test: F = 3.671, p = 0.01) swam faster during the non-spawning period than those during the spawning period, whereas Pseudocrossocheilus tridentis exhibited an opposite pattern (independent sample T test: F = −11.637, p = 0.012). There was no significant difference in Uind/SL between the two periods in the rest of the fish species (Figure 4 and Figure 6b). For the same species, considering Uind/W, only Onychostoma elongatum performed better during the non-spawning period than that during the spawning period (independent sample T test: F = 2.936, p = 0.026; Figure 4 and Figure 6c). For the same species, in terms of Uind/(W/SL), Acrossocheilus yunnanensis had a better swimming ability during the non-spawning period than that during the spawning period (independent sample T test: F = 2.384, p = 0.049), whereas Pseudocrossocheilus tridentis presented an opposite pattern (independent sample T test: F = −2.71, p = 0.027). There was no significant difference in Uind/(W/SL) between the two periods in the rest of the fish species (Figure 4 and Figure 6d).
Five fish species were adopted to test Ucrit during the non-spawning period (Abbottina rivularis, Acrossocheilus yunnanensis, Onychostoma elongatum, Sinocyclocheilus grahami and Hemibarbus maculatus), and four fish species were adopted to test the Ucrit during the spawning period (Abbottina rivularis, Acrossocheilus yunnanensis, Sinocyclocheilus grahami and Hemibarbus maculatus). There was a significant difference among the fish species between the two periods (one-way ANOVA for the non-spawning period: F = 4.841, p = 0.003; one-way ANOVA for the spawning period: F = 17.344, p = 0.001; Figure 7a) Hemibarbus maculatus had the greatest Ucrit during the non-spawning period, while Acrossocheilus yunnanensis exhibited the strongest performance in Ucrit during the spawning period. The Ucrit of fish did not exhibit a significant difference between the two periods, except for that Acrossocheilus yunnanensis swam faster during the non-spawning period (independent sample T test: F = −4.051, p = 0.014; Figure 4 and Figure 7a).
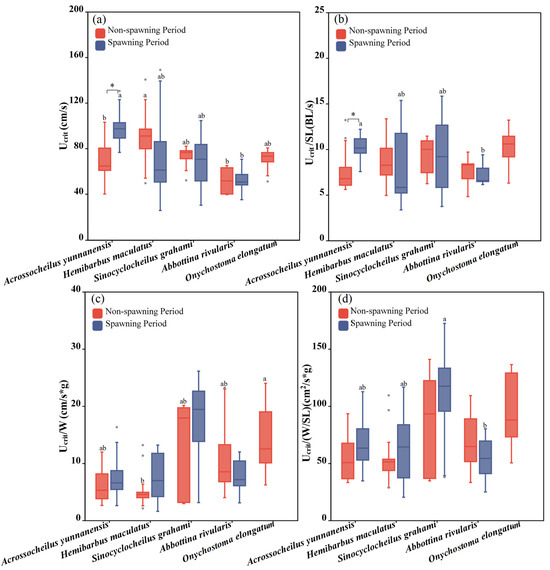
Figure 7.
Differences between the Ucrit, Ucrit/SL, Ucrit/W and Ucrit/(W/SL) of experimental fish across species and periods. (a–d) represent differences of Ucrit, Ucrit/SL, Ucrit/W and Ucrit/(W/SL) between two periods and seven studied fish, respectively. The boxes in the figure illustrate the range of lower and upper quartiles. The whiskers indicate the 1.5 interquartile range. The median values are represented by solid lines, respectively. The gray dots outside the boxes represent data that are outliers. * represents a statistically significant difference in Ucrit between the non-spawning and spawning periods (* indicates p < 0.05). Letters above boxes represents the results of post-hoc multiple comparisons (LSD or Tamhane’s T2 test, p < 0.05). Groups sharing no common letters differ significantly (“a” represents the highest mean value of this group, and “b” represents the lowest.), and there was no statistical difference among the groups with any of the same letters (such as “ab” and “a”, “b” and “ab”, etc.).
The significant difference in Ucrit/W among fish species was found during the non-spawning period (one-way ANOVA: FUcrit/W = 3.371, pUcrit/W = 0.045; Figure 7c), and Onychostoma elongatum had the highest Ucrit/W. There existed significant differences among the fish species during the spawning period in terms of Ucrit/SL and Ucrit/(W/SL) (one-way ANOVA: FUcrit/SL = 7.541, pUcrit/SL = 0.008; F Ucrit/(W/SL) = 3.491, pUcrit/(W/BL) = 0.029; Figure 7b,d). Acrossocheilus yunnanensis swam faster in terms of Ucrit/SL than other species and Sinocyclocheilus grahami exhibited the strongest Ucrit/(W/SL). For the same species, in terms of Ucrit/SL, Acrossocheilus yunnanensis performed better during the spawning period (independent sample T test: F = −3.077, p = 0.016; Figure 4 and Figure 7b), while there was no significant difference in Ucrit/W and Ucrit/(W/SL) between the two periods for all species (Figure 4 and Figure 7c,d).
