The Skin Photophores of Chauliodus sloani Bloch & Schneider, 1801 (Pisces: Stomiidae): A Morphological, Ultrastructural and Immunohistochemical Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Treatment
2.2. Histochemistry
2.3. Ultrastructure Analysis
2.4. Immunohistochemistry
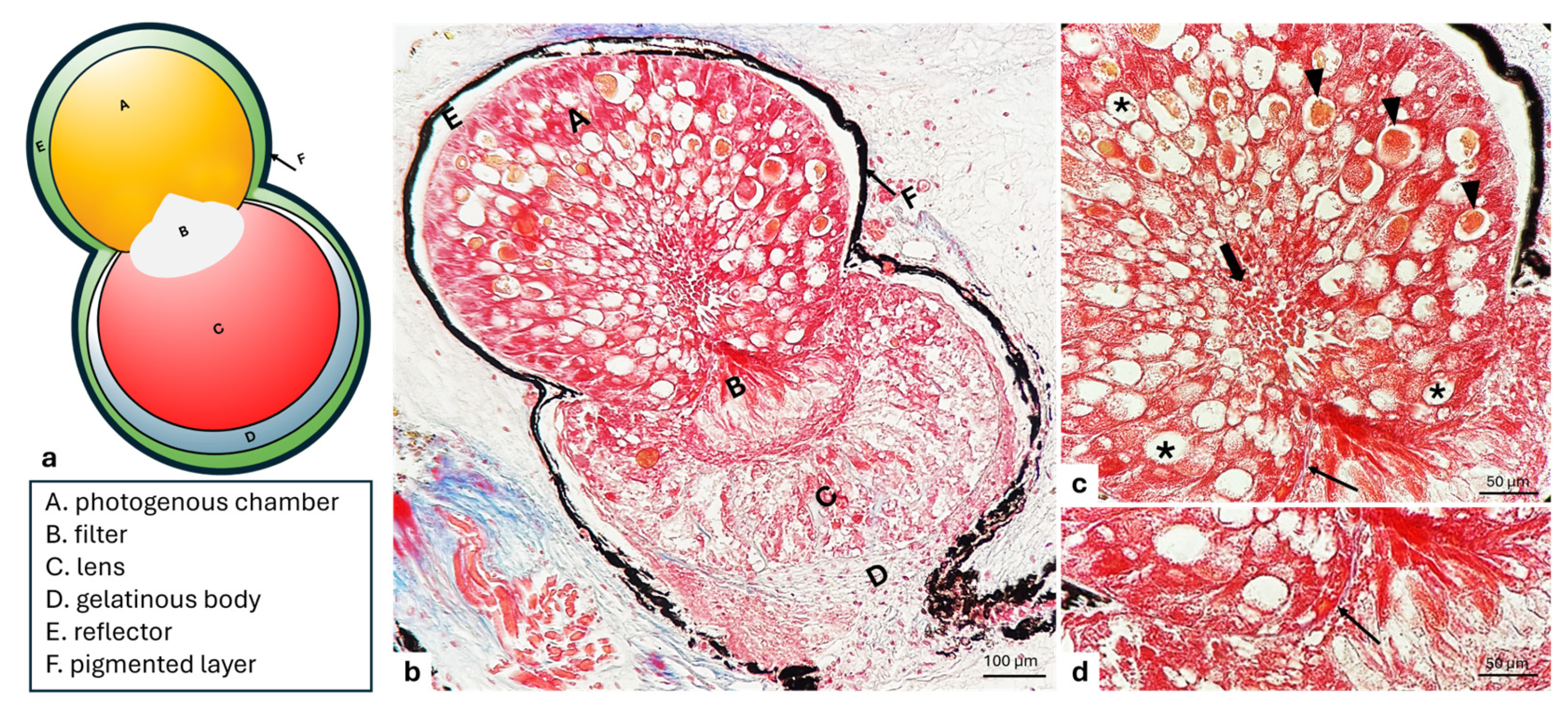
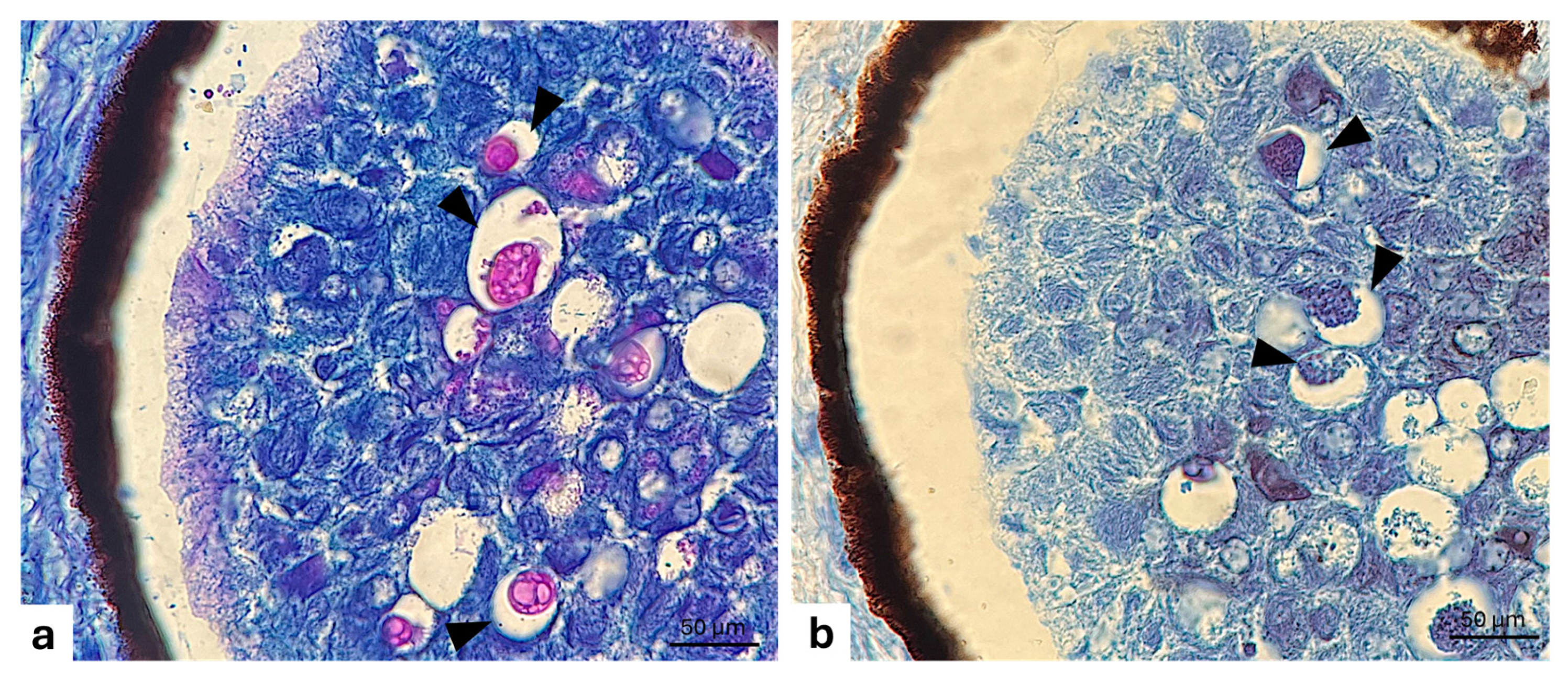
