Sperm Membrane: Molecular Implications and Strategies for Cryopreservation in Productive Species
Simple Summary
Abstract
1. Introduction
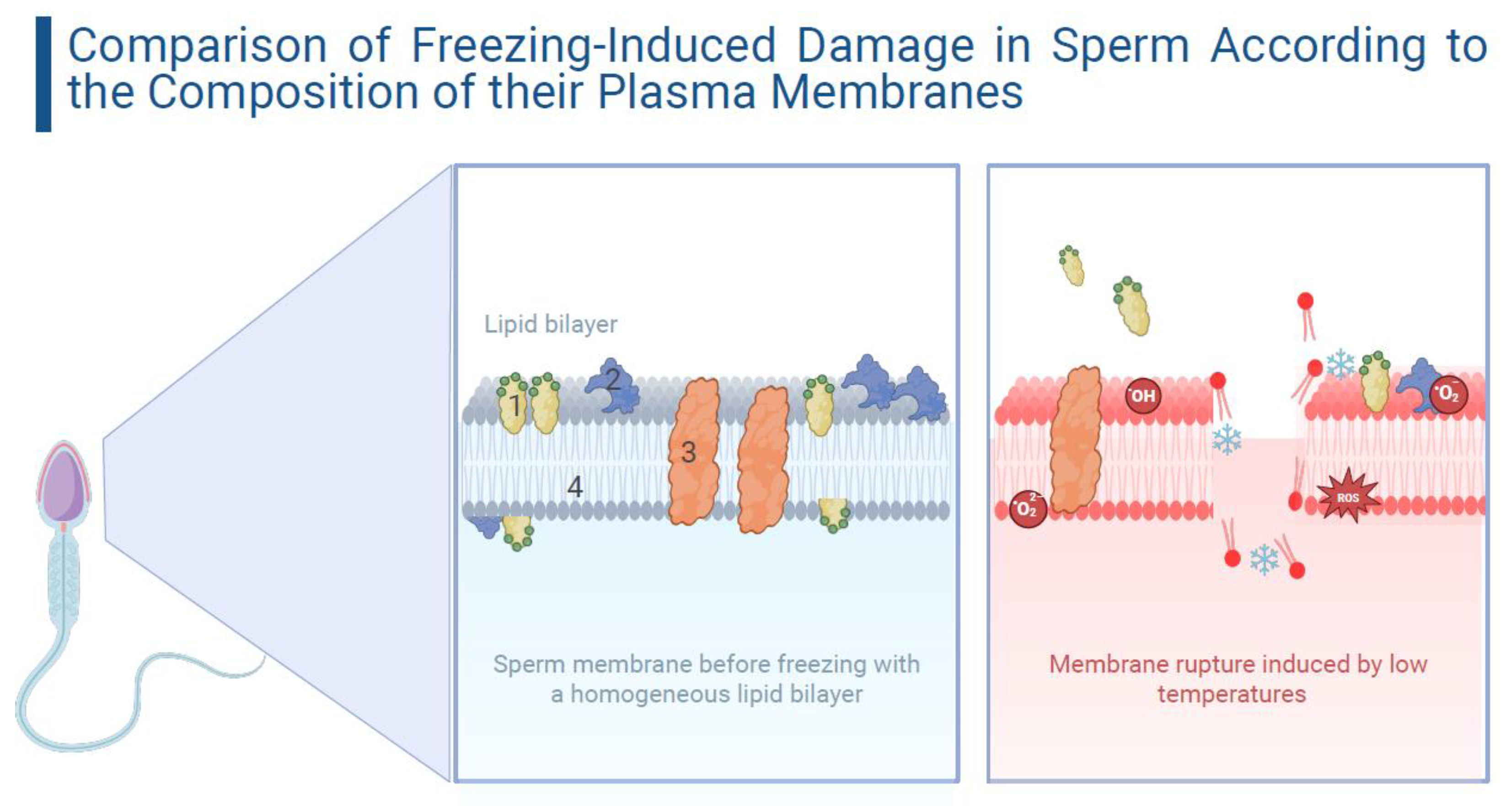
2. Impact on the Sperm Membrane During Cryopreservation
3. Specific Variations in Sperm Responses to Cryopreservation
3.1. Cryopreservation-Induced Damage in Ruminant Sperm: Focus on Bovine and Ovine Species
3.2. Cryopreservation-Induced Damage in Ruminant Sperm: Focus on Equine and Porcine Species
4. Strategies to Combat Sperm Membrane Damage
5. Future Projections
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Prasad, J.K.; Srivastava, N.; Ghosh, S.K. Strategies to minimize various stress-related freeze–thaw damages during conventional cryopreservation of mammalian spermatozoa. Biopreserv. Biobank. 2019, 17, 603–612. [Google Scholar] [CrossRef]
- Bailey, J.L.; Bilodeau, J.F.; Cormier, N. Sperm cryopreservation in domestic animals: A damaging and capacitating phenomenon. J. Androl. 2000, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Benko, F.; Fialková, V.; Žiarovská, J.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. In Vitro versus Cryo-Induced Capacitation of Bovine Spermatozoa, Part 2: Changes in the Expression Patterns of Selected Transmembrane Channels and Protein Kinase A. Int. J. Mol. Sci. 2022, 23, 14646. [Google Scholar] [CrossRef] [PubMed]
- Grötter, L.G.; Cattaneo, L.; Marini, P.E.; Kjelland, M.E.; Ferré, L.B. Recent advances in bovine sperm cryopreservation techniques with a focus on sperm post-thaw quality optimization. Reprod. Domest. Anim. 2019, 54, 655–665. [Google Scholar] [CrossRef]
- Barbas, J.P.; Mascarenhas, R.D. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank. 2009, 10, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Khalil, W.A.; El-harairy, M.A.; Zeidan, A.E.B.; Hassan, M.A.E.; Mohey-Elsaeed, O. Evaluation of bull spermatozoa during and after cryopreservation: Structural and ultrastructural insights. Int. J. Vet. Sci. Med. 2018, 6, S49–S56. [Google Scholar] [CrossRef]
- Ozimic, S.; Ban-Frangez, H.; Stimpfel, M. Sperm Cryopreservation Today: Approaches, Efficiency, and Pitfalls. Curr. Issues Mol. Biol. 2023, 45, 4716–4734. [Google Scholar] [CrossRef]
- Yánez-Ortiz, I.; Catalán, J.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim. Reprod. Sci. 2022, 246, 106904. [Google Scholar] [CrossRef]
- Sharafi, M.; Borghei-Rad, S.M.; Hezavehei, M.; Shahverdi, A.; Benson, J.D. Cryopreservation of sperm in domestic animals: A review of current challenges, applications, and prospective strategies. Animals 2022, 12, 3271. [Google Scholar] [CrossRef]
- Holt, W.V. Fundamental aspects of sperm cryobiology: The importance of species and individual differences. Theriogenology 2000, 53, 47–58. [Google Scholar] [CrossRef]
- Watson, P.F. The causes of reduced fertility with cryopreserved sperm. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef] [PubMed]
- García, W.; Tabarez, A.; Palomo, M.J. Effect of type of egg yolk and removal of seminal plasma on donor age and ram sperm cryopreservation. Anim. Reprod. Sci. 2017, 145, 1124–1132. [Google Scholar] [CrossRef]
- Catalán, J.; Llavanera, M.; Bonilla-Correal, S.; Papas, M.; Gacem, S.; Rodríguez-Gil, J.E.; Yeste, M.; Miró, J. Irradiating frozen-thawed stallion sperm with red-light increases their resilience to withstand post-thaw incubation at 38 °C. Theriogenology 2020, 157, 85–95. [Google Scholar] [CrossRef]
- Fraser, L.; Zasiadczyk, L.; Mogielnicka-Brzozowska, M. Variaciones de las características de calidad del esperma de diferentes eyaculados entre verracos tras la congelación-descongelación. Cells 2025, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Januskauskas, A.; Johannisson, A.; Rodriguez-Martinez, H. Subtle membrane changes in cryopreserved bull sperm in relation with sperm viability, chromatin structure, and field fertility. Theriogenology 2003, 60, 743–758. [Google Scholar] [CrossRef]
