Functional Requirement of Niacinamide for Blood Profiles, Antioxidant Status, and Intestinal Health in Finishing Pigs Fed a Low-Protein Diet
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Growth Performance
2.3. Sample Collection
2.4. Blood Profiles
2.5. Rectal SCFA Detection
2.6. Rectal Microbiota Sequencing
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Physiological Parameters
3.3. Serum Biochemical Parameters
3.4. Serum Antioxidant and Immune Parameters
3.5. Rectal SCFA
3.6. Microbiota of Rectal Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maiese, K.; Chong, Z.Z.; Hou, J.; Shang, Y.C. The vitamin niacinamide: Translating nutrition into clinical care. Molecules 2009, 14, 3446. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chong, Z.Z.; Maiese, K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient niacinamide. Front. Biosci. 2004, 9, 2500. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Amici, A.; Emanuelli, M.; Orsomando, G.; Raffaelli, N.; Ruggieri, S. Enzymology of NAD+ homeostasis in man. Cell. Mol. Life. Sci. 2004, 61, 19–34. [Google Scholar] [CrossRef]
- Hwang, E.S.; Song, S.B. Possible adverse effects of high-dose niacinamide: Mechanisms and safety assessment. Biomolecules 2020, 10, 687. [Google Scholar] [CrossRef]
- Maiese, K. Nicotinamide as a foundation for treating neurodegenerative disease and metabolic disorders. Curr. Neurovasc. Res. 2021, 18, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Stancek, M.; Schnell, R.; Ryden-Aulin, M. Analysis of escherichia coli nicotinate mononucleotide adenylyltransferase mutants in vivo and in vitro. BMC Biochem. 2005, 6, 16. [Google Scholar] [CrossRef]
- Delaroque, C.; Chervy, M.; Gewirtz, A.T.; Chassaing, B. Social overcrowding impacts gut microbiota, promoting stress, inflammation, and dysglycemia. Gut Microbes 2021, 13, 2000275. [Google Scholar] [CrossRef]
- Lee, I.K.; Kye, Y.C.; Kim, G.; Kim, H.W.; Gu, M.J.; Umboh, J.; Maaruf, K.; Kim, S.W.; Yun, C.H. Stress, nutrition, and intestinal immune responses in pigs- a review. Asian-Australas J. Anim. Sci. 2016, 29, 1075–1082. [Google Scholar] [CrossRef]
- Powick, W.C.; Ellis, N.R.; Et, A. Nicotinic acid deficiency and nicotinic acid requirement of young pigs on a purified diet. J. Anim. Sci. 1947, 6, 310–324. [Google Scholar] [CrossRef]
