Prenatal Factors Influencing Calf Morbidity and Mortality in Dairy Cattle: A Systematic Review of the Literature (2000–2024)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Heat Stress During Late Gestation
3.1.1. Study Selection
3.1.2. Study Characteristics
3.2. Nutrition of the Pregnant Cow
3.2.1. Study Selection
3.2.2. Study Characteristics
3.3. Body Condition of the Dam
3.3.1. Study Selection
3.3.2. Study Characteristics
3.4. Vaccination of the Dam
3.4.1. Study Selection
3.4.2. Study Characteristics
3.5. Parity
3.5.1. Study Selection
3.5.2. Study Characteristics
3.6. Twin Pregnancy
3.6.1. Study Selection
3.6.2. Study Characteristics
3.7. Methodological Strengths and Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| THI | Temperature–humidity index |
| IgG | Immunoglobulin G |
| BCS | Body condition score |
| BRD | Bovine respiratory disease |
| OR | Odds ratio |
| DCAD | Dietary cation-anion difference |
| HR | Hazards ratio |
| FTP | Failure of passive transfer |
| IRR | Incidence rate ratio |
References
- van der Fels-Klerx, H.; Saatkamp, H.; Verhoeff, J.; Dijkhuizen, A. Effects of bovine respiratory disease on the productivity of dairy heifers quantified by experts. Livest. Prod. Sci. 2002, 75, 157–166. [Google Scholar] [CrossRef]
- Bach, A. Associations between several aspects of heifer development and dairy cow survivability to second lactation. J. Dairy Sci. 2011, 94, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Chuck, G.M.; Mansell, P.D.; Stevenson, M.A.; Izzo, M.M. Early life events associated with first lactation reproductive performance in southwest Victorian pasture-based dairy herds. Aust. Vet. J. 2023, 102, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Pelaez, A.; Pritchard, D.G.; Pfeiffer, D.U.; Jones, E.; Honeyman, P.; Mawdsley, J.J. Calf mortality as a welfare indicator on British cattle farms. Vet. J. 2008, 176, 177–181. [Google Scholar] [CrossRef]
- Roche, S.M.; Genore, R.; Renaud, D.L.; Shock, D.A.; Bauman, C.; Croyle, S.; Barkema, H.W.; Dubuc, J.; Keefe, G.P.; Kelton, D.F. Short communication: Describing mortality and euthanasia practices on Canadian dairy farms. J. Dairy Sci. 2020, 103, 3599–3605. [Google Scholar] [CrossRef]
- Quigley, J.D. Invited review: An evaluation of EFSA opinion on calf welfare from a nutritional and management perspective. J. Dairy Sci. 2024, 107, 7483–7503. [Google Scholar] [CrossRef]
- Welk, A.; Otten, N.D.; Jensen, M.B. Invited review: The effect of milk feeding practices on dairy calf behavior, health, and performance-A systematic review. J. Dairy Sci. 2023, 106, 5853–5879. [Google Scholar] [CrossRef]
- Mee, J.F. Invited review: Bovine neonatal morbidity and mortality-Causes, risk factors, incidences, sequelae and prevention. Reprod. Domest. Anim. 2023, 58 (Suppl. S2), 15–22. [Google Scholar] [CrossRef]
- Cuttance, E.; Laven, R. Estimation of perinatal mortality in dairy calves: A review. Vet. J. 2019, 252, 105356. [Google Scholar] [CrossRef]
- Zablotski, Y.; Voigt, K.; Hoedemaker, M.; Müller, K.E.; Kellermann, L.; Arndt, H.; Volkmann, M.; Dachrodt, L.; Stock, A. Perinatal mortality in German dairy cattle: Unveiling the importance of cow-level risk factors and their interactions using a multifaceted modelling approach. PLoS ONE 2024, 19, e0302004. [Google Scholar] [CrossRef]
- Compton, C.W.R.; Heuer, C.; Thomsen, P.T.; Carpenter, T.E.; Phyn, C.V.C.; McDougall, S. Invited review: A systematic literature review and meta-analysis of mortality and culling in dairy cattle. J. Dairy Sci. 2017, 100, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Todd, C.G.; McGee, M.; Tiernan, K.; Crosson, P.; O’Riordan, E.; McClure, J.; Lorenz, I.; Earley, B. An observational study on passive immunity in Irish suckler beef and dairy calves: Tests for failure of passive transfer of immunity and associations with health and performance. Prev. Vet. Med. 2018, 159, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.F.; Chancellor, N.; Wathes, D.C. A Cohort Study Risk Factor Analysis for Endemic Disease in Pre-Weaned Dairy Heifer Calves. Animals 2021, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Godden, S.M.; Lombard, J.E.; Woolums, A.R. Colostrum Management for Dairy Calves. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 535–556. [Google Scholar] [CrossRef]
- Khan, M.A.; Lee, H.J.; Lee, W.S.; Kim, H.S.; Ki, K.S.; Hur, T.Y.; Suh, G.H.; Kang, S.J.; Choi, Y.J. Structural growth, rumen development, and metabolic and immune responses of Holstein male calves fed milk through step-down and conventional methods. J. Dairy Sci. 2007, 90, 3376–3387. [Google Scholar] [CrossRef]
- Lundborg, G.K.; Svensson, E.C.; Oltenacu, P.A. Herd-level risk factors for infectious diseases in Swedish dairy calves aged 0–90 days. Prev. Vet. Med. 2005, 68, 123–143. [Google Scholar] [CrossRef]
- Griffin, C.M.; Scott, J.A.; Karisch, B.B.; Woolums, A.R.; Blanton, J.R.; Kaplan, R.M.; Epperson, W.B.; Smith, D.R. A randomized controlled trial to test the effect of on-arrival vaccination and deworming on stocker cattle health and growth performance. Bov. Pract. 2018, 52, 26–33. [Google Scholar] [CrossRef]
- Keller, S.; Donat, K.; Söllner-Donat, S.; Wehrend, A.; Klassen, A. Beziehungen zwischen perinataler Mortalität und Management von Kühen vor und zur Kalbung in großen Milchviehherden. Tierarztl. Prax. Ausg. G. Grosstiere. Nutztiere. 2024, 52, 271–280. [Google Scholar] [CrossRef]
- Viidu, D.-A.; Mõtus, K. Implementation of a pre-calving vaccination programme against rotavirus, coronavirus and enterotoxigenic Escherichia coli (F5) and association with dairy calf survival. BMC Vet. Res. 2022, 18, 59. [Google Scholar] [CrossRef]
- Hoedemaker, M.; Ruddat, I.; Teltscher, M.K.; Essmeyer, K.; Kreienbrock, L. Influence of animal, herd and management factors on perinatal mortality in dairy cattle—A survey in Thuringia, Germany. Berl. Munch. Tierarztl. Wochenschr. 2010, 123, 130–136. [Google Scholar]
- Del Silva Río, N.; Stewart, S.; Rapnicki, P.; Chang, Y.M.; Fricke, P.M. An observational analysis of twin births, calf sex ratio, and calf mortality in Holstein dairy cattle. J. Dairy Sci. 2007, 90, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.P.A.; Tao, S.; Thompson, I.M.T.; Dahl, G.E. In utero heat stress decreases calf survival and performance through the first lactation. J. Dairy Sci. 2016, 99, 8443–8450. [Google Scholar] [CrossRef] [PubMed]
- Mee, J.F. Impacts of dairy cow nutrition precalving on calf health. JDS Commun. 2023, 4, 245–249. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Ferag, A.; Gherissi, D.E.; Khenenou, T.; Boughanem, A.; Moussa, H.H.; Kechroud, A.A.; Fares, M.A. Heat stress effect on fertility of two imported dairy cattle breeds from different Algerian agro-ecological areas. Int. J. Biometeorol. 2024, 68, 2515–2529. [Google Scholar] [CrossRef]
- FAO; IDF; IFCN. World Mapping of Animal Feeding Systems in the Dairy Sector; FAO: Rome, Italy, 2014; ISBN 978-92-5-108461-8. [Google Scholar]
- Zigo, F.; Vasil’, M.; Ondrašovičová, S.; Výrostková, J.; Bujok, J.; Pecka-Kielb, E. Maintaining Optimal Mammary Gland Health and Prevention of Mastitis. Front. Vet. Sci. 2021, 8, 607311. [Google Scholar] [CrossRef]
- Capper, J.L.; Cady, R.A. The effects of improved performance in the U.S. dairy cattle industry on environmental impacts between 2007 and 2017. J. Anim. Sci. 2020, 98, skz291. [Google Scholar] [CrossRef]
- Dohoo, I.R.; Martin, S.W.; Stryhn, H. Veterinary Epidemiologic Research; VER, Inc: Charlotte, PE, Canada, 2014; ISBN 978-0-919013-60-5. [Google Scholar]