Six fish species except for Discogobio yunnanensis were adopted to test the Uburst during different periods and a significant difference among fish species was found during the non-spawning period (one-way ANOVA: F = 3.001, p = 0.035) but not during the spawning period (one-way ANOVA: F = 2.849, p = 0.059). During the non-spawning period, Acrossocheilus yunnanensis and Abbottina rivularis had the smallest Uburst compared to the other three species (Figure 8a). For the same species, between the two periods, we did not find a significant difference in terms of Uburst (Figure 4 and Figure 8a).
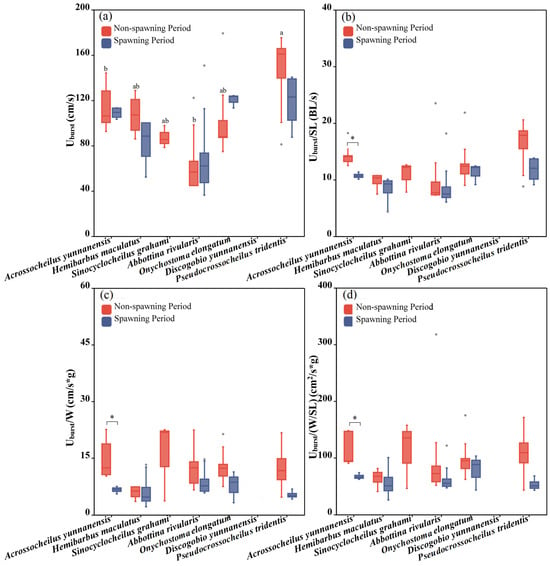
Figure 8.
Differences between the Uburst, Uburst/SL, Uburst/W and Uburst/(W/SL) of the experimental fish across species and periods. The boxes in the figure illustrate the range of lower and upper quartiles. (a–d) represent differences of Uburst, Uburst/SL, Uburst/W and Uburst/(W/SL) between two periods and seven studied fish, respectively. The whiskers indicate the 1.5 interquartile range. The median values are represented by solid lines, respectively. The gray dots outside the boxes represent data that are outliers. * represents a statistically significant difference in Uburst between the non-spawning and spawning periods (* indicates p < 0.05). Letters above boxes represents the results of post-hoc multiple comparisons (LSD or Tamhane’s T2 test, p < 0.05). Groups sharing no common letters differ significantly (“a” represents the highest mean value of this group, and “b” represents the lowest.), and there was no statistical difference among the groups with any of the same letters (such as “ab” and “a”, “b” and “ab”, etc.).
There existed no significant difference among the fish species during both periods in terms of Uburst/SL, Uburst/W and Uburst/(W/SL) (one-way ANOVA for the non-spawning period: FUburst/SL = 1.23, pUburst/SL = 0.332, FUburst/W = 3.348, pUburst/W = 0.063, FUburst/(W/SL) = 0.412, pUburst/(W/SL) = 0.835; one-way ANOVA for the spawning period: FUburst/SL = 0.963, pUburst/SL = 0.454, FUburst/W = 0.761, pUburst/W = 0.566, F Uburst/(W/SL) = 0.562, pUburst/(W/SL) = 0.693). For the same species, between the two periods in terms of Uburst/SL (independent sample T test: F = 3.249, p = 0.014), Uburst/W (independent sample T test: F = 3.349, p = 0.026) and Uburst/(W/SL) (independent sample T test: F = 3.483, p = 0.023), only Acrossocheilus yunnanensis showed a significant difference between the two periods, presenting a better swimming performance during the non-spawning period. We did not obtain the Uburst of Discogobio yunnanensis due to its Uburst exceeding the test range of the Loligo SY10800 used in this study. It is speculated that Discogobio yunnanensis had the strongest swimming performance in terms of Uburst.
3.5. Factors Influencing Swimming Performance
As shown in Figure 9a, the result of the Euclidean distance from the mantel test indicates that weight parameters (W and W/SL) were positively related to Ucrit (p < 0.05, R > 0) but negatively connected to Uburst (p < 0.05, R < 0). Weight parameters were more correlated with swimming ability than body length parameters (SL, FL and TL). The period was significantly correlated with Uind (p = 0.002), the sample site was significantly correlated with Uburst (p = 0.001) and the DO and water temperature exhibited a significantly positive correlation with Ucrit (for DO: p = 0.009, R = 0.195; for T: p = 0.008, R = 0.194). As shown in Figure 9b–d, the result of RF indicates that species, DO and T were the prominent factors affecting fish swimming ability in this study (% importance for species > 12.16, for DO > 7.98 and for T > 9.47). The sample site was a secondary important factor influencing Uburst and the period was also an important factor influencing Uind. Weight parameters were found to have greater effects on fish swimming speeds than the body length parameters. Generally speaking, the species identity was the main factor shaping the swimming ability, followed by environmental factors and morphological factors, combining the mantel test and RF.
