Ultrasonography of the Tympanic Bulla in Llamas and Alpacas: Techniques and Physiological Findings
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ultrasonographic Examination
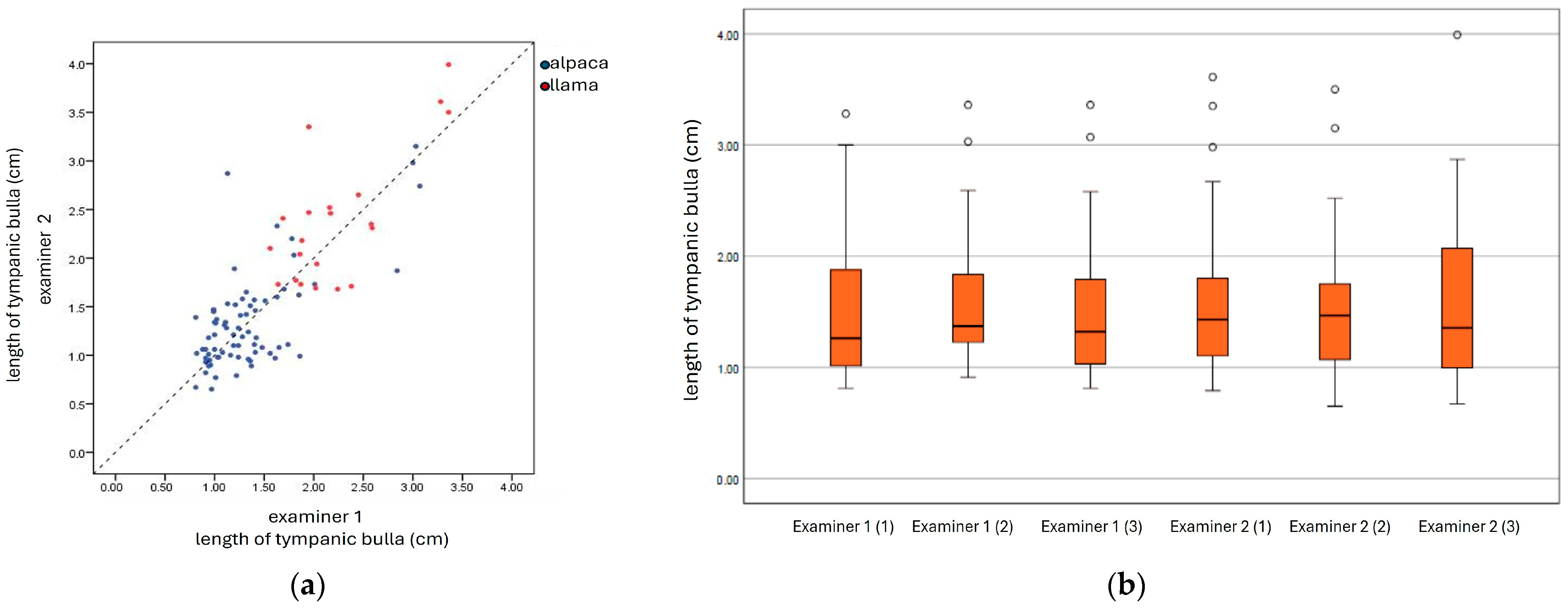
2.3. Statistical Analysis
3. Results
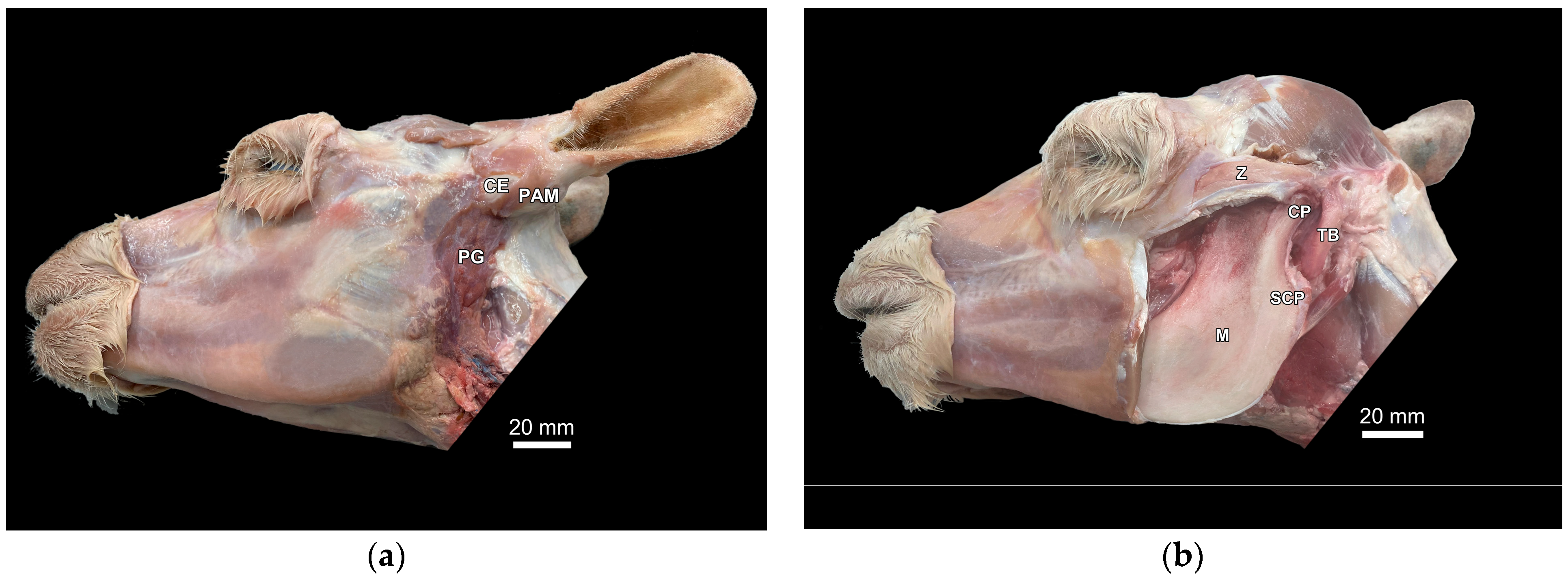
3.1. Identification of Anatomical Structures During Dissection and Ultrasonographic Appearance of Tympanic Bulla and Surrounding Structures in Cadaver Specimens
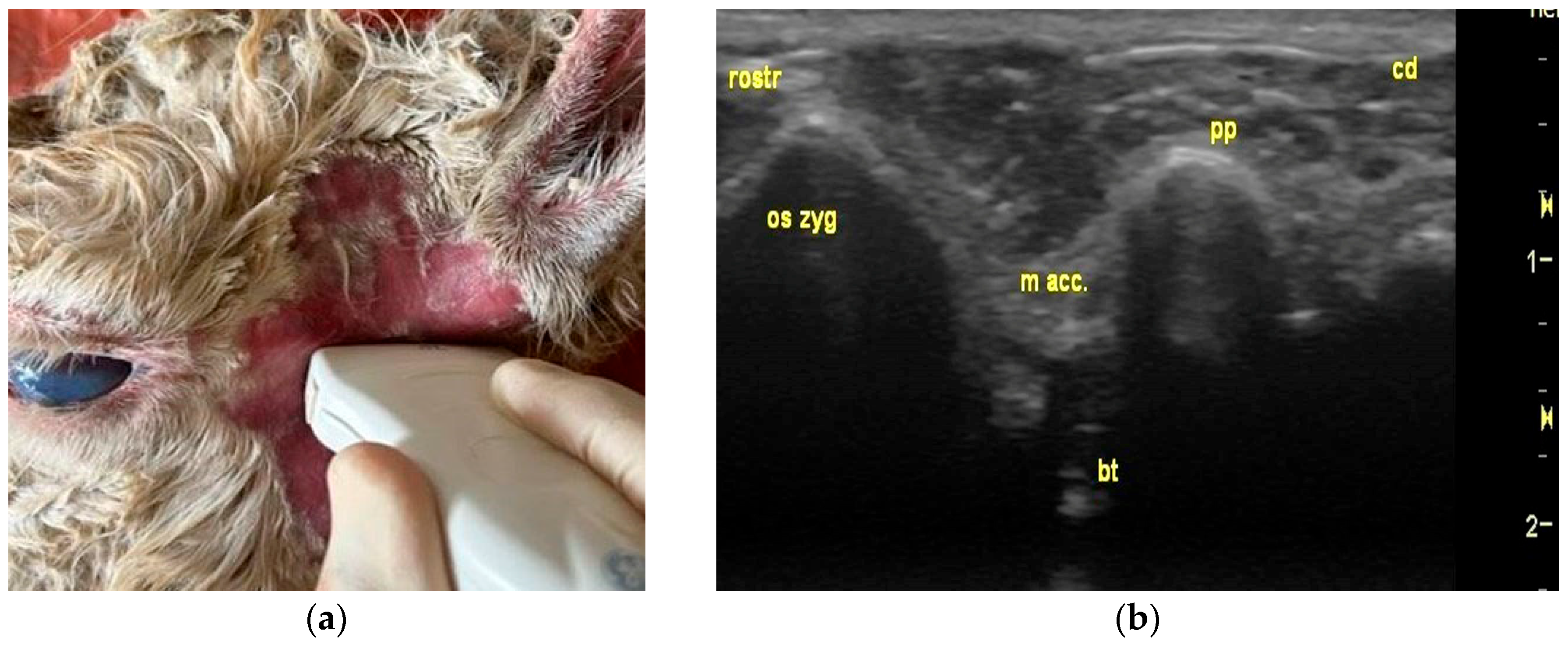
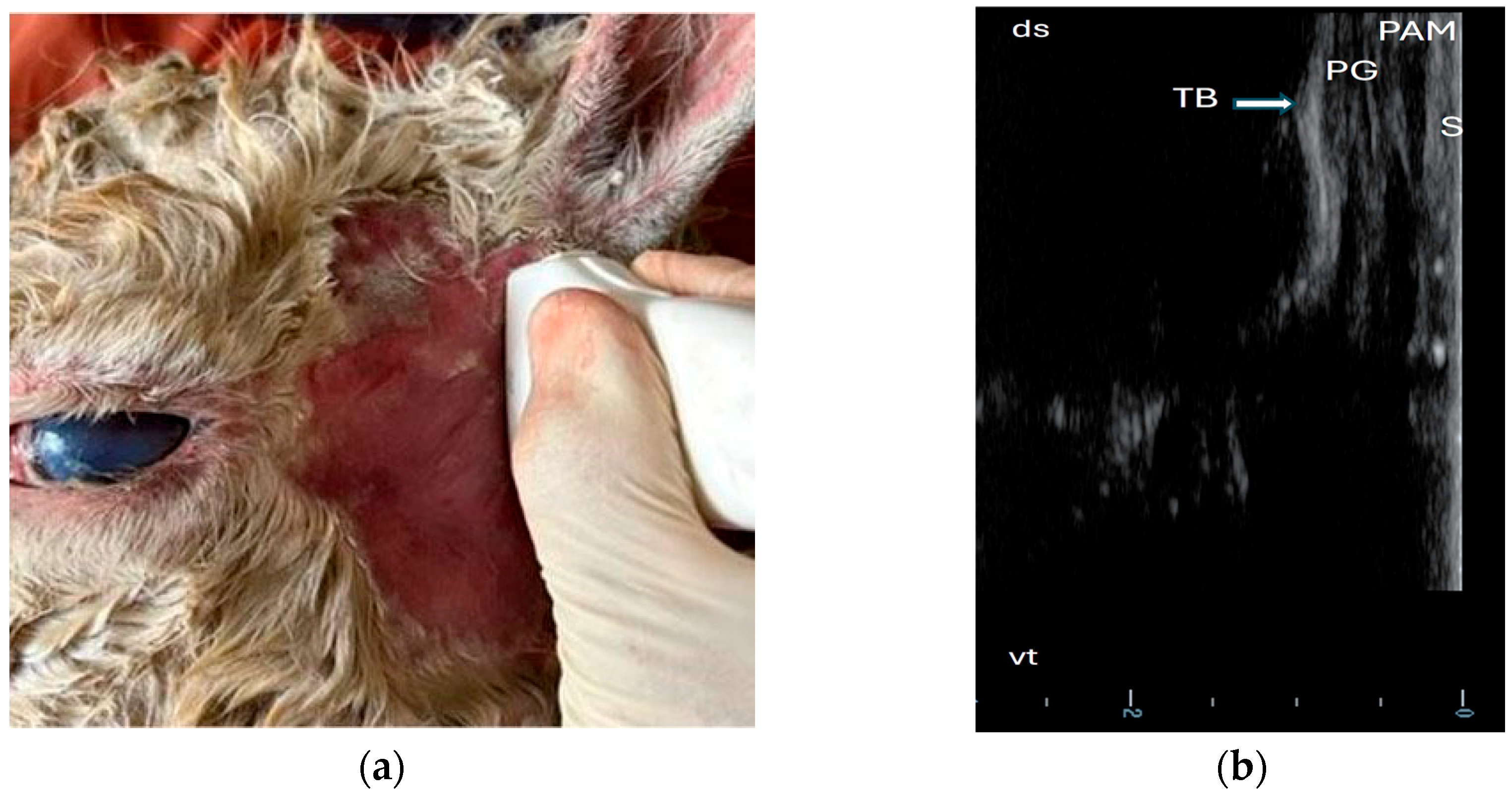
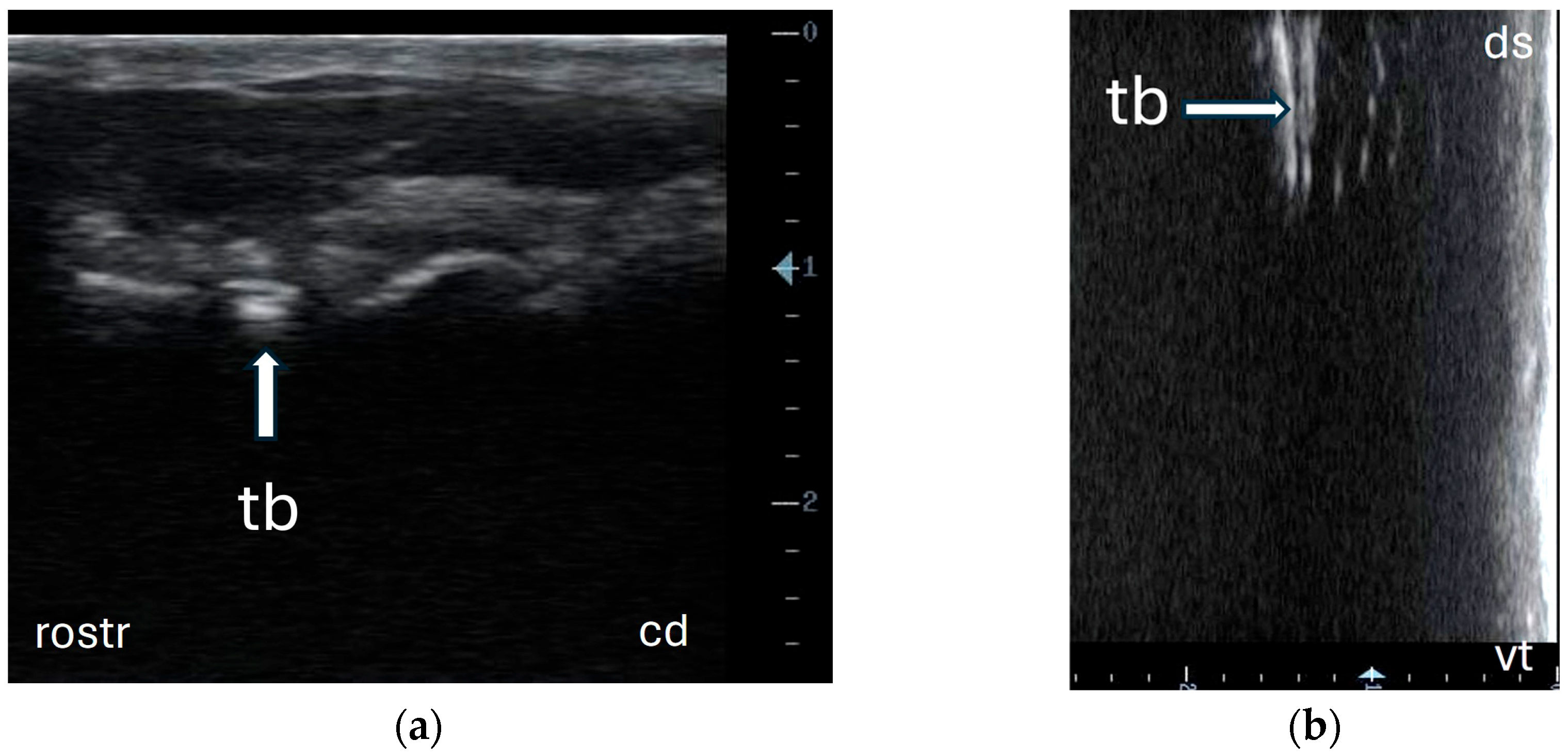
3.2. Ultrasonography of Tympanic Bulla and Surrounding Structures in Live Llamas and Alpacas and Measurement of Sonographically Visible Length of Bulla Wall
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| M | Mean |
| BCS | Body condition score |
| CT | Computed tomography |
| GSP | Good scientific practice |
| ICC | Intraclass correlation coefficient |
| MAX | Maximum |
| MHz | Mega Hertz |
| MIN | Minimum |
| SACs | South American camelids |
| SD | Standard deviation |
References
- Cebra, C.K.; Anderson, D.; Tibary, A.; VanSaun, R.; Johnson Larue, W. Llama and Alpaca Care Medicine Surgery, Reproduction, Nutrition and Healthcare, 1st ed.; Elsevier: St. Louis, MO, USA, 2014; pp. 455, 711–713. [Google Scholar]