- Ansari-Lari, M. Study of perinatal mortality and dystocia in dairy cows in Fars Province, Southern Iran. Int. J. Dairy Sci. 2007, 2, 85–89. [Google Scholar]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Trifković, J.; Jovanović, L.; Đurić, M.; Stevanović-Đorđević, S.; Milanović, S.; Lazarević, M.; Sladojević, Ž.; Kirovski, D. Influence of different seasons during late gestation on Holstein cows’ colostrum and postnatal adaptive capability of their calves. Int. J. Biometeorol. 2018, 62, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.D.; Dado-Senn, B.; Ouellet, V.; Dahl, G.E.; Laporta, J. Effect of late-gestation heat stress in nulliparous heifers on postnatal growth, passive transfer of immunoglobulin G, and thermoregulation of their calves. JDS Commun. 2021, 2, 165–169. [Google Scholar] [CrossRef]
- Kara, H.; Güven, M. Evaluation of colostrum quality and passive transfer immunity in terms of heat stress and disease incidence in Holstein cattle in Central Anatolia. Ankara Univ. Vet. Fak. 2024, 71, 481–486. [Google Scholar] [CrossRef]
- Mellado, M.; Arroyo, N.; García, J.E.; Arias, N.; Macías-Cruz, U.; Mellado, J. Climatic and calf-related risk factors associated with failure of transfer of passive immunity in Holstein calves in a hot environment. Trop. Anim. Health Prod. 2024, 56, 57. [Google Scholar] [CrossRef]
- Monteiro, A.P.A.; Tao, S.; Thompson, I.M.; Dahl, G.E. Effect of heat stress during late gestation on immune function and growth performance of calves: Isolation of altered colostral and calf factors. J. Dairy Sci. 2014, 97, 6426–6439. [Google Scholar] [CrossRef]
- Laporta, J.; Fabris, T.F.; Skibiel, A.L.; Powell, J.L.; Hayen, M.J.; Horvath, K.; Miller-Cushon, E.K.; Dahl, G.E. In utero exposure to heat stress during late gestation has prolonged effects on the activity patterns and growth of dairy calves. J. Dairy Sci. 2017, 100, 2976–2984. [Google Scholar] [CrossRef]
- Skibiel, A.L.; Fabris, T.F.; Corrá, F.N.; Torres, Y.M.; McLean, D.J.; Chapman, J.D.; Kirk, D.J.; Dahl, G.E.; Laporta, J. Effects of feeding an immunomodulatory supplement to heat-stressed or actively cooled cows during late gestation on postnatal immunity, health, and growth of calves. J. Dairy Sci. 2017, 100, 7659–7668. [Google Scholar] [CrossRef] [PubMed]
- Dado-Senn, B.; Vega Acosta, L.; Torres Rivera, M.; Field, S.L.; Marrero, M.G.; Davidson, B.D.; Tao, S.; Fabris, T.F.; Ortiz-Colón, G.; Dahl, G.E.; et al. Pre- and postnatal heat stress abatement affects dairy calf thermoregulation and performance. J. Dairy Sci. 2020, 103, 4822–4837. [Google Scholar] [CrossRef]
- Tang, C.; Liang, Y.; Guo, J.; Wang, M.; Li, M.; Zhang, H.; Arbab, A.A.I.; Karrow, N.A.; Yang, Z.; Mao, Y. Effects of Seasonal Heat Stress during Late Gestation on Growth Performance, Metabolic and Immuno-Endocrine Parameters of Calves. Animals 2022, 12, 716. [Google Scholar] [CrossRef]
- Yin, T.; Halli, K.; König, S. Direct genetic effects, maternal genetic effects, and maternal genetic sensitivity on prenatal heat stress for calf diseases and corresponding genomic loci in German Holsteins. J. Dairy Sci. 2022, 105, 6795–6808. [Google Scholar] [CrossRef]
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Increases in extreme heat stress in domesticated livestock species during the twenty-first century. Glob. Change Biol. 2021, 27, 5762–5772. [Google Scholar] [CrossRef] [PubMed]
- Zeppetello, L.R.V.; Raftery, A.E.; Battisti, D.S. Probabilistic projections of increased heat stress driven by climate change. Commun. Earth Environ. 2022, 3, 183. [Google Scholar] [CrossRef]
- Ghaffari, M.H. Developmental programming: Prenatal and postnatal consequences of hyperthermia in dairy cows and calves. Domest. Anim. Endocrinol. 2022, 80, 106723. [Google Scholar] [CrossRef]
- Giannone, C.; Bovo, M.; Ceccarelli, M.; Torreggiani, D.; Tassinari, P. Review of the Heat Stress-Induced Responses in Dairy Cattle. Animals 2023, 13, 3451. [Google Scholar] [CrossRef]
- Khan, I.; Mesalam, A.; Heo, Y.S.; Lee, S.-H.; Nabi, G.; Kong, I.-K. Heat Stress as a Barrier to Successful Reproduction and Potential Alleviation Strategies in Cattle. Animals 2023, 13, 2359. [Google Scholar] [CrossRef]
- Lewis, K.; Shewbridge Carter, L.; Bradley, A.; Dewhurst, R.; Forde, N.; Hyde, R.; Kaler, J.; March, M.D.; Mason, C.; O’Grady, L.; et al. Quantification of the effect of in utero events on lifetime resilience in dairy cows. J. Dairy Sci. 2024, 107, 4616–4633. [Google Scholar] [CrossRef]
- Ouellet, V.; Laporta, J.; Dahl, G.E. Late gestation heat stress in dairy cows: Effects on dam and daughter. Theriogenology 2020, 150, 471–479. [Google Scholar] [CrossRef]
- Cartwright, S.L.; Schmied, J.; Karrow, N.; Mallard, B.A. Impact of heat stress on dairy cattle and selection strategies for thermotolerance: A review. Front. Vet. Sci. 2023, 10, 1198697. [Google Scholar] [CrossRef]
- Parsad, R.; Ahlawat, S.; Bagiyal, M.; Arora, R.; Gera, R.; Chhabra, P.; Sharma, U.; Singh, A. Climate resilience in goats: A comprehensive review of the genetic basis for adaptation to varied climatic conditions. Mamm. Genome 2025, 36, 151–161. [Google Scholar] [CrossRef]
- Avendaño-Reyes, L.; Macías-Cruz, U.; Sánchez-Castro, M.A.; Anzures-Olvera, F.; Vicente-Pérez, R.; Mellado, M.; Zamorano-Algándar, R.; Robinson, P.H.; Castañeda-Bustos, V.J.; López-Baca, A. Effects of parity, seasonal heat stress, and colostrum collection time postpartum on colostrum quality of Holstein cattle in an arid region. Int. J. Biometeorol. 2024, 68, 427–434. [Google Scholar] [CrossRef]
- Samara, E.M.; Al-Badwi, M.A.; Abdoun, K.A.; Abdelrahman, M.M.; Okab, A.B.; Bahadi, M.A.; Al-Haidary, A.A. The interrelationship between macrominerals and heat stress in ruminants: Current perspectives and future directions—A review. Vet. Res. Commun. 2024, 49, 29. [Google Scholar] [CrossRef] [PubMed]
- Aragona, K.M.; Rice, E.M.; Engstrom, M.; Erickson, P.S. Effect of β-carotene supplementation to prepartum Holstein cows on colostrum quality and calf performance. J. Dairy Sci. 2021, 104, 8814–8825. [Google Scholar] [CrossRef]
- Prom, C.M.; Engstrom, M.A.; Drackley, J.K. Effects of prepartum supplementation of β-carotene on colostrum and calves. J. Dairy Sci. 2022, 105, 8839–8849. [Google Scholar] [CrossRef] [PubMed]
- Aragona, K.M.; Rice, E.M.; Engstrom, M.; Erickson, P.S. Supplementation of nicotinic acid to prepartum Holstein cows increases colostral immunoglobulin G, excretion of urinary purine derivatives, and feed efficiency in calves. J. Dairy Sci. 2020, 103, 2287–2302. [Google Scholar] [CrossRef]
- Chen, F.; Li, Y.; Shen, Y.; Guo, Y.; Zhao, X.; Li, Q.; Cao, Y.; Zhang, X.; Li, Y.; Wang, Z.; et al. Effects of prepartum zinc-methionine supplementation on feed digestibility, rumen fermentation patterns, immunity status, and passive transfer of immunity in dairy cows. J. Dairy Sci. 2020, 103, 8976–8985. [Google Scholar] [CrossRef]
- Gultepe, E.E.; Uyarlar, C.; Bayram, İ. Supplementation of Cr Methionine During Dry Period of Dairy Cows and Its Effect on Some Production and Biochemical Parameters During Early Lactation and on Immunity of Their Offspring. Biol. Trace Elem. Res. 2018, 186, 143–153. [Google Scholar] [CrossRef]
- Alharthi, A.S.; Batistel, F.; Abdelmegeid, M.K.; Lascano, G.; Parys, C.; Helmbrecht, A.; Trevisi, E.; Loor, J.J. Maternal supply of methionine during late-pregnancy enhances rate of Holstein calf development in utero and postnatal growth to a greater extent than colostrum source. J. Anim. Sci. Biotechnol. 2018, 9, 83. [Google Scholar] [CrossRef]
- Wang, H.; Elsaadawy, S.A.; Wu, Z.; Bu, D.P. Maternal Supply of Ruminally-Protected Lysine and Methionine During Close-Up Period Enhances Immunity and Growth Rate of Neonatal Calves. Front. Vet. Sci. 2021, 8, 780731. [Google Scholar] [CrossRef]
