Effect of Potassium–Magnesium Sulfate on Intestinal Dissociation and Absorption Rate, Immune Function, and Expression of NLRP3 Inflammasome, Aquaporins and Ion Channels in Weaned Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1
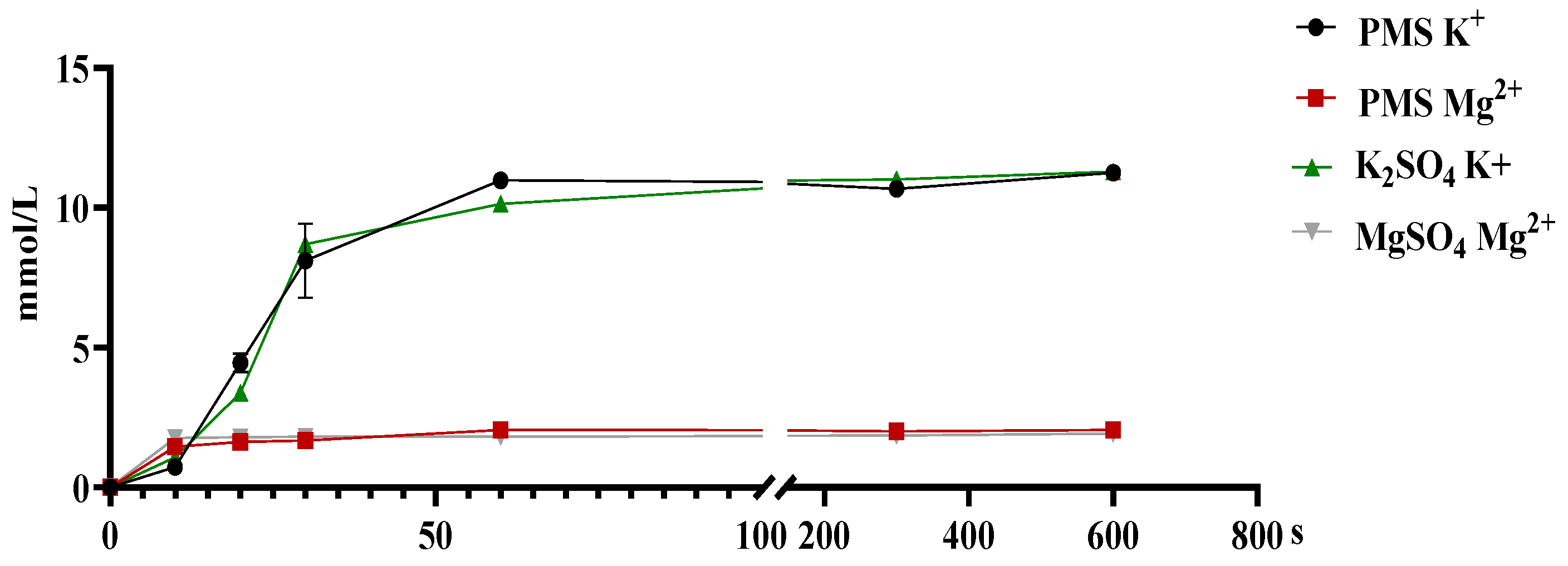
2.1.1. In Vitro Dissociation Assay
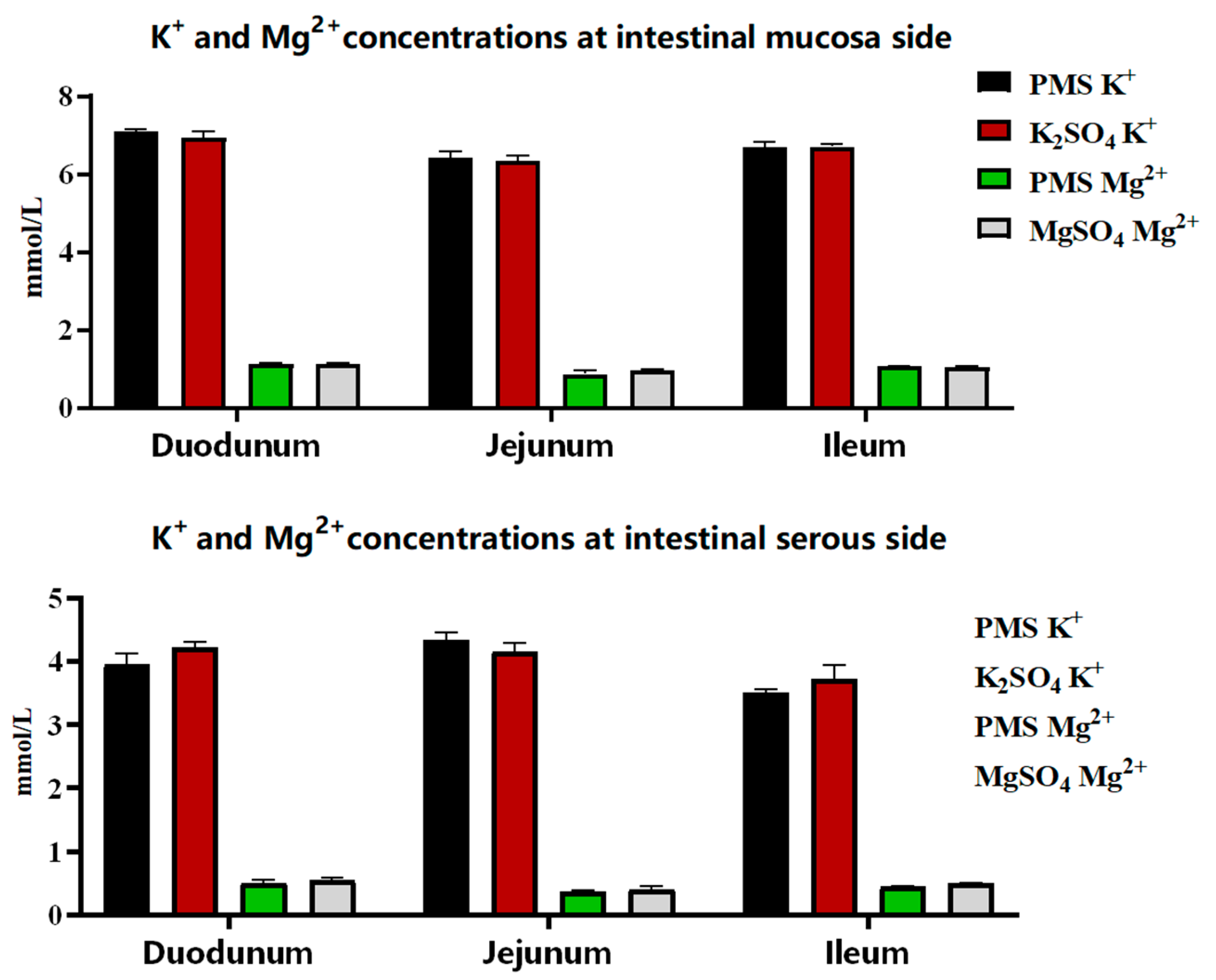
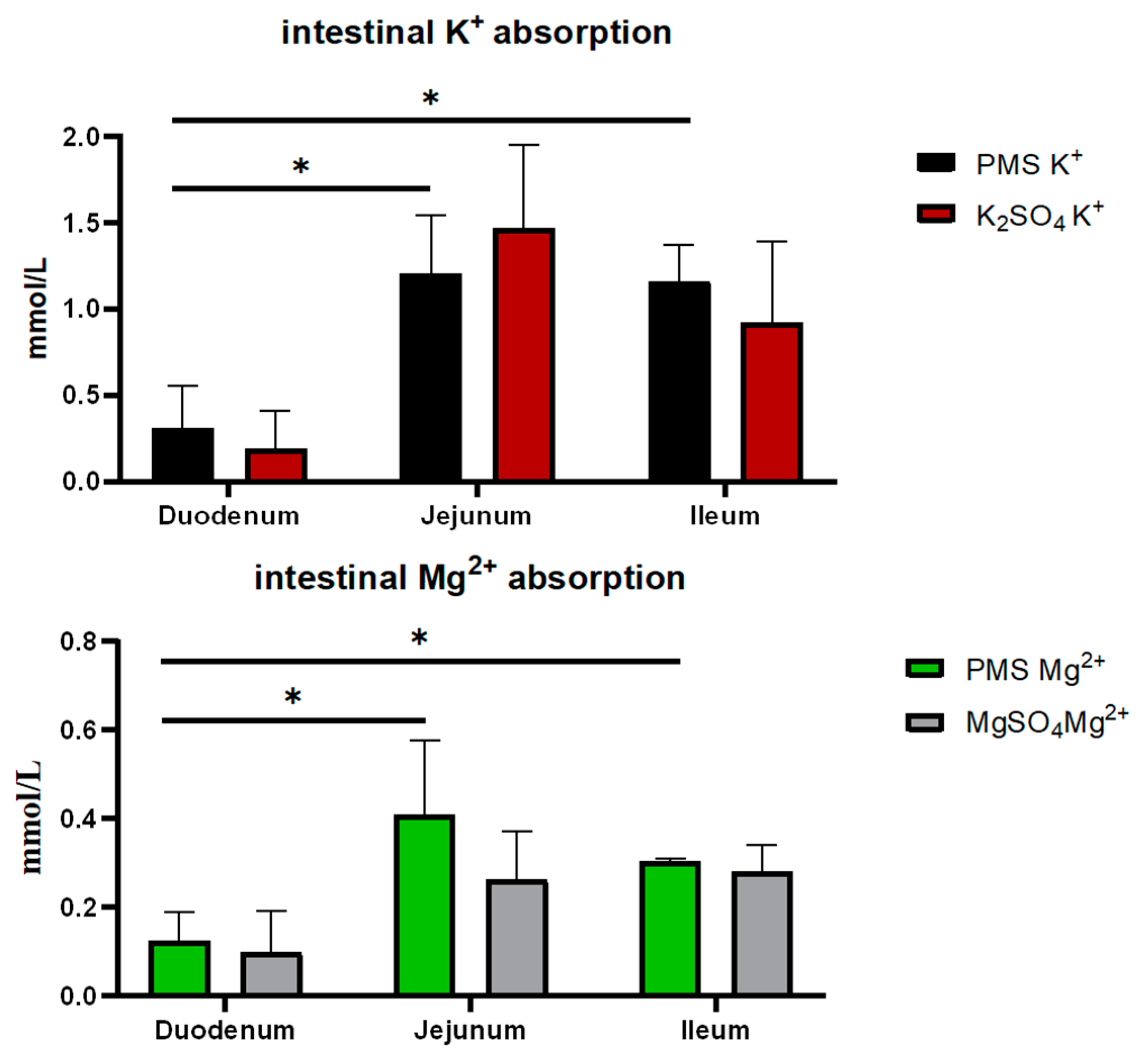
2.1.2. In Vitro Ussing Chamber Assay
2.2. Experiment 2
2.2.1. Animals, Diets, and Experimental Design
2.2.2. Sample Collection
2.2.3. Determinations of Serum Immune Parameters
2.2.4. RNA Extraction, cDNA Synthesis, and qPCR Assay
2.2.5. Statistical Analysis
3. Results
3.1. The Dissociation and Absorption of K+ and Mg2+ in PMS, Potassium Sulfate, and Magnesium Sulfate
