Preliminary Study of the Antimicrobial Capacity of the Cutaneous Mucus and Smear Cytology of the Epidermis in a Population of European eels (Anguilla anguilla, Linnaeus 1758)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Bacterial Growth
2.3. Antibacterial Plate Assay
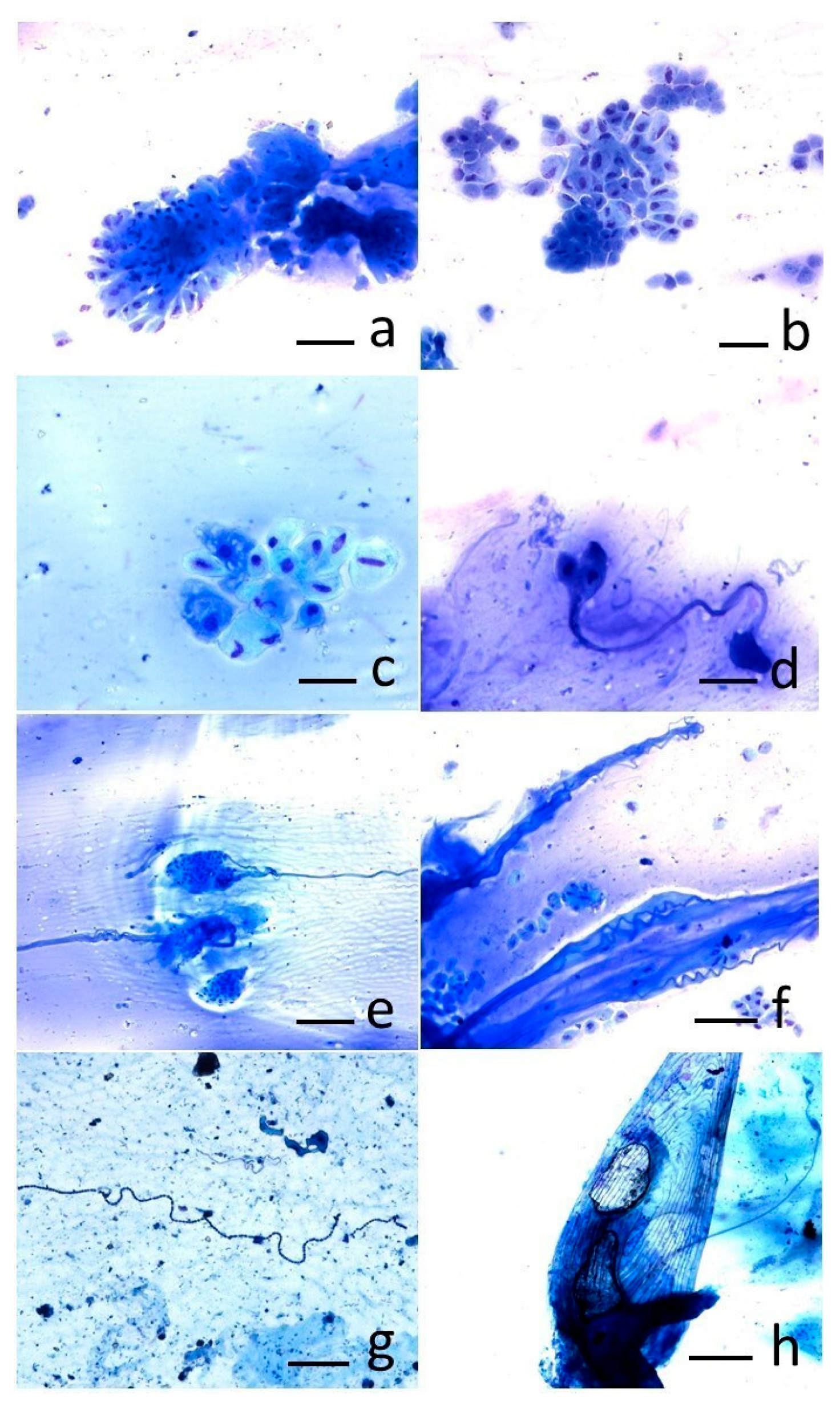
2.4. Cytological Analysis
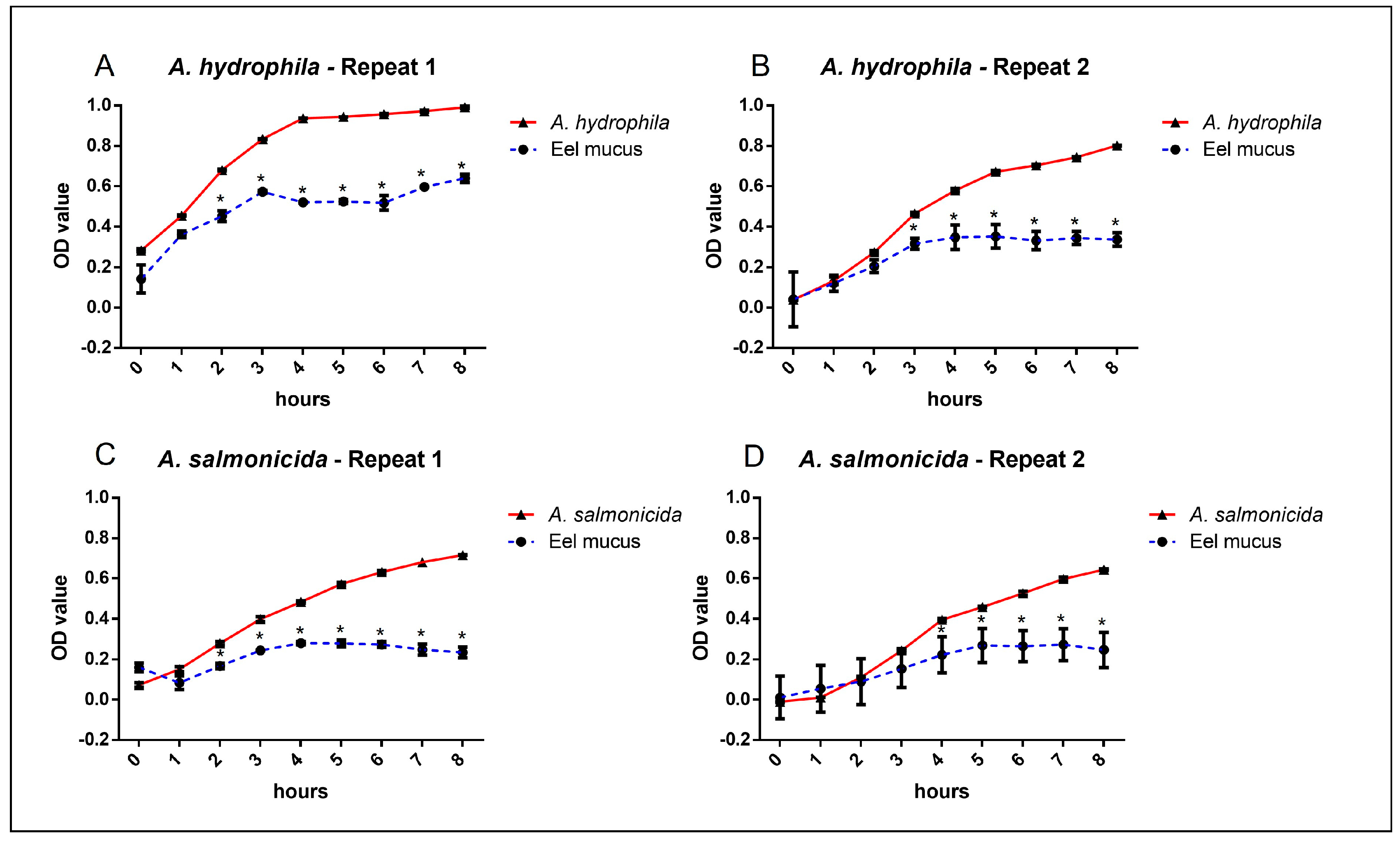
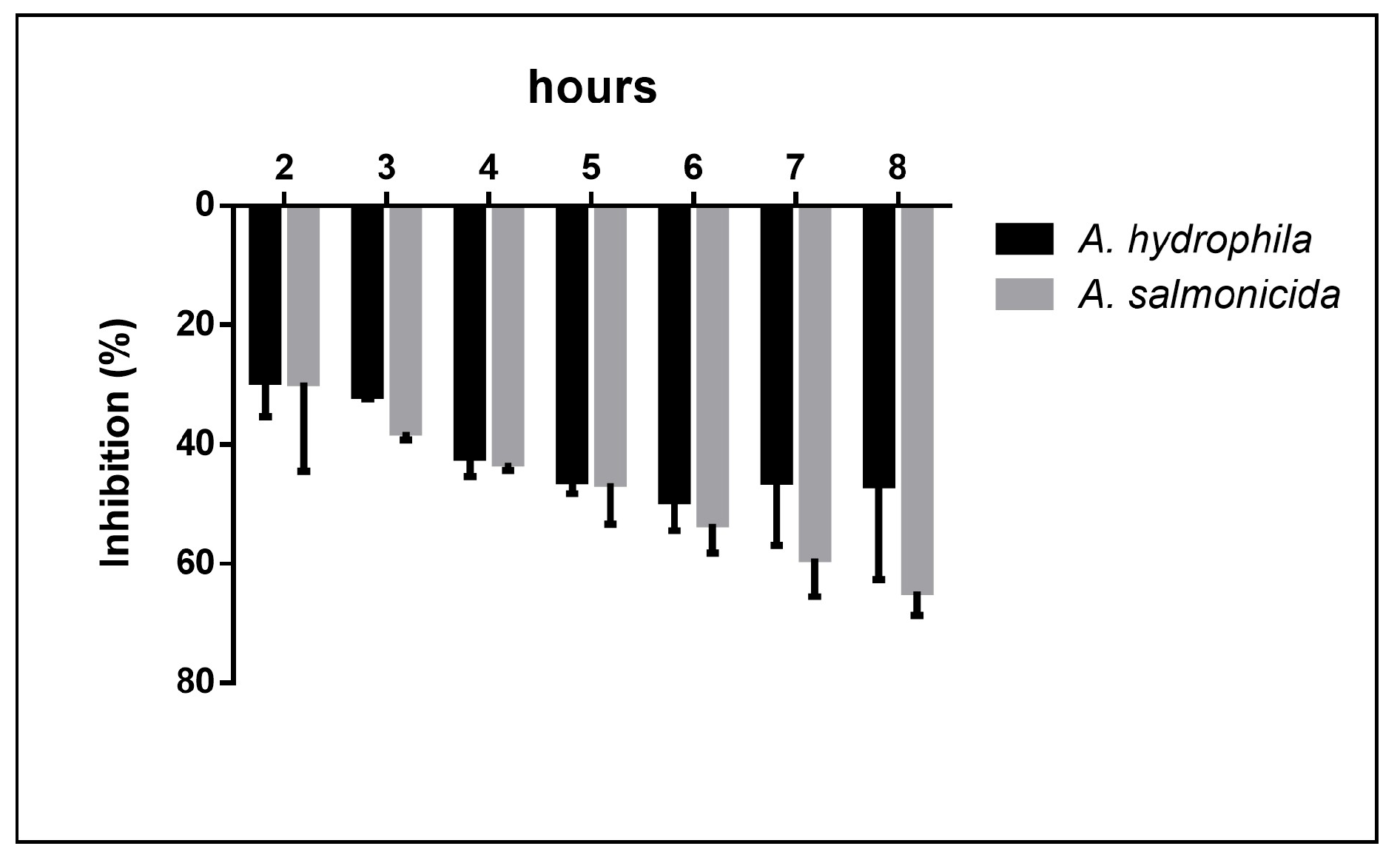
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guardiola, F.A.; Cuesta, A.; Abellán, E.; Meseguer, J.; Esteban, M.A. Comparative analysis of the humoral immunity of skin mucus from several marine teleost fish. Fish Shellfish Immunol. 2014, 40, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alacid, L.; Firmino, J.P.; Sanahuja, I.; Madrid, C.; Polo, J.; de Borba, M.R.; Balsalobre, C.; Gisbert, E.; Ibarz, A. Impact of dietary porcine blood by-products in meagre (Argyrosomus regius) physiology, evaluated by welfare biomarkers and the antibacterial properties of the skin mucus. Fish Shellfish Immunol. 2021, 118, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Sanahuja, I.; Fernández-Alacid, L.; Ordóñez-Grande, B.; Sánchez-Nuño, S.; Ramos, A.; Araujo, R.M.; Ibarz, A. Comparison of several non-specific skin mucus immune defences in three piscine species of aquaculture interest. Fish Shellfish Immunol. 