- Thomas, B.L.; Guadagnin, A.R.; Fehlberg, L.K.; Sugimoto, Y.; Shinzato, I.; Drackley, J.K.; Cardoso, F.C. Feeding rumen-protected lysine to dairy cows prepartum improves performance and health of their calves. J. Dairy Sci. 2022, 105, 2256–2274. [Google Scholar] [CrossRef]
- van Hese, I.; Goossens, K.; Vandaele, L.; Ampe, B.; Haegeman, A.; Opsomer, G. The effect of maternal supply of rumen-protected protein to Holstein Friesian cows during the dry period on the transfer of passive immunity and colostral microbial composition. J. Dairy Sci. 2023, 106, 8723–8745. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.; Guan, R.; Shi, K.; Wei, Z.; Liu, J.; Liu, H. Effects of Dietary Rumen-Protected Betaine Supplementation on Performance of Postpartum Dairy Cows and Immunity of Newborn Calves. Animals 2019, 9, 167. [Google Scholar] [CrossRef]
- Zenobi, M.G.; Bollatti, J.M.; Lopez, A.M.; Barton, B.A.; Hixson, C.L.; Maunsell, F.P.; Thatcher, W.W.; Miller-Cushon, K.; Santos, J.E.P.; Staples, C.R.; et al. Effects of maternal choline supplementation on performance and immunity of progeny from birth to weaning. J. Dairy Sci. 2022, 105, 9896–9916. [Google Scholar] [CrossRef]
- Jolazadeh, A.R.; Mohammadabadi, T.; Dehghan-Banadaky, M.; Chaji, M.; Garcia, M. Effect of supplementation fat during the last 3 weeks of uterine life and the preweaning period on performance, ruminal fermentation, blood metabolites, passive immunity and health of the newborn calf. Br. J. Nutr. 2019, 122, 1346–1358. [Google Scholar] [CrossRef]
- Garcia, M.; Greco, L.F.; Favoreto, M.G.; Marsola, R.S.; Martins, L.T.; Bisinotto, R.S.; Shin, J.H.; Lock, A.L.; Block, E.; Thatcher, W.W.; et al. Effect of supplementing fat to pregnant nonlactating cows on colostral fatty acid profile and passive immunity of the newborn calf. J. Dairy Sci. 2014, 97, 392–405. [Google Scholar] [CrossRef]
- Garcia, M.; Greco, L.F.; Favoreto, M.G.; Marsola, R.S.; Wang, D.; Shin, J.H.; Block, E.; Thatcher, W.W.; Santos, J.E.P.; Staples, C.R. Effect of supplementing essential fatty acids to pregnant nonlactating Holstein cows and their preweaned calves on calf performance, immune response, and health. J. Dairy Sci. 2014, 97, 5045–5064. [Google Scholar] [CrossRef]
- Petzold, M.; Meyer, U.; Kersten, S.; Breves, G.; Dänicke, S. Feeding conjugated linoleic acids and various concentrate proportions to late pregnant cows and its consequence on blood metabolites of calves. Livest. Sci. 2014, 161, 95–100. [Google Scholar] [CrossRef]
- Kovács, L.; Pajor, F.; Bakony, M.; Fébel, H.; Edwards, J.E. Prepartum Magnesium Butyrate Supplementation of Dairy Cows Improves Colostrum Yield, Calving Ease, Fertility, Early Lactation Performance and Neonatal Vitality. Animals 2023, 13, 1319. [Google Scholar] [CrossRef]
- Hall, J.A.; Bobe, G.; Vorachek, W.R.; Estill, C.T.; Mosher, W.D.; Pirelli, G.J.; Gamroth, M. Effect of supranutritional maternal and colostral selenium supplementation on passive absorption of immunoglobulin G in selenium-replete dairy calves. J. Dairy Sci. 2014, 97, 4379–4391. [Google Scholar] [CrossRef]
- Zimpel, R.; Nehme Marinho, M.; Almeida, K.V.; Ruiz, A.R.; Nelson, C.D.; Thatcher, W.W.; Santos, J.E.P. Effects of maternal level of dietary cation-anion difference fed to prepartum nulliparous cows on offspring acid-base balance, metabolism, and growth. J. Dairy Sci. 2021, 104, 8746–8764. [Google Scholar] [CrossRef]
- Collazos, C.; Lopera, C.; Santos, J.E.P.; Laporta, J. Effects of the level and duration of maternal diets with negative dietary cation-anion differences prepartum on calf growth, immunity, and mineral and energy metabolism. J. Dairy Sci. 2017, 100, 9835–9850. [Google Scholar] [CrossRef]
- Rajaeerad, A.; Ghorbani, G.R.; Khorvash, M.; Sadeghi-Sefidmazgi, A.; Mahdavi, A.H.; Rashidi, S.; Wilkens, M.R.; Hünerberg, M. Impact of a Ration Negative in Dietary Cation-Anion Difference and Varying Calcium Supply Fed before Calving on Colostrum Quality of the Dams and Health Status and Growth Performance of the Calves. Animals 2020, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- García, R.; González, M.R. Analysis of critical periods in the feeding of pregnant Holstein cows and their influence on calf performance. Technical note. Cuban J. Agric. Sci. 2003, 37, 365–367. [Google Scholar]
- Caixeta, L.S.; Omontese, B.O. Monitoring and Improving the Metabolic Health of Dairy Cows during the Transition Period. Animals 2021, 11, 352. [Google Scholar] [CrossRef]
- Waldon, N.; Nickles, K.; Parker, A.; Swanson, K.; Relling, A. A review of the effect of nutrient and energy restriction during late gestation on beef cattle offspring growth and development. J. Anim. Sci. 2023, 101, skac319. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, D.; Muralidharan, O.; Neves, P.A.; Bhutta, Z.A. Associations of Maternal Nutritional Status and Supplementation with Fetal, Newborn, and Infant Outcomes in Low-Income and Middle-Income Settings: An Overview of Reviews. Nutrients 2024, 16, 3725. [Google Scholar] [CrossRef]
- Keats, E.C.; Haider, B.A.; Tam, E.; Bhutta, Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 3, CD004905. [Google Scholar] [CrossRef]
- Ren, Y.; Zeng, Y.; Wu, Y.; Zhang, Q.; Xiao, X. Maternal methyl donor supplementation: A potential therapy for metabolic disorder in offspring. J. Nutr. Biochem. 2024, 124, 109533. [Google Scholar] [CrossRef]
- Kamada, H.; Nonaka, I.; Ueda, Y.; Murai, M. Selenium addition to colostrum increases immunoglobulin G absorption by newborn calves. J. Dairy Sci. 2007, 90, 5665–5670. [Google Scholar] [CrossRef]
- Kessler, E.C.; Bruckmaier, R.M.; Gross, J.J. Colostrum composition and immunoglobulin G content in dairy and dual-purpose cattle breeds. J. Anim. Sci. 2020, 98, skaa237. [Google Scholar] [CrossRef]
- Szenci, O. Correlations between muscle tone and acid-base balance in newborn calves: Experimental substantiation of a simple new score system proposed for neonatal status diagnosis. Acta Vet. Acad. Sci. Hung. 1982, 30, 79–84. [Google Scholar]
- Thornburg, K.L.; Valent, A.M. Maternal Malnutrition and Elevated Disease Risk in Offspring. Nutrients 2024, 16, 2614. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P.; Horst, R.L. Role of acid-base physiology on the pathogenesis of parturient hypocalcaemia (milk fever)—The DCAD theory in principal and practice. Acta Vet. Scand. Suppl. 2003, 97, 51–56. [Google Scholar]
- Berry, D.P.; Lee, J.M.; Macdonald, K.A.; Roche, J.R. Body condition score and body weight effects on dystocia and stillbirths and consequent effects on postcalving performance. J. Dairy Sci. 2007, 90, 4201–4211. [Google Scholar] [CrossRef]
- Mee, J.F.; Grant, J.; Sánchez-Miguel, C.; Doherty, M. Pre-Calving and Calving Management Practices in Dairy Herds with a History of High or Low Bovine Perinatal Mortality. Animals 2013, 3, 866–881. [Google Scholar] [CrossRef]
- Bahrami-Yekdangi, M.; Ghorbani, G.R.; Sadeghi-Sefidmazgi, A.; Mahnani, A.; Drackley, J.K.; Ghaffari, M.H. Identification of cow-level risk factors and associations of selected blood macro-minerals at parturition with dystocia and stillbirth in Holstein dairy cows. Sci. Rep. 2022, 12, 5929. [Google Scholar] [CrossRef] [PubMed]
- Gundelach, Y.; Essmeyer, K.; Teltscher, M.K.; Hoedemaker, M. Risk factors for perinatal mortality in dairy cattle: Cow and foetal factors, calving process. Theriogenology 2009, 71, 901–909. [Google Scholar] [CrossRef]