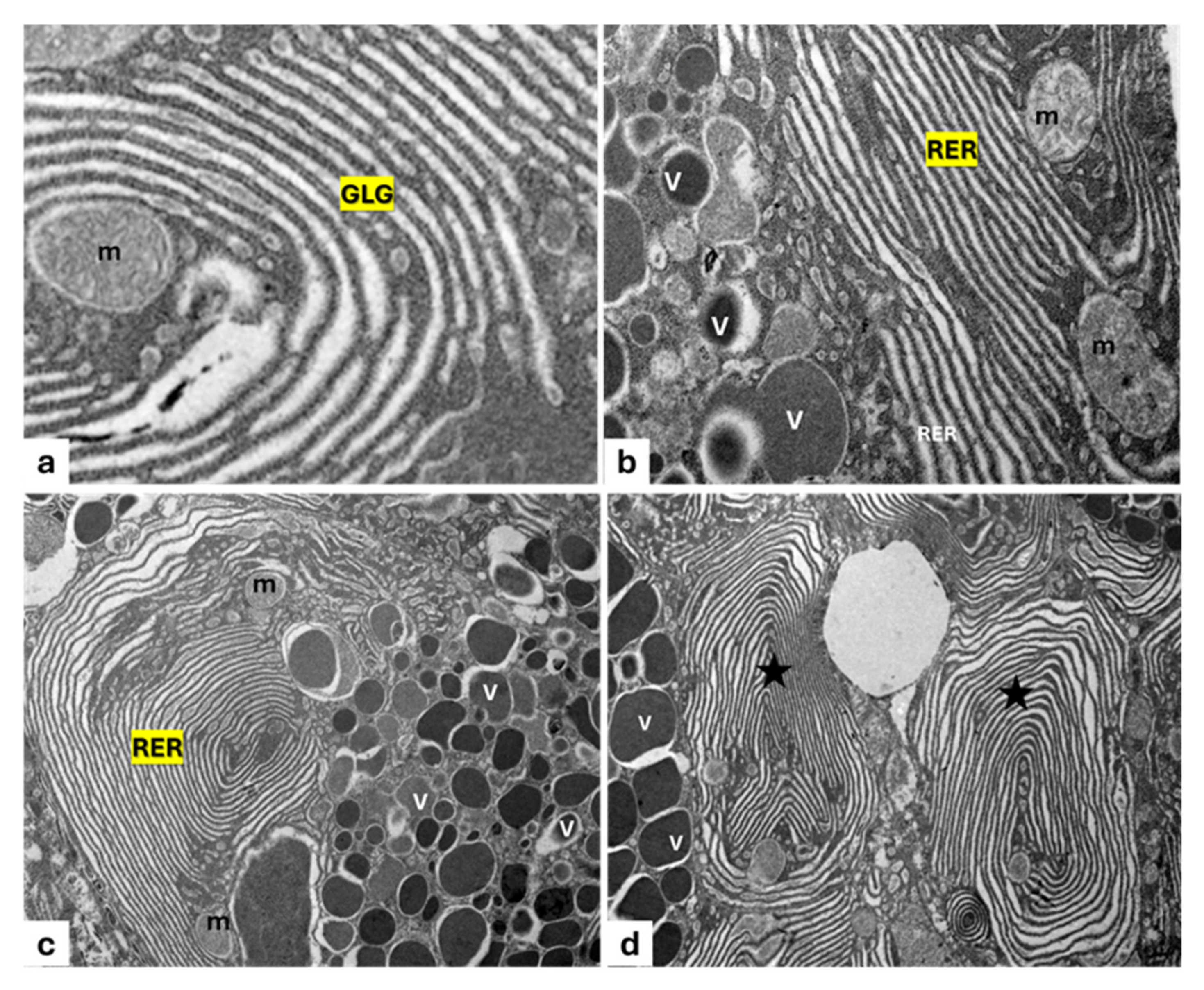
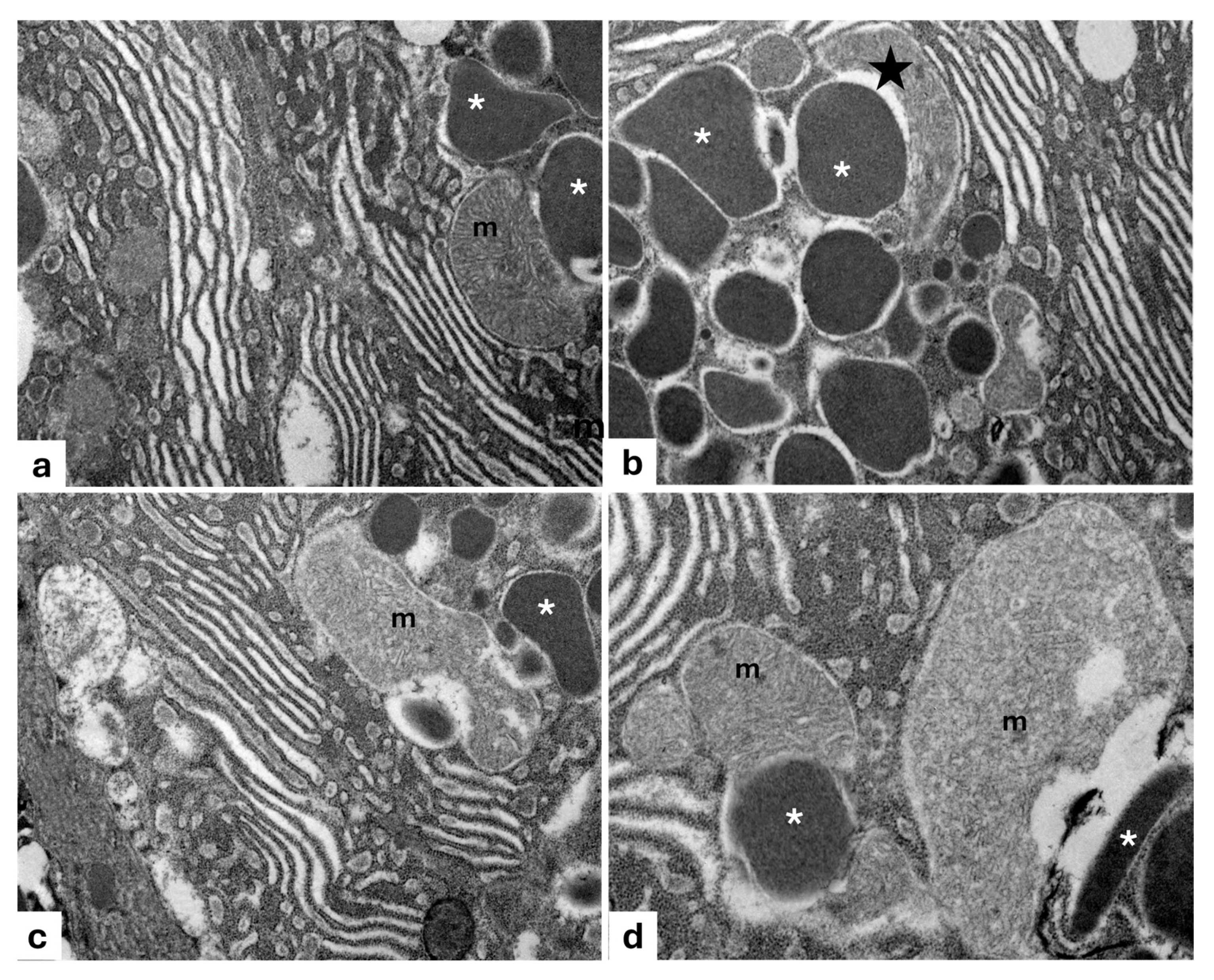
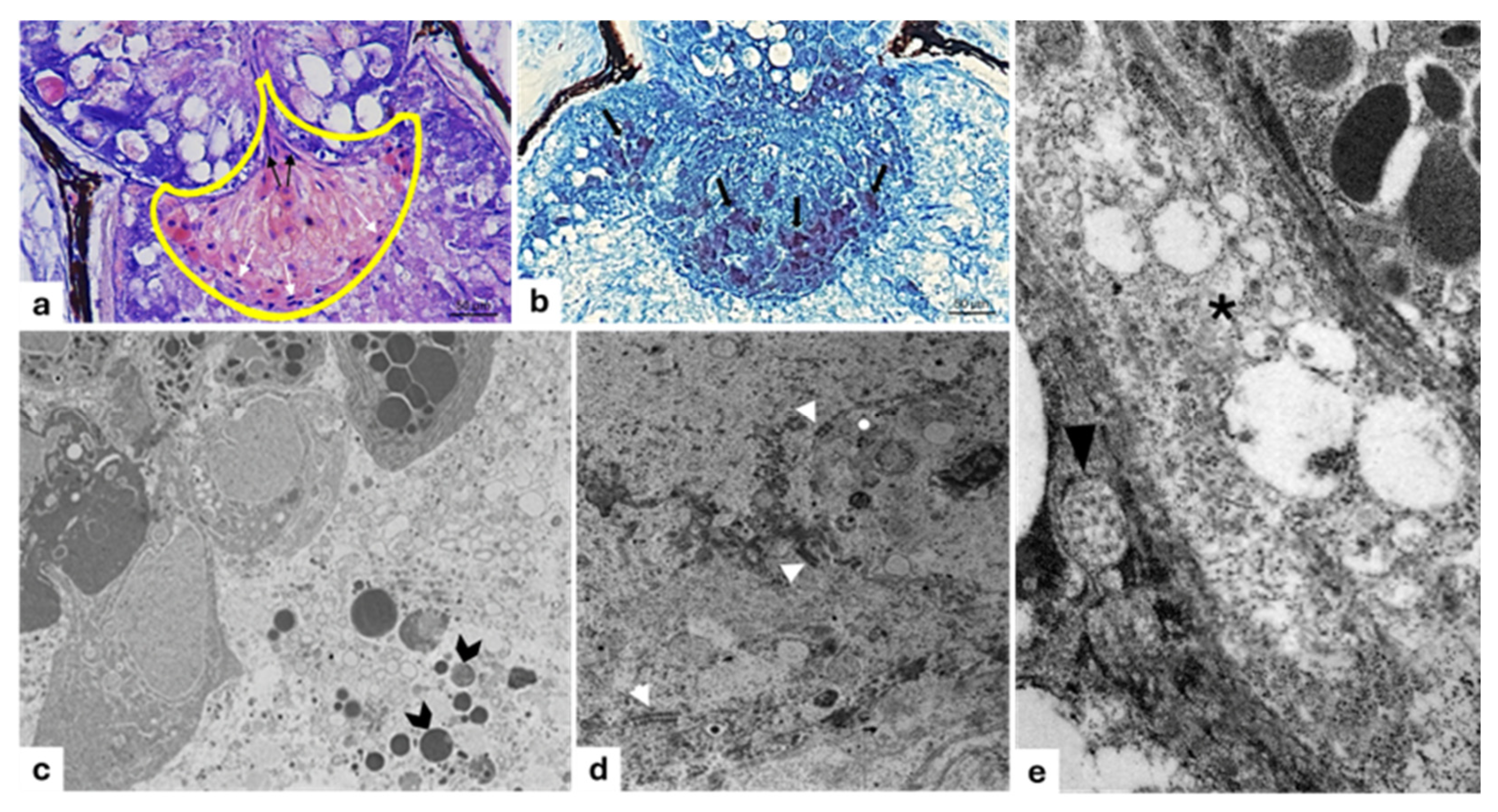
3. Results
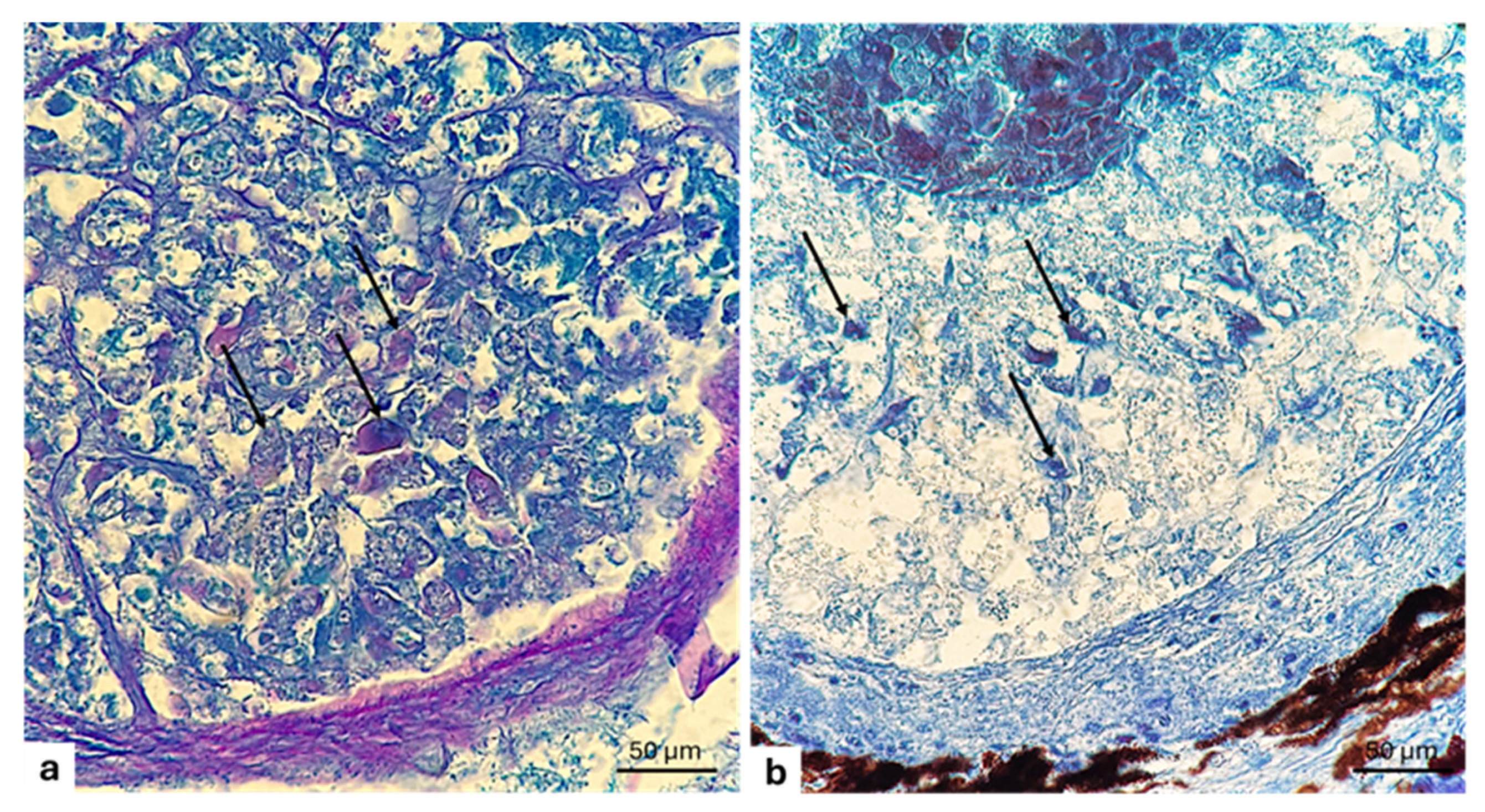
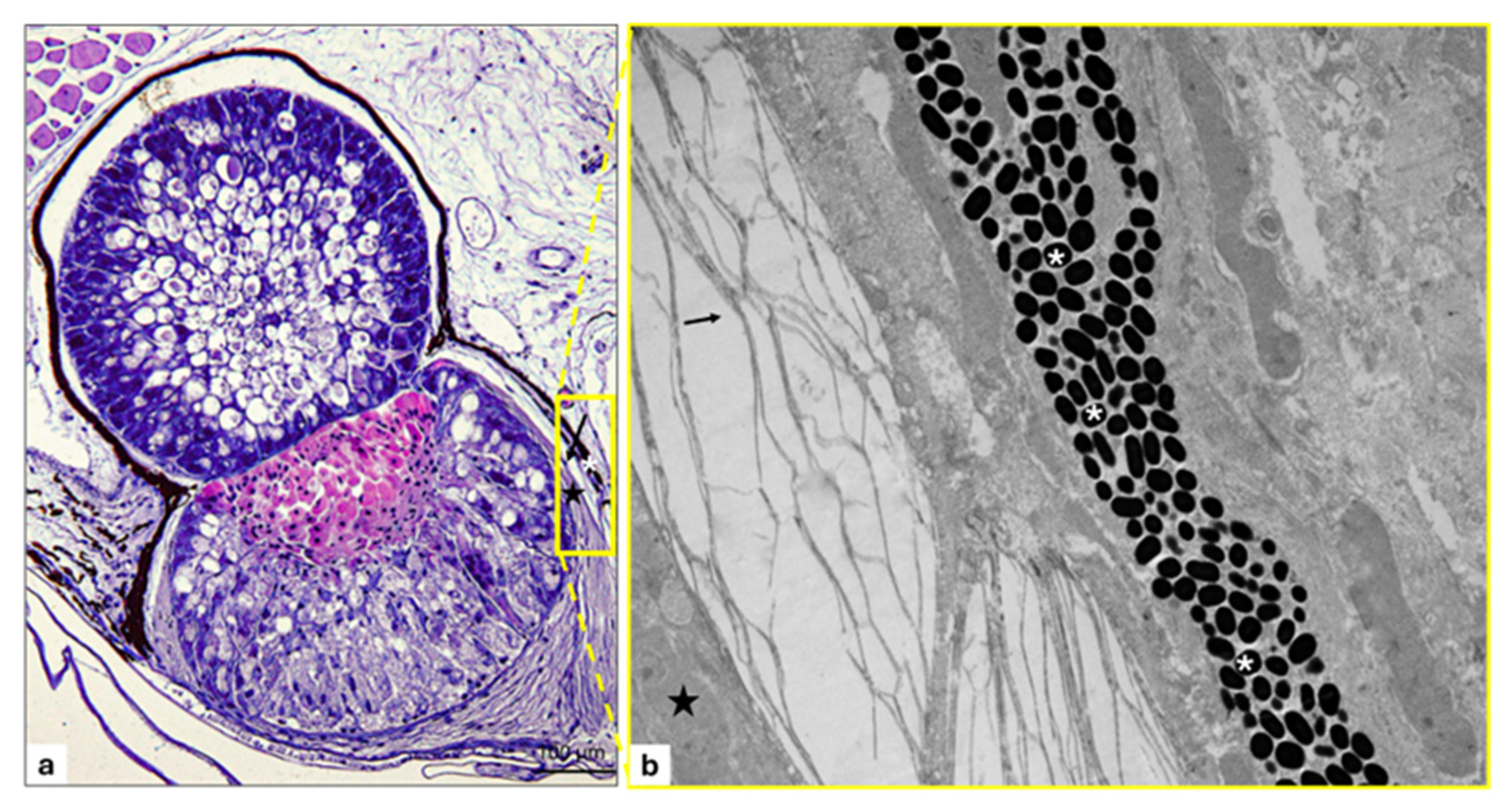
3.1. Filter
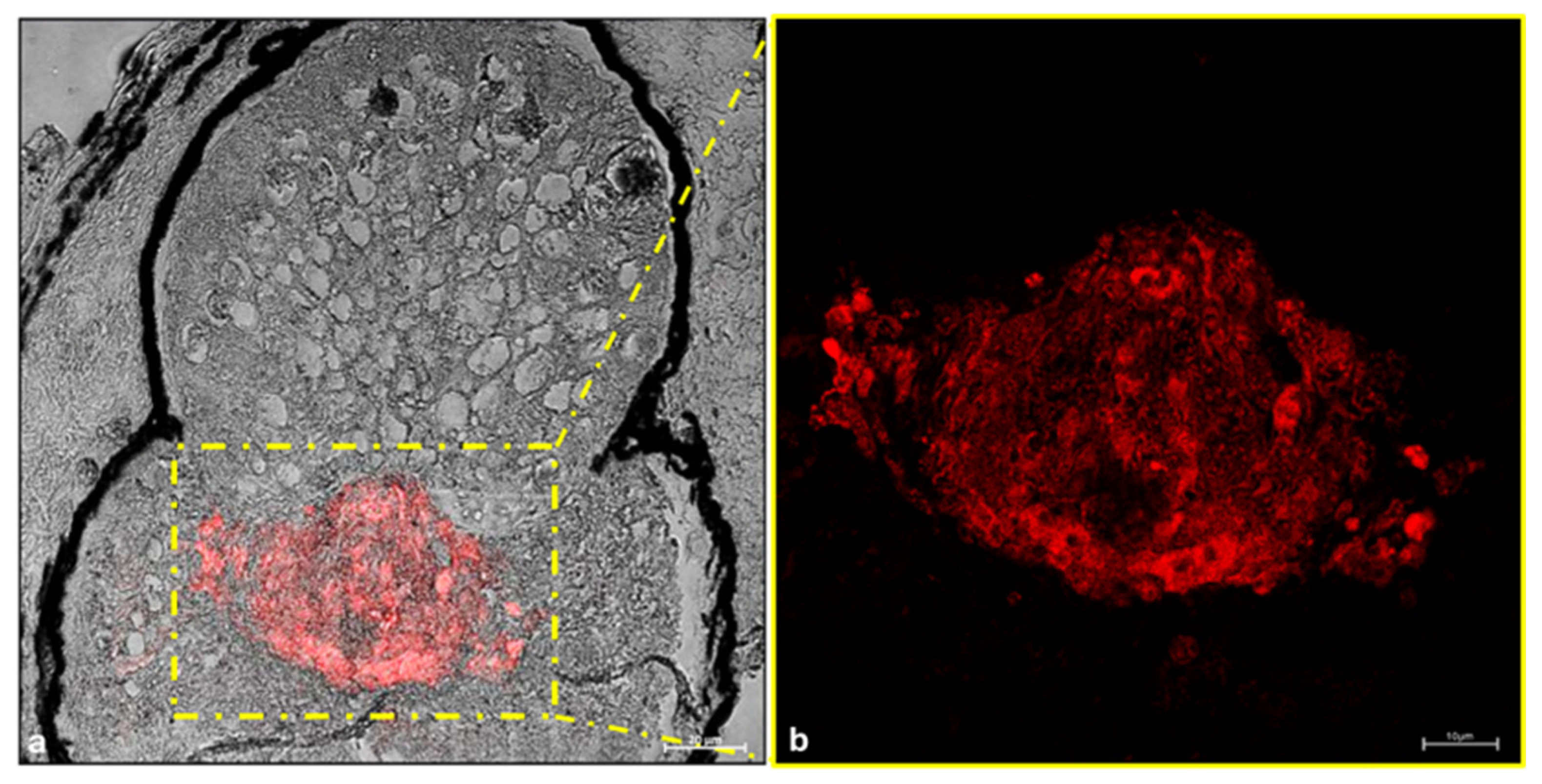
Immunohistochemistry
3.2. Lens
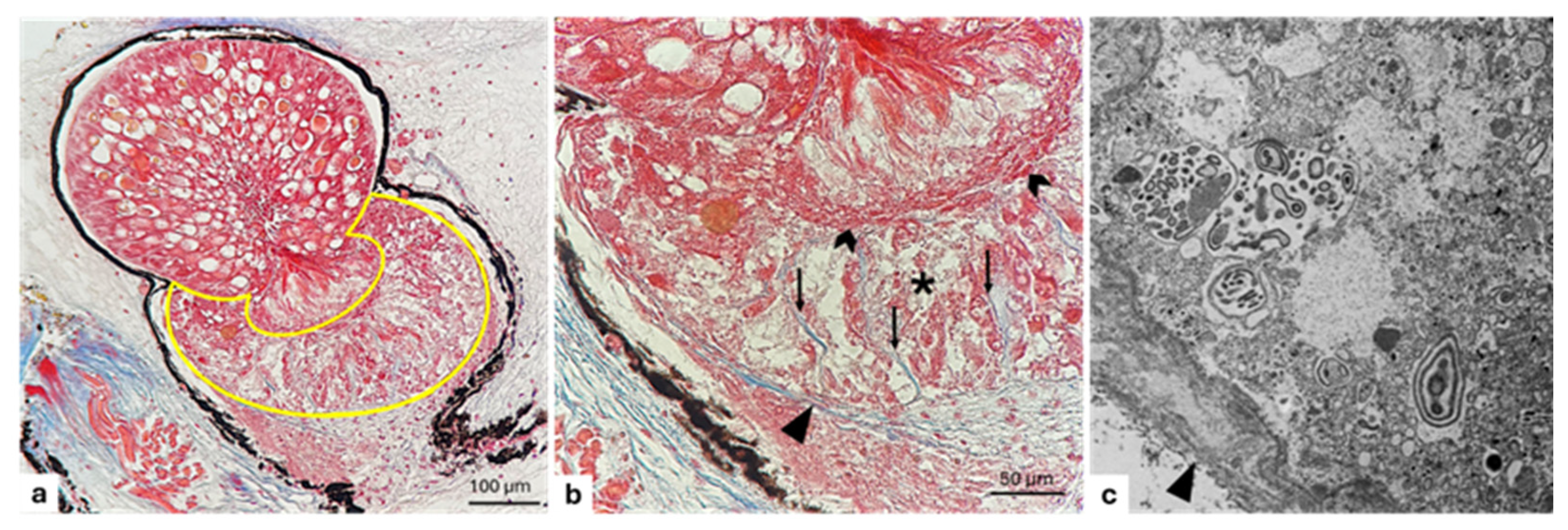
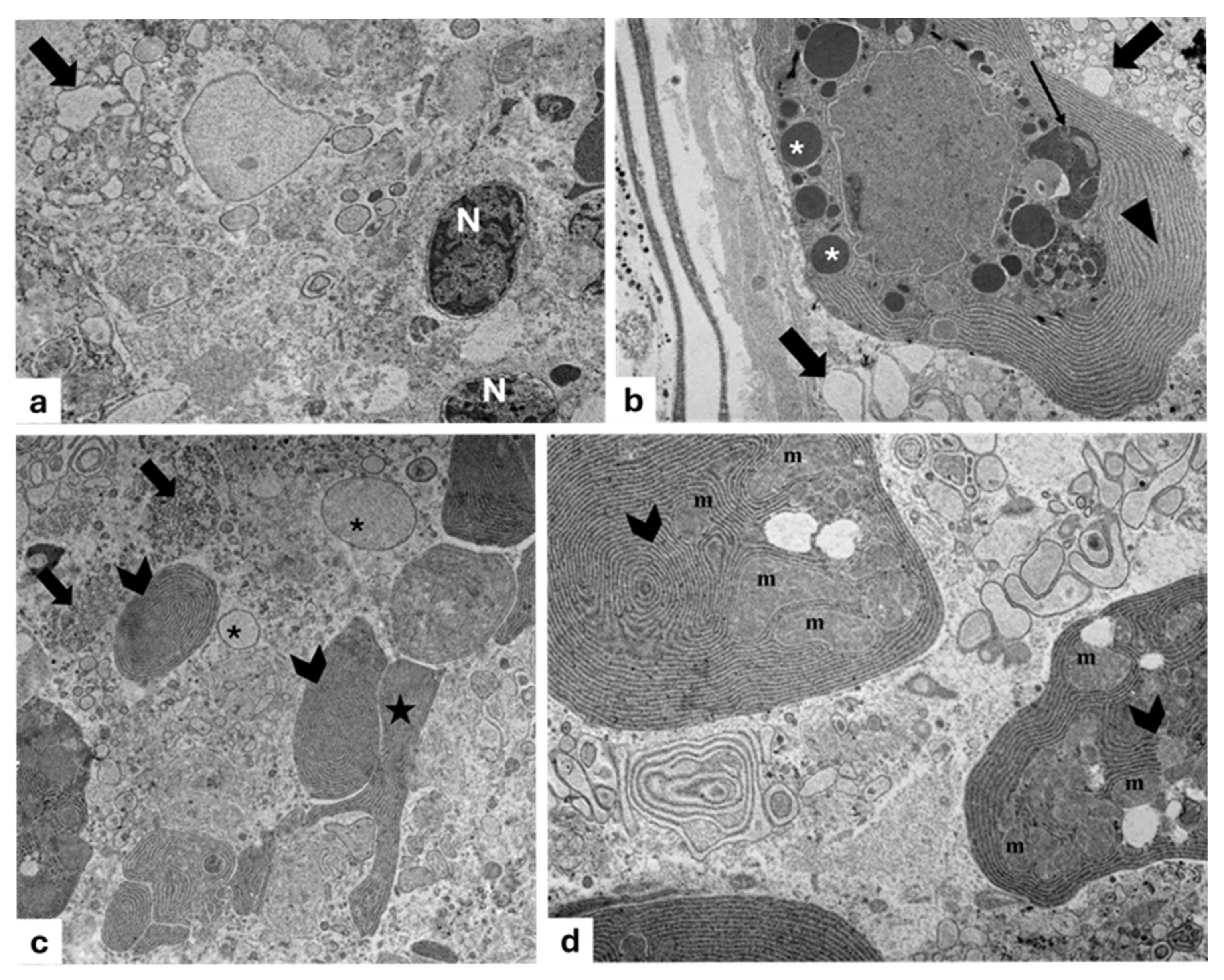
