Evaluating Good Husbandry Practices and Organic Fermented Additives for Coccidiosis Control in a Pilot Study Using Slow-Growing Broilers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Organic Fermented Additive Preparation
2.2. Metagenomic Analysis of the Organic Fermented Additive
2.3. Legal Framework
2.4. Parasites
2.5. Animals and Management
2.6. Experimental Design
2.6.1. Trial 1
2.6.2. Trial 2
2.7. Oocyst Quantification
2.8. Infection Parameters: Lesion Score, Microscopic Observation, Histopathological Analysis, and Clinical Signs of Coccidiosis
2.9. Production Parameters: Body Weight Gain, Feed Conversion, Productivity Index, and Anticoccidial Index
2.10. Statistical Analysis
3. Results
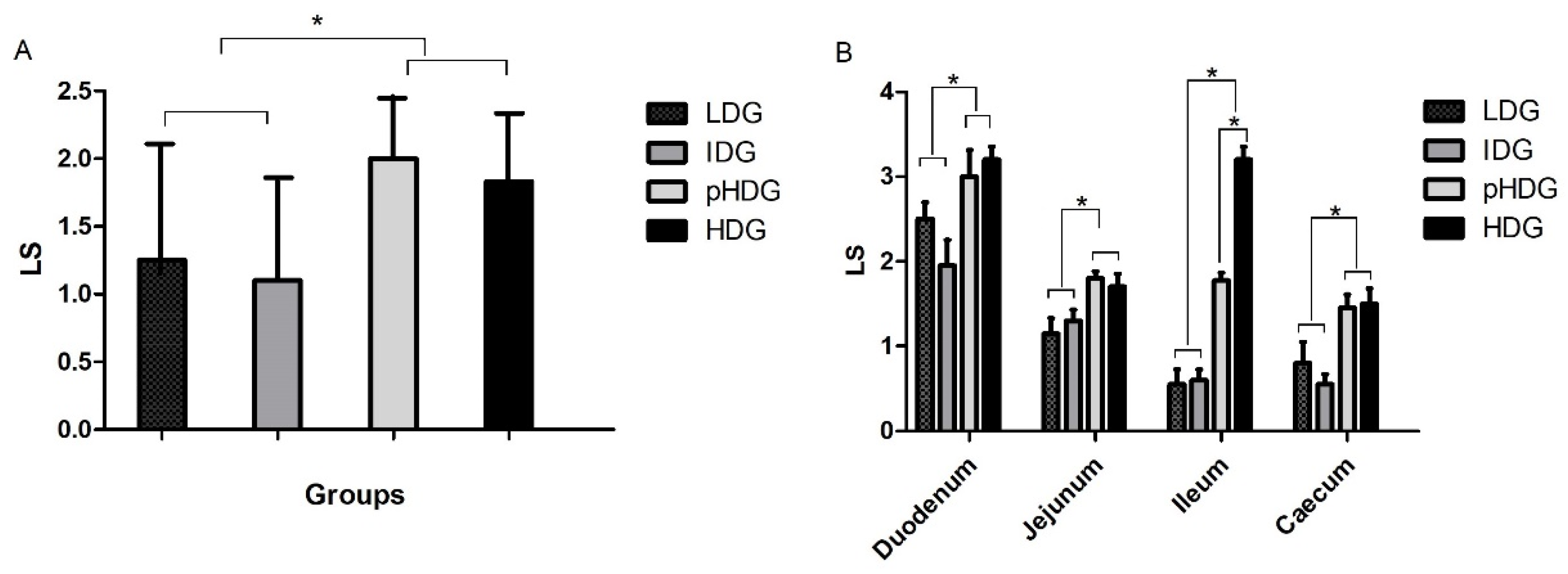
3.1. Trial 1: Susceptibility of Campero-INTA to Eimeria-Infection
3.1.1. Oocysts Excretion, Microscopic Lesions, and Clinical Signs of Coccidiosis
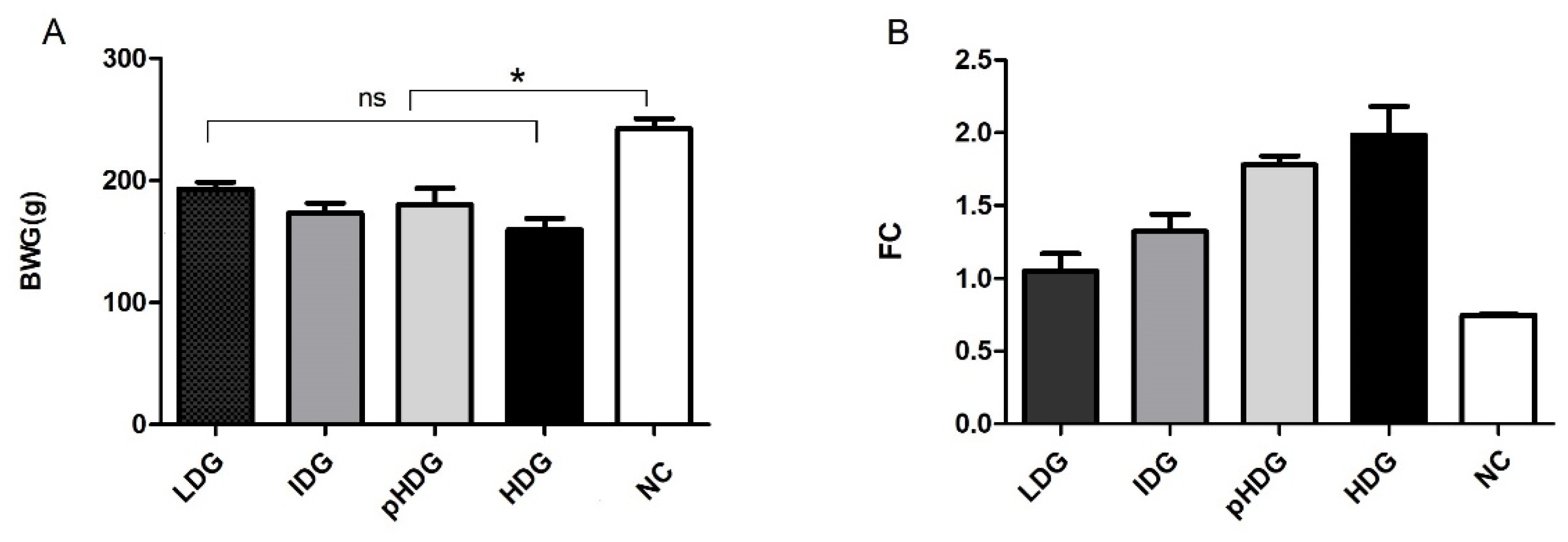
3.1.2. Body Weight Gain, Feed Conversion, and Productivity Index
3.2. Trial 2: Effect of Good Husbandry Practices and Organic Fermented Additive in Campero-INTA Broilers
3.2.1. Organoleptic Properties and Metagenomics of Organic Fermented Additive
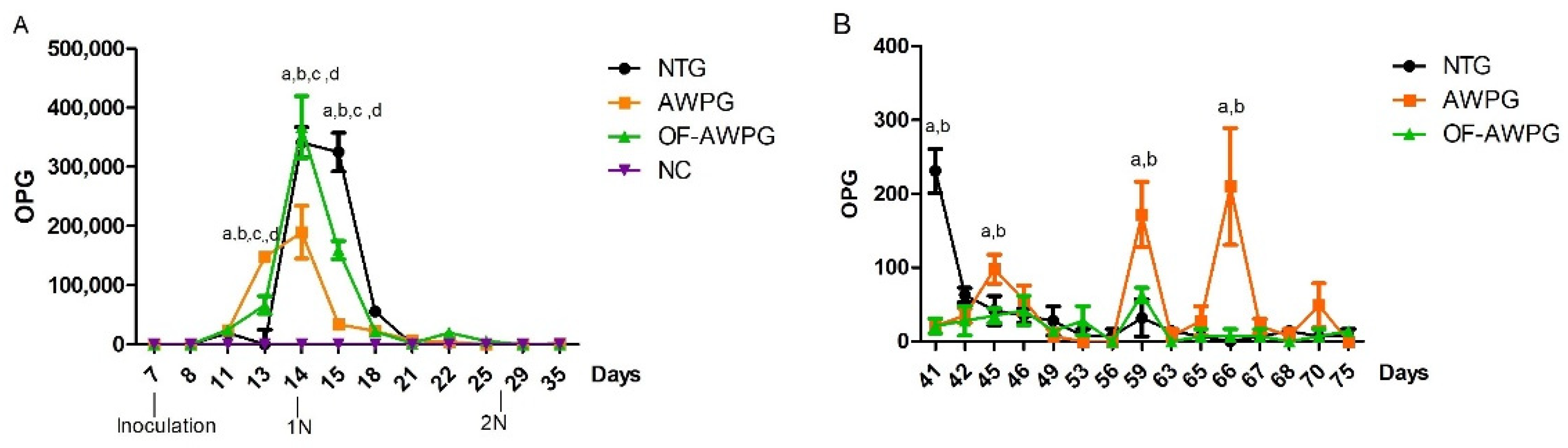
3.2.2. Clinical Signs of Coccidiosis and Mortality
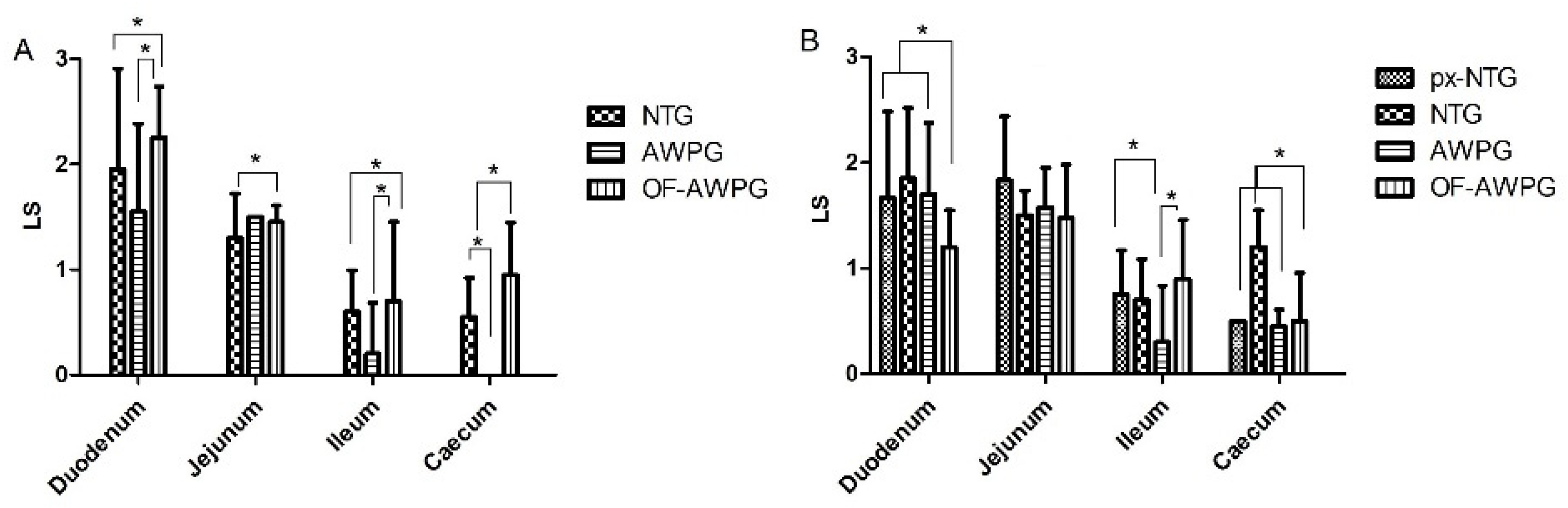
3.2.3. Oocysts Excretion, Lesion Scoring, and Microscopic Lesions
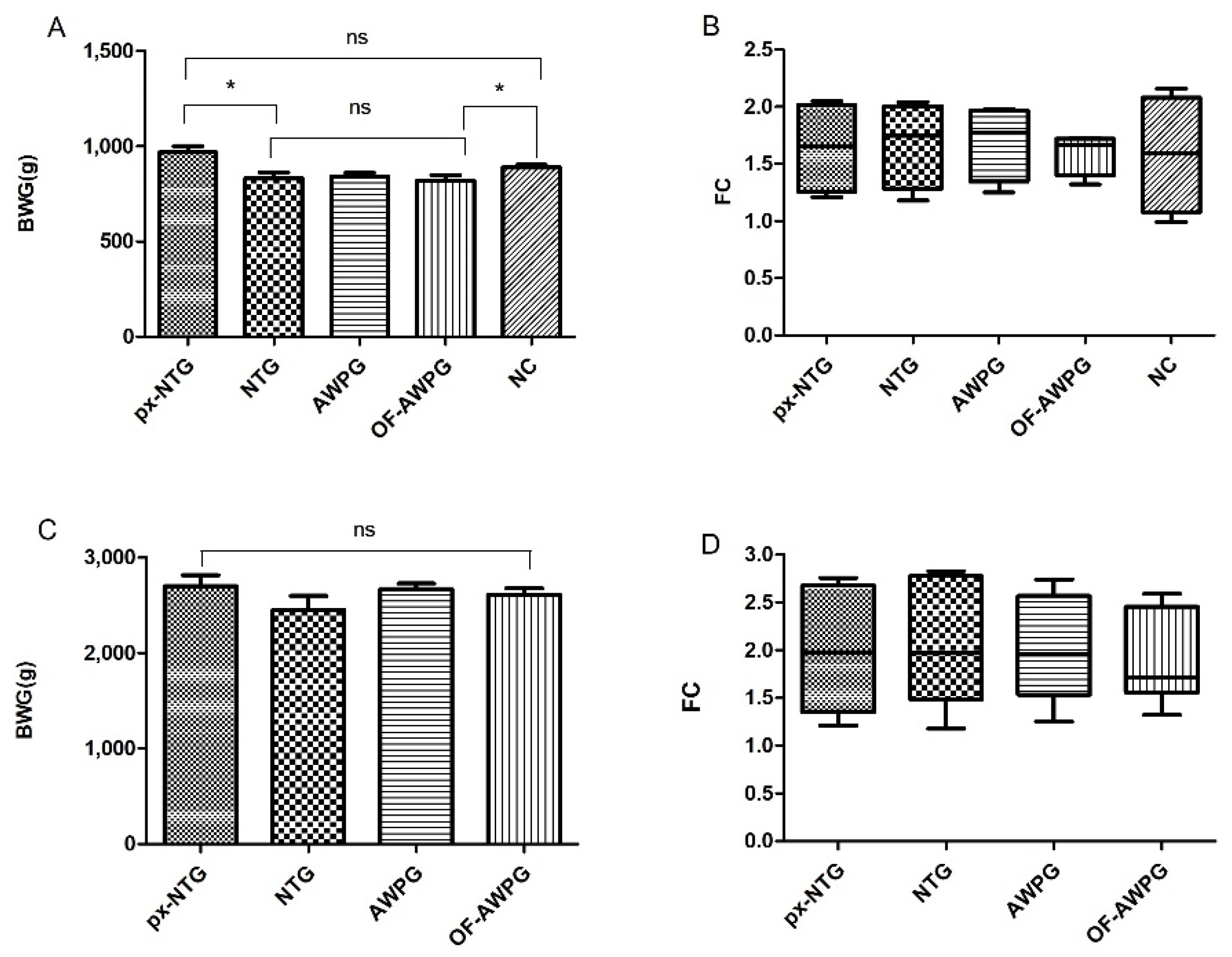
3.2.4. Body Weight and Feed Conversion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FPPS | Family poultry production systems |