- Szenci, O.; Abdelmegeid, M.K.; Solymosi, N.; Brydl, E.; Bajcsy, C.Á.; Biksi, I.; Kulcsár, M. Prediction of stillbirth in Holstein-Friesian dairy cattle by measuring metabolic and endocrine parameters during the peripartal period. Reprod. Domest. Anim. 2018, 53, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, B.T.; Piñeiro, J.M.; Barragan, A.A.; Relling, A.E.; Garcia-Guerra, A.; Schuenemann, G.M. Association of prepartum lying time with nonesterified fatty acids and stillbirth in prepartum dairy heifers and cows. J. Dairy Sci. 2020, 103, 11782–11794. [Google Scholar] [CrossRef]
- Abdullahoğlu, E.; Duru, S.; Özlüer, A.; Fİlya, İ. Factors affecting colostrum quality and calf passive transfer levels in Holstein cattle. Anim. Sci. Pap. Rep. 2019, 37, 29–39. [Google Scholar]
- Immler, M.; Büttner, K.; Gärtner, T.; Wehrend, A.; Donat, K. Maternal Impact on Serum Immunoglobulin and Total Protein Concentration in Dairy Calves. Animals 2022, 12, 755. [Google Scholar] [CrossRef]
- Kara, K.N. Relation between non-infectious factors and neonatal calf health status in dairy herd. Anim. Sci. J. 2020, 91, e13471. [Google Scholar] [CrossRef] [PubMed]
- Meier, K.K.; Stock, A.; Merle, R.; Arndt, H.; Dachrodt, L.; Hoedemaker, M.; Kellermann, L.; Knubben-Schweizer, G.; Volkmann, M.; Müller, K.-E. Risk factors for omphalitis in neonatal dairy calves. Front. Vet. Sci. 2024, 11, 1480851. [Google Scholar] [CrossRef] [PubMed]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A Body Condition Scoring Chart for Holstein Dairy Cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, E.; Dueholm, L.; Vink, D.; Andersen, J.E.; Jakobsen, E.B.; Illum-Nielsen, S.; Petersen, F.A.; Enevoldsen, C. Within- and across-person uniformity of body condition scoring in Danish Holstein cattle. J. Dairy Sci. 2006, 89, 3721–3728. [Google Scholar] [CrossRef]
- McGuirk, S.M. Disease management of dairy calves and heifers. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 139–153. [Google Scholar] [CrossRef]
- Berry, D.P.; Lonergan, P.; Butler, S.T.; Cromie, A.R.; Fair, T.; Mossa, F.; Evans, A.C.O. Negative influence of high maternal milk production before and after conception on offspring survival and milk production in dairy cattle. J. Dairy Sci. 2008, 91, 329–337. [Google Scholar] [CrossRef]
- Santman-Berends, I.M.G.A.; Nijhoving, G.H.; van Wuijckhuise, L.; Muskens, J.; Bos, I.; van Schaik, G. Evaluation of the association between the introduction of data-driven tools to support calf rearing and reduced calf mortality in dairy herds in the Netherlands. Prev. Vet. Med. 2021, 191, 105344. [Google Scholar] [CrossRef]
- Meganck, V.; Hoflack, G.; Piepers, S.; Opsomer, G. Evaluation of a protocol to reduce the incidence of neonatal calf diarrhoea on dairy herds. Prev. Vet. Med. 2015, 118, 64–70. [Google Scholar] [CrossRef]
- Dubrovsky, S.A.; van Eenennaam, A.L.; Karle, B.M.; Rossitto, P.V.; Lehenbauer, T.W.; Aly, S.S. Bovine respiratory disease (BRD) cause-specific and overall mortality in preweaned calves on California dairies: The BRD 10K study. J. Dairy Sci. 2019, 102, 7320–7328. [Google Scholar] [CrossRef]
- Trotz-Williams, L.A.; Wayne Martin, S.; Leslie, K.E.; Duffield, T.; Nydam, D.V.; Peregrine, A.S. Calf-level risk factors for neonatal diarrhea and shedding of Cryptosporidium parvum in Ontario dairy calves. Prev. Vet. Med. 2007, 82, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Al Mawly, J.; Grinberg, A.; Prattley, D.; Moffat, J.; Marshall, J.; French, N. Risk factors for neonatal calf diarrhoea and enteropathogen shedding in New Zealand dairy farms. Vet. J. 2015, 203, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Maier, G.U.; Love, W.J.; Karle, B.M.; Dubrovsky, S.A.; Williams, D.R.; Champagne, J.D.; Anderson, R.J.; Rowe, J.D.; Lehenbauer, T.W.; van Eenennaam, A.L.; et al. Management factors associated with bovine respiratory disease in preweaned calves on California dairies: The BRD 100 study. J. Dairy Sci. 2019, 102, 7288–7305. [Google Scholar] [CrossRef]
- Dubrovsky, S.A.; van Eenennaam, A.L.; Karle, B.M.; Rossitto, P.V.; Lehenbauer, T.W.; Aly, S.S. Epidemiology of bovine respiratory disease (BRD) in preweaned calves on California dairies: The BRD 10K study. J. Dairy Sci. 2019, 102, 7306–7319. [Google Scholar] [CrossRef]
- McCarthy, H.R.; Cantor, M.C.; Lopez, A.J.; Pineda, A.; Nagorske, M.; Renaud, D.L.; Steele, M.A. Effects of supplementing colostrum beyond the first day of life on growth and health parameters of preweaning Holstein heifers. J. Dairy Sci. 2024, 107, 3280–3291. [Google Scholar] [CrossRef]
- Lee, J.S.; Keil, M.; Ellick Wong, K.F.; Lee, H.K. The Role of Goal Source in Escalation of Commitment. Exp. Psychol. 2024, 71, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Arega, S.M.; Knobel, D.L.; Toka, F.N.; Conan, A. Non-specific effects of veterinary vaccines: A systematic review. Vaccine 2022, 40, 1655–1664. [Google Scholar] [CrossRef]
- Chambers, G.P.; Kelton, W.; Smolenski, G.; Cuttance, E. Impact of prepartum administration of a vaccine against infectious calf diarrhea on nonspecific colostral immunoglobulin concentrations of dairy cows. J. Anim. Sci. 2022, 100, skac212. [Google Scholar] [CrossRef]
- Brainard, J.; Hammer, C.C.; Hunter, P.R.; Katzer, F.; Hurle, G.; Tyler, K. Efficacy of halofuginone products to prevent or treat cryptosporidiosis in bovine calves: A systematic review and meta-analyses. Parasitology 2021, 148, 408–419. [Google Scholar] [CrossRef]
- Donlon, J.D.; McAloon, C.G.; Hyde, R.; Aly, S.; Pardon, B.; Mee, J.F. A systematic review of the relationship between housing environmental factors and bovine respiratory disease in preweaned calves—Part 2: Temperature, relative humidity and bedding. Vet. J. 2023, 300–302, 106032. [Google Scholar] [CrossRef]
- Donlon, J.D.; McAloon, C.G.; Hyde, R.; Aly, S.; Pardon, B.; Mee, J.F. A systematic review of the relationship between housing environmental factors and bovine respiratory disease in preweaned calves—Part 1: Ammonia, air microbial count, particulate matter and endotoxins. Vet. J. 2023, 300–302, 106031. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Zadeh, N.G.; Nejati-Javaremi, A.; Miraei-Ashtiani, S.R.; Kohram, H. An observational analysis of twin births, calf stillbirth, calf sex ratio, and abortion in Iranian holsteins. J. Dairy Sci. 2008, 91, 4198–4205. [Google Scholar] [CrossRef] [PubMed]
- Lombard, J.E.; Garry, F.B.; Tomlinson, S.M.; Garber, L.P. Impacts of dystocia on health and survival of dairy calves. J. Dairy Sci. 2007, 90, 1751–1760. [Google Scholar] [CrossRef]
- Mee, J.F.; Berry, D.P.; Cromie, A.R. Prevalence of, and risk factors associated with, perinatal calf mortality in pasture-based Holstein-Friesian cows. Animal 2008, 2, 613–620. [Google Scholar] [CrossRef]
- Brickell, J.S.; McGowan, M.M.; Pfeiffer, D.U.; Wathes, D.C. Mortality in Holstein-Friesian calves and replacement heifers, in relation to body weight and IGF-I concentration, on 19 farms in England. Animal 2009, 3, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Gulliksen, S.M.; Lie, K.I.; Løken, T.; Osterås, O. Calf mortality in Norwegian dairy herds. J. Dairy Sci. 2009, 92, 2782–2795. [Google Scholar] [CrossRef]
- Kayano, M.; Kadohira, M.; Stevenson, M.A. Risk factors for stillbirths and mortality during the first 24h of life on dairy farms in Hokkaido, Japan 2005-2009. Prev. Vet. Med. 2016, 127, 50–55. [Google Scholar] [CrossRef]
- Jukna, V.; Meškinytė, E.; Antanatis, R.; Paulauskas, A.; Juozaitienė, V. Determining the Association of the Dry Period Duration with Dystocia and Stillbirths in Dairy Cows by Considering Parity, Season, and Gestation Length. Animals 2024, 14, 1444. [Google Scholar] [CrossRef]