3.3. Gelatinous Body
3.4. Reflector
3.5. Pigmented Layer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibbs, R.; Whitehead, P.; Bauchot, M.; Hureau, J.; Nielsen, J.; Tortonese, E. Fishes of the North-Eastern Atlantic the Mediterranean; UNESCO: Paris, France, 1984; Volume 1, pp. 336–337. ISBN 92-3-002215-2. [Google Scholar]
- Kenaley, C.P. Exploring feeding behaviour in deep-sea dragonfishes (Teleostei: Stomiidae): Jaw biomechanics and functional significance of a loosejaw. Biol. J. Linn. Soc. 2012, 106, 224–240. [Google Scholar] [CrossRef]
- Herring, P. The Biology of the Deep Ocean; Oxford University Press: Oxford, UK, 2002. [Google Scholar] [CrossRef]
- Herring, P.J. Systematic distribution of bioluminescence in living organisms. J. Biolumin. Chemilumin. 1987, 1, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, P.; Ammendolia, G.; Cavallaro, M.; Consoli, P.; Esposito, V.; Malara, D.; Rao, I.; Romeo, T.; Andaloro, F. Influence of lunar phases, winds and seasonality on the stranding of mesopelagic fish in the Strait of Messina (Central Mediterranean Sea). Mar. Ecol. 2017, 38, e12459. [Google Scholar] [CrossRef]
- Cavallaro, M. Studio Morfologico ed Ultrastrutturale dei Fotofori in Diverse Specie di Pesci Mesopelagici dello Stretto di Messina. Ph.D. Thesis, University of Messina, Messina, Italy, 2017. Available online: https://tesidottorato.depositolegale.it/handle/20.500.14242/101323 (accessed on 19 May 2025).