| INTA | Instituto Nacional de Tecnología Agropecuaria |
| AE | Agroecology |
| LAB | Lactic acid bacteria |
| BWG | Body Weight Gain |
| rBWG | Relative Body Weight Gain |
| FC | Feed Conversion |
| LS | Lesion Score |
| PI | Productivity index |
| ACI | Anticoccidial Index |
| LDG | Low-dose group |
| IDG | Intermediate dose group |
| pHDG | pre-inoculated high-dose group |
| HDG | High dose group |
| VH | Villus height |
| CD | Crypt depth |
| AWP | Animal Welfare Practices |
| NTG | non-treated control group |
| AWP | animal welfare practice |
| AWPG | animal welfare practice group |
| OF-AWPG | AWP with organic fermented additive group |
| px-NTG | proximity non-challenged- non treated groups |
| NC | non-challenged-not treated group |
References
- OECD-FAO. Agricultural Outlook 2021–2030; OECD: Paris, France, 2021; pp. 163–177. [Google Scholar]
- Kumar, M.; Dahiya, S.P.; Ratwan, P. Backyard Poultry Farming in India: A Tool for Nutritional Security and Women Empowerment. Biol. Rhythm. Res. 2021, 52, 1476–1491. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of The United Nations. Family Poultry Development; FAO: Rome, Italy, 2014; Volume 12, pp. 1–25. [Google Scholar]
- Tomazic, M.L.; Britez, J.D.; Pisón-Martínez, M.L.; Barbano, P.; Canet, Z.; Trangoni, M.D.; Poklepovich, T.J.; Cubas, F.; Alegría-Morán, R.; Ramírez-Toloza, G.; et al. Chicken Coccidiosis in Peri-Urban Family Farming in Two South American Countries: Prevalence and Circulating Eimeria spp. Animals 2025, 15, 982. [Google Scholar] [CrossRef] [PubMed]