- Meyer, C.L.; Berger, P.J.; Koehler, K.J. Interactions among factors affecting stillbirths in Holstein cattle in the United States. J. Dairy Sci. 2000, 83, 2657–2663. [Google Scholar] [CrossRef]
- Atashi, H. Factors affecting stillbirth and effects of stillbirth on subsequent lactation performance in a Holstein dairy herd in Isfahan. Iran. J. Vet. Res. 2011, 12, 24–30, 84. [Google Scholar]
- Bayram, B.; Topal, M.; Aksakal, V.; Önk, K. Investigate the effects of non-genetic factors on calving difficulty and stillbirth rate in Holstein Friesian cattle using the CHAID analysis. Kafkas Uni. Vet. Fak. Derg. 2015, 21, 645–652. [Google Scholar]
- Hossein-Zadeh, N.G. Evaluation of the effect of twin births on the perinatal calf mortality and productive performance of Holstein dairy cows. Archiv Tierzucht 2010, 53, 256–265. [Google Scholar] [CrossRef]
- Azizzadeh, M.; Shooroki, H.F.; Kamalabadi, A.S.; Stevenson, M.A. Factors affecting calf mortality in Iranian Holstein dairy herds. Prev. Vet. Med. 2012, 104, 335–340. [Google Scholar] [CrossRef]
- MacFarlane, J.A.; Grove-White, D.H.; Royal, M.D.; Smith, R.F. Identification and quantification of factors affecting neonatal immunological transfer in dairy calves in the UK. Vet. Rec. 2015, 176, 625. [Google Scholar] [CrossRef]
- Lundborg, G.K.; Oltenacu, P.A.; Maizon, D.O.; Svensson, E.C.; Liberg, P.G.A. Dam-related effects on heart girth at birth, morbidity and growth rate from birth to 90 days of age in Swedish dairy calves. Prev. Vet. Med. 2003, 60, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Pithua, P.; Wells, S.J.; Godden, S.M.; Raizman, E.A. Clinical trial on type of calving pen and the risk of disease in Holstein calves during the first 90 d of life. Prev. Vet. Med. 2009, 89, 8–15. [Google Scholar] [CrossRef]
- Marcato, F.; van den Brand, H.; Kemp, B.; Engel, B.; Schnabel, S.K.; Hoorweg, F.A.; Wolthuis-Fillerup, M.; van Reenen, K. Effects of transport age and calf and maternal characteristics on health and performance of veal calves. J. Dairy Sci. 2022, 105, 1452–1468. [Google Scholar] [CrossRef]
- Mee, J.F. Prevalence and risk factors for dystocia in dairy cattle: A review. Vet. J. 2008, 176, 93–101. [Google Scholar] [CrossRef]
- Mee, J.F.; Sánchez-Miguel, C.; Doherty, M. Influence of modifiable risk factors on the incidence of stillbirth/perinatal mortality in dairy cattle. Vet. J. 2014, 199, 19–23. [Google Scholar] [CrossRef]
- Zaborski, D.; Grzesiak, W.; Szatkowska, I.; Dybus, A.; Muszynska, M.; Jedrzejczak, M. Factors affecting dystocia in cattle. Reprod. Domest. Anim. 2009, 44, 540–551. [Google Scholar] [CrossRef]
- Ettema, J.F.; Santos, J.E.P. Impact of age at calving on lactation, reproduction, health, and income in first-parity Holsteins on commercial farms. J. Dairy Sci. 2004, 87, 2730–2742. [Google Scholar] [CrossRef] [PubMed]
- Ring, S.C.; McCarthy, J.; Kelleher, M.M.; Doherty, M.L.; Berry, D.P. Risk factors associated with animal mortality in pasture-based, seasonal-calving dairy and beef herds. J. Anim. Sci. 2018, 96, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Staněk, S.; Nejedlá, E.; Fleischer, P.; Pechová, A.; Šlosárková, S. Prevalence of Failure of Passive Transfer of Immunity in Dairy Calves in the Czech Republic. Acta Univ. Agric. Silvic. Mendel. Brun. 2019, 67, 163–172. [Google Scholar] [CrossRef]
- Szelényi, Z.; Szenci, O.; Kovács, L.; Garcia-Ispierto, I. Practical Aspects of Twin Pregnancy Diagnosis in Cattle. Animals 2021, 11, 1061. [Google Scholar] [CrossRef]
- López-Gatius, F.; López-Béjar, M.; Fenech, M.; Hunter, R.H.F. Ovulation failure and double ovulation in dairy cattle: Risk factors and effects. Theriogenology 2005, 63, 1298–1307. [Google Scholar] [CrossRef]
- Sargeant, J.M.; Amezcua, M.D.R.; Rajic, A.; Waddell, L. A Guide to Conducting Systematic Reviews in Agri-Food Public Health; Public Health Agency of Canada: Guelph, ON, Canada, 2005. [Google Scholar]
- Moher, D.; Tetzlaff, J.; Tricco, A.C.; Sampson, M.; Altman, D.G. Epidemiology and reporting characteristics of systematic reviews. PLoS Med. 2007, 4, e78. [Google Scholar] [CrossRef]


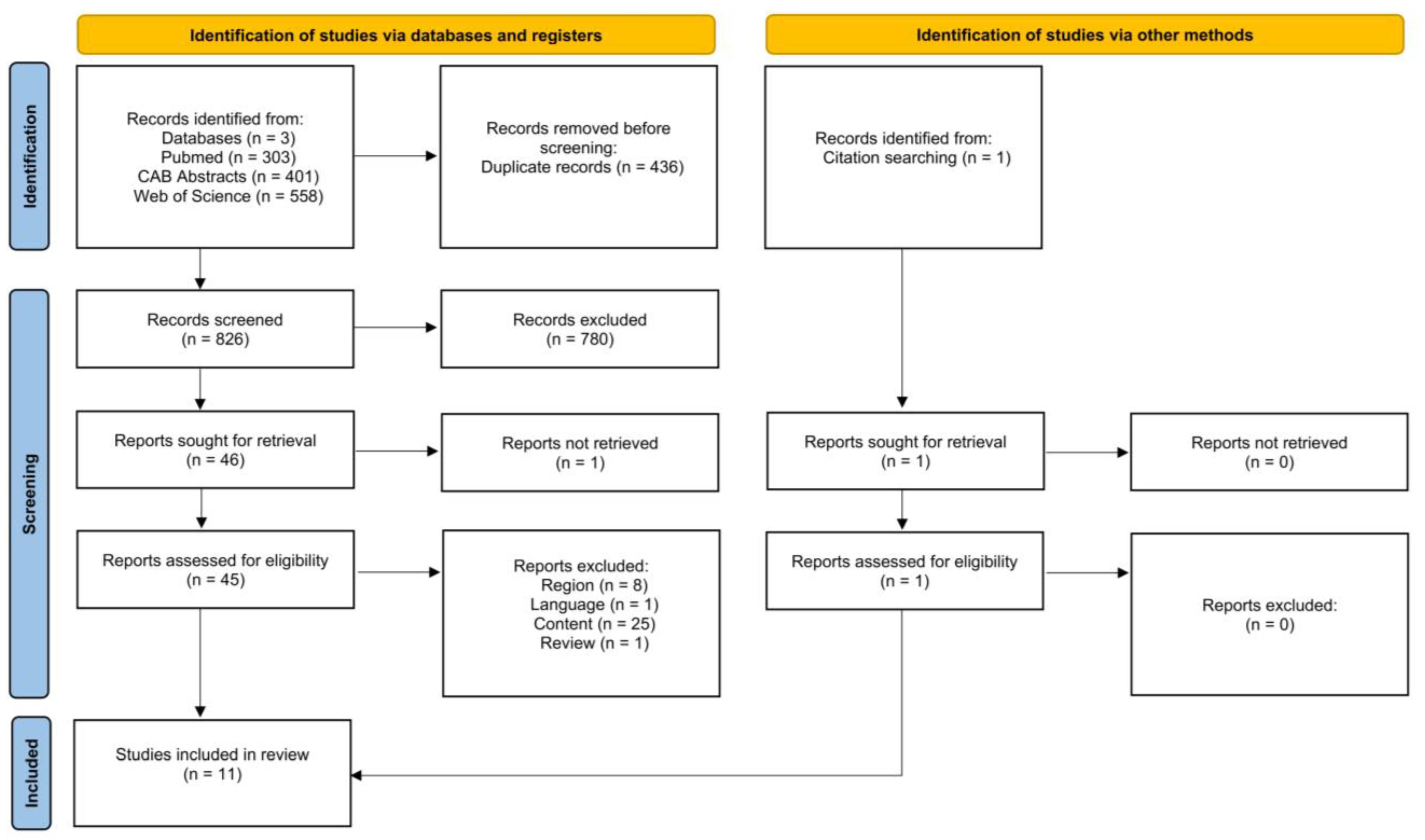



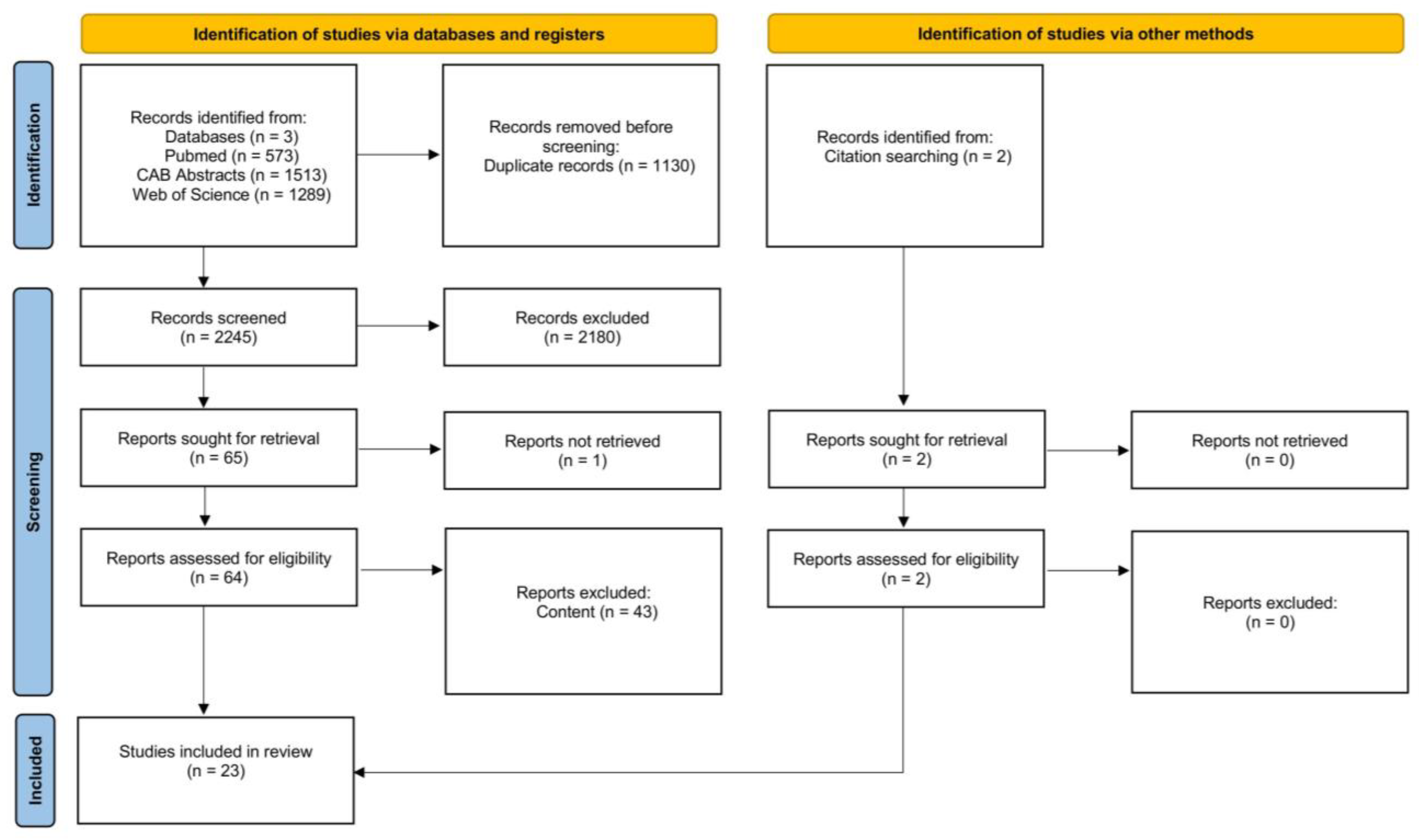

| Search Categories | Search Term |
|---|---|
| Heat stress of the dam | dairy AND cow AND heat stress OR climate OR summer AND gestation AND calf |
| Nutrition of the dam | dairy AND cow AND nutrition AND gestation AND calf |
| Body condition of the dam | dairy AND cow AND bcs OR body condition AND calf |
| Vaccination of the dam | dairy AND cow AND vaccination OR immunity AND calf |
| Parity | dairy AND cow AND parity OR lactation AND calf |
| Twin pregnancy | dairy AND cow AND twin pregnancy AND calf OR twins OR twinning AND calf |
| Author | Country | Study Design | Animals/ Herds | Breed | Outcome | Study Group | Control Group | Effect Estimate (95% CI) | p | Result | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [22] | USA | clinical trial | 146 | Holstein | Perinatal mortality (48 h after calving) | THI > 78 last 46 d of gestation | cows cooled last 46 d of gestation | − | 0.25 | = | − |
| [22] | USA | clinical trial | 146 | Holstein | Mortality bull calves up to 4 months | THI > 78 last 46 d of gestation | cows cooled last 46 d of gestation | − | 0.35 | = | − |
| [22] | USA | clinical trial | 146 | Holstein | Heifers leaving before puberty | THI > 78 last 46 d of gestation | cows cooled last 46 d of gestation | − | 0.26 | = | − |
| [22] | USA | clinical trial | 146 | Holstein | Heifers being culled before puberty due to sickness, malformation, growth retardation | THI > 78 last 46 d of gestation | cows cooled last 46 d of gestation | − | 0.03 | - | − |
| [33] | Serbia | Observation | 20/1 | Holstein | IgG calf serum (24 h after birth) | THI ~ 75 last 53 d of gestation | THI ~ 44 last 53 d of gestation | − | 0.036 | - | − |
| [34] | USA | Clinical trial | 19/1 | Holstein | IgG calf serum (56 d after birth) | THI ~ 77.3 last 60 d of gestation | cows cooled last 60 d of gestation | − | 0.03 | - | only heifer calves |
| [35] | Türkiye | Observation | 878/1 | Holstein | Correlation with serum brix (32–48 h after calving) | THI last 60 d of gestation | − | r = −0.14 | <0.001 | higher THI results in lesser serum brix | |
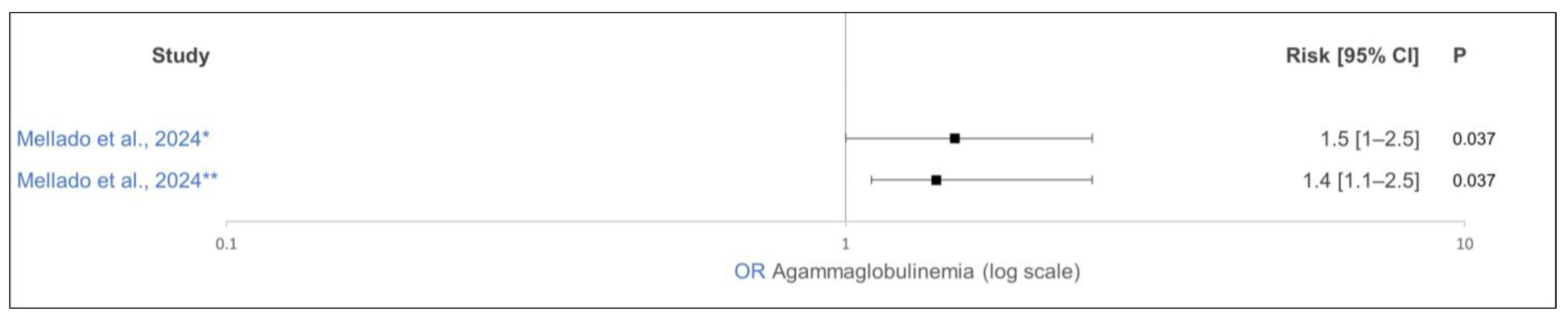
| [36] | Mexico | Observation | 4411/1 | Holstein | Agammaglobulinemia | average THI ≥ 80 last 90 d of gestation | average THI ≤ 70 last 90 d of gestation | OR 1.5 (1.0–2.5) | 0.037 | - | − |
| [36] | Mexico | Observation | 4411/1 | Holstein | Agammaglobulinemia | average THI 70–80 last 90 d of gestation | average THI ≤ 70 last 90 d of gestation | OR 1.4 (1.1–1.8) | 0.037 | - | − |
| [37] | USA | Clinical trial | 36 | Holstein | Calf Health Score | THI > 78 last 46 d of gestation | cows cooled last 46 d of gestation | − | − | = | − |
| [38] | USA | Clinical trial | 60 | Holstein | Calf Health Score | THI > 72 last 46 d of gestation | cows cooled last 46 d of gestation | − | − | = | − |
| [39] | USA | Clinical trial | 36 | Holstein | Calf Health Score | THI > 78 last 46 d of gestation | cows cooled last 46 d of gestation | − | − | = | − |
| [40] | USA | Clinical trial | 36 | Holstein | Calf Health Score | THI > 78 last 44 ± 5 d of gestation | cows cooled last 44 ± 5 d of gestation | − | − | = | − |
| [41] | China | Observation | 51/1 | Holstein | Incidence of diarrhea first 7 days of life | THI 70–74 last 33 d of gestation | THI 57–67 last 33 d of gestation | − | − | − | different seasons |
| [42] | Germany | Observation | 21,316/53 | Holstein | Morbidity (Omphalitis (O), Diarrhea (D), Respiratory disease (RD)) | THI last 56 d of gestation | − | − | − | last week of gestation increase of incidence 0.0044 (O), 0.012 (D), 0.0277 (RD) per increase of 1 THI unit |