- Whitehead, C.E.; Bedenice, D. Neurologic diseases in Llamas and Alpacas. Vet. Clin. North Am. Food Anim. Pract. 2009, 25, 385–405. [Google Scholar] [CrossRef] [PubMed]
- van Metre, D.C.; Barrington, G.M.; Parish, S.M.; Tumas, D.B. Otitis media/interna and suppurative meningoencephalomyelitis associated with Listeria monocytogenes infection in a llama. J. Am. Vet. Med. Assoc. 1991, 199, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Marriott, M.R.; Dart, A.J.; Macpherson, C.; Hodgson, D.R. Total ear canal ablation and lateral bulla osteotomy in an alpaca. Aust. Vet. J. 1999, 77, 301–302. [Google Scholar] [CrossRef]
- Koenig, J.B.; Watrous, B.J.; Kaneps, A.J.; Adams, J.G.; Parker, J.E. Otitis media in a llama. J. Am. Vet. Med. Assoc. 2001, 218, 1619–1623. [Google Scholar] [CrossRef]
- Choncha-Albornoz, I.; Stieger-Vanegas, S.M.; Cebra, C.K. Computed tomographic features of the osseus structures of the external acoustic meatus, tympanic cavity, and tympanic bulla of llamas (Lama glama). Am. J. Vet. Res. 2012, 73, 42–52. [Google Scholar] [CrossRef]