2019, 89, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alacid, L.; Sanahuja, I.; Ordóñez-Grande, B.; Sánchez-Nuño, S.; Viscor, G.; Gisbert, E.; Herrera, M.; Ibarz, A. Skin mucus metabolites in response to physiological challenges: A valuable non-invasive method to study teleost marine species. Sci. Total Environ. 2018, 644, 1323–1335. [Google Scholar] [CrossRef]
- Haenen, O. Major eel diseases in Europe: The past 30 years. In EELS: Biology, Monitoring, Management, Culture and Exploitation; Coulson, P., Don, A., Eds.; CABI: Wallingford, UK, 2019; pp. 257–279. [Google Scholar]
- Menanteau-Ledouble, S.; Kumar, G.; Saleh, M.; El-Matbouli, M. Aeromonas salmonicida: Updates on an old acquaintance. DAO 2016, 120, 49–68. [Google Scholar] [CrossRef]
- Hollinger, C.; Newton, A.L. Fish. In Veterinary Cytology; Sharkey, L.C., Radin, M.J., Seelig, D., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2021; pp. 876–881. [Google Scholar]
- Subramanian, S.; Ross, N.W.; MacKinnon, S.L. Comparison of antimicrobial activity in the epidermal mucus extracts of fish. Comp. Biochem. Physiol. Part B 2008, 150, 85–92. [Google Scholar] [CrossRef]