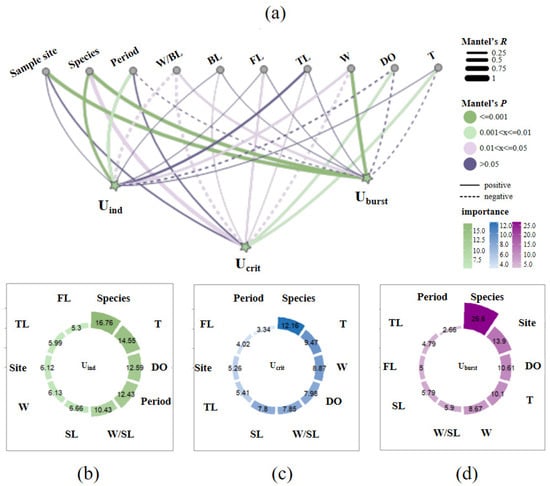
Figure 9.
Factors influencing fish swimming ability. (a) depicts the correlations between fish swimming speeds and impact factors from the mantel test result, indicated by R and p values. (b–d) depict the importance ranking of impact factors for Uind, Ucrit and Uburst from the RF result, indicated by percentage values.
4. Discussion
Fish swimming ability is generally associated with their morphological parameters, physiological needs and living environment [40]. A field experiment on fish swimming ability was launched to investigate the discrepancy in fish swimming speeds among different species, periods and environmental factors. Furthermore, we identified the key factors driving fish swimming ability, which could provide important technical support for the restoration of river connectivity and a reference for the conservation of rare and endemic fish in mountainous rivers.
4.1. The Effect of Morphological Factors on Fish Swimming Performance
In this study, the SL, FL, TL, as well as the W of fish showed a greater increase during the spawning period than during non-spawning period. This may be due to the fact that the fish are at the stage of sexual maturity and fully developed during the spawning period [41]. In this study, fish swimming ability was not strongly correlated with body length parameters (SL, FL, TL), with r being less than 0.6 in the fitting result, while weight parameters (W and W/SL) were found to have a better correlation with fish swimming speeds. Previous studies usually thought that body length parameters linearly correlated with fish swimming speeds [35,42,43], but our results suggest that weight-based metrics outperform length metrics in predicting swimming capacity.
Weight parameters in this study were positively related to Ucrit but negatively connected to Uburst. There is a complex relationship between muscle mass and drag force in fish and it varies with the fish shape and size [44,45]. For Uburst, greater weight increased the drag force required for bursting movements and thus reduced Uburst. Red muscle mass increases as a result of weight gain, and the larger red muscle mass allows for a more sustained release of energy available for aerobic exercise, providing sufficient fuel for Ucrit [46,47,48]. Aerobic enhancement squeezes anaerobic expenditure, and there exists a competitive relationship between them [49]. In addition, previous studies proved that lean body mass and certain fat mass are an important physical basis for anaerobic capacity, and we also suggest introducing lean body mass and fat mass as new factors to evaluate fish Uburst [50,51].
4.2. The Effect of Species on Fish Swimming Performance
Acrossocheilus yunnanensis and Onychostoma elongatum belong to the same genus of Acrossocheilus and have similar habits, and their swimming capabilities were also found to be relatively close to each other. The bodies of Acrossocheilus yunnanensis and Onychostoma elongatum are slender and streamlined, causing their susceptibility to flow velocities, and consequently resulting in lower Uind compared to other fish species [52]. Fish in the upper layer are more sensitive to the water current than those in the lower layer [52]. Abbottina rivularis, Discogobio yunnanensis and Pseudocrossocheilus tridentis have flattened thoraxes and abdomens, making them suitable for the bottom and less vulnerable to the current, and thus exhibiting greater Uind [53].
Fish in the lotic environment prefer to maintain aerobic and anaerobic exercise to resist current shocks [54,55]. Discogobio yunnanensis and Pseudocrossocheilus tridentis, belonging to the same subfamily of Labeoninae and being active in the lotic environment, have developed physiological and metabolic characteristics as well as morphological features suitable for their environment in the process of long-term evolution and adaptation. Discogobio yunnanensis and Pseudocrossocheilus tridentis living in the lotic environment have stronger swimming abilities and have stronger resistance to the water current. The Uburst of Discogobio yunnanensis even exceeded the test range of the instrument used in this study. Instruments with bigger test ranges should be considered to obtain its Uburst. Fish in the lentic environment typically prioritize maneuverability (Uind) rather than stronger swimming abilities (Uburst and Ucrit), often exhibiting deeper, laterally compressed bodies [9]. For example, Abbottina rivularis has a small body size with a big head, which is easily subject to a large current driving force, consequently resulting in a relatively weaker acceleration ability and a lower swimming ability.