- Galvan, N.; Middleton, J.R.; Cook, C.; Britt, L.G.; Kuroki, K. Otitis interna, media, and externa with destruction of the left tympanic bulla and subluxation and septic arthritis of the left temporomandibular joint in an alpaca (Vicugna pacos). Can. Vet. J. 2013, 54, 283–285. [Google Scholar] [PubMed]
- Franz, S.; Högler, S.; Gumpenberger, M.; Dadak, A. Intracranial abscess formation in an adult alpaca: A case report. BMC Vet. Res. 2019, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.P.; Mueller, T.; Clapp, K.S.; Darien, B.J.; Forrest, L.J.; Colopy, S.A. Modified ear canal ablation and lateral bulla osteotomy for management of otitis media in an alpaca. Vet. Surg. 2012, 41, 273–277. [Google Scholar] [CrossRef]
- Miesner, M.D. Examination of the ear. In Veterinary techniques in Llamas and Alpacas, 2nd ed.; Anderson, D.E., Miesner, M.D., Jones, M.L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2023; pp. 110–113. [Google Scholar]
- Blond, L.; Buczinski, S. Basis of ultrasound imaging and the main Artifacts in bovine Medicine. Vet. Clin. Food Anim. Pract. 2009, 25, 553–565. [Google Scholar] [CrossRef]