- NRC (National Research Council). Nutrient Requirements of Swine, 11th ed.; National Academic Press: Washington, DC, USA, 2012.
- Subuh, A.M.H.; Motl, M.A.; Fritts, C.A.; Waldroup, P.W. Use of various ratios of extruded full fat soybean meal and dehulled solvent extracted soybean meal in broiler diets. Int. J. Poult. Sci. 2002, 1, 9–12. [Google Scholar] [CrossRef]
- Clegg, K.M. Bound nicotinic acid in dietary wheaten products. Br. J. Nutr. 1963, 17, 325–329. [Google Scholar] [CrossRef]
- Luce, W.G.; Peo, E.J.; Hudman, D.B. Availability of niacin in wheat for swine. J. Nutr. 1966, 88, 39–44. [Google Scholar] [CrossRef]
- Ivers, D.J.; Veum, T.L. Effect of niacin additions to corn-soybean meal diets on performance of pigs from weaning to finishing. J. Anim. Sci. 1993, 71, 3383. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, X.; Qiu, Y.; Wang, L.; Shang, X.; Gao, K.; Yang, X.; Jiang, Z. Effect of niacin on growth performance, intestinal morphology, mucosal immunity and microbiota composition in weaned piglets. Animals 2021, 23, 2186. [Google Scholar] [CrossRef]
- Lan, T.; Cai, M.; Wang, S.; Lu, Y.; Tang, Z.; Tang, Q.; Gao, J.; Xu, Y.; Peng, X.; Sun, Z. Effects of adding nicotinamide to diets with normal and low protein levels on the immunity, antioxidant, and intestinal microbiota in growing-finishing pigs. J. Nutr. Biochem. 2025, 136, 109809. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, X.; Zhang, C.; Wang, Q.; Zhang, W.; Xie, J. Higher niacin intakes improve the lean meat rate of Ningxiang pigs by regulating lipid metabolism and gut microbiota. Front. Nutr. 2022, 9, 959039. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; Association of the Official Analytical Chemists: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Zhao, Y.; Liu, C.; Niu, J.; Cui, Z.; Zhao, X.; Li, W.; Zhang, Y.; Yang, Y.; Gao, P.; Guo, X.; et al. Impacts of dietary fiber level on growth performance, apparent digestibility, intestinal development, and colonic microbiota and metabolome of pigs. J. Anim. Sci. 2023, 101, 174. [Google Scholar] [CrossRef]
- Shen, Y.B.; Voilqué, G.; Odle, J.; Kim, S.W. Dietary L-tryptophan supplementation with reduced large neutral amino acids enhances feed efficiency and decreases stress hormone secretion in nursery pigs under social-mixing stress. J. Nutr. 2012, 142, 1540–1546. [Google Scholar] [CrossRef]