4.3. The Effect of Life Period on Fish Swimming Performance
The Uind of all fish species in this study increased to some extent during the spawning period compared to the non-spawning period, and significant differences between the two periods were found in Hemibarbus maculatus, Discogobio yunnanensis and Pseudocrossocheilus tridentis. We speculate that it is mainly due to the mutual adaptation between the environment and fish. During the long process of natural selection, fish species undergo self-evolution to adapt to the changes in the environment [56]. The spawning period for fish in the Qingshui River basin is from April to May, which also belongs to the rainy season of the river. During this period the amount of water in the river rises and the flow velocity increases, creating an increasing Uind of fish in the basin during the spawning period [57].
The locomotion performance of fish is usually dynamic with their life history [58], and special attention should be paid to the life history effects concerning the evaluation of fish swimming ability. Changes in metabolic activities during the spawning and non-spawning period are crucial to fish swimming ability [4]. Fish swimming performance is mainly fueled by the rapid catabolism of energy storage substances (e.g., ATP, phosphocreatine, glycogen, etc.) from muscles [5]. Fish use more energy to help with reproduction during the spawning period and reduce their energy consumption for the rest of their activities [59]. In this study, the Uburst of fish during the spawning period was lower than during the non-spawning period. There also existed a certain degree of reduction in Uburst/SL, Uburst/W and Uburst/(W/SL) during the spawning period compared to the non-spawning period for all the fish species. The reduced capacity for muscle productivity in fish was found during the spawning period due to the long-term non-use of biological muscles [60], and this functional reduction due to muscle wastage could explain the changes in Uburst between the two different periods in this study. However, the significant increases in body size for fish during the spawning period allow them to achieve a balance between escape and reproduction, resulting in a not-statistically significant difference in Uburst between the non-spawning and spawning periods.
4.4. Application and Future Work
This study quantified the swimming behavior of typical endemic fish species in Southwest China and analyzed the impacts of biological characteristics and environmental factors on fish swimming performance. These findings provide theoretical references and foundational data to support the conservation of rare and endemic fish resources and the restoration of connectivity in mountainous rivers in Southwest China. For instance, a growing number of fish passage facilities in impaired rivers have been constructed, aimed at restoring upstream and downstream connectivity in China [23]; however, many of them have performed ineffectively, because they were not developed for endemic fish species [61,62,63]. Data on fish swimming ability can assist in determining the appropriate hydraulic conditions for fish migration, and can subsequently help to optimize the design of fish passage facilities to guarantee the attractiveness and pass efficiency [64,65,66].
Fish swimming ability is usually related to biological characteristics and external environmental factors [40], but the driving force of fish swimming ability is currently unclear. The results of the RF test and mantel test in this study showed that the species had a prominent influence on fish swimming ability among all the factors. The species difference was the result of long-term natural evolution and profoundly affected the fish behavior. Environmental factors also had an important impact on fish swimming performance, following closely behind the species. This study evaluated the impact of two important environmental factors (DO and T) on fish swimming ability. However, fish metabolic activities are still affected by many other factors, such as photoperiod and light intensity [67,68], developmental stage [69], sex [70], population density [71], the size of the swimming ability test system [4], water flow, etc. Future works should incorporate the above factors together to construct a comprehensive prediction model of swimming ability. In addition, although abundant data on the swimming performance of endemic fish species in the Qingshui River were obtained in this study through the field experiment, some tests involved small sample sizes (e.g., n = 3 for some species–period combinations) due to logistical challenges in fish collecting. Future studies should aim for larger sample sizes to validate the findings in this study.
5. Conclusions
Understanding the changes in fish swimming ability and identifying the driving factors behind these changes are of pressing importance. Seven endemic fish species from the Qingshui River were taken as the objects in this study to explore the effects of biological characteristics and environmental factors on fish swimming ability. The main conclusions were as follows:
(1) Body length minimally affected Uind but positively correlated with Ucrit and Uburst. Weight parameters (W and W/SL) showed stronger correlations with swimming performance than body length alone.
(2) Fish adapted to lotic environments (e.g., Discogobio yunnanensis) exhibited superior Ucrit and Uburst, while streamlined species displayed heightened flow sensitivity.
(3) There were significant differences in fish swimming abilities between the spawning period and non-spawning period. The Uind, Uind/SL, Uind/W and Uind/(W/SL) of Discogobio yunnanensis and Pseudocrossocheilus tridentis were higher during the spawning period, and this was similar to the Ucrit and Ucrit/SL of Acrossocheilus yunnanensis. The Uburst of all fish species was smaller during the spawning period.
(4) The swimming ability was mainly influenced by the species identity, followed by environmental conditions and morphological factors. Species, DO and T were the prominent factors affecting the three types of swimming ability in this study. The sample site was a secondary important factor influencing Uburst and the period was also an important factor influencing Uind.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani15121819/s1.
Author Contributions
Conceptualization, Y.W.; methodology, Z.Z., Q.W., G.C. and X.L.; formal analysis, J.R., G.C. and X.L.; investigation, Z.Z. and Q.W.; data curation, Z.Z. and Q.W.; writing—original draft preparation, J.R.; writing—review and editing, Y.W., R.L. and K.L.; supervision, R.L. and K.L.; funding acquisition, R.L. and K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science & Technology Fundamental Resources Investigation Program (2022FY100203), the National Natural Science Foundation of China (U2240212, 52179075) and the Sichuan Province Ecological Environment Protection Science and Technology Project Plan (2023HB01).