- MinAgro, INTA. Manual de Avicultura. Available online: https://www.argentina.gob.ar/sites/default/files/manual_de_avicultura_2oano.pdf (accessed on 3 June 2025).
- OIE. Terrestrial Animal Health Code—Animal Welfare and Broiler Chicken Production Systems; WOHA: Singapore, 2022. [Google Scholar]
- Wlaźlak, S.; Pietrzak, E.; Biesek, J.; Dunislawska, A. Modulation of the Immune System of Chickens a Key Factor in Maintaining Poultry Production—A Review. Poult. Sci. 2023, 102, 102785. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, G.M.; Canet, Z.E.; Cantaro, H.; Miquel, M.C.; Melo, J.E.; Miller, M.M.; Berres, M.E.; Fulton, J.E. Mhc-B Haplotypes in “Campero-Inta” Chicken Synthetic Line. Poult. Sci. 2019, 98, 5281–5286. [Google Scholar] [CrossRef]
- Bonino, M.F.; Canet, Z.E. El Pollo y el Huevo Campero; Instituto Nacional de Tecnología Agropecuaria: Pergamino, Argentina, 1999; pp. 1–39. ISBN 9875210080. [Google Scholar]
- Stadig, L.M.; Rodenburg, T.B.; Reubens, B.; Aerts, J.; Duquenne, B.; Tuyttens, F.A.M. Effects of Free-Range Access on Production Parameters and Meat Quality, Composition and Taste in Slow-Growing Broiler Chickens. Poult. Sci. 2016, 95, 2971–2978. [Google Scholar] [CrossRef]
- Chapman, H.D. Milestones in Avian Coccidiosis Research: A Review. Am. Hist. Rev. 2014, 119, 501–511. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Agroecology and the Emergence of a Post COVID-19 Agriculture. Agric. Hum. Values 2020, 37, 525–526. [Google Scholar] [CrossRef]