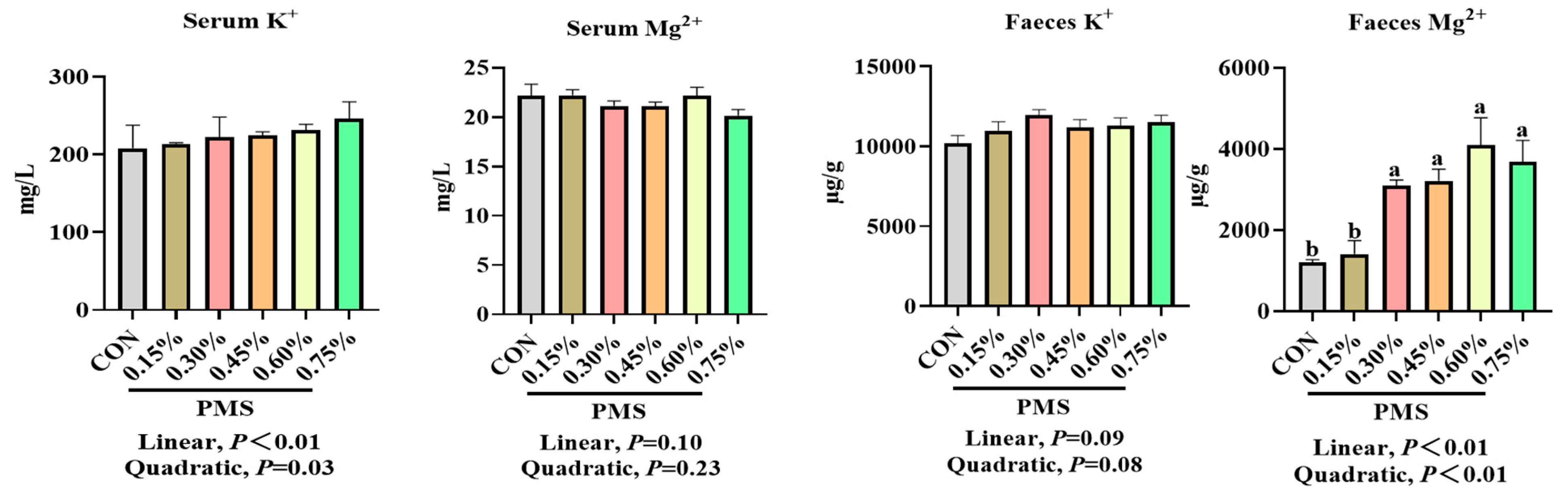
3.2. Effects of Dietary Supplementation with PMS on Serum and Fecal Potassium and Magnesium Ion Concentrations in Weaned Piglets
3.3. The Effect of Dietary Supplementation with PMS on Serum Immune Indicators of Weaned Piglets
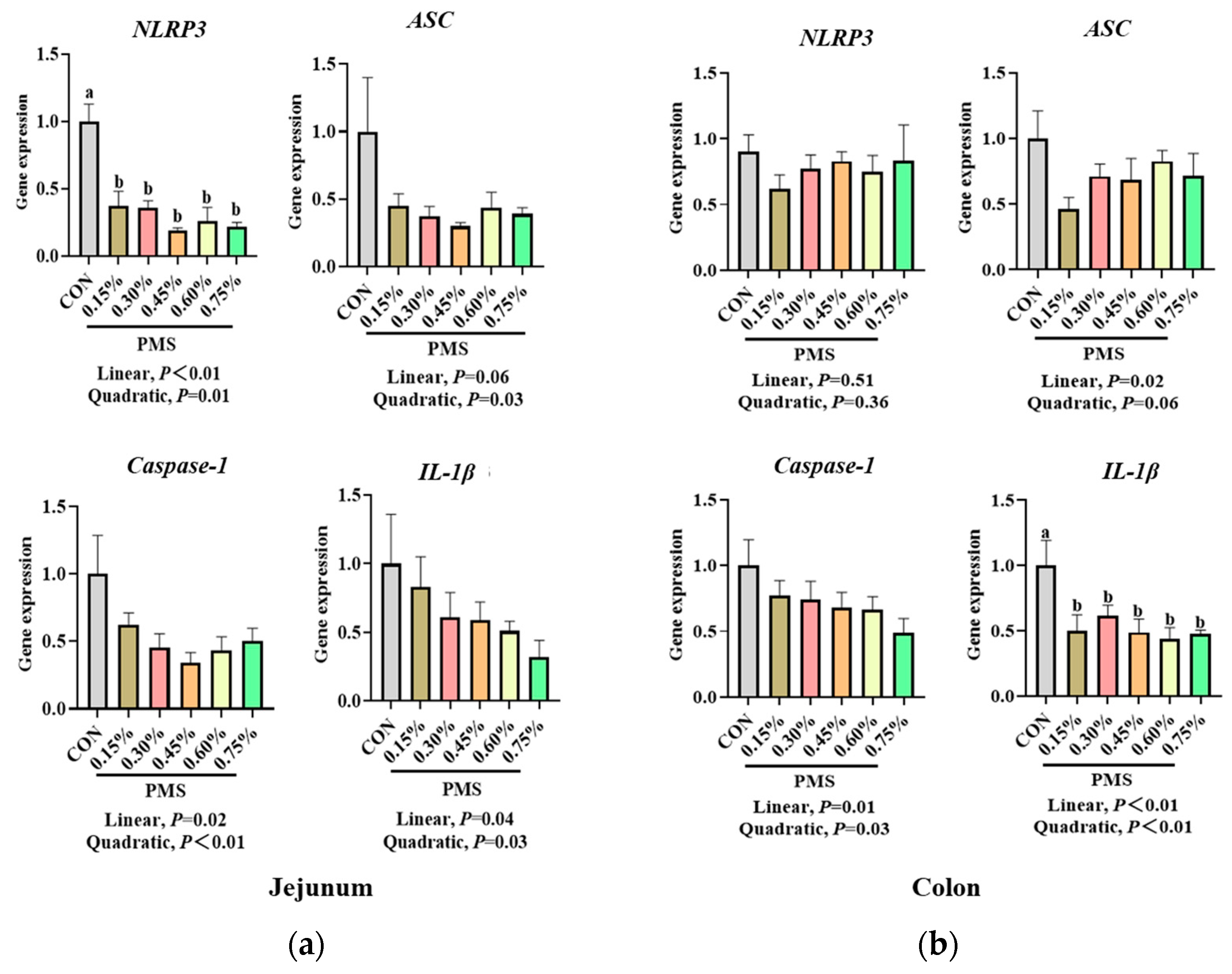
3.4. Effect of Dietary Supplementation with PMS on Intestinal Expression of NLRP3 Inflammasome and Cytokines in Weaned Piglets
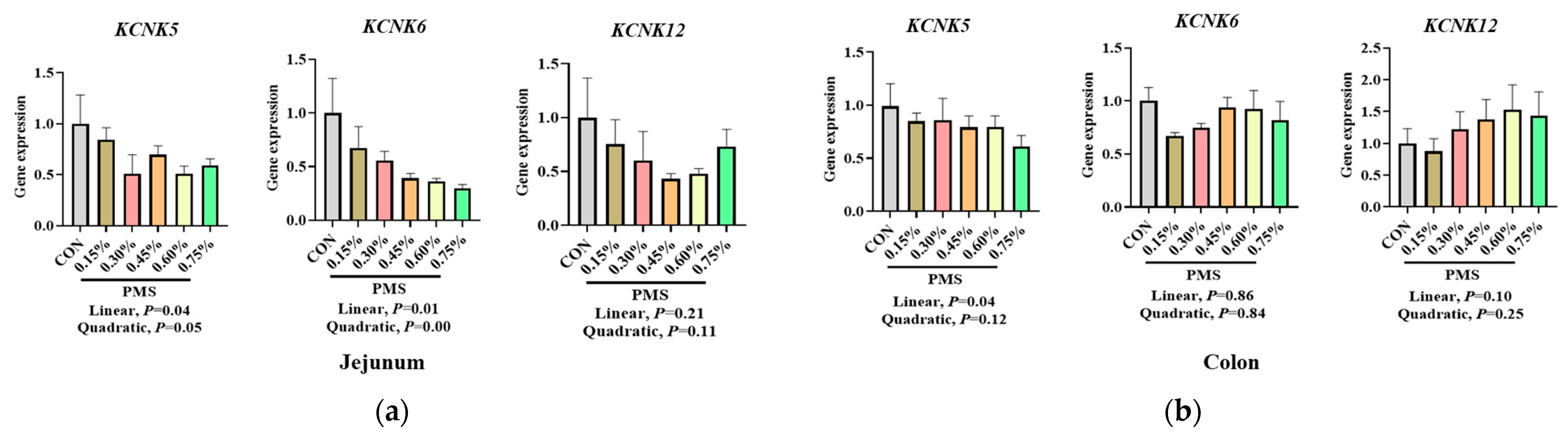
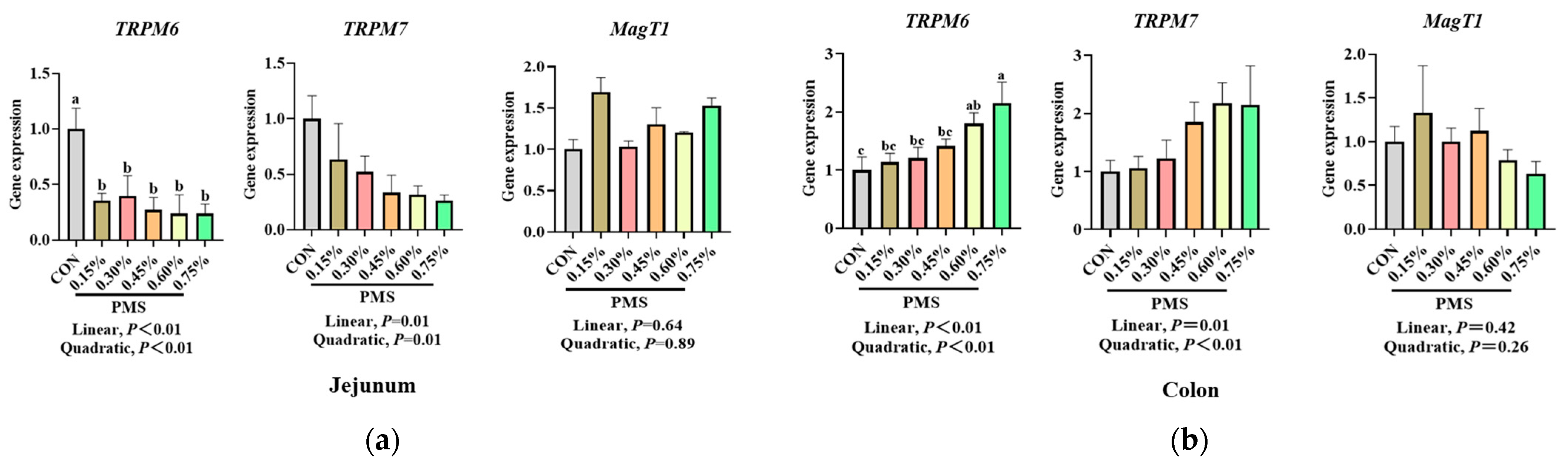
3.5. Effect of Dietary Supplementation with PMS on Intestinal Expression Levels of Potassium and Magnesium Ion Channels in Weaned Piglets