Institutional Review Board Statement
The experimental procedures involving animals in this study received approval from the Animal Experiment Ethics Committee of Sichuan University. All experimental methods were conducted in strict accordance with the Experimental Animal Management Regulations as stipulated by the State Council of the People’s Republic of China, ensuring the ethical and humane treatment of the experimental subjects.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study (Department of Agriculture of Yunnan Province).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Zhiguang Zhang was employed by the company Beijing Engineering Corporation Limited, Power Construction Corporation of China. Author Qi Wei was employed by the company Sichuan Water Development Investigation, Design & Research Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Uind | Induced Swimming Speed |
| Ucrit | Critical Swimming Speed |
| Uburst | Burst Swimming Speed |
| SL | Standard Body Length |
| FL | Fork Length |
| W | Weight |
| W/SL | Weight-to-Standard-Body-Length Ratio |
| TL | Total Length |
| DO | Dissolved Oxygen |
| T | Temperature |
| RF | Random Forest |
| QSH | Qingshuihe Hydropower Station |
| BSJ | Bisongjiu Hydropower Station |
| GLI | Gelei I Hydropower Station |
| GLII | Gelei II Hydropower Station |
| SB | Shibie Hydropower Station |
| HPY | Houpayan Hydropower Station |
| BD | Bada Hydropower Station |
References
- Wolter, C.; Arlinghaus, R. Navigation impacts on freshwater fish assemblages: The ecological relevance of swimming performance. Rev. Fish Biol. Fish. 2003, 13, 63–89. [Google Scholar] [CrossRef]
- Wang, Z.L.; Mao, K.; Du, W.; Cai, M.; Li, X. Diluted concentrations of methamphetamine in surface water induce behavior disorder, transgenerational toxicity, and ecosystem-level consequences of fish. Water Res. 2020, 184, 116164. [Google Scholar] [CrossRef] [PubMed]
- He, D.R.; Cai, H.C. Fish Behavior; Xiamen University Press: Xiamen, China, 1998; pp. 12–18. [Google Scholar]
- Deslauriers, D. Factors Influencing Swimming Performance and Behaviour of the Shortnose Sturgeon (Acipenser brevirostrum). Bachelor’s Thesis, University of New Brunswick, Department of Biology, Fredericton, NB, Canada, 2011. [Google Scholar]
- Brett, J.R.; Glass, N.R. Metabolic rates and critical swimming speeds of Sockeye salmon (Oncorhynchus nerka) in relation to size and temperature. J. Fish. Res. Board Can. 1973, 30, 379–387. [Google Scholar] [CrossRef]
- Milligan, C.L. Metabolic recovery from exhaustive exercise in rainbow trout. Comp. Biochem. Physiol. Part A Physiol. 1996, 113, 51–60. [Google Scholar] [CrossRef]
- Plaut, I. Critical swimming speed: Its ecological relevance. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 131, 41–50. [Google Scholar] [CrossRef]
- Gregory, T.R.; Wood, C.M. Individual variation and interrelationships between swimming performance, growth rate, and feeding in juvenile rainbow trout (Oncorhynchus mykiss). J. Can. Sci. Halieut. Aquat. 1998, 55, 1583–1590. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Cao, Z.D.; Fu, S.J. Flow velocity selection and its relationship to locomotive energetic metabolism in Chinese bream (Parabramis pekinensis) and pale chub (Zacco platypus). Acta Ecol. Sin. 2016, 36, 4187–4194. [Google Scholar]
- Facey, D.E.; Grossman, G.D. The metabolic cost of maintaining position four North American stream fishes: Effects of season and velocity. Physiol. Zool. 1990, 63, 757–776. [Google Scholar] [CrossRef]
- Facey, D.E.; Grossman, G.D. The relationship between water velocity, energetic costs, and microhabitat use in four North American stream fishes. Hydrobiologia 1992, 239, 1–6. [Google Scholar] [CrossRef]
- Yoshida, M.; Matsuura, K.; Uematsu, K. Developmental changes in the swimming behavior and underlying motoneuron activity in the larval angelfish, Pterophyllum scalare. Zool. Sci. 1996, 13, 229–234. [Google Scholar] [CrossRef]
- Koehn, J.D.; Harrington, D.J. Environmental conditions and timing for the spawning of Murray cod (Maccullochella peelii peelii) and the endangered trout cod (M. macquariensis) in southeastern Australian rivers. River Res. Appl. 2006, 22, 327–342. [Google Scholar] [CrossRef]