- Rubio-Guillén, J.L.; Quintero-Moreno, A.A.; González Villalobos, D.M. Effect of cryopreservation on plasma membrane and acrosomal integrity of bull spermatozoa. Rev. Cient. 2009, 19, 382–389. [Google Scholar]
- Dias Maziero, R.R.; Guasti, P.N.; Monteiro, G.A.; Avanzi, B.R.; Hartwig, F.P.; Lisboa, F.P.; Papa, F.O. Evaluation of sperm kinetics and plasma membrane integrity of frozen equine sperm in different storage volumes and freezing conditions. J. Equine Vet. Sci. 2013, 33, 165–168. [Google Scholar] [CrossRef]
- Mocé, E.; Blanch, E.; Tomás, C.; Graham, J.K. Use of cholesterol in sperm cryopreservation: Present moment and perspectives to future. Reprod. Domest. Anim. 2010, 45 (Suppl. 2), 57–66. [Google Scholar] [CrossRef]
- Pini, T.; Leahy, T.; de Graaf, S.P. Sublethal sperm freezing damage: Manifestations and solutions. Theriogenology 2018, 118, 172–181. [Google Scholar] [CrossRef]
- Bevers, E.M.; Comfurius, P.; Dekkers, D.W.; Zwaal, R.F. Lipid translocation across the plasma membrane of mammalian cells. Biochim. Biophys. Acta 1999, 1439, 317–330. [Google Scholar] [CrossRef]
- Drobnis, E.Z.; Crowe, L.M.; Berger, T.; Anchordoguy, T.J.; Overstreet, J.W.; Crowe, J.H. Cold shock damage is due to lipid phase transitions in cell membranes: A demonstration using sperm as a model. J. Exp. Zool. 1993, 265, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Parks, J.E.; Lynch, D.V. Lipid composition and thermotropic phase behavior of boar, bull, stallion, and rooster sperm membranes. Cryobiology 1992, 29, 255–266. [Google Scholar] [CrossRef]
- Medeiros, C.M.; Forell, F.; Oliveira, A.T.; Rodrigues, J.L. Current status of sperm cryopreservation: Why isn’t it better? Theriogenology 2002, 57, 327–344. [Google Scholar] [CrossRef]
- Lemma, A. Effect of Cryopreservation on Sperm Quality and Fertility; Manafi, M., Ed.; Artificial Insemination in Farm Animals; InTech Europe: Debre Zeit, Ethiopia, 2011; pp. 191–216. [Google Scholar]
- Cabrera, T.; Ramires-Neto, C.; Belaz, K.R.A.; Freitas-Dell’aqua, C.P.; Zampieri, D.; Tata, A.; Eberlin, M.N.; Alvarenga, M.A.; Souza, F.F. Influence of spermatozoal lipidomic profile on the cryoresistance of frozen spermatozoa from stallions. Theriogenology 2018, 108, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Kadirvel, G.; Kumar, S.; Kumaresan, A.; Kathiravan, P. Capacitation status of fresh and frozen-thawed buffalo spermatozoa in relation to cholesterol level, membrane fluidity and intracellular calcium. Anim. Reprod. Sci. 2009, 116, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Ushiyama, A.; Ishikawa, N.; Tajima, A.; Asano, A. Comparison of membrane characteristics between freshly ejaculated and cryopreserved sperm in the chicken. J. Poult. Sci. 2016, 53, 305–312. [Google Scholar] [CrossRef]
- Buhr, M.M.; Curtis, E.F.; Kakuda, N.S. Composition and behavior of head membrane lipids of fresh and cryopreserved boar sperm. Cryobiology 1994, 31, 224–238. [Google Scholar] [CrossRef]
- Schiller, J.; Arnhold, J.; Glander, H.-J.; Arnold, K. Lipid analysis of human spermatozoa and seminal plasma by MALDI-TOF mass spectrometry and NMR spectroscopy—effects of freezing and thawing. Chem. Phys. Lipids 2000, 106, 145–156. [Google Scholar] [CrossRef]
- Zampini, R.; Castro-González, X.A.; Scandura, M.; Sari, L.M.; Diaz, A.V.; Martin, A.; Argañaraz, M.E.; Apichela, S.A. Cryopreservation modifies the distribution of the prostate-derived lectin SL15 on the llama (Lama glama) sperm. Theriogenology 2023, 202, 93–102. [Google Scholar] [CrossRef]