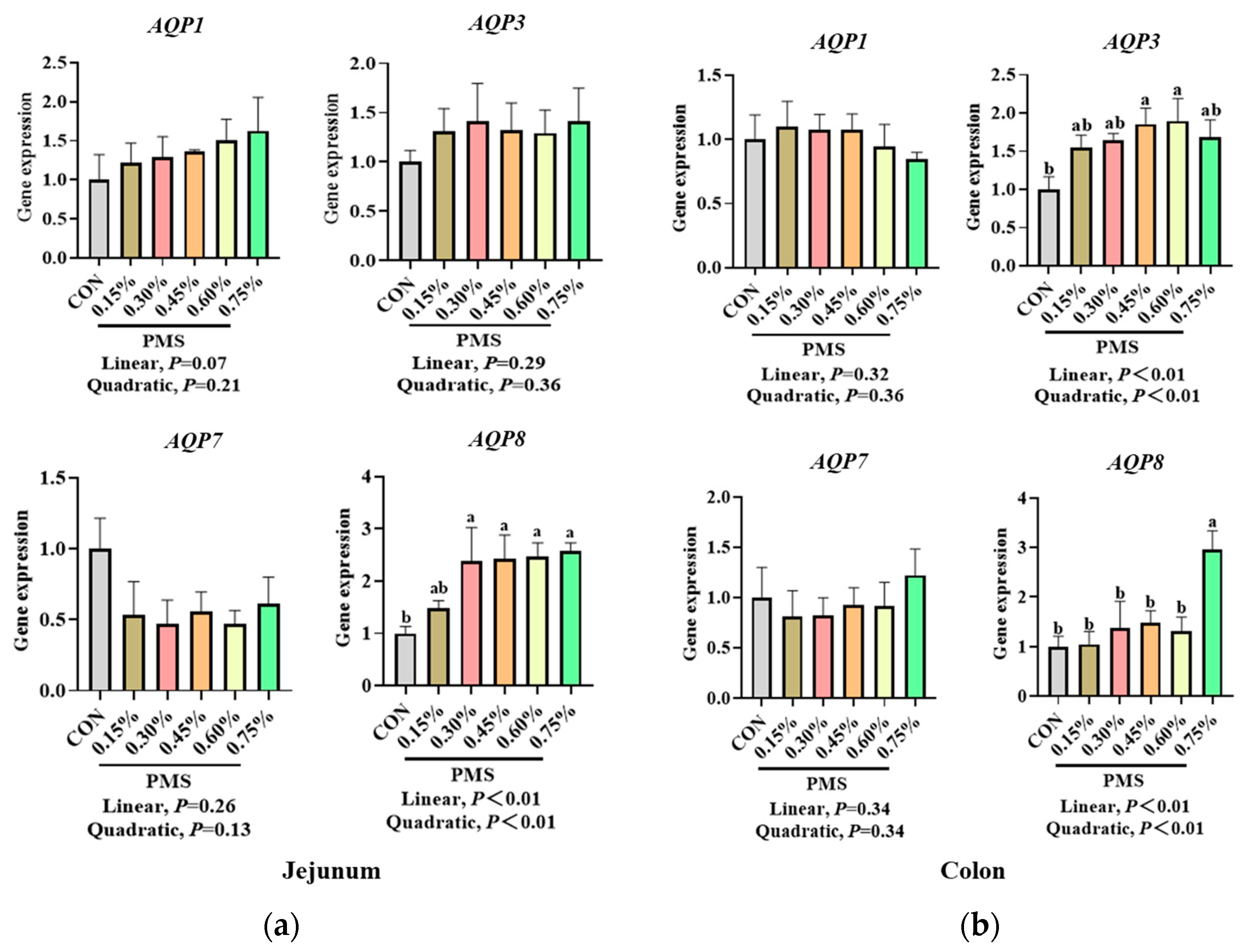
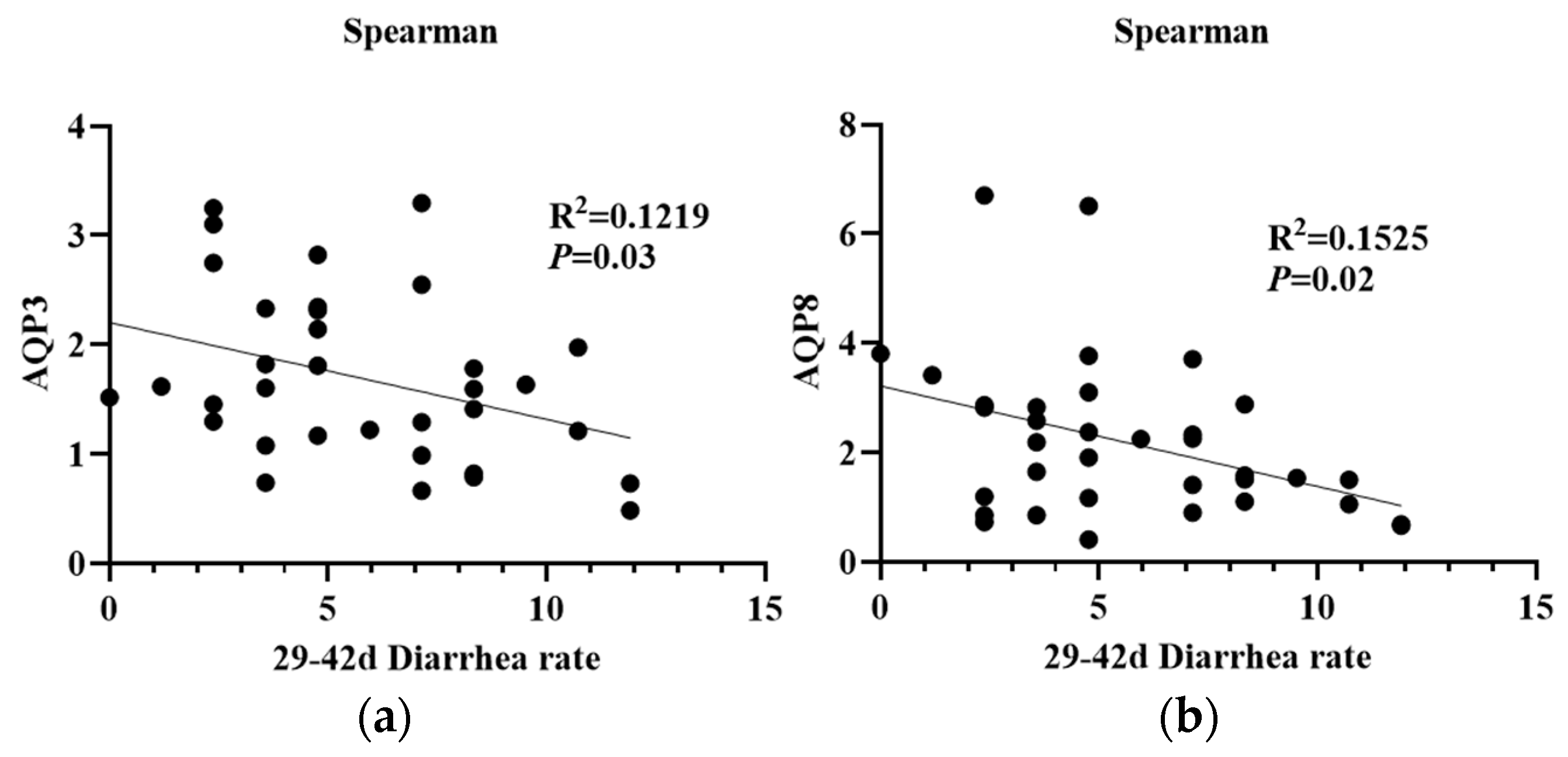
3.6. Effect of Dietary Supplementation with PMS on Intestinal Expression of AQPs in Weaned Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayaraman, B.; Nyachoti, C. Husbandry practices and gut health outcomes in weaned piglets: A review. Anim. Nutr. 2017, 3, 205–211. [Google Scholar] [CrossRef]
- Zheng, L.; Duarte, M.E.; Loftus, A.S.; Kim, S.W. Intestinal health of pigs upon weaning: Challenges and nutritional intervention. Front. Vet. Sci. 2021, 8, 628258. [Google Scholar] [CrossRef]
- Lukaski, H. Vitamin and mineral status: Effects on physical performance. Nutrition 2004, 20, 632–644. [Google Scholar] [CrossRef]
- Sampath, V.; Sureshkumar, S.; Seok, W.J.; Kim, I.H. Role and functions of micro and macro-minerals in swine nutrition: A short review. J. Anim. Sci. Technol. 2023, 65, 479–489. [Google Scholar] [CrossRef]
- Weglicki, W.; Quamme, G.; Tucker, K.; Haigney, M.; Resnick, L. Potassium, magnesium, and electrolyte imbalance and complications in disease management. Clin. Exp. Hypertens. 2005, 27, 95–112. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium in disease prevention and overall health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef]
- Ahmed, F.; Mohammed, A. Magnesium: The forgotten electrolyte-A review on hypomagnesemia. Med. Sci. 2019, 7, 56. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012.