- Brodie, E.D. Behavioral modification as a means of reducing the cost of reproduction. Am. Nat. 1989, 134, 225–238. [Google Scholar] [CrossRef]
- Jebria, N.B.; Carmigniani, R.; Drouineau, H.; Oliveira, E.D. Coupling 3D hydraulic simulation and fish telemetry data to characterize the behaviour of migrating smolts approaching a bypass. J. Ecohydraul. 2023, 8, 144–157. [Google Scholar] [CrossRef]
- Koya, Y.; Inoue, M.; Naruse, T.; Sawaguchi, S. Dynamics of oocyte and embryonic development during ovarian cycle of the viviparous mosquitofish Gambusia affinis. Fish. Sci. 2000, 66, 63–70. [Google Scholar] [CrossRef]
- Liermann, C.R.; Nilsson, C.; Robertson, J.; Ng, R.Y. Implications of dam obstruction for global freshwater fish diversity. BioScience 2012, 62, 539–548. [Google Scholar] [CrossRef]
- Latrubesse, E.M.; Arima, E.Y.; Dunne, T.; Park, E.; Baker, V.R.; D’Horta, F.M.; Wight, C.; Wittmann, F.; Zuanon, J.; Baker, P.A.; et al. Damming the rivers of the Amazon basin. Nature 2017, 546, 363–369. [Google Scholar] [CrossRef]
- Chaudhari, S.; Brown, E.; Quispe-Abad, R.; Moran, E.; Müller, N.; Pokhrel, Y. In-stream turbines for rethinking hydropower development in the Amazon basin. Nat. Sustain. 2021, 4, 680–687. [Google Scholar] [CrossRef]
- Mensinger, M.A.; Blomberg, E.J.; Zydlewski, J.D. The consequences of dam passage for downstream-migrating American eel in the Penobscot river, Maine. Can. J. Fish. Aquat. Sci. 2021, 78, 1181–1192. [Google Scholar] [CrossRef]
- Cheong, T.S.; Kavvas, M.L.; Anderson, E.K. Evaluation of adult white sturgeon swimming abilities and applications to fishway design. Environ. Biol. Fishes 2006, 77, 197–208. [Google Scholar] [CrossRef]
- Zhang, L.; Pang, M.; Bahaj, A.B.S.; Yang, Y.; Wang, C. Small hydropower development in China: Growing challenges and transition strategy. Renew. Sustain. Energy Rev. 2021, 137, 110653. [Google Scholar] [CrossRef]
- Shi, X.; Kynard, B.; Liu, D.; Qiao, Y.; Chen, Q. Development of fish passage in China. Fisheries 2015, 40, 161–169. [Google Scholar] [CrossRef]
- Bestgen, K.R.; Mefford, B.; Bundy, J.M.; Walford, C.D.; Compton, R.I. Swimming performance and fishway model passage success of rio grande silvery minnow. Trans. Am. Fish. Soc. 2010, 139, 433–448. [Google Scholar] [CrossRef]
- Mai, Y.; Wang, X.; Peng, S.; Tie, H.; Cai, Y.; Chen, H.M.; Peng, M.; Wang, Y.; Li, H.; Zeng, Y.; et al. Supporting Evidence for the “Ten-Year Fishing Ban”: Different Modes of Fishing and Pollution Induce a Fish Diversity Decline between the Pearl River and Its Estuary. ACS EST Water 2023, 3, 2590–2603. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Pan, Z.; Sun, D.; Zou, J. Microplastics in wild freshwater fish of different feeding habits from Beijiang and Pearl River Delta regions, south China. Chemosphere 2020, 258, 127345. [Google Scholar] [CrossRef]
- Huang, H.; Yan, Z. Present situation and future prospect of hydropower in China. Renew. Sustain. Energy Rev. 2009, 13, 1652–1656. [Google Scholar] [CrossRef]
- Li, X.Z.; Chen, Z.J.; Fan, X.C.; Cheng, Z.J. Hydropower development situation and prospects in china. Renew. Sustain. Energy Rev. 2018, 82 Pt 1, 232–239. [Google Scholar] [CrossRef]
- Mao, X. Review of fishway research in China. Ecol. Eng. 2018, 115, 91–95. [Google Scholar] [CrossRef]
- O’Connor, J.; Hale, R.; Mallen-Cooper, M. Developing performance standards in fish passage: Integrating ecology, engineering and socio-economics. Ecol. Eng. J. Ecotechnol. 2022, 182, 106732. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Wang, Y.; Hao, Y.; Li, K.; Wang, H.; Liang, R. Restoration of a fish-attracting flow field downstream of a dam based on the swimming ability of endemic fishes: A case study in the upper Yangtze River basin. J. Environ. Manag. 2023, 345, 118694. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, T.Y.; Feng, J.J.; He, T.; Li, R. Study on Habitat Simulation and Substrate Rehabilitation Techniques for Spawning Grounds of Schizothorax prenanti. SICHUAN Environ. 2024, 43, 84–93. [Google Scholar]