- Kofler, J.; Steiner, A.; Starke, A.; Nuss, K. Ultrasonographic imaging of bone lesions. In Ultrasonography of the Bovine Musculoskeletal System. Indications, Examination Protocols, Findings, 1st ed.; Kofler, J., Ed.; Schlütersche: Hannover, Germany, 2021; pp. 175–192. [Google Scholar]
- Dickie, A.M.; Doust, R.; Cromarty, L.; Johnson, V.S.; Sullivan, M.; Boyd, J.S. Ultrasound imaging of the canine tympanic bulla. Res. Vet. Sci. 2003, 75, 121–126. [Google Scholar] [CrossRef]
- Griffiths, L.G.; Sullivan, M.; O’Neill, T.; Reid, S.W.J. Ultrasonography versus radiography for detection of fluid in the canine tympanic bulla. Vet. Radiol. Ultrasound 2003, 44, 210–213. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.; Hall, J.; Cranfield, F.; Sullivan, M. Anatomy and ultrasonographic appearance of the tympanic bulla and associated structures in the rabbit. Vet. J. 2007, 173, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, V.B.; Babkine, M.; Nichols, S.; Desroches, A. Ultrasound evaluation of tympanic bulla in calves. Can. Vet. J. 2012, 53, 849–854. [Google Scholar] [CrossRef]
- King, A.M.; Posthumus, J.; Hammond, G.; Sullivan, M. Comparison of ultrasonography, radiography and a single computed tomography slice for the identification of fluid within the tympanic bulla of rabbit cadavers. Vet. J. 2012, 193, 493–497. [Google Scholar] [CrossRef]