| Author | Country | Study Design | Animals/Herds | Breed | Outcome | Study Group | Control Group | Effect Estimate (95% CI) | p | Result | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [54] | USA | Clinical trial | 18 | Holstein | IgG calf serum (24 h after birth) | β-carotene supplementation in last 28 d of gestation | no supplementation | − | 0.59 | = | − |
| [55] | USA | Clinical trial | 94 | Holstein | Total protein calf serum (24 h after birth) | β-carotene supplementation in last 28 d of gestation | no supplementation | − | 0.63 | = | − |
| [56] | USA | Clinical trial | 36 | Holstein | IgG calf serum (24 h after birth) | Nicotinic acid supplementation in last 28 d of gestation | no supplementation | − | 0.86 | = | − |
| [57] | China | Clinical trial | 40 | Holstein | IgG calf serum (24 h after birth) | Zn-Methionine supplementation in last 60 d of gestation | no supplementation | − | − | = | − |
| [58] | Türkiye | Clinical trial | 45 | Holstein | IgG calf serum (24 h after birth) | Cr-Methionine supplementation in last 60 d of gestation | no supplementation or injection of levamisole | − | − | = | − |
| [59] | USA | Clinical trial | 81 | Holstein | Fecal score of the calves over first 9 weeks | Methionine supplementation in last 28 d of gestation | no supplementation | − | − | = | − |
| [60] | China | Clinical trial | 120 | Holstein | IgG calf serum (24 h after birth) | Methionine and/or Lysine supplementation in last 21 d of gestation | no supplementation | − | <0.01 | + | Only heifer calves |
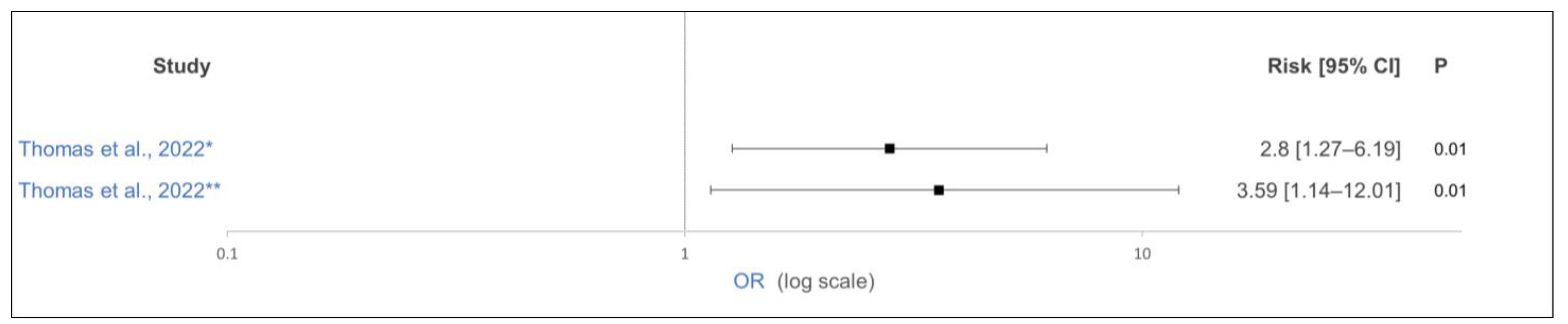
| [61] | USA | Clinical trial | 78 | Holstein | Medication in first 8 weeks of life (Electrolytes or antibiotics) | no supplementation | Lysine supplementation in last 26 d of gestation | OR 2.8 (1.27–6.19) | 0.01 | − | Only bull calves |
| [61] | USA | Clinical trial | 78 | Holstein | Antibiotics first 8 weeks of life | no supplementation | Lysine supplementation in last 26 d of gestation | OR 3.69 (1.14–12.01) | 0.01 | − | Only bull calves |
| [62] | Belgium | Clinical trial | 74 | Holstein | IgG calf serum (72 h after birth) | Rumen-protected protein supplementation in last 45 d of gestation | no supplementation | − | − | = | − |
| [63] | China | Clinical trial | 24 | Holstein | IgG calf serum (24 h after birth) | Rumen-protected betaine supplementation in last 45 d of gestation | no supplementation | − | <0.05 | + | Only heifer calves |
| [64] | USA | Clinical trial | 111 | Holstein | IgG calf serum (24–36 h after birth) | Choline supplementation in last 28 d of gestation | no supplementation | − | − | = | − |
| [65] | Iran | Clinical trial | 120 | Holstein | IgG calf serum (24 h after birth) | Soybean oil or fish oil supplementation in last 21 d of gestation | no supplementation | − | <0.01 | + | − |
| [66] | USA | Clinical trial | 78 | Holstein | IgG calf serum (24 h after birth) | Essential or conjugated fatty acids supplementation in last 56 d of gestation | no supplementation | − | 0.09 | = | − |
| [67] | USA | Clinical trial | 96 | Holstein | IgG calf serum (24 h after birth) | Essential fatty acids supplementation in last 56 d of gestation | no supplementation | − | 0.31 | = | − |
| [68] | Germany | Clinical trial | 21 | Holstein | Total protein calf serum (24 h after birth) | Conjugated linoleic acids supplementation in last 21 d of gestation | Fat supplementation | − | − | = | − |
| [69] | Hungary | Clinical trial | 219 | Holstein | Calf vitality at birth | Magnesium butyrate supplementation in last 23 d of gestation | no supplementation | − | 0.001 | + | − |
| [69] | Hungary | Clinical trial | 219 | Holstein | IgG calf serum; perinatal Mortality; Morbidity | Magnesium butyrate supplementation in last 23 d of gestation | no supplementation | − | − | = | − |
| [70] | USA | Clinical trial | 60 | Holstein | IgG calf serum (48 h after birth) | Selenium yeast supplementation in last 56 d of gestation | no supplementation | − | 0.03 | + | − |
| [71] | USA | Clinical trial | 132 | Holstein | IgG calf serum; Morbidity; Mortality; | Feeding DCAD- in last 22 of gestation | no DCAD | − | − | = | − |
| [72] | USA | Clinical trial | 60 | Holstein | IgG calf serum; Morbidity; Mortality; | Feeding DCAD- in last 21 or 42 d of gestation | no DCAD | − | − | = | − |
| [73] | Iran | Clinical trial | 12 | Holstein | Days with abnormal fecal score | Feeding DCAD- in last 21 d of gestation | no DCAD | − | <0.01 | − | Only heifer calves |
| [74] | Cuba | Clinical trial | 260 | Holstein | Diarrhea (first 90 d of life) | ≤75% of energy requirement in last 90 d of gestation | ≥85% of energy requirement in last 90 d of gestation | − | <0.05 | − | − |
| Author | Country | Study Design | Animals/Herds | Breed | Outcome | Study Group | Control Group | Effect Estimate (95% CI) | p | Result | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [85] | New Zealand | Observation | 2384.1 | Dairy | Perinatal mortality (48 h after calving) | BCS | − | − | − | = | − |
| [86] | Ireland | Observation | −0.30 | Dairy | Perinatal mortality (48 h after calving) | BCS in Heifers ≥ 3.75 | BCS in Heifers ≤ 3 | OR 0.053 (0.005–0.53) | <0.0001 | + | − |
| [86] | Ireland | Observation | −0.30 | Dairy | Perinatal mortality (48 h after calving) | BCS in Heifers 3.25–3.5 | BCS in Heifers ≥ 3.75 | OR 104.153 (16.004–677) | <0.0001 | − | − |
| [87] | Iran | Observation | 14,546.3 | Dairy | Perinatal mortality (48 h after calving) | BCS | − | − | − | = | − |
| [18] | Germany | Observation | −0.97 | Dairy | Perinatal mortality (48 h after calving) | BCS < 3.25 | BCS 3.25–3.75 | OR 3.41 (0.51–6.31) | 0.022 | − | − |
| [18] | Germany | Observation | −0.97 | Dairy | Perinatal mortality (48 h after calving) | BCS > 3.75 | BCS 3.25–3.75 | OR 2 (0.03–3.98) | 0.047 | − | − |
| [88] | Germany | Observation | 411.1 | Holstein | Perinatal mortality (24 h after calving) | BCS | − | − | − | = | − |
| [89] | Hungary | Observation | 155.3 | Holstein | Perinatal mortality (24 h after calving) | BCS | − | − | − | = | − |
| [90] | USA | Observation | 1044.3 | Holstein | Perinatal mortality (24 h after calving) | BCS | − | − | − | = | − |
| [91] | Türkiye | Observation | 354.2 | Holstein | IgG calf serum (36 h after birth) | BCS | − | − | − | = | − |
| [92] | Germany | Observation | 551.124 | Dairy | Total protein calf serum (3 d after birth) | BCS | − | − | − | = | − |
| [93] | Türkiye | Observation | 517.1 | Holstein | Calf Health Score 0 and 1 (good) until 28 d after calving. | BCS 3–3.75 | BCS < 3 or BCS > 3.75 | OR 1.59 | 0.001 | + | − |
| [94] | Germany | Observation | 3445.567 | Dairy | Omphalitis | BCS < 3.25 | BCS 3.25–3.75 | OR 1.38 (1.06–1.79) | 0.016 | − | − |
| [94] | Germany | Observation | 3445.567 | Dairy | Omphalitis | BCS > 3.75 | BCS 3.25–3.75 | OR 1.37 (1.00–1.86) | 0.045 | − | − |
| Author | Country | Study Design | Animals/Herds | Breed | Outcome | Study Group | Control Group | Effect Estimate (95% CI) | p | Result | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [19] | Estonia | Observation | −/15 | Dairy | Calf mortality due to diarrhea (21 d) | Vaccination of the dam against neonatal calf diarrhoe (NCD) + feeding transition milk at least 14 d | Same herds before implementing vaccination | HR 0.72 (0.63–0.81) | <0.001 | + | − |
| [19] | Estonia | Observation | −/15 | Dairy | Calf mortality due to diarrhea (21 d) | Vaccination of the dam against NCD + feeding transition milk up to 14 d | Same herds before implementing vaccination | HR 0.24 (0.14–0.41) | <0.001 | + | − |