- Genovese, S.; Guglielmo, L. Spiaggiamenti di fauna abissale nello Stretto di Messina. Atti Soc. Peloritana Sci. Fis. Mat. E Nat. 1971, 17, 331–370. Available online: https://hdl.handle.net/11570/1703968 (accessed on 19 May 2025).
- Battaglia, P.; Andaloro, F.; Consoli, P.; Esposito, V.; Malara, D.; Musolino, S.; Pedà, C.; Romeo, T. Feeding habits of the Atlantic bluefin tuna, Thunnus thynnus (L. 1758), in the central Mediterranean Sea (Strait of Messina). Helgol. Mar. Res. 2013, 67, 97–107. [Google Scholar] [CrossRef]
- Battaglia, P.; Malara, D.; Romeo, T.; Andaloro, F. Relationships between otolith size and fish size in some mesopelagic and bathypelagic species from the Mediterranean Sea (Strait of Messina, Italy). Sci. Mar. 2010, 74, 605–612. [Google Scholar] [CrossRef]
- Azzellino, A.; Conley, D.; Vicinanza, D.; Kofoed, J.P. Marine renewable energies: Perspectives and implications for marine ecosystems. Sci. World J. 2013, 2013, 547563. [Google Scholar] [CrossRef]
- Evans, C.W.; Pace, L.; Cziko, P.A.; Marsh, A.G.; Cheng, C.-H.C.; DeVries, A.L. Metabolic energy utilization during development of Antarctic naked dragonfish (Gymnodraco acuticeps). Polar Biol. 2006, 29, 519–525. [Google Scholar] [CrossRef]
- Briglia, M.; Cavallaro, M.; Abbate, F.; Guerrera, M.C.; Levanti, M.; Cometa, M.; Montalbano, G.; Germanà, A.; Laurà, R. Preliminary data on the structure and ultrastructure of the viper fish photophores, Chauliodus sloani Bloch & Schneider, 1801 (family: Stomiidae). In Proceedings of the 74th Congress of the Italian Society of Veterinary Sciences SISVET, Virtual, 23–26 June 2021; p. 1. Available online: https://hdl.handle.net/11570/3205971 (accessed on 19 May 2025).
- Eduardo, L.N.; Lucena-Frédou, F.; Mincarone, M.M.; Soares, A.; Le Loc’h, F.; Frédou, T.; Ménard, F.; Bertrand, A. Trophic ecology, habitat, and migratory behaviour of the viperfish Chauliodus sloani reveal a key mesopelagic player. Sci. Rep. 2020, 10, 20996. [Google Scholar] [CrossRef]
- Davis, A.L.; Thomas, K.N.; Goetz, F.E.; Robison, B.H.; Johnsen, S.; Osborn, K.J. Ultra-black Camouflage in Deep-Sea Fishes. Curr. Biol. 2020, 30, 3470–3476.e3473. [Google Scholar] [CrossRef]
- Marshall, J.; Johnsen, S. 11 Camouflage in Marine Fish. Animal Camouflage: Mechanisms and Function; Cambridge University Press: Cambridge, UK, 2011; pp. 186–211. [Google Scholar] [CrossRef]
- Harvey, E.N. The luminous organs of fishes. In The Physiology of Fishes; Brown, M.E., Ed.; Elsevier/Academic Press: New York, NY, USA, 1957; Volume 3, pp. 345–366. [Google Scholar]
- Baguet, F. Les Photophores des Poissons Lumineux; Pergamon Press: Oxford, UK, 1977. [Google Scholar]
- Herring, P.J. Bioluminescence of marine organisms. Nature 1977, 267, 788–793. [Google Scholar] [CrossRef]
- Harvey, E.N. The Mechanism of Light Production in Animals. Science 1916, 44, 208–209. [Google Scholar] [CrossRef]
- Harvey, E.N. Luminescent organisms. Am. Sci. 1952, 40, 468–481. [Google Scholar]
- Denton, E.; Gilpin-Brown, J.; Roberts, B. On the organization and function of the photophores of Argyropelecus. J. Physiol. 1969, 204, 38P–39P. [Google Scholar]
- Denton, E.; Gilpin-Brown, J.; Wright, P. On the filters’ in the photophores of mesopelagic fish and on a fish emitting red light and especially sensitive to red light. J. Physiol. 1970, 208, 72P–73P. [Google Scholar]
- Denton, E.J.; Herring, P.J.; Widder, E.A.; Latz, M.F.; Case, J.F. The roles of filters in the photophores of oceanic animals and their relation to vision in the oceanic environment. Proc. R. Soc. London Ser. B. Biol. Sci. 1985, 225, 63–97. [Google Scholar] [CrossRef]
- Zaccone, G.; Abelli, L.; Salpietro, L.; Zaccone, D.; Macrì, B.; Marino, F. Nervous control of photophores in luminescent fishes. Acta Histochem. 2011, 113, 387–394. [Google Scholar] [CrossRef] [PubMed]
- O’day, W.T. The Histology and Fine-Structure of Some Bioluminescent Organs in Deep-Sea Fishes; University of Southern California: Los Angeles, CA, USA, 1972. [Google Scholar]
- Anctil, M. Stimulation of bioluminescence in lanternfishes (Myctophidae). II. Can. J. Zool. 1972, 50, 233–237. [Google Scholar] [CrossRef]
- Anctil, M. Luminescent organs of Myctophid fishes: Structural and histochemical aspects. Can. J. Zool. 1972, 50, 233–237. [Google Scholar]
- Anctil, M.; Case, J.F. The caudal luminous organs of lanternfishes: General innervation and ultrastructure. Am. J. Anat. 1977, 149, 1–21. [Google Scholar] [CrossRef]