- Palaksha, K.J.; Shin, G.W.; Kim, Y.R.; Jung, T.S. Evaluation of non-specific immune components from the skin mucus of ol-ive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2008, 24, 479–488. [Google Scholar] [CrossRef]
- Fuochi, V.; Li Volti, G.; Camiolo, G.; Tiralongo, F.; Giallongo, C.; Distefano, A.; Petronio Petronio, G.; Barbagallo, I.; Viola, M.; Furneri, P.M.; et al. Antimicrobial and anti-proliferative effects of skin mucus derived from Dasyatis pastinaca (Linnaeus, 1758). Mar. Drugs 2017, 15, 342. [Google Scholar] [CrossRef]
- Ebron, N.; Julien, S.; Orange, N.; Auperin, B.; Molle, G. Isolation and characterization of novel glycoproteins from fish epidermal mucus: Correlation between their pore-forming properties and their antibacterial activities. Biochim. Biophys. Acta 2000, 1467, 271–280. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tasumi, S.; Tsutsui, S.; Okamoto, M.; Suetake, H. Molecular diversity of skin mucus lectins in fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 136, 723–730. [Google Scholar] [CrossRef]
- Zheng, C.C.; Cai, X.Y.; Huang, M.M.; Mkingule, I.; Sun, C.; Qian, S.C.; Wu, Z.J.; Han, B.N.; Fei, H. Effect of biological additives on Japanese eel (Anguilla japonica) growth performance, digestive enzymes activity and immunology. Fish Shellfish Immunol. 2019, 84, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; He, L.; Wu, L.; Xiao, Y.; Zhai, S.; Yan, Q. Immunization of a novel bivalent outer membrane protein simultaneously resisting Aeromonas hydrophila, Edwardsiella anguillarum and Vibrio vulnificus infection in European eels (Anguilla anguilla). Fish Shellfish Immunol. 2020, 97, 46–57. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wu, L.; Tang, Y.; Lin, P.; Zhai, S.; Xiao, Y.; Guo, S. Immunization of a novel outer membrane protein from Aeromonas hydrophila simultaneously resisting A. hydrophila and Edwardsiella anguillarum infection in European eels (Angullia angullia). Fish Shellfish Immunol. 2020, 97, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.A.; Liles, M.R.; Hossain, M.J.; Griffin, M.J.; Hemstreet, W.G. 1.2.9 Motile Aeromonas Septicemia. In American Fisheries Society-Fish Health Section Blue Book; American Fisheries Society: Washington, DC, USA, 2014; pp. 1–11. Available online: https://units.fisheries.org/fhs/wp-content/uploads/sites/30/2017/08/1.2.9-Motile-Aeromonas-Septicemia-2014.pdf (accessed on 28 April 2025).