- Pinotti, L.; Manoni, M.; Ferrari, L.; Tretola, M.; Cazzola, R.; Givens, I. The contribution of dietary magnesium in farm animals and human nutrition. Nutrients 2021, 13, 509. [Google Scholar] [CrossRef]
- Spears, J.W. Evaluation of trace mineral sources. Vet. Clin. North Am. Food Anim. Pract. 2023, 39, 413–424. [Google Scholar] [CrossRef]
- D’Souza, D.N.; Warner, R.D.; Leury, B.J.; Dunshea, F.R. The effect of dietary magnesium aspartate supplementation on pork quality. J. Anim. Sci. 1998, 76, 104–109. [Google Scholar] [CrossRef]
- Zang, J.; Chen, J.; Tian, J.; Wang, A.; Liu, H.; Hu, S.; Che, X.; Ma, Y.; Wang, J.; Wang, C.; et al. Effects of magnesium on the performance of sows and their piglets. J. Anim. Sci. Biotechnol. 2014, 5, 39. [Google Scholar] [CrossRef]
- Plush, K.; Weaver, A.; Staveley, L.; van Wettere, W. Maternal magnesium sulfate supplementation in a pre-farrow diet improves factors important for piglet viability. Animals 2018, 8, 185. [Google Scholar] [CrossRef]
- Ahmad, T.; Khalid, T.; Mushtaq, T.; Mirza, M.A.; Nadeem, A.; Babar, M.E.; Ahmad, G. Effect of potassium chloride supplementation in drinking water on broiler performance under heat stress conditions. Poult. Sci. 2008, 87, 1276–1280. [Google Scholar] [CrossRef]
- Ansari, I.; Khalaji, S.; Hedayati, M. Potassium phosphate and potassium carbonate administration by feed or drinking water improved broiler performance, bone strength, digestive phosphatase activity and phosphorus digestibility under induced heat stress conditions. Trop. Anim. Health Prod. 2020, 52, 591–600. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, L.; Bai, R.; Cui, L.; Han, H.; Han, Y.; Sun, W.; Li, Y.; Jiang, X.; Li, X.; et al. Dietary supplementation with different types of potassium and magnesium during late gestation and lactation modulates the reproductive performance, antioxidant capacity, and immune function of sows. Animals 2023, 13, 2183. [Google Scholar] [CrossRef]
- Cao, S.; Huang, K.; Wen, X.; Gao, J.; Cui, B.; Yao, K.; Zhan, X.; Hu, S.; Wu, Q.; Xiao, H.; et al. Dietary supplementation with potassium-magnesium sulfate modulates the antioxidant capacity, immunity, and gut microbiota in weaned piglets. Front. Microbiol. 2022, 13, 961989. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Gong, T.; Yang, Y.; Jin, T.; Jiang, W.; Zhou, R. Orchestration of NLRP3 inflammasome activation by ion fluxes. Trends Immunol. 2018, 39, 393–406. [Google Scholar] [CrossRef]
- Li, C.; Chen, M.; He, X.; Ouyang, D. A mini-review on ion fluxes that regulate NLRP3 inflammasome activation. Acta Biochim. Biophys. Sin. 2021, 53, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, J.; Liu, T.; Cheng, W.; Wang, Y.; Ding, S.; Wang, R. A novel mechanism for NLRP3 inflammasome activation. Metabol. Open. 2022, 13, 100166. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Wang, H.; Ma, L.; Su, W.; Liu, Y.; Xie, N.; Liu, J. NLRP3 inflammasome in health and disease (Review). Int. J. Mol. Med. 2025, 55, 48. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Luo, Z.; Yu, C.; Wei, Y.; Zhang, Z.; Han, Y.; Zhang, H.; Zhang, J.; Xu, W.; Xu, J. Effect of microbe-derived antioxidants on intestinal oxidative stress, NLRP3 inflammasome, morphologic structure, and growth performance in weanling piglets. J. Food. Sci. 2025, 90, e70064. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Du, X.; Huang, Z.; Jia, G.; Zhao, H. Dihydromyricetin attenuates lipopolysaccharide-induced intestinal injury in weaned piglets by regulating oxidative stress and inhibiting NLRP3 inflammasome. J. Anim. Sci. 2025, 103, skaf114. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, N.; Wen, D.; Guo, P.; Liu, Y.; Fu, S.; Ye, C.; Wu, Z.; Qiu, Y. Baicalin attenuates lipopolysaccharide-induced intestinal inflammatory injury via suppressing PARP1-mediated NF-κB and NLRP3 signalling pathway. Toxicon 2024, 239, 107612. [Google Scholar] [CrossRef]
- Verkman, A.S.; Mitra, A.K. Structure and function of aquaporin water channels. Am. J. Physiol. Renal Physiol. 2000, 278, F13–F28. [Google Scholar] [CrossRef]
- Deng, Z.; Zhao, Y.Y.; Ma, Z.Y.; Zhang, M.L.; Wang, H.; Yi, Z.Q.; Tuo, B.G.; Li, T.L.; Liu, X.M. Pathophysiological role of ion channels and transporters in gastrointestinal mucosal diseases. Cell Mol. Life Sci. 2021, 78, 8109–8125. [Google Scholar] [CrossRef]
- Laforenza, U. Water channel proteins in the gastrointestinal tract. Mol. Aspects Med. 2012, 33, 642–650. [Google Scholar] [CrossRef]
- Ikarashi, N.; Kon, R.; Sugiyama, K. Aquaporins in the colon as a new therapeutic target in diarrhea and constipation. Int. J. Mol. Sci. 2016, 17, 1172. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Carlson, P.; Chedid, V.; Vijayvargiya, P.; Burton, D.; Busciglio, I. Aquaporin expression in colonic mucosal biopsies from irritable bowel syndrome with diarrhea. Clin. Transl. Gastroenterol. 2019, 10, e00019. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Li, Y.; Xue, C.; Dong, N.; Bi, C.; Shan, A. Aquaporin: Targets for dietary nutrients to regulate intestinal health. J. Anim. Physiol. Anim. Nutr 2022, 106, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yin, J.; Xu, K.; Han, H.; Liu, Z.; Wang, C.; Li, T.; Yin, Y. Protein level and infantile diarrhea in a postweaning piglet model. Mediators Inflamm. 2020, 2020, 1937387. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Y. Drug sorption onto and release from soy protein fibers. J. Mater. Sci. Mater. Med. 2009, 20, 2477–2486. [Google Scholar] [CrossRef]