| [19] | Estonia | Observation | −/15 | Dairy | Calf mortality due to diarrhea (21 d) | Vaccination of the dam against NCD not consequent | Same herds before implementing vaccination | HR 1.61 (1.21–2.15) | <0.001 | − | − |
| [100] | Netherlands | observation | −/13,000 | Dairy | Postnatal mortality (3–14 d) | Vaccination of the dam against NCD | No vaccination | IRR 0.91 (0.88–0.93) | <0.001 | + | − |
| [100] | Netherlands | observation | −/13,000 | Dairy | Preweaned mortality (15–55 d) | Vaccination of the dam against NCD | No vaccination | IRR 1.07 (1.03–1.11) | <0.01 | − | − |
| [101] | Belgium, Netherlands | observation | 523/24 | Dairy | Mortality (first 21 d of life) | Vaccination of the dam against NCD + use of halofuginone lactate for 7 days | No vaccination, no halofuginone lactate | − | − | = | − |
| [102] | USA | observation | 11,465/5 | Dairy | Mortality due to BRD till weaning | Vaccination of the dam with a live vaccine against BRD | No vaccination | OR 0.328 (0.13–0.829) | 0.018 | + | − |
| [102] | USA | observation | 11,465/5 | Dairy | Overall mortality till weaning | Vaccination of the dam with a live vaccine against BRD | No vaccination | OR 0.549 (0.414–0.727) | <0.001 | + | − |
| [92] | Germany | observation | 551/124 | Dairy | Total protein calf serum (3 d after birth) | Vaccination of the dam against NCD | No vaccination | − | − | = | − |
| [101] | Belgium, Netherlands | observation | 523/24 | Dairy | Morbidity NCD first 21 d of life | Vaccination of the dam against NCD + use of halofuginone lactate for 7 days | No vaccination, no halofuginone lactate | OR 0.26 (0.12–0.6) | <0.01 | + | − |
| [103] | Canada | observation | 1045/11 | Dairy | Morbidity NCD first 30 d of life | Vaccination of the dam against NCD | No vaccination | − | − | = | − |
| [104] | New Zealand | observation | 1283/97 | Dairy | liquid faeces in 9-to-21-day-old calves | Vaccination of the dam against NCD | No vaccination | OR 0.2 (0.1–0.9) | 0.03 | + | − |
| [105] | USA | observation | 4253/95 | Dairy | BRD till weaning | Vaccination of the dam against BRD (live or inactivated) | No vaccination | − | − | = | − |
| [106] | USA | observation | 11,300/5 | Dairy | BRD till weaning | Vaccination of the dam with a modified live vaccine against BRD | No vaccination | HR 0.326 (0.263–0.405) | <0.001 | + | − |
| [106] | USA | observation | 11,300/5 | Dairy | BRD till weaning | Vaccination of the dam with an inactivated vaccine against BRD | No vaccination | HR 0.847 (0.739–0.971) | <0.001 | + | − |
| Author | Country | Study Design | Animals/Herds | Breed | Outcome | Study Group | Control Group | Effect Estimate (95% CI) | p | Result | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|
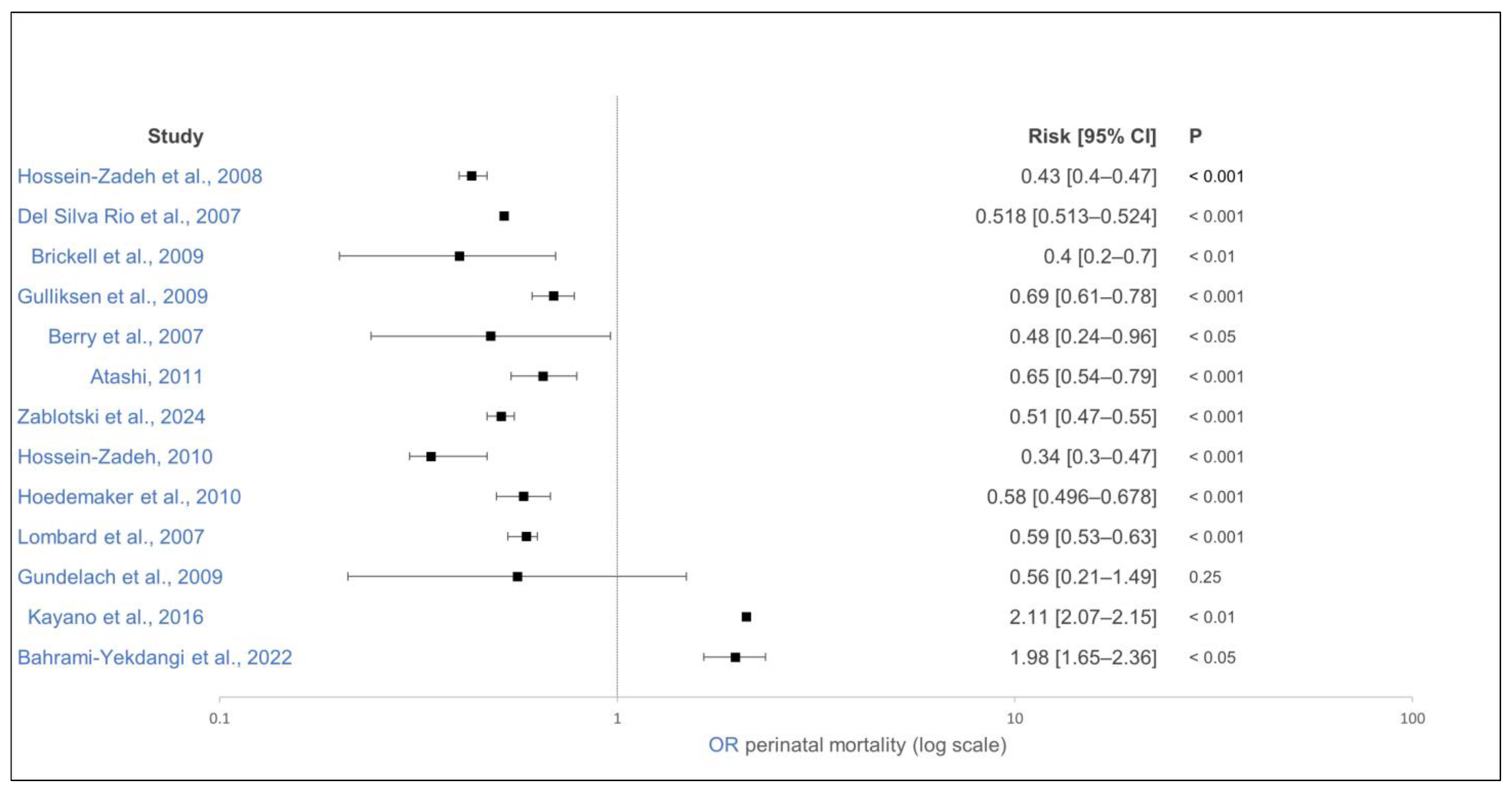
| [114] | Iran | Observation | 104,572/16 | Holstein | Perinatal mortality * | Parity = 2 | Parity = 1 | OR 0.43 (0.4–0.47) | <0.001 | + | − |
| [114] | Iran | Observation | 104,572/16 | Holstein | Perinatal mortality * | Parity = 3 | Parity = 1 | OR 0.41 (0.37–0.45) | <0.001 | + | − |
| [114] | Iran | Observation | 104,572/16 | Holstein | Perinatal mortality * | Parity ≥ 4 | Parity = 1 | OR 0.44 (0.41–0.48) | <0.001 | + | − |
| [31] | Iran | Observation | 2831/64 | Dairy | Perinatal mortality (1 h after birth) | Parity ≥ 2 | Parity = 1 | − | − | = | − |
| [21] | USA | Observation | 1,164,233/4103 | Holstein | Perinatal mortality (24 h after birth) | Parity = 2 | Parity = 1 | OR 0.518 (0.513–0.524) | <0.001 | + | − |
| [21] | USA | Observation | 1,164,233/4103 | Holstein | Perinatal mortality (24 h after birth) | Parity ≥ 3 | Parity = 1 | OR 0.526 (0.521–0.53) | <0.001 | + | − |
| [115] | USA | Observation | 7788/3 | Holstein | Perinatal mortality (24 h after birth) | Parity ≥ 2 | Parity = 1 | OR 0.59 (0.53–0.63) | <0.001 | + | − |
| [116] | Ireland | Observation | 305,531/− | Holstein | Perinatal mortality (24 h after birth) | Parity ≥ 2 | Parity = 1 | − | <0.05 | + | − |
| [117] | England | Observation | 1097/19 | Holstein | Perinatal mortality (24 h after birth) | Parity ≥ 2 | Parity = 1 | OR 0.4 (0.2–0.7) | <0.01 | + | − |
| [118] | Norway | Observation | 246,156/14,423 | Dairy | Perinatal mortality (24 h after birth) | Parity = 2 | Parity = 1 | OR 0.69 (0.61–0.78) | <0.001 | + | − |
| [118] | Norway | Observation | 246,156/14,423 | Dairy | Perinatal mortality (24 h after birth) | Parity = 3 | Parity = 1 | OR 0.7 (0.61–0.8) | <0.001 | + | − |
| [118] | Norway | Observation | 246,156/14,423 | Dairy | Perinatal mortality (24 h after birth) | Parity ≥ 4 | Parity = 1 | OR 0.68 (0.59–0.78) | <0.001 | + | − |
| [88] | Germany | Observation | 463/1 | Holstein | Perinatal mortality (24 h after birth) | Parity ≥ 2 | Parity = 1 | OR 0.56 (0.21–1.49) | 0.25 | = | − |
| [20] | Germany | Observation | 13,158/46 | Dairy | Perinatal mortality (24 h after birth) | Parity ≥ 2 | Parity = 1 | OR 0.58 (0.496–0.678) | <0.001 | + | − |
| [119] | Japan | Observation | 1,281,737/5,172 | Dairy | Perinatal mortality (24 h after birth) | Parity ≥ 2 | Parity = 1 | OR 2.11 (2.07–2.15) | <0.01 | − | − |
| [120] | Lithuania | Observation | 3861/1 | Holstein | Perinatal mortality (24 h after birth) | Parity ≥ 3 | Parity = 2 | OR 0.71 (0.47–0.972) | 0.043 | + | − |
| [121] | USA | Observation | 666,341/− | Holstein | Perinatal mortality (48 h after birth) | Parity = 2 or 3 | Parity = 1 | − | <0.001 | + | − |
| [85] | New Zealand | Observation | 2384/1 | Dairy | Perinatal mortality (48 h after birth) | Parity = 2 | Parity = 1 | OR 0.48 (0.24–0.96) | <0.05 | + | − |
| [85] | New Zealand | Observation | 2384/1 | Dairy | Perinatal mortality (48 h after birth) | Parity = 3 | Parity = 1 | OR 0.43 (0.21–0.89) | <0.05 | + | − |
| [85] | New Zealand | Observation | 2384/1 | Dairy | Perinatal mortality (48 h after birth) | Parity = 4 | Parity = 1 | OR 0.44 (0.26–0.75) | <0.05 | + | − |
| [85] | New Zealand | Observation | 2384/1 | Dairy | Perinatal mortality (48 h after birth) | Parity ≥ 5 | Parity = 1 | OR 0.56 (0.35–0.89) | <0.05 | + | − |
| [122] | Iran | Observation | 12,283/1 | Holstein | Perinatal mortality (48 h after birth) | Parity = 2 | Parity = 1 | OR 0.65 (0.54–0.79) | <0.001 | + | − |
| [122] | Iran | Observation | 12,283/1 | Holstein | Perinatal mortality (48 h after birth) | Parity = 3 | Parity = 1 | OR 0.57 (0.45–0.74) | <0.001 | + | − |
| [122] | Iran | Observation | 12,283/1 | Holstein | Perinatal mortality (48 h after birth) | Parity ≥ 4 | Parity = 1 | OR 0.75 (0.56–0.98) | 0.04 | + | − |
| [123] | Türkiye | Observation | 947/1 | Holstein | Perinatal mortality (48 h after birth) | Parity ≥ 2 | Parity = 1 | − | <0.05 | + | − |
| [87] | Iran | Observation | 51,405/3 | Holstein | Perinatal mortality (48 h after birth) | Parity = 2 | Parity = 1 | OR 1.98 (1.65–2.36) | <0.05 | − | − |
| [87] | Iran | Observation | 51,405/3 | Holstein | Perinatal mortality (48 h after birth) | Parity = 3 | Parity = 1 | OR 1.84 (1.48–2.29) | <0.05 | − | − |
| [87] | Iran | Observation | 51,405/3 | Holstein | Perinatal mortality (48 h after birth) | Parity ≥ 4 | Parity = 1 | OR 2.2 (1.69–2.86) | <0.05 | − | − |
| [10] | Germany | Observation | 133,942/721 | Dairy | Perinatal mortality (48 h after birth) | Parity = 2 | Parity = 1 | OR 0.51 (0.47–0.55) | <0.001 | + | − |
| [10] | Germany | Observation | 133,942/721 | Dairy | Perinatal mortality (48 h after birth) | Parity ≥ 3 | Parity = 1 | OR 0.6 (0.56–0.64) | <0.001 | + | − |
| [10] | Germany | Observation | 133,942/721 | Dairy | Perinatal mortality (48 h after birth) | Parity ≥ 3 | Parity = 2 | OR 1.18 (1.09–1.28) | <0.001 | − | − |