- Phocas, F.; Belloc, C.; Bidanel, J.; Delaby, L.; Dourmad, J.Y.; Dumont, B.; Ezanno, P.; Fortun-Lamothe, L.; Foucras, G.; Frappat, B.; et al. Review: Towards the Agroecological Management of Ruminants, Pigs and Poultry through the Development of Sustainable Breeding Programmes: I-Selection Goals and Criteria. Animal 2016, 10, 1749–1759. [Google Scholar] [CrossRef]
- Pinard-Van Der Laan, M.H.; Monvoisin, J.L.; Pery, P.; Hamet, N.; Thomas, M. Comparison of Outbred Lines of Chickens for Resistance to Experimental Infection with Coccidiosis (Eimeria tenella). Poult. Sci. 1998, 77, 185–191. [Google Scholar] [CrossRef]
- Du, S.; Song, Z.; Cen, Y.; Fan, J.; Li, P.; Si, H.; Hu, D. Susceptibility and Cecal Microbiota Alteration to Eimeria-Infection in Yellow-Feathered Broilers, Arbor Acres Broilers and Lohmann Pink Layers. Poult. Sci. 2024, 103, 103824. [Google Scholar] [CrossRef]
- Zhi, W.; Chen, H.; Bai, B.; Jia, Z.; Pan, X.; Wang, B.; Kong, R.; Liu, Q.; Ma, C.; Ma, D. Combined Oral Immunization with Probiotics Entercoccus Faecalis Delivering Surface-Anchored Eimeria Tenella Proteins Provide Protective Efficacies against Homologous Infection in Chickens. Front. Immunol. 2022, 13, 1042143. [Google Scholar] [CrossRef] [PubMed]
- Gramaglia, C. Suplementación Animal con un Enfoque Agroecológico. Available online: https://www.ciap.org.ar/Sitio/Archivos/INTA_CRCordoba_EEAManfredi_Gramaglia_C_Suplementacion_animal_con_un.pdf (accessed on 30 May 2025).