- Matte, J.J.; Corrent, E.; Simongiovanni, A. Tryptophan metabolism, growth responses, and postprandial insulin metabolism in weaned piglets according to the dietary provision of niacin (vitamin B) and tryptophan. J. Anim. Sci. 2016, 94, 1961. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.T.; Tan, L.M.; You, C.Y.; Lan, T.Y.; Li, W.X.; Xu, Y.T. Effects of dietary niacinamide and CP concentrations on the nitrogen excretion, growth performance, and meat quality of pigs. Animal 2023, 17, 100869. [Google Scholar] [CrossRef]
- Surjana, D.; Halliday, G.M.; Damian, D.L. Role of niacinamide in DNA damage, mutagenesis, and DNA repair. J. Nucleic Acids 2010, 2010, 157591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Zhang, Y.; Fei, M.; Li, Z.; Ren, D.; Wang, C.; Wei, X. Nicotinamide benefited amino acid metabolism and rumen fermentation pattern to improve growth performance of growing lambs. Anim. Biosci. 2024, 37, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cui, Z.; Wei, X.; Wang, J.; Yao, J.; Cai, C.; Wang, J. Nicotinamide supplementation alters plasma lipidomic profiles of peripartal dairy cows. Anim. Sci. J. 2023, 94, e13857. [Google Scholar] [CrossRef] [PubMed]
- Wohlrab, J.; Kreft, D. Niacinamide—Mechanisms of action and its topical use in dermatology. Skin. Pharmacol. Physiol. 2014, 27, 311. [Google Scholar] [CrossRef]
- Yuan, K.; Shaver, R.D.; Bertics, S.J.; Espineira, M.; Grummer, R.R. Effect of rumen-protected niacin on lipid metabolism, oxidative stress, and performance of transition dairy cows. J. Dairy Sci. 2012, 95, 2673. [Google Scholar] [CrossRef]
- Wang, Z.; Yi, Z.; Wang, Q.; Yin, L.; Li, J.; Xie, J. Effect of different levels of niacin on serum biochemical parameters, antioxidant status, cytokine levels, inflammatory gene expression and colonic microbial composition in weaned piglets. Animals 2022, 12, 3018. [Google Scholar] [CrossRef]
- Zhen, R.; Liu, C.; Wei, C.; Luo, Y.; Hu, X.; Liu, G.; Yi, H.; Huang, Y. Effect of different dosages of sodium butyrate and niacin on growth, faecal microbiota and Vitamin B metabolism in weaned piglets. J. Appl. Microbiol. 2022, 132, 4466–4475. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Xiong, Y.; Wu, Q.; Jiang, Z. Effects of niacin on intestinal immunity, microbial community and intestinal barrier in weaned piglets during starvation. Int. Immunopharmacol. 2021, 95, 107584. [Google Scholar] [CrossRef]