- Varghese, T.; Divyashree, B.C.; Roy, S.C.; Roy, K.S. Loss of heat shock protein 70 from apical region of buffalo (Bubalus bubalis) sperm head after freezing and thawing. Theriogenology 2016, 85, 828–834. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Wang, K.; Zhang, Y.; Zhao, X. Loss of protein kinase 2 subunit alpha 2 (CK2α’) effect ram sperm function after freezing and thawing process. Anim. Reprod. Sci. 2017, 181, 9–15. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, F.E.; Chen, H.C.; Colenbrander, B.; Verkleij, A.J. Cold-induced ultrastructural changes in bull and boar sperm plasma membranes. Cryobiology 1990, 27, 171–183. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina Chávez, D.A.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto Morales, A.; Montoro, V.; et al. Sperm cryopreservation in ruminants: Understanding the molecular changes induced by the cryopreservation process to optimize sperm quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef]
- Leahy, T.; Gadella, B.M. New insights into the regulation of cholesterol efflux from the sperm membrane. Asian J. Androl. 2015, 17, 561–567. [Google Scholar]
- Ushiyama, A.; Tajima, A.; Ishikawa, N.; Asano, A. Modification of membrane cholesterol and desmosterol in chicken spermatozoa improves post-thaw survival and prevents impairment of sperm function after cryopreservation. Reprod. Fertil. Dev. 2018, 30, 591–599. [Google Scholar] [CrossRef]
- Crockett, E.L. Cholesterol function in plasma membranes from ectotherms: Membrane-specific roles in adaptation to temperature. Am. Zool. 1998, 38, 291–304. [Google Scholar] [CrossRef]
- Karunakaran, M.; Devanathan, T.G. Evaluation of bull sperm for fertility- associated protein, in vitro characters and fertility. J. Appl. Anim. Res. 2017, 45, 136–144. [Google Scholar] [CrossRef]
- Kolyada, M.N.; Osipova, V.P.; Berberova, N.T. Use of cryoprotectors and antioxidants in sturgeon sperm cryopreservation. Cryobiology 2023, 111, 30–39. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; De Lamirande Ey Gagnon, C. Función positiva de las especies reactivas de oxígeno en la capacitación espermática de mamíferos: Activación y modulación de eventos de fosforilación. Biol. Med. Radic. Libres 2006, 41, 528–540. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Redox regulation of mammalian sperm capacitation. Asian J. Androl. 2015, 17, 583–590. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Ma, B.; Nixon, B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J. Androl. 2015, 17, 633–639. [Google Scholar] [CrossRef]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Virk, G.; Ong, C.; Du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants 2022, 11, 306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Henkel, R.; Sengupta, P.; Agarwal, A. Rol Fisiológico de Las ROS en la Función Espermática. Infertilidad Masculina; Parekattil, S., Esteves, S., Agarwal, A., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef]
- Said, T.M.; Gaglani, A.; Agarwal, A. Implication of apoptosis in sperm cryoinjury. Reprod. Biomed. Online 2010, 21, 456–462. [Google Scholar] [CrossRef]
- Makker, K.; Agarwal, A.; Sharma, R. Oxidative stress & male infertility. Indian J. Med. Res. 2009, 129, 357–367. [Google Scholar]
- Avello, M.; Suwalsky, M. Radicales libres, antioxidantes naturales y mecanismos de protección. Atenea 2006, 494, 161–172. [Google Scholar] [CrossRef]
- Bennett, D.A.; White, I.G. Influence of the cholesterol content of mammalian spermatozoa on susceptibility to cold-shock. Cryobiology 2015, 14, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Lessard, C.; Parent, S.; Leclerc, P.; Bailey, J.L.; Sullivan, R. Cryopreservation alters the levels of the bull sperm surface protein P25b. J. Androl. 2000, 21, 700–707. [Google Scholar] [CrossRef]
- Upadhyay, V.R.; Ramesh, V.; Dewry, R.K.; Kumar, G.; Raval, K.; Patoliya, P. Implications of cryopreservation on structural and functional attributes of bovine spermatozoa: An overview. Andrologia 2021, 53, e14154. [Google Scholar] [CrossRef]
- Ugur, M.R.; Saber Abdelrahman, A.; Evans, H.C.; Gilmore, A.A.; Hitit, M.; Arifiantini, R.I.; Purwantara, B.; Kaya, A.; Memili, E. Avances en la criopreservación de esperma de toro. Front. Vet. Sci. 2019, 6, 268. [Google Scholar]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on sperm functions. Vet. Med. Int. 2010, 2010, 686137. [Google Scholar]