- Food and Agriculture Organization of the United Nations. Family Farming Knowledge Platform Suplementación Animal con un Enfoque Agroecológico, 2022. Available online: https://www.fao.org/family-farming/detail/es/c/1604417/ (accessed on 3 June 2025).
- Lu, J.; Breitwieser, F.P.; Thielen, P.; Salzberg, S.L. Bracken: Estimating Species Abundance in Metagenomics Data. PeerJ Comput. Sci. 2017, 3, e104. [Google Scholar] [CrossRef] [PubMed]
- Breitwieser, F.P.; Salzberg, S.L. Pavian: Interactive Analysis of Metagenomics Data for Microbiome Studies and Pathogen Identification. Bioinformatics 2020, 36, 1303–1304. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.; Pagotto, A.; Furtado, M.; Katsuyama, Â.; Madeira, A.B.; Gruber, A. A Multiplex PCR Assay for the Simultaneous Detection and Discrimination of the Seven Eimeria Species That Infect Domestic Fowl. Parasitology 2003, 127, 317–325. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W. Anticoccidial Drugs: Lesion Scoring Techniques in Battery and Floor-Pen Experiments with Chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- De Pablos, L.M.; Dos Santos, M.F.B.; Montero, E.; Garcia-Granados, A.; Parra, A.; Osuna, A. Anticoccidial Activity of Maslinic Acid against Infection with Eimeria Tenella in Chickens. Parasitol. Res. 2010, 107, 601–604. [Google Scholar] [CrossRef]
- Morisawa, Y.; Kataoka, M.; Kitano, N.; Matsuzawa, T. Studies on Anticoccidial Agents. 10. Synthesis and Anticoccidial Activity of 5-Nitronicotinamide and Its Analogs. J. Med. Chem. 1977, 20, 129–133. [Google Scholar] [CrossRef]
- Geng, T.; Ruan, X.; Xie, Y.; Shen, B.; Fang, R.; Zhao, J.; Zhou, Y. Anticoccidial Activity of a Botanical Natural Product Based on Eucalyptus, Apigenin and Eugenol against Eimeria Tenella in Broiler Chickens. Parasites Vectors 2024, 17, 327. [Google Scholar] [CrossRef]
- Bangoura, B.; Daugschies, A. Eimeria. Parasitic Protozoa of Farm Animals and Pet; Florin-Christensen, M., Schittger, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 55–102. [Google Scholar]
- Répérant, J.-M.; Dardi, M.; Pagès, M.; Thomas-Hénaff, M. Pathogenicity of Eimeria praecox Alone or Associated with Eimeria acervulina in Experimentally Infected Broiler Chickens. Vet. Parasitol. 2012, 187, 333–336. [Google Scholar] [CrossRef]
- Flores, R.A.; Nguyen, B.T.; Cammayo, P.L.T.; Fernandez-Colorado, C.P.; Roy, A.; Kim, S.; Kim, W.; Min, W. Comparative Analysis of Evaluation Parameters in E. Acervulina, E. Maxima and E. Tenella-Infected Broilers. J. Vet. Sci. 2022, 23, e91. [Google Scholar] [CrossRef]
- Zhou, B.; Jia, L.; Wei, S.; Ding, H.; Yang, J.; Wang, H. Effects of Eimeria tenella Infection on the Barrier Damage and Microbiota Diversity of Chicken Cecum. Poult. Sci. 2020, 99, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Rysman, K.; Eeckhaut, V.; Croubels, S.; Maertens, B.; Van Immerseel, F. Iohexol Is an Intestinal Permeability Marker in Broilers under Coccidiosis Challenge. Poult. Sci. 2023, 102, 102690. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Huang, S.; Huang, Y.; Bai, X.; Zhang, R.; Lei, Y.; Wang, X. Effects of Eimeria Challenge on Growth Performance, Intestine Integrity, and Cecal Microbial Diversity and Composition of Yellow Broilers. Poult. Sci. 2024, 103, 104470. [Google Scholar] [CrossRef] [PubMed]
- Belote, B.L.; Soares, I.; Sanches, A.W.D.; De Souza, C.; Scott-Delaunay, R.; Lahaye, L.; Kogut, M.H.; Santin, E. Applying Different Morphometric Intestinal Mucosa Methods and the Correlation with Broilers Performance under Eimeria Challenge. Poult. Sci. 2023, 102, 102849. [Google Scholar] [CrossRef]