- Luecke, R.W.; Mcmillen, W.N.; Et, A. The relationship of nicotinic acid, tryptophane and protein in the nutrition of the pig. J. Nutr. 1947, 33, 251. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Ghatge, M.S.; Safo, M.K. Hemoglobin: Structure, function and allostery. Subcell. Biochem. 2020, 94, 345. [Google Scholar] [CrossRef]
- Huang, G.; Mei, X.; Hu, J. The antioxidant activities of natural polysaccharides. Curr. Drug. Targets. 2017, 18, 1296. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.; Grenier, D. Understanding the virulence of streptococcus suis: A veterinary, medical, and economic challenge. Med. Mal. Infect. 2018, 48, 159. [Google Scholar] [CrossRef] [PubMed]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567. [Google Scholar] [CrossRef]
- Duarte, M.E.; Kim, S.W. Phytobiotics from oregano extracts enhance the intestinal health and growth performance of pigs. Antioxidants 2022, 11, 2066. [Google Scholar] [CrossRef]
- Wei, P.; Sun, W.; Hao, S.; Deng, L.; Zou, W.; Wu, H.; Lu, W.; He, Y. Dietary supplementation of crossbred pigs with glycerol, vitamin C, and niacinamide alters the composition of gut flora and gut flora-derived metabolites. Animals 2024, 14, 2198. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Yin, D.; Wu, W.; Sun, X.; Zhang, Y.; Guo, X.; Chen, J.; Yuan, J. Effect of supplementation of nicotinamide and sodium butyrate on the growth performance, liver mitochondrial function and gut microbiota of broilers at high stocking density. Food. Funct. 2019, 10, 7081–7090. [Google Scholar] [CrossRef] [PubMed]
- Bannantine, J.P.; Bayles, D.O.; Waters, W.R.; Palmer, M.V.; Stabel, J.R.; Paustian, M.L. Early antibody response against mycobacterium avium subspecies paratuberculosis antigens in subclinical cattle. Proteome. Sci. 2008, 6, 5. [Google Scholar] [CrossRef]
- Barnett, A.M.; Roy, N.C.; Cookson, A.L.; McNabb, W.C. Metabolism of caprine milk carbohydrates by probiotic bacteria and Caco−2:HT29(−)MTX epithelial co-cultures and their impact on intestinal barrier integrity. Nutrients 2018, 10, 949. [Google Scholar] [CrossRef]