- Clarke, L.L. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1151–G1166. [Google Scholar] [CrossRef]
- Wijtten, P.J.; van der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef]
- He, L.; Yin, Y.; Li, T.; Huang, R.; Xie, M.; Wu, Z.; Wu, G. Use of the Ussing chamber technique to study nutrient transport by epithelial tissues. Front. Biosci. (Landmark Ed.) 2013, 18, 1266–1274. [Google Scholar]
- Ryan, M.P. Interrelationships of magnesium and potassium homeostasis. Miner. Electrolyte Metab. 1993, 19, 290–295. [Google Scholar]
- Agarwal, R.; Afzalpurkar, R.; Fordtran, J.S. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 1994, 107, 548–571. [Google Scholar] [CrossRef]
- McDonough, A.A.; Youn, J.H. Potassium homeostasis: The knowns, the unknowns, and the health benefits. Physiology 2017, 32, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.H. Gut sensing of potassium intake and its role in potassium homeostasis. Semin. Nephrol. 2013, 33, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.H.; McDonough, A.A. Recent advances in understanding integrative control of potassium homeostasis. Annu. Rev. Physiol. 2009, 71, 381–401. [Google Scholar] [CrossRef]
- Hegazy, E.; Schwenk, M. Choline uptake by isolated enterocytes of guinea pig. J. Nutr. 1984, 114, 2217–2220. [Google Scholar] [CrossRef] [PubMed]
- Woodard, J.P.; Chen, W.; Keku, E.O.; Liu, S.C.; Lecce, J.G.; Rhoads, J.M. Altered jejunal potassium (Rb+) transport in piglet rotavirus enteritis. Am. J. Physiol. 1993, 265, G388–G393. [Google Scholar] [CrossRef]
- Schonewille, J.T.; Everts, H.; Jittakhot, S.; Beynen, A.C. Quantitative prediction of magnesium absorption in dairy cows. J. Dairy Sci. 2008, 91, 271–278. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Z.; Jiang, Z. Expression, distribution and role of aquaporin water channels in human and animal stomach and intestines. Int. J. Mol. Sci. 2016, 17, 1399. [Google Scholar] [CrossRef]
- Zhu, C.; Nie, X.; Lu, Q.; Jiang, Z. Roles and regulation of aquaporin-3 in maintaining the gut health: An updated review. Front Physiol. 2023, 14, 1264570. [Google Scholar] [CrossRef]
- Ikarashi, N.; Mochiduki, T.; Takasaki, A.; Usui, S.; Hirano, K. A mechanism by which the osmotic laxative magnesium sulphate increases the intestinal aquaporin 3 expression in HT-29 cells. Life Sci. 2011, 88, 194–200. [Google Scholar] [CrossRef]
- Okahira, M.; Kubota, M.; Iguchi, K.; Usui, S.; Hirano, K. Regulation of aquaporin 3 expression by magnesium ion. Eur. J. Pharmacol. 2008, 588, 26–32. [Google Scholar] [CrossRef]
- Rabolli, V.; Wallemme, L.; Lo Re, S.; Uwambayinema, F.; Palmai-Pallag, M.; Thomassen, L.; Tyteca, D.; Octave, J.N.; Marbaix, E.; Lison, D.; et al. Critical role of aquaporins in interleukin 1β (IL-1β)-induced inflammation. J. Biol. Chem. 2014, 289, 13937–13947. [Google Scholar] [CrossRef] [PubMed]
- Kalita, A.; Das, M. Aquaporins (AQPs) as a marker in the physiology of inflammation and its interaction studies with garcinol. Inflammopharmacology 2024, 32, 1575–1592. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Lin, L.; Chen, X.; Chen, L.; Yang, J.; Chen, Y.; Qian, D.; Zeng, Y.; Xu, Y. PPAR-γ/NF-kB/AQP3 axis in M2 macrophage orchestrates lung adenocarcinoma progression by upregulating IL-6. Cell Death Dis. 2024, 15, 532. [Google Scholar] [CrossRef] [PubMed]
- Peplowski, M.A.; Vegso, A.J.; Iablokov, V.; Dicay, M.; Zaheer, R.S.; Renaux, B.; Proud, D.; Hollenberg, M.D.; Beck, P.L.; MacNaughton, W.K. Tumor necrosis factor α decreases aquaporin 3 expression in intestinal epithelial cells through inhibition of constitutive transcription. Physiol. Rep. 2017, 5, e13451. [Google Scholar] [CrossRef]
- Livingston, M.L.; Landon, C.D.; Barnes, H.J.; Brake, J.; Livingston, K.A. Dietary potassium and available phosphorous on broiler growth performance, carcass characteristics, and wooden breast. Poult. Sci. 2019, 98, 2813–2822. [Google Scholar] [CrossRef]
- Kim, C.H.; Paik, I.K.; Kil, D.Y. Effects of increasing supplementation of magnesium in diets on productive performance and eggshell quality of aged laying hens. Biol. Trace Elem. Res. 2013, 151, 38–42. [Google Scholar] [CrossRef]
- O’Driscoll, K.; O’Gorman, D.M.; Taylor, S.; Boyle, L.A. The influence of a magnesium-rich marine extract on behaviour, salivary cortisol levels and skin lesions in growing pigs. Animal 2013, 7, 1017–1027. [Google Scholar] [CrossRef]
- Belkameh, M.M.; Sedghi, M.; Azarfar, A. The Effect of different levels of dietary magnesium on eggshell quality and laying hen’s performance. Biol. Trace Elem. Res. 2021, 199, 1566–1573. [Google Scholar] [CrossRef]
- Schonewille, J.T. Magnesium in dairy cow nutrition: An overview. Plant Soil 2013, 368, 167–178. [Google Scholar] [CrossRef]
- Villa-Bellosta, R. Dietary magnesium supplementation improves lifespan in a mouse model of progeria. EMBO Mol. Med. 2020, 12, e12423. [Google Scholar] [CrossRef]
- Galland, L. Magnesium and immune function: An overview. Magnesium 1988, 7, 290–299. [Google Scholar] [PubMed]