- De Verdal, H.; Mignon-Grasteau, S.; Jeulin, C.; Le Bihan-Duval, E.; Leconte, M.; Mallet, S.; Martin, C.; Narcy, A. Digestive Tract Measurements and Histological Adaptation in Broiler Lines Divergently Selected for Digestive Efficiency. Poult. Sci. 2010, 89, 1955–1961. [Google Scholar] [CrossRef]
- Conway, D.P.; McKenzie, M.E.; Dayton, A.D. Relationship of Coccidial Lesion Scores and Weight Gain in Infections of Eimeria acervulina, E. Maxima and E. Tenella in Broilers. Avian Pathol. 1990, 19, 489–496. [Google Scholar] [CrossRef]
- Sharma, V.D.; Fernando, M.A. Effect of Eimeria Acervulina Infection on Nutrient Retention with Special Reference to Fat Malabsorption in Chickens. Can. J. Comp. Med. 1975, 39, 146–154. [Google Scholar]
- Rochell, S.J.; Parsons, C.M.; Dilger, R.N. Effects of Eimeria acervulina Infection Severity on Growth Performance, Apparent Ileal Amino Acid Digestibility, and Plasma Concentrations of Amino Acids, Carotenoids, and A1-Acid Glycoprotein in Broilers. Poult. Sci. 2016, 95, 1573–1581. [Google Scholar] [CrossRef]
- Teng, P.-Y.; Yadav, S.; Castro, F.L.D.S.; Tompkins, Y.H.; Fuller, A.L.; Kim, W.K. Graded Eimeria Challenge Linearly Regulated Growth Performance, Dynamic Change of Gastrointestinal Permeability, Apparent Ileal Digestibility, Intestinal Morphology, and Tight Junctions of Broiler Chickens. Poult. Sci. 2020, 99, 4203–4216. [Google Scholar] [CrossRef]
- Teng, P.-Y.; Choi, J.; Tompkins, Y.; Lillehoj, H.; Kim, W. Impacts of Increasing Challenge with Eimeria maxima on the Growth Performance and Gene Expression of Biomarkers Associated with Intestinal Integrity and Nutrient Transporters. Vet. Res. 2021, 52, 81. [Google Scholar] [CrossRef]
- Sakkas, P.; Oikeh, I.; Blake, D.P.; Nolan, M.J.; Bailey, R.A.; Oxley, A.; Rychlik, I.; Lietz, G.; Kyriazakis, I. Does Selection for Growth Rate in Broilers Affect Their Resistance and Tolerance to Eimeria Maxima? Vet. Parasitol. 2018, 258, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Swinkels, W.J.C.; Post, J.; Cornelissen, J.B.; Engel, B.; Boersma, W.J.A.; Rebel, J.M.J. Immune Responses to an Eimeria acervulina Infection in Different Broilers Lines. Vet. Immunol. Immunopathol. 2007, 117, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ballou, A.L.; Ali, R.A.; Mendoza, M.A.; Ellis, J.C.; Hassan, H.M.; Croom, W.J.; Koci, M.D. Development of the Chick Microbiome: How Early Exposure Influences Future Microbial Diversity. Front. Vet. Sci. 2016, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Villa, R.E.; Azimonti, G.; Bonos, E.; Christensen, H.; Durjava, M.; Dusemund, B.; Gehring, R.; Glandorf, B.; Kouba, M.; et al. Safety and Efficacy of a Feed Additive Consisting of Lentilactobacillus buchneri DSM 32651 for All Animal Species (BioCC OÜ). EFS2 2024, 22, e9029. [Google Scholar] [CrossRef]
- Cheon, M.-J.; Lim, S.-M.; Lee, N.-K.; Paik, H.-D. Probiotic Properties and Neuroprotective Effects of Lactobacillus buchneri KU200793 Isolated from Korean Fermented Foods. Int. J. Mol. Sci. 2020, 21, 1227. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, Y.; Fan, Y.; Dong, Y.; Gai, Z. Genome Sequence and Evaluation of Safety and Probiotic Potential of Lacticaseibacillus paracasei LC86 and Lacticaseibacillus casei LC89. Front. Microbiol. 2025, 15, 1501502. [Google Scholar] [CrossRef]
- Abdelazez, A.; Abdelmotaal, H.; Zhu, Z.T.; Fang-Fang, J.; Sami, R.; Zhang, L.J.; Al-Tawaha, A.R.; Meng, X.C. Potential Benefits of Lactobacillus plantarum as Probiotic and Its Advantages in Human Health and Industrial Applications: A Review. Adv. Environ. Biol 2018, 12, 16–27. [Google Scholar] [CrossRef]