- Huang, L.; Qin, Y.; Yan, Q.; Lin, G.; Huang, L.; Huang, B.; Huang, W. MinD plays an important role in Aeromonas hydrophila adherence to Anguilla japonica mucus. Gene 2015, 565, 275–281. [Google Scholar] [CrossRef]
- Qin, Y.; Lin, G.; Chen, W.; Xu, X.; Yan, Q. Flagellar motility is necessary for Aeromonas hydrophila adhesion. Microb. Pathog. 2016, 98, 160–166. [Google Scholar] [CrossRef]
- Lin, G.; Chen, W.; Su, Y.; Qin, Y.; Huang, L.; Yan, Q. Ribose operon repressor (RbsR) contributes to the adhesion of Aeromonas hydrophila to Anguilla japonica mucus. Microbiologyopen 2017, 6, e00451. [Google Scholar] [CrossRef]
- Esteve, C.; Biosca, E.G.; Amaro, C. Virulence of Aeromonas hydrophila and some other bacteria isolated from European eels Anguilla anguilla reared in fresh water. DAO 1993, 16, 15–20. [Google Scholar] [CrossRef]
- Esteve, C.; Alcaide, E. Influence of diseases on the wild eel stock: The case of Albufera Lake. Aquaculture 2009, 289, 143–149. [Google Scholar] [CrossRef]
- Parchemin, C.; Tapissier-Bontemps, N.; Sasal, P.; Faliex, E. Anguilla sp. diseases diagnoses and treatments: The ideal methods at the crossroads of conservation and aquaculture purposes. J. Fish Dis. 2022, 45, 943–969. [Google Scholar] [CrossRef]
- Pedersen, K.; Garcia, J.A.; Larsen, J.L. Aeromonas salmonicida a potential pathogen in modern eel (Anguilla anguilla) farming? Bull. Eur. Ass Fish Pathol. 1999, 19, 127–129. [Google Scholar]
- Esteban, M.Á. An overview of the immunological defenses in fish skin. ISRN Immunol. 2012, 2012, 853470. [Google Scholar] [CrossRef]
- Gómez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Easy, R.H.; Ross, N.W. Changes in Atlantic salmon (Salmo salar) epidermal mucus proteins during infection with the salmon louse (Lepeophtheirus salmonis). Comp. Biochem. Physiol. Part D Genom. Proteom. 2009, 4, 159–167. [Google Scholar] [CrossRef]
- Brinchmann, M.F. Immune relevant molecules identified in the skin mucus of fish using-omics technologies. Mol. Biosyst. 2016, 12, 2056–2063. [Google Scholar] [CrossRef]
- Rainger, G.E.; Rowley, A.F. Antibacterial activity in the mucus of rainbow trout, Oncorhynchus mykiss (Walbaum), against Aeromonas salmonicida. J. Fish Dis. 1993, 16, 395–401. [Google Scholar] [CrossRef]
- Dash, S.; Das, S.K.; Samal, J.; Thatoi, H. Epidermal mucus, a major determinant in fish health: A review. Iran. J. Vet. Res. 2018, 19, 72–81. [Google Scholar]
- Pethkar, M.R.; Lokhande, M.V. Antifungal activity of skin mucus of three cultivable fish species (Catla-catla, Cirrhinus mrigala and Anguilla anguilla). Int. J. Zool. Stud. 2017, 2, 1–3. [Google Scholar]
- Hussain, A.; Kumar, M.; Mukhopadhyay, K.; Dutta, A.; Sachan, S.G. Antimicrobial peptides in Clarias batrachus epidermal mucus: Characterization and therapeutic potential. Fish Shellfish Immunol. 2025, 159, 110191. [Google Scholar] [CrossRef]
- Conforto, E.; Vílchez-Gómez, L.; Parrinello, D.; Parisi, M.G.; Esteban, M.Á.; Cammarata, M.; Guardiola, F.A. Role of mucosal immune response and histopathological study in European eel (Anguilla anguilla L.) intraperitoneal challenged by Vibrio anguillarum or Tenacibaculum soleae. Fish Shellfish Immunol. 2021, 114, 330–339. [Google Scholar] [CrossRef]