- Pereira, R.; Sá, R.; Barros, A.; Sousa, M. Major regulatory mechanisms involved in sperm motility. Asian J. Androl. 2017, 19, 5–14. [Google Scholar]
- Santiago-Moreno, J.; Toledano-Díaz, A.; Castaño, C.; Velázquez, R.; Bóveda, P.; O’Brien, E.; Peris-Frau, P.; Pequeño, B.; Martínez-Madrid, B.; Esteso, M.C. Review: Sperm cryopreservation in wild small ruminants: Morphometric, endocrine and molecular basis of cryoresistance. Animal 2023, 17 (Suppl. 1), 100741. [Google Scholar] [CrossRef] [PubMed]
- Maylem, E.R.S.; Rivera, S.M.; Ramos, G.E.; Atabay, E.C.; Venturina, E.V.; Atabay, E.P. Changes in heat shock protein 70 (HSP70) in water buffalo spermatozoa revealed a capacitation-like event upon cryopreservation. Asian J. Agric. Biol. 2021, 3, 202007412. [Google Scholar]
- Rosyada, Z.N.A.; Ulum, M.F.; Tumbelaka, L.I.T.A.; Solihin, D.D.; Purwantara, B.; Memili, E. Implications of sperm heat shock protein 70-2 in bull fertility. Vet. World 2022, 15, 1456–1466. [Google Scholar] [CrossRef]
- Porras Vargas, J.L.; Maldonado Castro, G.A.; Rodriguez Molano, C.E. Evaluation Of IATF Protocols With Frozen Vs Refrigerated Sperm in Brahman Cattle. Cienc. Desarro. 2024, 15, 23–28. [Google Scholar] [CrossRef]
- Anel, L.; Alvarez, M.; Martinez-Pastor, F.; Garcia-Macias, V.; Anel, E.; de Paz, P. Improvement Strategies in Ovine Artificial Insemination. Reprod. Domest. Anim. 2006, 41 (Suppl. S2), 30–42. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.; Kasimanickam, R. Laparoscopic artificial insemination in sheep: Review and cost benefit analysis. Clinical Theriogenology 2025, 17, 1–16. [Google Scholar] [CrossRef]
- Sun, W.; Jiang, S.; Su, J.; Zhang, J.; Bao, X.; Ding, R.; Shi, P.; Li, S.; Wu, C.; Zhao, G.; et al. The effects of cryopreservation on the acrosome structure, enzyme activity, motility, and fertility of bovine, ovine, and goat sperm. Anim. Reprod. 2021, 17, e20200219. [Google Scholar] [CrossRef]
- Salmon, V.M.; Castonguay, F.; Demers-Caron, V.; Leclerc, P.; Bailey, J.L. Cholesterol-loaded cyclodextrin improves ram sperm cryoresistance in skim milk-extender. Anim. Reprod. Sci. 2017, 177, 1–11. [Google Scholar] [CrossRef]
- Falchi, L.; Galleri, G.; Zedda, M.T.; Pau, S.; Bogliolo, L.; Ariu, F.; Ledda, S. Liquid storage of ram sperm for 96 h: Effects on kinematic parameters, membranes and DNA integrity, and ROS production. Livest. Sci. 2018, 207, 1–6. [Google Scholar] [CrossRef]
- Al-Essawe, E.M.; Johannisson, A.; Wulf, M.; Aurich, C.; Morrell, J.M. Addition of seminal plasma to thawed stallion spermatozoa did not repair cryoinjuries. Anim. Reprod. Sci. 2018, 196, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, A.E.; Bodu, M.; Bucak, M.N.; Ağır, V.; Özcan, A.; Keskin, N.; İli, P.; Topraggaleh, T.R.; Sidal, H.; Başpınar, N.; et al. The synergistic effect of trehalose and low concentrations of cryoprotectants can improve post-thaw ram sperm parameters. Cryobiology 2020, 95, 157–163. [Google Scholar] [CrossRef]
- Moore, A.I.; Squires, E.L.; y Graham, J.K. Efecto del plasma seminal en la criopreservación de +espermatozoides equinos. Theriogenology 2005, 63, 2372–2381. [Google Scholar] [CrossRef]
- Aurich, J.; Kuhl, J.; Tichy, A.; Aurich, C. Efficiency of Sperm Cryopreservation in Stallions. Animals 2020, 10, 1033. [Google Scholar] [CrossRef]
- Oliveira RAde Wolf, C.A.; Viu MAde, O.; Gambarini, M.L. Addition of glutathione to an extender for frozen equine sperm. J. Equine Vet. Sci. 2013, 33, 1148–1152. [Google Scholar] [CrossRef]
- Jimenez, S.; del Alamo, M.M.R.; Álvarez-Rodríguez, M.; Olegario Hidalgo, C.; Peña, A.I.; Muiño, R.; Mogas, T. In vitro assessment of egg yolk-, soya bean lecithin- and liposome-based extenders for cryopreservation of dairy bull sperm. Anim. Reprod. Sci. 2020, 215, 106315. [Google Scholar]