- Gilbert, M.J.H.; Barbarich, J.M.; Casselman, M.; Kasurak, A.V.; Higgs, D.M.; Tierney, K.B. The role of substrate holding in achieving critical swimming speeds: A case study using the invasive round goby (Neogobius melanostomus). Environ. Biol. Fishes 2016, 99, 793–799. [Google Scholar] [CrossRef]
- Ke, S.; Yang, S.; Tu, Z.; Soomro, S.-E.; Ji, H.; Li, D.; Xu, J.; Qi, H.; Shi, X. Swimming performance of a threatened native fish (Gymnocypris przewalskii) informs fishway design in Qinghai Lake. Hydrobiologia 2025. [Google Scholar] [CrossRef]
- Hammer, C. Fatigue and exercise tests with fish. Comp. Biochem. Physiol. Part A Physiol. 1995, 112, 1–20. [Google Scholar] [CrossRef]
- Song, B.L. Effects of Water Current on Swimming Activity, Growth and Ecophysiological Aspect of Young Barbodes schwanenfeldi. Ph.D. Thesis, Jinan University, Guangzhou, China, 2008. [Google Scholar]
- Jain, K.E.; Birtwell, I.K.; Farrell, A.P.; Jain, K.E.; Birtwell, I.K.; Farrell, A.P. Repeat swimming performance of mature sockeye salmon following a brief recovery period: A proposed measure of fish health and water quality. Can. J. Zool. 1998, 76, 1488–1496. [Google Scholar] [CrossRef]
- Penghan, L.Y.; Cao, D.Z.; Fu, S.J. Effect of starvation on swimming performance of juvenile carp. Chin. J. Ecol. 2014, 33, 2756–2760. [Google Scholar]
- Brett, J.R. The Respiratory metabolism and swimming performance of young Sockeye Salmon. J. Fish. Res. Board Can. 1964, 21, 1183–1226. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, H.; Deng, L.J.; Li, T.C.; Yang, K.; Fu, S.J.; Song, Z.B. Improved aerobic and anaerobic swimming performance after exercise training and detraining in Schizothorax wangchiachii: Implications for fisheries releases. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 245, 110698. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.; Froese, R.; Liang, C.; Müller, J.; Sorensen, P. Post-spawning growth acceleration in fish as a result of reduced live weight and thus, increased food conversion efficiency. Environ. Biol. Fishes 2023, 106, 2031–2043. [Google Scholar] [CrossRef]
- Peake, S.; Beamish, F.W.H.; McKinley, R.S.; Scruton, D.A.; Katopodis, C. Relating swimming performance of lake sturgeon, Acipenser fulvescens, to fishway design. Can. J. Fish. Aquat. Sci. 1997, 54, 1361–1366. [Google Scholar] [CrossRef]
- Verhille, C.E.; Poletto, J.B.; Cocherell, D.E.; DeCourten, B.; Baird, S.; Cech, J.J., Jr.; Fangue, N.A. Larval green and white sturgeon swimming performance in relation to water-diversion flows. Conserv. Physiol. 2014, 2, cou031. [Google Scholar] [CrossRef][Green Version]
- Alvarez, D.; Metcalfe, N.B. Catch-up growth and swimming performance in threespine sticklebacks (Gasterosteus aculeatus): Seasonal changes in the cost of compensation. Can. J. Fish. Aquat. Sci. 2005, 62, 2169–2176. [Google Scholar] [CrossRef]
- James, R.S.; Johnston, I.A. Scaling of muscle performance during escape responses in the fish Myoxocephalus scorpius L. J. Exp. Biol. 1998, 201, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Johnson, D.; Mandal, P.; Gan, M.; Yuan, X.; Tu, Z.; Huang, Y. Effect of exhaustive exercise on the swimming capability and metabolism of juvenile Siberian sturgeon. Trans. Am. Fish. Soc. 2015, 144, 532–538. [Google Scholar] [CrossRef]
- Plaut, I. Does pregnancy affect swimming performance of female Mosquitofish, Gambusia affinis? Funct. Ecol. 2002, 16, 290–295. [Google Scholar] [CrossRef]
- Rome, L.C.; Funke, R.P.; Alexander, R.M. The influence of temperature on muscle velocity and sustained performance in swimming carp. J. Exp. Biol. 1990, 154, 163–178. [Google Scholar] [CrossRef]
- Dowson, M.N.; Nevill, M.E.; Lakomy, H.K.A.; Nevill, A.M.; Hazeldine, R.J. Modelling the relationship between isokinetic muscle strength and sprint running performance. J. Sports Sci. 1998, 16, 257–265. [Google Scholar] [CrossRef]
- Mayhew, J.L.; Hancock, K.; Rollison, L.; Ball, T.E.; Bowen, J.C. Contributions of strength and body composition to the gender difference in anaerobic power. J. Sports Med. Phys. Fit. 2001, 41, 33. [Google Scholar]
- Young, W.; Mclean, B.; Ardagna, J. Relationship between strength qualities and sprinting performance. J. Sports Med. Phys. Fit. 1995, 35, 13–19. [Google Scholar]