- Trapani, V.; Petito, V.; Di Agostini, A.; Arduini, D.; Hamersma, W.; Pietropaolo, G.; Luongo, F.; Arena, V.; Stigliano, E.; Lopetuso, L.R.; et al. Dietary magnesium alleviates experimental murine colitis through upregulation of the transient receptor potential melastatin 6 channel. Inflamm. Bowel Dis. 2018, 24, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Kumar, S.; Hussain, A.; Mishra, N.; Garg, A.; Gowda, B.H.J.; Farid, A.; Gupta, G.; Dua, K.; Taghizadeh-Hesary, F. A narrative review on the role of magnesium in immune regulation, inflammation, infectious diseases, and cancer. J. Health Popul. Nutr. 2023, 42, 74. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A. Magnesium deficit—Overlooked cause of low vitamin D status? BMC Med. 2013, 11, 229. [Google Scholar] [CrossRef]
- Orhan, C.; Er, B.; Deeh, P.B.D.; Bilgic, A.A.; Ojalvo, S.P.; Komorowski, J.R.; Sahin, K. Different sources of dietary magnesium supplementation reduces oxidative stress by regulation Nrf2 and NF-κB signaling pathways in high-fat diet rats. Biol. Trace Elem. Res. 2021, 199, 4162–4170. [Google Scholar] [CrossRef]
- Hou, W.X.; Cheng, S.Y.; Liu, S.T.; Shi, B.M.; Shan, A.S. Dietary supplementation of magnesium sulfate during late gestation and lactation affects the milk composition and immunoglobulin levels in sows. Asian-Australas. J. Anim. Sci 2014, 27, 1469–1477. [Google Scholar]
- Wang, P.; Zhang, J.; Tian, Y.; Yu, B.; He, J.; Yu, J.; Zheng, P. Weaning stress aggravates defense response and the vurden of protein metabolism in low-birth-weight piglets. Animals 2025, 15, 1369. [Google Scholar] [CrossRef]
- Cross, S.N.; Nelson, R.A.; Potter, J.A.; Norwitz, E.R.; Abrahams, V.M. Magnesium sulfate differentially modulates fetal membrane inflammation in a time-dependent manner. Am. J. Reprod. Immunol. 2018, 80, e12861. [Google Scholar] [CrossRef]
- López-Baltanás, R.; Rodríguez-Ortiz, M.E.; Canalejo, A.; Díaz-Tocados, J.M.; Herencia, C.; Leiva-Cepas, F.; Torres-Peña, J.D.; Ortíz-Morales, A.; Muñoz-Castañeda, J.R.; Rodríguez, M.; et al. Magnesium supplementation reduces inflammation in rats with induced chronic kidney disease. Eur. J. Clin. Investig. 2021, 51, e13561. [Google Scholar] [CrossRef]
- Wang, H.H.; Huang, C.R.; Lin, H.C.; Lin, H.A.; Chen, Y.J.; Tsai, K.J.; Shih, C.T.; Huang, K.Y.; Ojcius, D.M.; Tsai, M.H.; et al. Magnesium-enriched deep-sea water inhibits NLRP3 inflammasome activation and dampens inflammation. Heliyon 2024, 10, e35136. [Google Scholar] [CrossRef]
- Xie, C.; Li, X.; Zhu, J.; Wu, J.; Geng, S.; Zhong, C. Magnesium isoglycyrrhizinate suppresses LPS-induced inflammation and oxidative stress through inhibiting NF-κB and MAPK pathways in RAW264.7 cells. Bioorg. Med. Chem. 2019, 27, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Di, A.; Xiong, S.; Ye, Z.; Malireddi, R.K.S.; Kometani, S.; Zhong, M.; Mittal, M.; Hong, Z.; Kanneganti, T.D.; Rehman, J.; et al. The TWIK2 potassium efflux channel in macrophages mediates NLRP3 inflammasome-induced inflammation. Immunity. 2018, 49, 56–65.e4. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Huang, Y.; Chen, S.; Xu, R.; Xu, L.; Qiu, J.; Shi, F.; Liu, S.; Zha, Q.; Ouyang, D.; et al. Dextran sodium sulfate potentiates NLRP3 inflammasome activation by modulating the KCa3.1 potassium channel in a mouse model of colitis. Cell. Mol. Immunol. 2022, 19, 925–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, Y. Intracellular potassium ion measurements by inductively coupled plasma optical emission spectrometer (ICP-OES). Methods Mol. Biol. 2022, 2459, 85–92. [Google Scholar]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef]
- Immanuel, C.N.; Teng, B.; Dong, B.E.; Gordon, E.M.; Luellen, C.; Lopez, B.; Harding, J.; Cormier, S.A.; Fitzpatrick, E.A.; Schwingshackl, A.; et al. Two-pore potassium channel TREK-1 (K2P2.1) regulates NLRP3 inflammasome activity in macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2024, 326, L367–L376. [Google Scholar] [CrossRef]
- Pardo, L.; Stühmer, W. The roles of K+ channels in cancer. Nat. Rev. Cancer 2014, 14, 39–48. [Google Scholar] [CrossRef]
- Göb, E.; Bittner, S.; Bobak, N.; Kraft, P.; Göbel, K.; Langhauser, F.; Homola, G.A.; Brede, M.; Budde, T.; Meuth, S.G.; et al. The two-pore domain potassium channel KCNK5 deteriorates outcome in ischemic neurodegeneration. Pflugers Arch. 2015, 467, 973–987. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, J.; Yu, J.; Su, J. Dietary intakes of calcium, iron, magnesium, and potassium elements and the risk of colorectal cancer: A Meta-Analysis. Biol. Trace Elem. Res. 2019, 189, 325–335. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, L.; Tan, B.; Li, G.; Huang, B.; Xiong, X.; Li, F.; Kong, X.; Liu, G.; Yin, Y. Developmental changes in intercellular junctions and Kv channels in the intestine of piglets during the suckling and post-weaning periods. J. Anim. Sci. Biotechnol. 2016, 7, 4. [Google Scholar] [CrossRef]
- Pietropaolo, G.; Pugliese, D.; Armuzzi, A.; Guidi, L.; Gasbarrini, A.; Rapaccini, G.L.; Wolf, F.I.; Trapani, V. Magnesium absorption in intestinal cells: Evidence of cross-talk between EGF and TRPM6 and novel implications for cetuximab therapy. Nutrients 2020, 12, 3277. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Trapani, V.; Petito, V.; Reddel, S.; Pietropaolo, G.; Graziani, C.; Masi, L.; Gasbarrini, A.; Putignani, L.; Scaldaferri, F.; et al. Dietary magnesium alleviates experimental murine colitis through modulation of gut microbiota. Nutrients 2021, 13, 4188. [Google Scholar] [CrossRef] [PubMed]