- Aurich, C.; Ortega Ferrusola, C.; Peña Vega, F.J.; Schrammel, N.; Morcuende, D.; Aurich, J. Seasonal changes in the sperm fatty acid composition of Shetland pony stallions. Theriogenology 2018, 107, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, S.; Rodrigues, A.S.; Henriques, L.; Batista, C.; Ramalho-Santos, J. Seasonal functional relevance of sperm characteristics in equine spermatozoa. Theriogenology 2010, 73, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.J.; Arias, M.E.; Fuentes, F.; Muñoz, E.; Bernecic, N.; Fair, S.; Felmer, R. Cellular and Molecular Consequences of Stallion Sperm Cryopreservation: Recent Approaches to Improve Sperm Survival. J. Equine Vet. Sci. 2023, 126, 104499. [Google Scholar] [CrossRef]
- Gonzalez-Castro, R.A.; Whitcomb, L.A.; Pinsinski, E.C.; Carnevale, E.M. Cryopreservation of equine spermatozoa reduces plasma membrane integrity and phospholipase C zeta 1 content as associated with oocyte activation. Andrology 2024, 12, 918–931. [Google Scholar] [CrossRef]
- Bubenickova, F.; Šichtař, J.; Nováčková, L.; Sirohi, J.; Šimoník, O. Structure of sperm subpopulations of stallion spermatozoa after thawing differs between good and bad freezers. Czech J. Animology 2020, 65, 403–410. [Google Scholar] [CrossRef]
- Hernández-Avilés, C.; Ramírez-Agámez, L.; Love, C.C. The relationship between post-thaw sperm quality parameters and blastocyst production following Intracytoplasmic Sperm Injection (ICSI) of in vitro-matured equine oocytes. Theriogenology 2025, 244, 117500. [Google Scholar] [CrossRef]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef]
- Knox, R.V. Fertility of frozen boar sperm when used for artificial insemination. Domest. Animat. Reprod. 2015, 50 (Suppl. 2), 90–97. [Google Scholar] [CrossRef]
- Yeste, M.; Rodríguez-Gil, J.E.; Bonet, S. Artificial insemination with frozen-thawed boar sperm. Mol. Reprod. Dev. 2017, 84, 802–813. [Google Scholar] [CrossRef]
- Casas, I.; Sancho, S.; Ballester, J.; Briz, M.; Pinart, E.; Bussalleu, E.; Yeste, M.; Fàbrega, A.; Rodríguez-Gil, J.E.; Bonet, S. The HSP90AA1 sperm content and the prediction of the boar ejaculate freezability. Theriogenology 2010, 74, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Cerolini, S.; Maldjian, A.; Surai, P.; Noble, R. Viability, susceptibility to peroxidation and fatty acid composition of boar sperm during liquid storage. Anim. Reprod. Sci. 2000, 58, 99–111. [Google Scholar] [CrossRef]
- Leahy, T.; Gadella, B.M. Sperm surface changes and physiological consequences induced by sperm handling and storage. Reproduction 2011, 142, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Vadnais, M.L.; Althouse, G.C. Characterization of capacitation, cryoinjury, and the role of seminal plasma in porcine sperm. Theriogenology 2011, 76, 1508–1516. [Google Scholar] [CrossRef]
- Flores, E.; Fernández-Novell, J.M.; Peña, A.; Rigau, T.; Rodríguez-Gil, J.E. Cryopreservation-induced alterations in boar spermatozoa mitochondrial function are related to changes in the expression and location of midpiece mitofusin-2 and actin network. Theriogenology 2010, 74, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, A.; Siqueira, A.P.; Hossain, M.S.; Johannisson, A.; Eriksson, I.; Wallgren, M.; Bergqvist, A.S. Quantification of kinetic changes in protein tyrosine phosphorylation and cytosolic Ca2+ concentration in boar spermatozoa during cryopreservation. Reprod. Fertil. Dev. 2012, 24, 531–542. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Moran, J.M.; Madejon, L.; Ortega Ferrusola, C.; Pena, F.J. Nitric oxide induces caspase activity in boar spermatozoa. Theriogenology 2008, 70, 91–96. [Google Scholar] [CrossRef]
- Pezo, F.; Yeste, M.; Zambrano, F.; Uribe, P.; Risopatrón, J.; Sánchez, R. Antioxidants and their effect on the oxidative/nitrosative stress of frozen-thawed boar sperm. Cryobiology 2021, 98, 5–11. [Google Scholar] [CrossRef]
- Santiso, R.; Tamayo, M.; Gosálvez, J.; Johnston, S.; Mari, A.; Fernández, C.; Losada, C.; Fernández, J.L. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis DNA Fragmentation Dynamics Allows the Assessment of Cryptic Sperm Damage in Human: Evaluation of Exposure to Ionizing Radiation, Hyperthermia, Acidic pH and Nitric Oxide. Mutat. Res. 2012, 734, 41–49. [Google Scholar] [CrossRef]