- Gosselin, V.B.; Babkine, M.; Francoz, D. Ultrasonography of the Tympanic bullae and larynx in cattle. Vet. Clin. N. Am. Food Anim. Pract. 2016, 32, 119–131. [Google Scholar] [CrossRef]
- Sharsher, A.; Ali, S.; Rashed, R.; Abedellaah, B. Ultrasound imaging of tympanic bulla and the surrounding structures in donkey (Equus asinus). J. Curr. Vet. Res. 2020, 2, 21–27. [Google Scholar] [CrossRef]
- Coeuriot, C.T.N.; Guise, L.; Casing, C.C.; Meregalli, R.; Fusellier, M.S. Tympanic bullae ultrasonography is feasible in nonsedated healthy rabbits (Oryctolagus cuniculus). J. Am. Vet. Med. Assoc. 2022, 260, 1934–1939. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.; Weinrauch, S.A.; Doust, R.; Hammond, G.; Yam, P.S.; Sullivan, M. Comparison of ultrasonography, radiography and a single computed tomography slice for fluid identification within the feline tympanic bulla. Vet. J. 2007, 173, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, V.B.; Babkine, M.; Gains, M.J.; Nichols, S.; Arsenault, J.; Francoz, D. Validation of an ultrasound imaging technique of the tympanic bullae for the diagnosis of otitis media in calves. J. Vet. Intern. Med. 2014, 28, 1594–1601. [Google Scholar] [CrossRef]