- Kim, Y.B.; Park, J.; Lee, H.-G.; Song, J.-Y.; Kim, D.-H.; Ji, W.; Joo, S.S.; Kim, M.; Jung, J.Y.; Kim, M.; et al. Dietary Probiotic Lacticaseibacillus paracasei NSMJ56 Modulates Gut Immunity and Microbiota in Laying Hens. Poult. Sci. 2024, 103, 103505. [Google Scholar] [CrossRef]
- Kang, X.; Li, X.-D.; Zhou, H.-Y.; Wang, F.; Lin, L.-B. Genome-Wide and 16S rRNA Sequencing-Based Analysis on the Health Effects of Lacticaseibacillus paracasei XLK401 on Chicks. Microorganisms 2023, 11, 2140. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Dong, S.; Wang, Q.; Zhong, Q. Simulated Gastrointestinal Digestion and Fecal Fermentation Characteristics of Exopolysaccharides Synthesized by Schleiferilactobacillus harbinensis Z171. J. Agric. Food Chem. 2024, 72, 19748–19765. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, X.; Wu, Z.; Dong, S.; Zhong, Q. In Vitro Cholesterol-Lowering Bioactivity, Synthetic Pathway, and Structural Characterization of Exopolysaccharide Synthesized by Schleiferilactobacillus harbinensis Z171. J. Agric. Food Chem. 2025, 73, 3737–3751. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Wang, J.; Wang, Y.; Gao, Y.; Wang, W. Protective Effects of Potential Probiotics Lacticaseibacillus rhamnosus SN21-1 and Lactiplantibacillus plantarum SN21-2 against Salmonella typhimurium Infection in Broilers. Poult. Sci. 2024, 103, 104207. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, H.; Wang, J.; Liu, H.; Wang, H.; Zhu, Y.; Wang, X.; Zhang, Y.; Xiang, W. Characterization of a LAL-Type Regulator NemR in Nemadectin Biosynthesis and Its Application for Increasing Nemadectin Production in Streptomyces cyaneogriseus. Sci. China Life Sci. 2019, 62, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, C.; Zhang, B.; He, H.; Jin, P.; Wang, J.; Zhang, J.; Wang, X.; Xiang, W. Complete Genome Sequence of Streptomyces cyaneogriseus ssp. Noncyanogenus, the Thermotolerant Producer of Commercial Antibiotics Nemadectin. J. Biotechnol. 2015, 204, 1–2. [Google Scholar] [CrossRef]
- Skowron, K.; Budzyńska, A.; Grudlewska-Buda, K.; Wiktorczyk-Kapischke, N.; Andrzejewska, M.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Two Faces of Fermented Foods—The Benefits and Threats of Its Consumption. Front. Microbiol. 2022, 13, 845166. [Google Scholar] [CrossRef]
- Dottavio, A.; Fernandez, R.; Librera, J.E.; Antruejo, A.; Canet, Z.; Di Masso, R. Eficiencia alimenticia en machos y hembras de dos híbridos experimentales de tres vías de pollos camperos. Revista Ciencia Veterinaria 2013, 15, 25–38. [Google Scholar] [CrossRef]
- FAO. Understanding and Integrating Gender Issues in Livestock Proyects and Programmes; FAO: Rome, Italy, 2013. [Google Scholar]
- Ahmed, S.; Begum, M.; Khatun, A.; Gofur, M.R.; Azad, M.T.A.; Kabir, A.; Haque, T.S. Family Poultry (FP) as a Tool for Improving Gender Equity and Women’s Empowerment in Developing Countries: Evidence from Bangladesh. Eur. J. Agric. Food Sci. 2021, 3, 37–44. [Google Scholar] [CrossRef]
- Portillo Salgado, R.; Vazquez Martinez, I. Género y Seguridad Alimentaria: Rol e Importancia de La Mujer En La Avicultura de Traspatio En Tetela de Ocampo, Puebla, México. Temas De Cienc. Tecnología 2019, 23, 33–40. [Google Scholar]
- Di Pillo, F.; Anríquez, G.; Alarcón, P.; Jimenez-Bluhm, P.; Galdames, P.; Nieto, V.; Schultz-Cherry, S.; Hamilton-West, C. Backyard Poultry Production in Chile: Animal Health Management and Contribution to Food Access in an Upper Middle-Income Country. Prev. Vet. Med. 2019, 164, 41–48. [Google Scholar] [CrossRef]

| Ingredients | Rations | |
|---|---|---|
| Name | Starter (1–28 Days) * | Finisher (36–75 Days) * |
| Monocalcium phosphate 22.7% | 1.450 | 0.840 |
| Soybean expeller | 41.900 | 28.400 |
| Yellow corn | 54.050 | 68.060 |