- Uribe, P.; Boguen, R.; Treulen, F.; Sanchez, R.; Villegas, J.V. Peroxynitrite-mediated nitrosative stress decreases motility and mitochondral membrane potential in human spermatozoa. Mol. Hum. Reprod. 2015, 21, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Pilane, C.M.; Bopape, M.A.; Mapeka, M.H.; Netshirovha, T.R. Assessment of the Susceptibility of Boar Sperm to Oxidative Stress. Open J. Anim. Sci. 2016, 6, 123–130. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, X.; Ali, M.A.; Qin, Z.; Zhang, Y.; Zeng, C. piR-121380 Is Involved in Cryo-Capacitation and Regulates Post-Thawed Boar Sperm Quality Through Phosphorylation of ERK2 via Targeting PTPN7. Front. Cell Dev. Biol. 2022, 9, 792994. [Google Scholar] [CrossRef]
- Chanapiwat, P.; Olanratmanee, E.O.; Kaeoket, K.; Tummaruk, P. Conception rate and litter size in multiparous sows after intrauterine insemination using frozen-thawed boar sperm in a commercial swine herd in Thailand. J. Vet. Med. Sci. 2014, 76, 1347–1351. [Google Scholar] [CrossRef]
- Pytlík, J.; Stádník, L.; Ducháček, J.; Codl, R. Comparative study of pregnancy rate of dairy cows inseminated with fresh or frozen-thawed sperm. Acta Univ. Agric. Silvic. Mendel. Brun. 2020, 68, 573–581. [Google Scholar] [CrossRef]
- Donovan, A.; Hanrahan, J.P.; Kummen, E.; Duffy, P.; Boland, M.P. Fertility in the ewe following cervical insemination with fresh or frozen–thawed sperm at a natural or synchronised oestrus. Anim. Reprod. Sci. 2004, 84, 359–368. [Google Scholar] [CrossRef]
- Olesen, I. Effects of cervical insemination with frozen sperm on fertility and litter size of Norwegian sheep. Livest. Prod. Sci. 1993, 37, 169–184. [Google Scholar] [CrossRef]
- Lewis, N.; Morganti, M.; Collingwood, F.; Grove-White, D.H.; McGregor Argo, C. Use of a single dose of frozen–thawed sperm for post-ovulatory mating at a commercial artificial insemination centre: Pregnancy rates and post-mating intrauterine fluid accumulation compared with chilled or fresh sperm insemination. J. Equine Vet. Sci. 2015, 35, 882–887. [Google Scholar] [CrossRef]
- Karatzas, G.; Karagiannidis, A.; Varsakeli, S.; Brikas, P. Fertility of fresh and frozen-thawed goat sperm during the nonbreeding season. Theriogenology 1997, 48, 1049–1059. [Google Scholar] [CrossRef]
- Rodríguez-Gil, J.E.; Silvers, G.; Flores, E.; Jesús Palomo, M.; Ramírez, A.; Montserrat Rivera, M.; Castro, M.; Brito, M.; Bücher, D.; Correa, J.; et al. Expression of the GM-CSF receptor in ovine spermatozoa: GM-CSF effect on sperm viability and motility of sperm subpopulations after the freezing-thawing process. Theriogenology 2007, 67, 1359–1370. [Google Scholar] [CrossRef]
- Vilanova, L.T.; Rauch, M.C.; Mansilla, A.; Zambrano, A.; Brito, M.; Werner, E.; Alfaro, V.; Cox, J.F.; Concha, I.I. Expression of granulocyte-macrophage colony stimulating factor (GM-CSF) in male germ cells: GM-CSF enhances sperm motility. Theriogenology 2003, 60, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, A.; Noli, C.; Rauch, M.C.; Werner, E.; Brito, M.; Amthauer, R.; Slebe, J.C.; Vera, J.C.; Concha, I.I. Expression of GM-CSF receptors in male germ cells and their role in signaling for increased glucose and vitamin C transport. J. Cell Biochem. 2001, 80, 625–634. [Google Scholar] [CrossRef]
- Tanhaye Kalate Sabz, F.; Amjadi, F.S.; Zandieh, Z.; Hosseini, E.; Aflatoonian, R.; Tabatabaei, M.; Mohammadian, M.; Ashrafi, M. GM-CSF (granulocyte-macrophage colony-stimulating factor) treatment improves sperm parameters in men with oligoasthenoteratospermia via PI3K/AKT pathway. Andrologia 2022, 54, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Whitty, A.L.; Kind, K.L.; Dunning, K.R.; McPherson, N.O.; Nottle, M.B. Treatment with GM-CSF of frozen bovine sperm improves function, fertilization, and subsequent embryo development. Theriogenology 2025, 235, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Navratil, A.M.; Bliss, S.P.; Berghorn, K.A.; Haughian, J.M.; Farmerie, T.A.; Graham, J.K.; Clay, C.M.; Roberson, M.S. Constitutive localization of the gonadotropin-releasing hormone (GnRH) receptor in low-density membrane microdomains is necessary for GnRH signaling to ERK. J. Biol. Chem. 2003, 278, 31593–31602. [Google Scholar] [CrossRef]