- Deng, L.; Hao, S.; Zou, W.; Wei, P.; Sun, W.; Wu, H.; Lu, W.; He, Y. Effects of supplementing growing-finishing crossbred pigs with glycerin, vitamin C, and niacinamide on carcass characteristics and meat quality. Animals 2023, 13, 3635. [Google Scholar] [CrossRef]
- Yi, Z.; Tan, X.; Wang, Q.; Huang, P.; Li, Y.; Ding, X. Dietary niacin affects intestinal morphology and functions via modulating cell proliferation in weaned piglets. Food. Funct. 2021, 12, 7402–7414. [Google Scholar] [CrossRef]
| Items | Diet |
|---|---|
| Ingredients | |
| Corn | 80.14 |
| Soybean meal, 46% CP | 6.39 |
| Wheat bran | 8.82 |
| Limestone | 0.89 |
| CaHPO4 | 0.55 |
| NaCl | 0.70 |
| L-Lys HCl (78%) | 0.33 |
| DL-Met (99%) | 0.04 |
| L-Thr (98.5%) | 0.12 |
| L-Trp (98%) | 0.02 |
| Premix 1 | 2.00 |
| Total | 100 |
| Analyzed composition | |
| Dry matter | 87.67 |
| Crude protein | 11.58 |
| Neutral detergent fiber | 8.24 |
| Ether extract | 2.29 |
| Ca | 0.55 |
| Total P | 0.54 |
| Calculated composition | |
| Net energy, kcal/kg | 2450 |
| SID Lys | 0.60 |
| SID Met | 0.20 |
| SID Thr | 0.42 |
| SID Trp | 0.11 |
| Items | Treatments | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NAM 30 | NAM 130 | NAM 230 | NAM 330 | NAM30 vs. NAM130 | NAM130 vs. NAM230 | Linear | Quad | ||
| Initial BW, kg | 80.50 | 80.03 | 80.25 | 80.63 | 7.89 | 0.967 | 0.984 | 0.986 | 0.998 |
| Final BW, kg | 102.75 | 103.13 | 103.88 | 106.13 | 7.78 | 0.973 | 0.947 | 0.741 | 0.941 |
| ADG, g/d | 742 | 770 | 788 | 850 | 58 | 0.732 | 0.837 | 0.177 | 0.398 |
| ADFI, kg/d | 2.79 | 2.92 | 2.70 | 3.06 | 0.18 | 0.372 | 0.158 | 0.246 | 0.323 |
| G:F | 0.27 | 0.26 | 0.29 | 0.28 | 0.01 | 0.944 | 0.382 | 0.451 | 0.698 |
| Items | Treatments | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NAM 30 | NAM 130 | NAM 230 | NAM 330 | NAM30 vs. NAM130 | NAM130 vs. NAM230 | Linear | Quad | ||
| WBC, ×109/L | 12.31 | 13.97 | 14.31 | 13.61 | 1.56 | 0.441 | 0.880 | 0.474 | 0.554 |
| LYM, ×109/L | 6.71 | 7.79 | 9.21 | 7.64 | 2.99 | 0.370 | 0.277 | 0.294 | 0.177 |
| NEU, ×109/L | 3.95 | 4.25 | 3.19 | 4.38 | 0.75 | 0.774 | 0.341 | 0.864 | 0.806 |
| EOS, ×109/L | 0.35 | 0.55 | 0.55 | 0.33 | 0.15 | 0.309 | 0.982 | 0.930 | 0.303 |
| BASO, ×109/L | 0.04 | 0.04 | 0.05 | 0.03 | 0.02 | 0.768 | 0.619 | 0.601 | 0.808 |
| RBC, ×1012/L | 7.65 | 8.14 | 8.22 | 7.98 | 0.41 | 0.385 | 0.884 | 0.493 | 0.489 |