| Premix INI-TERM 1.4% CLADAN | 1.400 | 1.400 |
| Oyster Shell | 1.200 | 0.800 |
| Soybean oil | NA | 0.500 |
| Groups | LS | VH (µm) | CD (µm) | VH–CD | BWG (g) | FC (g) | PI |
|---|---|---|---|---|---|---|---|
| LDG | 2.50 ± 0.62 a | 984.49 ± 143.77 a | 264.41 ± 82.65 a | 3.78 a | 176.75 ± 58.74 a | 1.05 ± 0.17 | 16.83 |
| IDG | 1.95 ± 1.00 a | 927.16 ± 97.84 a | 285.64 ± 71.65 a | 3.24 a | 167.33 ± 63.47 a | 1.18 ± 0.27 | 14.18 |
| pHDG | 3.00 ± 1.00 a | 946.66 ± 52.76 a | 298.41 ± 68.26 a | 3.17 a | 168.50 ± 56.68 a | 1.78 ± 0.08 | 9.47 |
| HDG | 3.20 ± 0.63 b | 860.31 ± 137.13 b | 286.12 ± 42.22 a | 2.88 a | 158.25 ± 47.24 a | 1.98 ± 0.28 | 7.99 |
| NC | 0.00 ± 0.00 c | 999.50 ± 70.39 a | 163.23 ± 81.16 b | 6.12 b | 290.00 ± 63.68 b | 0.75 ± 0.02 | 29.29 |
| Groups | rBWG | S% | LI | OI | ACI | Efficacy |
|---|---|---|---|---|---|---|
| 6.5 dpi | ||||||
| AWPG | 106.88 | 100 | 9.00 | 20 | 177.9 | Good |
| OF-AWPG | 98.40 | 100 | 12.80 | 40 | 145.4 | Moderate |
| NTG | 100.00 | 100 | 11.00 | 40 | 149.0 | Moderate |
| 19.5 dpi | ||||||
| AWPG | 104.02 | 100 | 10.10 | 20 | 173.9 | Good |
| OF-AWPG | 101.07 | 100 | 11.00 | 10 | 180.6 | High |
| NTG | 100.00 | 100 | 13.20 | 40 | 146.7 | Moderate |
| Period (Days) | px-NTG | NTG | AWPG | OF-AWPG | NC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | A | M | F | A | M | F | A | M | F | A | M | F | A | |
| 1–35 | 12.2 ± 0.8 | 11.1 ± 0.9 | 11.6 ± 0.9 | 10.2 ± 1.3 | 9.8 ± 0.7 | 10.0 ± 1.0 | 10.9 ± 1.2 | 9.5 ± 0.8 | 10.2 ± 1.0 | 10.4 ± 1.0 | 9.5 ± 0.9 | 9.9 ± 1.0 | 12.7 ± 0.5 | 11.1 ± 0.4 | 11.9 ± 0.5 |
| 36–75 | 54.7 ± 2.9 | 43.9 ± 2.7 | 49.3 ± 2.8 | 49.3 ± 6.8 | 42.9 ± 3.3 | 46.1 ± 5.1 | 52.8 ± 4.2 | 41.1 ± 2.6 | 46.9 ± 3.4 | 50.1 ± 3.6 | 41.5 ± 3.0 | 45.8 ± 3.3 | NA | NA | NA |
| Parameter | px-NTG | NTG | AWPG | OF-AWPG |
|---|---|---|---|---|
| BWG | 2697.75 ± 234.37 | 2448.10 ± 335.36 | 2671.10 ± 130.94 | 2611.04 ± 150.98 |
| FC | 2.66 ± 0.18 | 2.80 ± 0.36 | 2.51 ± 0.10 | 2.40 ± 0.12 |
| IP | 101.42 | 87.43 | 106.42 | 108.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, A.E.; Britez, J.D.; Pisón-Martínez, M.L.; Delgado, F.O.; Balbiani, F.; Berardo, C.C.; Gramaglia, C.; Cuba, F.; Poklepovich, T.J.; Moreno, C.; et al. Evaluating Good Husbandry Practices and Organic Fermented Additives for Coccidiosis Control in a Pilot Study Using Slow-Growing Broilers. Animals 2025, 15, 1752. https://doi.org/10.3390/ani15121752
Rodriguez AE, Britez JD, Pisón-Martínez ML, Delgado FO, Balbiani F, Berardo CC, Gramaglia C, Cuba F, Poklepovich TJ, Moreno C, et al. Evaluating Good Husbandry Practices and Organic Fermented Additives for Coccidiosis Control in a Pilot Study Using Slow-Growing Broilers. Animals. 2025; 15(12):1752. https://doi.org/10.3390/ani15121752
Chicago/Turabian StyleRodriguez, Anabel E., Jesica D. Britez, María Luz Pisón-Martínez, Fernando O. Delgado, Facundo Balbiani, Cecilia C. Berardo, César Gramaglia, Facundo Cuba, Tomás J. Poklepovich, Claudia Moreno, and et al. 2025. "Evaluating Good Husbandry Practices and Organic Fermented Additives for Coccidiosis Control in a Pilot Study Using Slow-Growing Broilers" Animals 15, no. 12: 1752. https://doi.org/10.3390/ani15121752
APA StyleRodriguez, A. E., Britez, J. D., Pisón-Martínez, M. L., Delgado, F. O., Balbiani, F., Berardo, C. C., Gramaglia, C., Cuba, F., Poklepovich, T. J., Moreno, C., Francinelli, G., Morici, G., Arias, M., Schapiro, J., Barbano, P., & Tomazic, M. L. (2025). Evaluating Good Husbandry Practices and Organic Fermented Additives for Coccidiosis Control in a Pilot Study Using Slow-Growing Broilers. Animals, 15(12), 1752. https://doi.org/10.3390/ani15121752