| [124] | Iran | Observation | 1,163,594/2552 | Holstein | Perinatal mortality * | Parity = 2 | Parity = 1 | OR 0.34 (0.3–0.47) | <0.001 | + | − |
| [124] | Iran | Observation | 1,163,594/2552 | Holstein | Perinatal mortality * | Parity = 3 | Parity = 1 | OR 0.31 (0.27–0.35) | <0.001 | + | − |
| [124] | Iran | Observation | 1,163,594/2552 | Holstein | Perinatal mortality * | Parity ≥ 4 | Parity = 1 | OR 0.39 (0.36–0.43) | <0.001 | + | − |
| [115] | USA | Observation | 7788/3 | Holstein | Mortality (1d–120 d) | Parity = 1 | Parity ≥ 2 | OR 0.9 (0.8–1.1) | 0.375 | = | only heifer calves |
| [115] | USA | Observation | 7788/3 | Holstein | Mortality (0 h–120 d) | Parity = 1 | Parity ≥ 2 | OR 1.2 (1.1–1.2) | <0.001 | − | only heifer calves |
| [115] | USA | Observation | 7788/3 | Holstein | Mortality (0 h–30 d) | Parity = 1 | Parity ≥ 2 | HR 1.2 (1–1.4) | 0.07 | = | only heifer calves |
| [125] | Iran | Observation | 4097/10 | Holstein | Mortality (90 d) | Parity = 1 | Parity ≥ 2 | − | − | = | − |
| [118] | Norway | Observation | 246,156/14,423 | Dairy | Mortality (1d–7 d) | Parity = 2 | Parity = 1 | OR 0.9 (0.85–0.95) | <0.001 | + | − |
| [118] | Norway | Observation | 246,156/14,423 | Dairy | Mortality (1d–7 d) | Parity = 3 | Parity = 1 | OR 0.89 (0.81–0.93) | <0.001 | + | − |
| [118] | Norway | Observation | 246,156/14,423 | Dairy | Mortality (1d–7 d) | Parity ≥ 4 | Parity = 1 | OR 0.83 (0.78–0.89) | <0.001 | + | − |
| [118] | Norway | Observation | 246,156/14,423 | Dairy | Mortality (8d–30 d) | Parity = 2 | Parity = 1 | OR 0.92 (0.87–0.98) | <0.05 | + | − |
| [118] | Norway | Observation | 246,156/14,423 | Dairy | Mortality (8d–30 d) | Parity = 3 | Parity = 1 | OR 0.9 (0.84–0.97) | <0.05 | + | − |
| [118] | Norway | Observation | 246,156/14,423 | Dairy | Mortality (8d–30 d) | Parity ≥ 4 | Parity = 1 | OR 0.85 (0.74–0.96) | <0.001 | + | − |
| [126] | England | Observation | 392/7 | Holstein | IgG calf plasma (1–7 d after birth) | Parity | − | − | − | = | − |
| [115] | USA | Observation | 7788/3 | Holstein | Morbidity (120 d) | Parity = 1 | Parity ≥ 2 | OR 0.8 (0.7–0.9) | <0.001 | + | Only heifer calves |
| [115] | USA | Observation | 7788/3 | Holstein | Respiratory disease (120 d) | Parity = 1 | Parity ≥ 2 | OR 0.8 (0.8–0.8) | <0.001 | + | Only heifer calves |
| [127] | Sweden | Observation | 3081/122 | Dairy | Respiratory disease (90 d) | Parity | − | − | − | = | Only heifer calves |
| [128] | USA | Observation | 449/3 | Holstein | Respiratory disease (90 d) | Parity = 1 | Parity ≥ 2 | OR 0.29 (0.09–0.91) | 0.03 | + | − |
| [115] | USA | Observation | 7788/3 | Holstein | Diarrhea (120 d) | Parity = 1 | Parity ≥ 2 | OR 0.8 (0.6–1.1) | <0.001 | + | Only heifer calves |
| [127] | Sweden | Observation | 3081/122 | Dairy | Diarrhea (90 d) | Parity | − | − | − | = | Only heifer calves |
| [128] | USA | Observation | 449/3 | Holstein | Diarrhea (90 d) | Parity | − | − | − | = | − |
| [129] | Netherlands | Observation | 683/13 | Holstein | Omphalitis | Parity = 2 | Parity = 3 | − | 0.02 | + | − |
| Author | Country | Study Design | Animals/Herds | Breed | Outcome | Study Group | Control Group | Effect Estimate (95% CI) | p | Result | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|
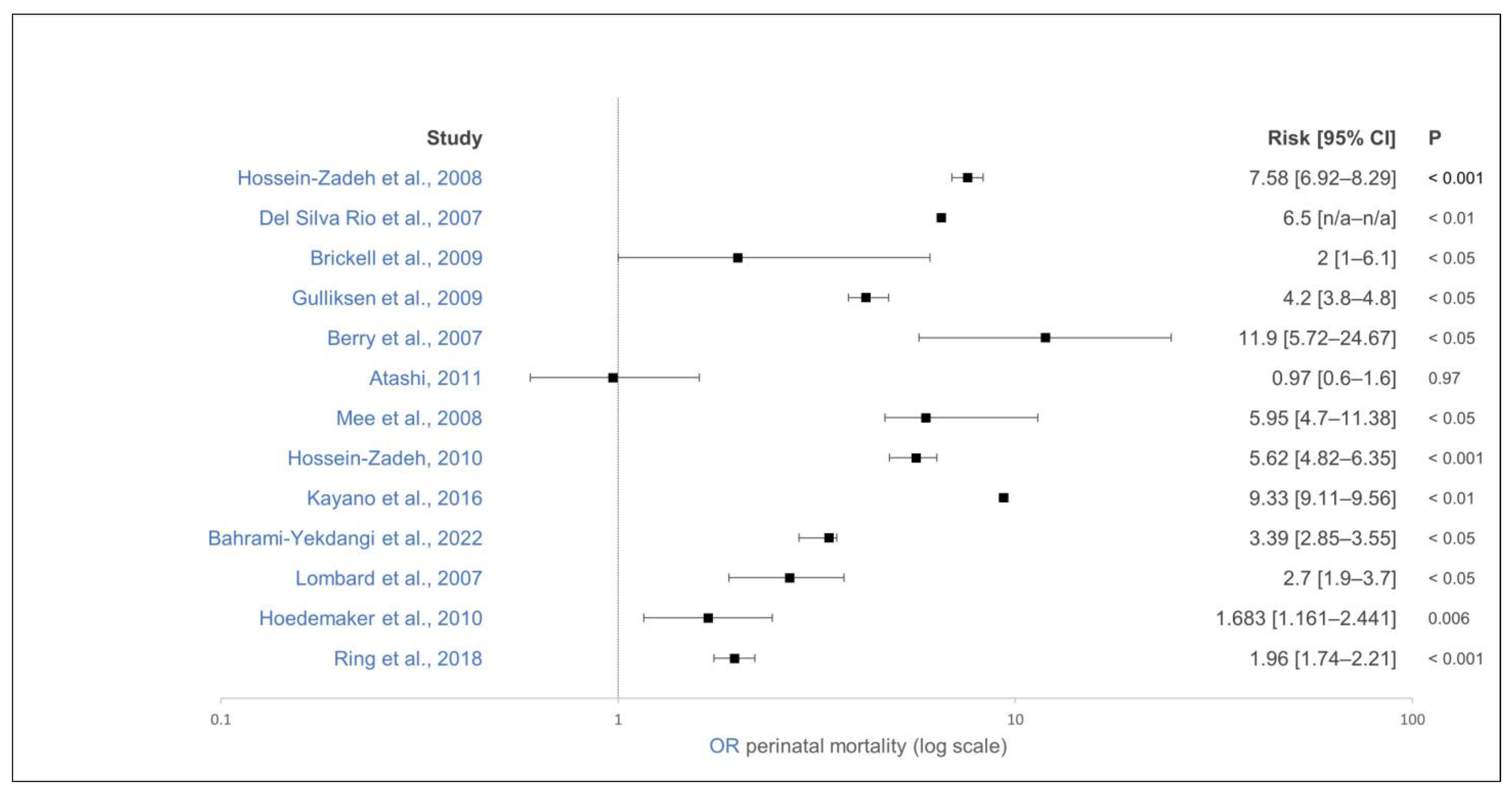
| [114] | Iran | Observation | 104,572/16 | Holstein | Perinatal mortality * | Twin pregnancy | Singelton calf | OR 7.58 (6.92–8.29) | <0.001 | − | − |
| [133] | USA | Observation | 1905/3 | Holstein | Perinatal mortality * | Twin pregnancy | Singelton calf | − | <0.01 | − | Primiparous |
| [124] | Iran | Observation | 1,163,594/2,552 | Holstein | Perinatal mortality * | Twin pregnancy | Singelton calf | OR 5.62 (4.82–6.35) | <0.001 | − | − |
| [21] | USA | Observation | 1,164,233/4103 | Holstein | Perinatal mortality (24 h after calving) | Twin pregnancy | Singelton calf | OR 6.5 | <0.01 | − | − |
| [115] | USA | Observation | 7788/3 | Holstein | Perinatal mortality (24 h after calving) | Twin pregnancy | Singelton calf | OR 2.7 (1.9–3.7) | <0.05 | − | − |
| [116] | Ireland | Observation | 304,531/− | Holstein | Perinatal mortality (24 h after calving) | Twin pregnancy | Singelton calf | OR 13.36 (11.03–16.21) | <0.05 | − | Primiparous |
| [116] | Ireland | Observation | 304,531/− | Holstein | Perinatal mortality (24 h after calving) | Twin pregnancy | Singelton calf | OR 5.95–9.6 (4.7–11.38) | <0.05 | − | Multiparous |
| [117] | England | Observation | 1097/19 | Holstein | Perinatal mortality (24 h after calving) | Twin pregnancy | Singelton calf | OR 2 (1–6.1) | <0.05 | − | − |
| [118] | Norway | Observation | 246,156/14,423 | Dairy | Perinatal mortality (24 h after calving) | Twin pregnancy | Singelton calf | OR 4.2 (3.8–4.8) | <0.05 | − | − |
| [20] | Germany | Observation | 13,158/46 | Dairy | Perinatal mortality (24 h after calving) | Twin pregnancy | Singelton calf | OR 1.683 (1.161–2.441) | 0.006 | − | − |
| [119] | Japan | Observation | 1,281,737/5,172 | Dairy | Perinatal mortality (24 h after calving) | Twin pregnancy | Singelton calf | OR 9.33 (9.11–9.56) | <0.01 | − | − |
| [85] | New Zealand | Observation | 2384/1 | Dairy | Perinatal mortality (48 h after calving) | Twin pregnancy | Singelton calf | OR 11.9 (5.72–24.67) | <0.05 | − | − |
| [122] | Iran | Observation | 12,283/1 | Holstein | Perinatal mortality (48 h after calving) | Twin pregnancy | Singelton calf | OR 0.98 (0.6–1.6) | 0.97 | = | − |
| [134] | Ireland | Observation | 11,256,112/29,299 | Dairy | Perinatal mortality (48 h after calving) | Twin pregnancy | Singelton calf | OR 1.96 (1.74–2.21) | <0.001 | − | − |
| [87] | Iran | Observation | 14,546/3 | Holstein | Perinatal mortality (48 h after calving) | Twin pregnancy | Singelton calf | OR 3.39 (2.85–3.55) | <0.05 | − | − |
| [118] | Norway | Observation | 246,156/14,423 | Dairy | Mortality first week of life | Twin pregnancy | Singelton calf | OR 1.3 (1.2–1.5) | <0.05 | − | − |
| [102] | USA | Observation | 11,465/5 | Dairy | Mortality till weaning | Twin pregnancy | Singelton calf | OR 1.688 (1.105–2.578) | 0.015 | − | − |
| [126] | England | Observation | 392/7 | Holstein | Total protein calf plasma (1–7 d after birth) | Twin pregnancy | Singelton calf | − | − | = | − |
| [135] | Czech Republic | Observation | 1175/33 | Dairy | FTP | Twin pregnancy | Singelton calf | − | 0.28 | = | − |
| [36] | Mexico | Observation | 4409/1 | Holstein | Adequate transfer of passive immunity | Singleton calf | Twin pregnancy | OR 1.4 (1.1 –1.8) | 0.0074 | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzebiatowski, L.; Wehrle, F.; Freick, M.; Donat, K.; Wehrend, A. Prenatal Factors Influencing Calf Morbidity and Mortality in Dairy Cattle: A Systematic Review of the Literature (2000–2024). Animals 2025, 15, 1772. https://doi.org/10.3390/ani15121772
Trzebiatowski L, Wehrle F, Freick M, Donat K, Wehrend A. Prenatal Factors Influencing Calf Morbidity and Mortality in Dairy Cattle: A Systematic Review of the Literature (2000–2024). Animals. 2025; 15(12):1772. https://doi.org/10.3390/ani15121772
Chicago/Turabian StyleTrzebiatowski, Lukas, Frederike Wehrle, Markus Freick, Karsten Donat, and Axel Wehrend. 2025. "Prenatal Factors Influencing Calf Morbidity and Mortality in Dairy Cattle: A Systematic Review of the Literature (2000–2024)" Animals 15, no. 12: 1772. https://doi.org/10.3390/ani15121772
APA StyleTrzebiatowski, L., Wehrle, F., Freick, M., Donat, K., & Wehrend, A. (2025). Prenatal Factors Influencing Calf Morbidity and Mortality in Dairy Cattle: A Systematic Review of the Literature (2000–2024). Animals, 15(12), 1772. https://doi.org/10.3390/ani15121772