- Yuan, X.; Tu, Z.Y.; Han, J.C.; Shi, X.T.; Liu, G.Y.; Huang, Y.P. Effects of Flow Rate on Swimming Behavior and Energy Consumption of Carassius auratus. J. Hydroecol. 2011, 32, 103–109. [Google Scholar]
- Kieffer, J.D.; Arsenault, L.M.; Litvak, M.K. Behaviour and performance of juvenile shortnose sturgeon Acipenser brevirostrum at different water velocities. J. Fish Biol. 2009, 74, 674–682. [Google Scholar] [CrossRef]
- Fu, S.J.; Cao, Z.D.; Yan, G.J.; Fu, C.; Pang, X. Integrating environmental variation, predation pressure, phenotypic plasticity and locomotor performance. Oecologia 2013, 173, 343–354. [Google Scholar] [CrossRef]
- Yan, G.J.; He, X.K.; Cao, Z.D.; Fu, S.J. An interspecific comparison between morphology and swimming performance in cyprinids. J. Evol. Biol. 2013, 26, 1802–1815. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.G.; Zeng, X.; Cai, R.Y.; Peng, J.L.; Fu, S.J. Effects of Temperature and Reproductive Status on the Fast-start Swimming Performance of Guppy. J. Chongqing Norm. Univ. (Nat. Sci.) 2017, 34, 28–32. [Google Scholar]
- Zhang, H.; Zeng, C.J.; Li, T.; He, S.F.; Mo, K.L.; Yang, P.S.; Chen, Q.W. Ecological Flow in the Mid-lower Hanjiang River Based on Spawning Demands of the Four Major Chinese Carps. J. Hydroecol. 2022, 43, 1–8. [Google Scholar]
- Huang, Q.F.; Deng, C.K.; Xia, J.Y.; Yan, H.J.; Xia, J.G. Geometric morphology of Brachymystax tsinlingensis and sympatric Phoxinus lagowskii: Life-history stage effects and interspecific differences. Chin. J. Ecol. 2024, 43, 922–929. [Google Scholar]
- Yang, H.; Cao, Z.D.; Fu, S.J. Swimming performance and energy metabolism of male and female crucian carps (Carassius auratus) during their III reproduction phase. Chin. J. Ecol. 2012, 31, 2606–2612. [Google Scholar]
- Videler, J.J. Fish Swimming; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Dudgeon, D. River regulation in Southern China: Ecological implications, conservation and environmental management. Regul. Rivers Res. Manag. 1995, 11, 35–54. [Google Scholar] [CrossRef]
- Dudgeon, D. River rehabilitation for conservation of fish biodiversity in monsoonal Asia. Ecol. Soc. 2005, 10, 15–34. [Google Scholar] [CrossRef]
- Zheng, J.; Han, D.; Hu, W.; Wang, X.; Zhang, X. Fish swimming performance related to fishway design. J. Hydroecol. 2010, 3, 104–110. [Google Scholar]
- Piper, A.T.; Wright, R.M.; Kemp, P.S. The influence of attraction flow on upstream passage of European eel (Anguilla anguilla) at intertidal barriers. Ecol. Eng. 2012, 44, 329–336. [Google Scholar] [CrossRef]
- Silva, A.T.; Baerum, K.M.; Hedger, R.D.; Baktoft, H.; Fjeldstad, H.P.; Gjelland, K.; Økland, F.; Forseth, T. The effects of hydrodynamics on the three-dimensional downstream migratory movement of Atlantic salmon. Sci. Total Environ. 2019, 705, 135773. [Google Scholar] [CrossRef] [PubMed]
- Vowles, A.S.; Kemp, P.S. Effects of light on the behaviour of brown trout (Salmo trutta) encountering accelerating flow: Application to downstream fish passage. Ecol. Eng. 2012, 47, 247–253. [Google Scholar] [CrossRef]
- Gray, D.W.; Goldstein, A.H.; Lerdau, M.T. The influence of light environment on photosynthesis and basal methylbutenol emission from Pinus ponderosa. Plant Cell Environ. 2005, 28, 1463–1474. [Google Scholar] [CrossRef]
- Vowles, A.S.; Anderson, J.J.; Gessel, M.H.; Williams, J.G.; Kemp, P.S. Effects of avoidance behaviour on downstream fish passage through areas of accelerating flow when light and dark. Anim. Behav. 2014, 92, 101–109. [Google Scholar] [CrossRef]
- Deng, C.K.; Huang, Q.F.; Li, P.; Xia, J.Y.; Xia, J.G. Comparative studies on burst swimming performance of Brachymystax tsinlingensis and sympatric Phoxinus lagowskii in different life history stages. Acta Ecol. Sin. 2024, 44, 3999–4008. [Google Scholar]
- Smith, R.L.; Paul, A.J.; Paul, J.M. Seasonal changes in energy and the energy cost of spawning in Gulf of Alaska Pacific cod. J. Fish Biol. 1990, 36, 307–316. [Google Scholar] [CrossRef]
- Claireaux, G.; Lagardère, J.-P. Influence of temperature, oxygen and salinity on the metabolism of the European sea bass. J. Sea Res. 1999, 42, 157–168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).