- Chubanov, V.; Gudermann, T.; Schlingmann, K.P. Essential role for TRPM6 in epithelial magnesium transport and body magnesium homeostasis. Pflugers Arch. 2005, 451, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Inoue, K.; Leng, T.; Guo, S.; Xiong, Z.G. TRPM7 channels regulate glioma stem cell through STAT3 and Notch signaling pathways. Cell. Signal. 2014, 26, 2773–2781. [Google Scholar] [CrossRef]
- Zou, Z.G.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, magnesium, and signaling. Int. J. Mol. Sci. 2019, 20, 1877. [Google Scholar] [CrossRef]
| Item | 1–28 Days | 29–42 Days |
|---|---|---|
| Ingredient, % | ||
| Corn | 34.05 | 56.18 |
| Expanded corn | 12.00 | 13.00 |
| Fermented soybean meal | 10.00 | 10.00 |
| Soybean meal | 5.00 | 6.00 |
| Expanded soybean | 11.00 | 3.67 |
| Fish meal | 3.00 | 3.00 |
| Whey powder | 15.00 | ~ |
| Whey protein concentrate | 1.00 | ~ |
| Soybean oil | 1.50 | 1.00 |
| Sucrose | 2.00 | 2.00 |
| Calcium citrate | 1.40 | ~ |
| CaCO3 | ~ | 1.10 |
| CaHPO4 | 0.60 | 0.60 |
| L-lys-HCl | 0.60 | 0.60 |
| DL-Met | 0.15 | 0.15 |
| L-Thr | 0.20 | 0.20 |
| L-Trp | 0.05 | 0.05 |
| NaCl | 0.30 | 0.30 |
| 50% Choline chloride | 0.15 | 0.15 |
| Premix 1 | 2.00 | 2.00 |
| Total | 100 | 100 |
| Nutrient level 2 | ||
| DE, MJ/kg | 15.06 | 14.60 |
| CP, % | 18.70 | 17.20 |
| SID Lys, % | 1.54 | 1.35 |
| SID Met, % | 0.47 | 0.45 |
| SID Met+Cys, % | 0.78 | 0.74 |
| SID Thr, % | 0.93 | 0.83 |
| SID Trp, % | 0.27 | 0.24 |
| Ca, % | 0.70 | 0.76 |
| Total P, % | 0.55 | 0.53 |
| STTD P, % | 0.36 | 0.31 |
| K, % | 1.03 | 0.68 |
| Mg, % | 0.19 | 0.15 |
| Na, % | 0.30 | 0.16 |
| Cl, % | 0.56 | 0.36 |
| dEB, mmol/kg | 211 | 125 |
| Primer 1 | Sequence (5′ to 3′) | NCBI Access Number |
|---|---|---|
| β-actin | F: TCTGGCACCACACCTTCT R: TGATCTGGGTCATCTTCTCAC | XM_021086047.1 |
| NLRP3 | F: CCTTCAGGCTGATTCAGGAG R: GACTCTTGCCGCTATCCATC | NM_001256770.2 |
| Caspase-1 | F: ATCTCACCGCTTCGGACATGGCTAT R: GTATTTCTTCCCACAAATGCCAGCC | NM_214162.1 |
| ASC | F: GCCGACGAGCTCAAGAAGTT R: TCCTTCATGCCGATGTCACG | AB873106.1 |
| IL-1β | F: TCTGCATGAGCTTTGTGCAAG R: ACAGGGCAGACTCGAATTCAAC | XM_021085847.1 |
| KCNK5 | F: GGCACGTATCTCACCATCCC R: TGATGGCCTCTTCCCTACGA | XM_001928254.4 |
| KCNK6 | F: GGGAGATGCAGAGGCTTGTT R: CCAGTTTCAATCACCCCCGA | XM_003127105.4 |
| KCNK12 | F: AACGTAGCACCAACTAGCGG R: CACTTCCTCGCTTCCCTGTTT | XM_005674606.3 |
| TRPM6 | F: TGGTGGAGCATATCGCAGTAG R: AGGCTGGGCAGTTCTAATGA | XM_021064975.1 |
| TRPM7 | F: CTCTCACGTGGTCTGTCTTCA R: ATTGTGCGACCAACTCCTCC | XM_021095614.1 |
| MagT1 | F: TATCGTATCCAAAGGTGGGGG R: GTAGGGAGACAGAATACACCTGA | XM_003135205.6 |
| AQP1 | F: AGACACTCTGACAAGCTGGC R: GTCAAGGGAGTGGGTGATGG | XM_021078524.1 |
| AQP3 | F: TGACCTTCGCTATGTGCTTCC R: GTCCAAGTGTCCAGAGGGGTAG | NM_001110172.1 |
| AQP7 | F: CGTGACCTCTACCCACAACC R: TGGGGAGGGGGTCACAAATA | XM_013980184.2 |
| AQP8 | F: AGGAGGGCTCATCAGGTTCT R: CTCAGCTTCACCGTCCCTTT | NM_001112683.1 |
| Item | PMS Supplementation, % | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.15 | 0.30 | 0.45 | 0.60 | 0.75 | Group | Linear | Quadratic | |
| IgG (μg/mL) | 0.25 ± 0.06 | 0.23 ± 0.09 | 0.28 ± 0.08 | 0.47 ± 0.11 | 0.40 ± 0.14 | 0.54 ± 0.13 | 0.14 | 0.01 | 0.03 |
| IgM (mg/mL) | 0.43 ± 0.02 | 0.39 ± 0.19 | 0.59 ± 0.08 | 0.67 ± 0.05 | 0.41 ± 0.11 | 0.59 ± 0.05 | 0.27 | 0.30 | 0.43 |
| IgA (mg/mL) | 11.56 ± 1.76 | 12.64 ± 3.17 | 17.10 ± 3.44 | 18.60 ± 3.66 | 14.45 ± 2.28 | 21.76 ± 3.25 | 0.09 | 0.10 | 0.28 |
| IL-1β (ng/mL) | 0.53 ± 0.09 | 0.42 ± 0.10 | 0.38 ± 0.11 | 0.32 ± 0.06 | 0.40 ± 0.04 | 0.08 ± 0.07 | 0.38 | 0.04 | 0.12 |
| IL-2 (pg/mL) | 20.09 ± 3.28 | 18.82 ± 3.18 | 19.35 ± 4.75 | 18.82 ± 3.03 | 23.33 ± 3.14 | 31.45 ± 4.98 | 0.09 | 0.01 | 0.02 |
| IL-8 (pg/mL) | 0.96 ± 0.13 | 0.74 ± 0.24 | 1.08 ± 0.28 | 1.06 ± 0.18 | 0.76 ± 0.24 | 1.10 ± 0.21 | 0.72 | 0.68 | 0.92 |
| IL-10 (pg/mL) | 14.90 ± 1.70 a | 9.64 ± 0.53 b | 9.24 ± 0.48 b | 9.44 ± 2.05 b | 15.28 ± 2.37 a | 15.61 ± 1.59 a | 0.03 | 0.28 | 0.01 |
| TNF-α (ng/mL) | 0.14 ± 0.04 | 0.10 ± 0.04 | 0.13 ± 0.04 | 0.11 ± 0.20 | 0.13 ± 0.01 | 0.11 ± 0.3 | 0.48 | 0.28 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Huang, K.; Wen, X.; Gao, K.; Yang, X.; Jiang, Z.; Cao, S.; Wang, L. Effect of Potassium–Magnesium Sulfate on Intestinal Dissociation and Absorption Rate, Immune Function, and Expression of NLRP3 Inflammasome, Aquaporins and Ion Channels in Weaned Piglets. Animals 2025, 15, 1751. https://doi.org/10.3390/ani15121751
Zhu C, Huang K, Wen X, Gao K, Yang X, Jiang Z, Cao S, Wang L. Effect of Potassium–Magnesium Sulfate on Intestinal Dissociation and Absorption Rate, Immune Function, and Expression of NLRP3 Inflammasome, Aquaporins and Ion Channels in Weaned Piglets. Animals. 2025; 15(12):1751. https://doi.org/10.3390/ani15121751
Chicago/Turabian StyleZhu, Cui, Kaiyong Huang, Xiaolu Wen, Kaiguo Gao, Xuefen Yang, Zongyong Jiang, Shuting Cao, and Li Wang. 2025. "Effect of Potassium–Magnesium Sulfate on Intestinal Dissociation and Absorption Rate, Immune Function, and Expression of NLRP3 Inflammasome, Aquaporins and Ion Channels in Weaned Piglets" Animals 15, no. 12: 1751. https://doi.org/10.3390/ani15121751
APA StyleZhu, C., Huang, K., Wen, X., Gao, K., Yang, X., Jiang, Z., Cao, S., & Wang, L. (2025). Effect of Potassium–Magnesium Sulfate on Intestinal Dissociation and Absorption Rate, Immune Function, and Expression of NLRP3 Inflammasome, Aquaporins and Ion Channels in Weaned Piglets. Animals, 15(12), 1751. https://doi.org/10.3390/ani15121751