| HGB, g/L | 149.50 | 161.67 | 155.33 | 152.25 | 3.03 | 0.049 | 0.302 | 0.847 | 0.193 |
| PLT, ×109/L | 169.75 | 222.33 | 166.67 | 223.25 | 42.32 | 0.370 | 0.374 | 0.466 | 0.775 |
| Items | Treatments | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NAM 30 | NAM 130 | NAM 230 | NAM 330 | NAM30 vs. NAM130 | NAM130 vs. NAM230 | Linear | Quad | ||
| ALB, g/L | 42.90 | 43.50 | 42.03 | 41.70 | 0.85 | 0.632 | 0.259 | 0.192 | 0.384 |
| TP, g/L | 72.20 | 72.17 | 70.13 | 72.37 | 2.70 | 0.993 | 0.609 | 0.893 | 0.902 |
| GLB, g/L | 29.30 | 28.67 | 28.10 | 30.67 | 2.61 | 0.868 | 0.882 | 0.748 | 0.776 |
| GLU, mmol/L | 5.84 | 4.45 | 4.89 | 4.78 | 0.52 | 0.192 | 0.560 | 0.275 | 0.279 |
| BUN, mmol/L | 5.72 | 5.52 | 5.38 | 6.27 | 0.51 | 0.788 | 0.857 | 0.499 | 0.445 |
| TC, mmol/L | 2.95 | 2.83 | 2.53 | 2.86 | 0.17 | 0.648 | 0.257 | 0.511 | 0.381 |
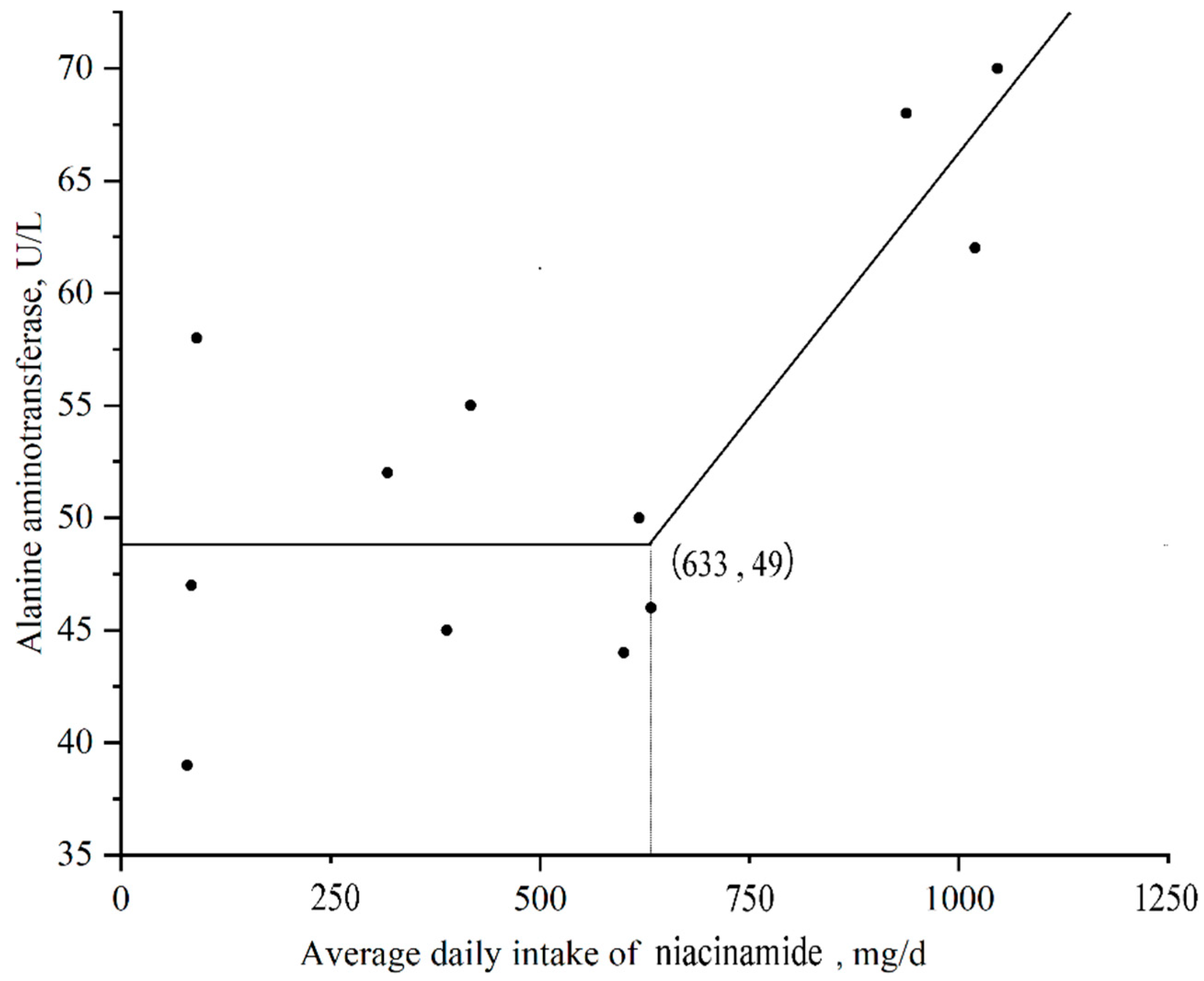
| ALT, U/L | 48.00 | 46.67 | 50.67 | 66.67 | 3.46 | 0.601 | 0.438 | 0.032 | 0.017 |
| ALP, U/L | 129.33 | 135.33 | 144.33 | 170.67 | 13.91 | 0.467 | 0.659 | 0.090 | 0.200 |
| Cr, μmol/L | 120.67 | 118.67 | 118.00 | 120.67 | 9.39 | 0.884 | 0.961 | 0.986 | 0.966 |
| Items | Treatments | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NAM 30 | NAM 130 | NAM 230 | NAM 330 | NAM30 vs. NAM130 | NAM130 vs. NAM230 | Linear | Quad | ||
| SOD, pg/mL | 216.48 | 234.82 | 210.27 | 207.04 | 25.97 | 0.631 | 0.523 | 0.632 | 0.818 |
| GSH-px, pmol/mL | 65.95 | 67.30 | 76.09 | 73.87 | 3.60 | 0.798 | 0.123 | 0.066 | 0.180 |
| T-AOC, U/mL | 7.07 | 6.61 | 5.01 | 6.22 | 0.65 | 0.633 | 0.119 | 0.212 | 0.239 |
| MDA, nmol/L | 1.55 | 1.22 | 1.74 | 1.42 | 0.05 | 0.013 | 0.001 | 0.776 | 0.961 |
| CAT, ng/L | 64.66 | 77.32 | 61.77 | 79.40 | 6.40 | 0.200 | 0.125 | 0.396 | 0.674 |
| IL-1β, ng/L | 108.42 | 90.54 | 121.66 | 128.11 | 7.69 | 0.148 | 0.024 | 0.034 | 0.162 |
| IL-6, ng/L | 1029.37 | 1051.87 | 1170.73 | 961.64 | 91.76 | 0.867 | 0.386 | 0.846 | 0.474 |
| TNF-α, pg/mL | 325.98 | 353.25 | 376.90 | 334.61 | 41.47 | 0.654 | 0.697 | 0.782 | 0.663 |
| IgG, μg/mL | 493.56 | 421.18 | 461.18 | 460.06 | 36.89 | 0.203 | 0.465 | 0.719 | 0.600 |
| IgA, μg/mL | 46.26 | 40.87 | 33.58 | 39.63 | 3.49 | 0.307 | 0.179 | 0.138 | 0.109 |
| Items | Treatments | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NAM 30 | NAM 130 | NAM 230 | NAM 330 | NAM30 vs. NAM130 | NAM130 vs. NAM230 | Linear | Quad | ||