- Purdy, P.H.; Graham, J.K. Effect of cholesterol addition to bull sperm membranes on sperm capacitation, acrosome reaction, and fertility. Biol. Reprod. 2004, 71, 522–527. [Google Scholar] [CrossRef]
- Yadav, H.P.; Kumar, A.; Shah, N.; Chauhan, D.S.; Saxena, A.; Yadav, S.; Swain, D.K. Effect of cholesterol-loaded cyclodextrin supplementation on tyrosine phosphorylation and apoptosis-like changes in frozen-thawed Hariana bull spermatozoa. Theriogenology 2017, 96, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Anzar, M.; Rajapaksha, K.; Boswall, L. Egg yolk-free cryopreservation of bull sperm. PLoS ONE 2019, 14, e0223977. [Google Scholar] [CrossRef]
- Baňas, Š.; Benko, F.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. Epicatechin Prevents Cryocapacitation of Bovine Spermatozoa through Antioxidant Activity and Stabilization of Transmembrane Ion Channels. Int. J. Mol. Sci. 2023, 24, 2510. [Google Scholar] [CrossRef]
- Baňas, Š.; Tvrdá, E.; Benko, F.; Ďuračka, M.; Čmiková, N.; Lukáč, N.; Kačániová, M. Kaempferol as an alternative cryosupplement for bovine spermatozoa: Cytoprotective and membrane-stabilizing effects. Rev. Int. Cienc. Mol. 2024, 25, 4129. [Google Scholar] [CrossRef]
- Ďuračka, M.; Debacker, M.; Bučko, O.; Lukáč, N.; Tvrdá, E. The effect of kaempferol and naringenin may improve in vitro quality of stored porcine sperm. J. Cent. Eur. Agric. 2019, 20, 1069–1075. [Google Scholar] [CrossRef]
- Paucar Quito, J.E.; Alvarado Alvarado, J.C.; Moscoso Piedra, A.L.; Maldonado Cornejo, M.E. Evaluation of three commercial diluents on post-thaw ram sperm. Polo Conoc. 2015, 10, 2542–2566. [Google Scholar]
- Fang, X.; Huang, L.L.; Xu, J.; Ma, C.Q.; Chen, Z.H.; Zhang, Z.; Liao, C.H.; Zheng, S.X.; Huang, P.; Xu, W.M.; et al. Proteomics and single-cell RNA analysis of Akap4-knockout mice model confirm indispensable role of Akap4 in spermatogenesis. Dev. Biol. 2019, 454, 118–127. [Google Scholar] [CrossRef]
- Blommaert, D.; Sergeant, N.; Delehedde, M.; Jouy, N.; Mitchell, V.; Franck, T.; Donnay, I.; Lejeune, J.P.; Serteyn, D. Expression, localization, and concentration of A-kinase anchor protein 4 (AKAP4) and its precursor (proAKAP4) in equine sperm: Promising marker correlated to the total and progressive motility in thawed spermatozoa. Theriogenology 2019, 131, 52–60. [Google Scholar] [CrossRef]
- Kwon, W.S.; Oh, S.A.; Kim, Y.J.; Rahman, M.S.; Park, Y.J.; Pang, M.G. Proteomic approaches for profiling negative fertility markers in inferior boar spermatozoa. Sci. Rep. 2015, 5, 13821. [Google Scholar] [CrossRef]
- Rahamim Ben-Navi, L.; Almog, T.; Yao, Z.; Seger, R.; Naor, Z. A-Kinase Anchoring Protein 4 (AKAP4) is an ERK1/2 substrate and a switch molecule between cAMP/PKA and PKC/ERK1/2 in human spermatozoa. Sci. Rep. 2016, 6, 37922. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Bernstein, I.R.; Cafe, S.L.; Delehedde, M.; Sergeant, N.; Anderson, A.L.; Trigg, N.A.; Eamens, A.L.; Lord, T.; Dun, M.D.; et al. A Kinase Anchor Protein 4 Is Vulnerable to Oxidative Adduction in Male Germ Cells. Front. Cell Dev. Biol. 2019, 7, 319. [Google Scholar] [CrossRef]
- Riesco, M.F.; Anel-López, L.; Neila-Montero, M.; Palacín-Martínez, C.; Montes-Garrido, R.; Álvarez, M.; de Paz, P.; Anel, L. ProAKAP4 as novel molecular marker of sperm quality in ram: An integrative study in fresh, cooled and cryopreserved sperm. Biomolecules 2020, 10, 1046. [Google Scholar] [CrossRef]
- Alharbi, Y.M.; Ali, M.; Alharbi, M.S. Impact of the Antioxidant Hydroxytyrosol on the Quality of Post-Thawed Stallion Sperm. Vet. Med. Int. 2024, 2024, 6558480. [Google Scholar] [CrossRef]