- Dickie, A.M.; Doust, R.; Cromarty, L.; Johnson, V.S.; Sullivan, M.; Boyd, J.S. Comparison of ultrasonography, radiography and a single computed tomography slice for the identification of fluid within the canine tympanic bulla. Res. Vet. Sci. 2003, 75, 209–216. [Google Scholar] [CrossRef]
- Doust, R.; King, A.; Hammond, G. Assessment of middle ear disease in the dog: A comparison of diagnostic imaging modalities. J. Small Anim. Pract. 2007, 48, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.H. Topographische-Anatomische Untersuchungen am Kopf des Alpakas (Vicugna pacos). Ph.D. Thesis, Department of Veterinary Sciences, Veterinary Faculty, Chair of Anatomy, Histology and Embryology, Ludwig-Maximilians-University Munich, Munich, Germany, 2020. [Google Scholar]
- Windschnurer, I.; Eibl, C.; Franz, S.; Gilhofer, E.M.; Waiblinger, S. Alpaca and llama behaviour during handling and its associations with caretaker attitudes and human-animal contact. Appl. Anim. Behav. Sci. 2020, 226, 104989. [Google Scholar] [CrossRef]
- Van Saun, R.J. Body Condition Scoring of Llamas and Alpacas. Available online: https://extension.psu.edu/body-condition-scoring-of-llamas-and-alpacas (accessed on 12 June 2025).