| TVFAs, mmol/L | 43.87 | 38.61 | 44.80 | 52.57 | 4.75 | 0.557 | 0.572 | 0.174 | 0.233 |
| Acetic acid, mmol/L | 24.33 | 21.22 | 22.59 | 29.43 | 3.49 | 0.458 | 0.786 | 0.136 | 0.083 |
| Propionic acid, mmol/L | 10.07 | 8.42 | 10.77 | 11.06 | 1.73 | 0.425 | 0.357 | 0.416 | 0.574 |
| Butyric acid, mmol/L | 4.88 | 5.05 | 7.27 | 7.12 | 1.66 | 0.930 | 0.366 | 0.086 | 0.241 |
| Isobutyric acid, mmol/L | 0.65 | 0.52 | 0.53 | 0.69 | 0.11 | 0.353 | 0.975 | 0.697 | 0.274 |
| Items | Treatments | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NAM 30 | NAM 130 | NAM 230 | NAM 330 | NAM30 vs. NAM130 | NAM130 vs. NAM230 | Linear | Quad | ||
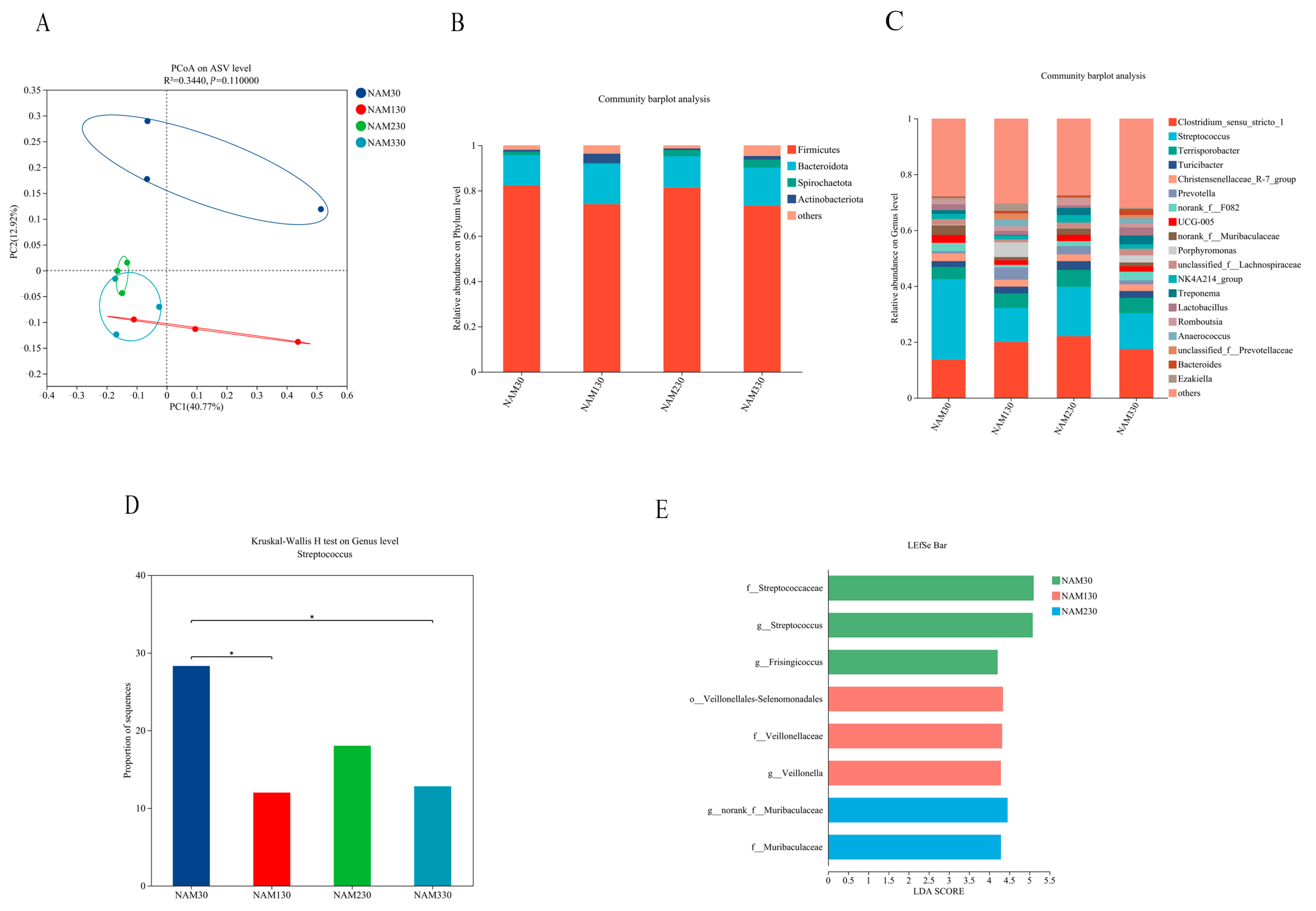
| Shannon index | 2.96 | 3.31 | 3.15 | 3.56 | 0.13 | 0.149 | 0.481 | 0.044 | 0.146 |
| Simpson index | 0.17 | 0.09 | 0.11 | 0.07 | 0.02 | 0.023 | 0.408 | 0.022 | 0.063 |
| Chao index | 107 | 108 | 98 | 110.33 | 2.70 | 0.869 | 0.126 | 0.999 | 0.499 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Tang, F.; Shi, Y.; Tan, Q.; Ju, Q.; Yang, Z.; Yang, G.; Gao, P.; Kim, S.W.; Xi, L.; et al. Functional Requirement of Niacinamide for Blood Profiles, Antioxidant Status, and Intestinal Health in Finishing Pigs Fed a Low-Protein Diet. Animals 2025, 15, 1813. https://doi.org/10.3390/ani15121813
Zhao Y, Tang F, Shi Y, Tan Q, Ju Q, Yang Z, Yang G, Gao P, Kim SW, Xi L, et al. Functional Requirement of Niacinamide for Blood Profiles, Antioxidant Status, and Intestinal Health in Finishing Pigs Fed a Low-Protein Diet. Animals. 2025; 15(12):1813. https://doi.org/10.3390/ani15121813
Chicago/Turabian StyleZhao, Yan, Fangli Tang, Yunlong Shi, Qinyu Tan, Qingxin Ju, Ziyi Yang, Guanqing Yang, Pengfei Gao, Sung Woo Kim, Lin Xi, and et al. 2025. "Functional Requirement of Niacinamide for Blood Profiles, Antioxidant Status, and Intestinal Health in Finishing Pigs Fed a Low-Protein Diet" Animals 15, no. 12: 1813. https://doi.org/10.3390/ani15121813
APA StyleZhao, Y., Tang, F., Shi, Y., Tan, Q., Ju, Q., Yang, Z., Yang, G., Gao, P., Kim, S. W., Xi, L., Cao, G., & Li, B. (2025). Functional Requirement of Niacinamide for Blood Profiles, Antioxidant Status, and Intestinal Health in Finishing Pigs Fed a Low-Protein Diet. Animals, 15(12), 1813. https://doi.org/10.3390/ani15121813