- Smith, V.J.; Fernandes, J.M.O.; Kemp, G.D. Antimicrobial peptides in teleost fish: A review. Fish Shellfish Immunol. 2015, 45, 805–814. [Google Scholar] [CrossRef]
- Fernandes, J.M.O.; Kemp, G.D.; Smith, V.J. Quorum sensing and quorum quenching in aquatic organisms: Implications for disease control. J. Fish Dis. 2021, 44, 1291–1303. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.S. Fish health aspects in grouper aquaculture. Aquaculture 2011, 320, 1–21. [Google Scholar] [CrossRef]
- Salinas, I. The mucosal immune system of teleost fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Hellio, C.; Pons, A.M.; Beaupoil, C.; Bourgougnon, N.; Le Gal, Y. Antibacterial, antifungal and cytotoxic activities of extracts from fish epidermis and epidermal mucus. Int. J. Antimicrobial. Agents 2002, 20, 214–219. [Google Scholar] [CrossRef]
- Benhamed, S.; Guardiola, F.A.; Mars, M.; Esteban, M.Á. Pathogen bacteria adhesion to skin mucus of fishes. Vet. Microbiol. 2014, 171, 1–12. [Google Scholar] [CrossRef]
- Elliott, D.G. Functional Morphology of the Integumentary System in Fishes. In Encyclopedia of Fish Physiology: From Genome to Environment; Farrell, A.P., Ed.; Academic Press: San Diego, CA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 1, pp. 476–488. [Google Scholar]
- Morini, M.; Gobbo, F.; Rinnovati, R.; Romagnoli, N.; Peli, A.; Massarenti, C.; Spadari, A.; Pietra, M. Bronchoalveolar lavage cytology in severe equine asthma: Cytocentrifugated versus sediment smear preparations. Vet. Sci. 2023, 10, 527. [Google Scholar] [CrossRef]
- Vezza, P.R.; Montgomery, E.A. Images in clinical medicine. Curschmann’s spirals. N. Engl. J. Med. 1998, 339, 1043. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpe, E.; Ciulli, S.; Morini, M.; Gentile, L.; Casalini, A.; Gentilezza, C.; Mandrioli, L. Preliminary Study of the Antimicrobial Capacity of the Cutaneous Mucus and Smear Cytology of the Epidermis in a Population of European eels (Anguilla anguilla, Linnaeus 1758). Animals 2025, 15, 1810. https://doi.org/10.3390/ani15121810
Volpe E, Ciulli S, Morini M, Gentile L, Casalini A, Gentilezza C, Mandrioli L. Preliminary Study of the Antimicrobial Capacity of the Cutaneous Mucus and Smear Cytology of the Epidermis in a Population of European eels (Anguilla anguilla, Linnaeus 1758). Animals. 2025; 15(12):1810. https://doi.org/10.3390/ani15121810
Chicago/Turabian StyleVolpe, Enrico, Sara Ciulli, Maria Morini, Laura Gentile, Antonio Casalini, Chiara Gentilezza, and Luciana Mandrioli. 2025. "Preliminary Study of the Antimicrobial Capacity of the Cutaneous Mucus and Smear Cytology of the Epidermis in a Population of European eels (Anguilla anguilla, Linnaeus 1758)" Animals 15, no. 12: 1810. https://doi.org/10.3390/ani15121810
APA StyleVolpe, E., Ciulli, S., Morini, M., Gentile, L., Casalini, A., Gentilezza, C., & Mandrioli, L. (2025). Preliminary Study of the Antimicrobial Capacity of the Cutaneous Mucus and Smear Cytology of the Epidermis in a Population of European eels (Anguilla anguilla, Linnaeus 1758). Animals, 15(12), 1810. https://doi.org/10.3390/ani15121810