- Eibl, C.; Franz, S. Ultrasonography of kidney and spleen in clinically healthy llamas and alpacas. Acta Vet. Scand. 2021, 63, 4. [Google Scholar] [CrossRef] [PubMed]
| Llamas: Measured Parameter | Gender | N | M | SD | Min. | Max. |
|---|---|---|---|---|---|---|
| Age | Male | 8 | 10.4 | 6.36 | 2 | 19 |
| Female | 12 * | 8.8 | 8.75 | 3 | 15 | |
| BCS | Male | 8 | 3.1 | 0.17 | 3 | 3.5 |
| Female | 13 | 2.8 | 0.24 | 2.5 | 3 |
| Alpacas: Measured Parameter | Gender | N | M | SD | Min. | Max. |
|---|---|---|---|---|---|---|
| Age | Male | 21 | 7.4 | 3.62 | 1 | 17 |
| Female | 29 | 7.5 | 4.21 | 1 | 13 | |
| BCS | Male | 21 | 2.9 | 0.35 | 2 | 3 |
| Female | 29 | 2.7 | 0.44 | 2 | 3.5 |
| L-TB (cm) | Gender | N | M (cm) | SD (cm) | Min. (cm) | Max. (cm) |
|---|---|---|---|---|---|---|
| Llamas | Male | 8 | 1.53 | 0.36 | 1.15 | 2.24 |
| Female | 13 | 1.80 | 0.51 | 1.16 | 3.28 | |
| Alpacas | Male | 21 | 1.43 | 0.46 | 0.63 | 2.44 |
| Female | 29 | 1.36 | 0.30 | 0.64 | 1.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giebl, R.; Maierl, J.; Tichy, A.; Eibl, C.; Dadak, A.; Franz, S. Ultrasonography of the Tympanic Bulla in Llamas and Alpacas: Techniques and Physiological Findings. Animals 2025, 15, 1762. https://doi.org/10.3390/ani15121762
Giebl R, Maierl J, Tichy A, Eibl C, Dadak A, Franz S. Ultrasonography of the Tympanic Bulla in Llamas and Alpacas: Techniques and Physiological Findings. Animals. 2025; 15(12):1762. https://doi.org/10.3390/ani15121762
Chicago/Turabian StyleGiebl, Rainer, Johann Maierl, Alexander Tichy, Cassandra Eibl, Agnes Dadak, and Sonja Franz. 2025. "Ultrasonography of the Tympanic Bulla in Llamas and Alpacas: Techniques and Physiological Findings" Animals 15, no. 12: 1762. https://doi.org/10.3390/ani15121762
APA StyleGiebl, R., Maierl, J., Tichy, A., Eibl, C., Dadak, A., & Franz, S. (2025). Ultrasonography of the Tympanic Bulla in Llamas and Alpacas: Techniques and Physiological Findings. Animals, 15(12), 1762. https://doi.org/10.3390/ani15121762