- Du, M.; Liu, Y.; Zhang, L.; Li, X.; Wang, N.; He, Q.; Cao, J.; Zhao, B.; Shi, Y.; Li, B.; et al. The effect of resveratrol on the cryopreservation of Mongolian horse sperm. Arch. Anim. Breed. 2025, 68, 27–41. [Google Scholar] [CrossRef]
- Loomis, P.R.; Graham, E.F. Effects of cryopreservation on sperm quality and fertility in horses. Theriogenology 2008, 69, 468–475. [Google Scholar]
- Kuleshova, L.L.; Lopata, A. Vitrification can be more favorable than slow cooling. Fertil. Steril. 2002, 78, 449–454. [Google Scholar] [CrossRef]
- Wowk, B. Thermodynamic aspects of vitrification. Cryobiology 2010, 60, 11–22. [Google Scholar] [CrossRef]
- Consuegra, C.; Crespo, F.; Dorado, J.; Diaz-Jimenez, M.; Pereira, B.; Ortiz, I.; Hidalgo, M. Vitrification of stallion sperm using 0.25 ml straws: Effect of volume, concentration and carbohydrates (sucrose/trehalose/raffinose). Anim. Reprod. Sci. 2019, 206, 69–77. [Google Scholar] [CrossRef]
- Hidalgo, M. Sperm vitrification in horses and donkeys. J. Equine Vet. Sci. 2025, 145, 105340. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Wu, Y.Y.; Chen, M.C. Predicting the cryotolerance of boar sperm through antioxidant stress. Reprod. Domest. Anim. 2024, 59, e14554. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiao, H.; Cheng, X.; Zhang, L.; Zhang, S.; Liu, G.; Meng, F.; Zhan, F.; Yang, F. Proteomic Analysis of Differences in the Freezability of Porcine Sperm Identifies α-Amylase As a Key Protein. J. Proteome Res. 2024, 23, 2641–2650. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Chen, Y.; Zhao, Y.; Tan, M.; He, B. Protective Effects of Betaine on Boar Sperm Quality during Liquid Storage and Transport. Animals 2024, 14, 2711. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.M.; Cheong, H.T.; Park, C.K.; Lee, S.H. Effect of Magnetized Freezing Extender on Membrane Damages, Motility, and Fertility of Boar Sperm Following Cryopreservation. Animals 2023, 13, 634. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, M.; Zhang, J.; Li, S.; Ma, S.; Jiang, S.; Jiang, Y.; Li, X. Polydopamine-based nano-protectant for prolonged boar sperm preservation by eliminating ROS and regulating protein phosphorylation via D2DR-mediated cAMP/PKA signaling pathway. J. Nanobiotechnol. 2025, 23, 151. [Google Scholar] [CrossRef]
- Hallberg, H.; Eklund, M.; Sundell, L.; Wången, G. Evaluation of AndroStar Premium for sperm storage at 4 °C and its effects on DNA integrity and fertilizing ability. J. Reprod. Biotechnol. 2024, 29, 112–120. [Google Scholar]

| Species | Cholesterol/Phospholipid | Membrane Fluidity | Susceptibility to Cold Shock | Post-Thaw Motility (%) | Main Cryopreservation Damage |
|---|---|---|---|---|---|
| Bovine | +++ | + | + | >50–70% | Minimal functional changes |
| Ovine | ++ | ++ | ++ | 40–60% | Membrane fragmentation, loss of acrosome |
| Stallion | ++ | ++ | ++ | 30–60% | Oxidative stress, membrane and DNA damage |
| Boar | + | +++ | +++ | <30% | Cold shock, apoptosis, mitochondrial damage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, M.; Leal, K.; Pezo, F.; Contreras, M.J. Sperm Membrane: Molecular Implications and Strategies for Cryopreservation in Productive Species. Animals 2025, 15, 1808. https://doi.org/10.3390/ani15121808
Castro M, Leal K, Pezo F, Contreras MJ. Sperm Membrane: Molecular Implications and Strategies for Cryopreservation in Productive Species. Animals. 2025; 15(12):1808. https://doi.org/10.3390/ani15121808
Chicago/Turabian StyleCastro, Macarena, Karla Leal, Felipe Pezo, and María José Contreras. 2025. "Sperm Membrane: Molecular Implications and Strategies for Cryopreservation in Productive Species" Animals 15, no. 12: 1808. https://doi.org/10.3390/ani15121808
APA StyleCastro, M., Leal, K., Pezo, F., & Contreras, M. J. (2025). Sperm Membrane: Molecular Implications and Strategies for Cryopreservation in Productive Species. Animals, 15(12), 1808. https://doi.org/10.3390/ani15121808