- Cavallaro, M.; Guerrera, M.C.; Abbate, F.; Levanti, M.B.; Laurà, R.; Ammendolia, G.; Malara, D.; Stipa, M.G.; Battaglia, P. Morphological, ultrastructural and immunohistochemical study on the skin ventral photophores of Diaphus holti Tåning, 1918 (Family: Myctophidae). Acta Zool. 2020, 102, 405–411. [Google Scholar] [CrossRef]
- Aragona, M.; Porcino, C.; Guerrera, M.C.; Montalbano, G.; Laurà, R.; Cometa, M.; Levanti, M.; Abbate, F.; Cobo, T.; Capitelli, G.; et al. The BDNF/TrkB Neurotrophin System in the Sensory Organs of Zebrafish. Int. J. Mol. Sci. 2022, 23, 2621. [Google Scholar] [CrossRef]
- Aragona, M.; Porcino, C.; Guerrera, M.C.; Montalbano, G.; Laurà, R.; Levanti, M.; Abbate, F.; Cobo, T.; Capitelli, G.; Calapai, F.; et al. Localization of BDNF and Calretinin in Olfactory Epithelium and Taste Buds of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2022, 23, 4696. [Google Scholar] [CrossRef] [PubMed]
- Lauriano, E.; Guerrera, M.; Laurà, R.; Capillo, G.; Pergolizzi, S.; Aragona, M.; Abbate, F.; Germanà, A. Effect of light on the calretinin and calbindin expression in skin club cells of adult zebrafish. Histochem. Cell Biol. 2020, 154, 495–505. [Google Scholar] [CrossRef]
- Aragona, M.; Briglia, M.; Porcino, C.; Mhalhel, K.; Cometa, M.; Germanà, P.G.; Montalbano, G.; Levanti, M.; Laurà, R.; Abbate, F.; et al. Localization of Calretinin, Parvalbumin, and S100 Protein in Nothobranchius guentheri Retina: A Suitable Model for the Retina Aging. Life 2023, 13, 2050. [Google Scholar] [CrossRef]
- Aragona, M.; Porcino, C.; Guerrera, M.C.; Montalbano, G.; Levanti, M.; Abbate, F.; Laurà, R.; Germanà, A. Localization of Neurotrophin Specific Trk Receptors in Mechanosensory Systems of Killifish (Nothobranchius guentheri). Int. J. Mol. Sci. 2021, 22, 10411. [Google Scholar] [CrossRef]
- Aragona, M.; Mhalhel, K.; Pansera, L.; Montalbano, G.; Guerrera, M.C.; Levanti, M.; Laurà, R.; Abbate, F.; Vega, J.A.; Germanà, A. Localization of Piezo 1 and Piezo 2 in Lateral Line System and Inner Ear of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2024, 25, 9204. [Google Scholar] [CrossRef]
- Cavallaro, M.; Aragona, M.; Germanà, A. Morphological and immunohistochemical study on photophores of Gonostoma denudatum Rafinesque, 1810 (Fam: Gonostomatidae). Atti Accad. Peloritana Pericolanti Cl. Sci. Med. Biol. 2021, 109, 1–7. [Google Scholar] [CrossRef]
- Cavallaro, M.; Mammola, C.L.; Verdiglione, R. Structural and ultrastructural comparison of photophores of two species of deep-sea fishes: Argyropelecus hemigymnus and Maurolicus muelleri. J. Fish Biol. 2004, 64, 1552–1567. [Google Scholar] [CrossRef]
- Cavallaro, M.; Battaglia, P.; Laurà, R.; Guerrera, M.C.; Abbate, F.; Germanà, A. The morphology of photophores in the garrick, Cyclothone braueri (Family: G onostomatidae): An ultrastructure study. Acta Zool. 2015, 96, 296–300. [Google Scholar] [CrossRef]
- Cavallaro, M.; Battaglia, P.; Guerrera, M.C.; Abbate, F.; Levanti, M.B.; Ammendolia, G.; Andaloro, F.; Germanà, A.; Laurà, R. Structure and ultrastructure study on photophores of the Madeira lanternfish, Ceratoscopelus maderensis (Lowe, 1839), Pisces: Myctophidae. Acta Zool. 2019, 100, 89–95. [Google Scholar] [CrossRef]
- Bassot, J.-M. Caractères cytologiques des cellules lumineuses chez quelques Télèostéens. Comptes Rendus Hebd. Seances l’Acad. Sci. 1960, 250, 3878–3880. [Google Scholar]
- Martin, R.P.; Carr, E.M.; Sparks, J.S. Variation in lanternfish (Myctophidae) photophore structure: A comprehensive comparative analysis. PLoS ONE 2024, 19, e0310976. [Google Scholar] [CrossRef]
- Denton, E.; Herring, P.J. On the filters in the ventral photophores of mesopelagic animals [proceedings]. J. Physiol. 1978, 284, 42P. [Google Scholar] [PubMed]
- Gur, D.; Palmer, B.A.; Weiner, S.; Addadi, L. Light Manipulation by Guanine Crystals in Organisms: Biogenic Scatterers, Mirrors, Multilayer Reflectors and Photonic Crystals. Adv. Funct. Mater. 2017, 27, 1603514. [Google Scholar] [CrossRef]
- Paitio, J.; Yano, D.; Muneyama, E.; Takei, S.; Asada, H.; Iwasaka, M.; Oba, Y. Reflector of the body photophore in lanternfish is mechanistically tuned to project the biochemical emission in photocytes for counterillumination. Biochem. Biophys. Res. Commun. 2020, 521, 821–826. [Google Scholar] [CrossRef]
- Paitio, J.; Oba, Y.; Meyer-Rochow, V.B. Bioluminescent fishes and their eyes. In Luminescence—An Outlook on the Phenomena Their Applications; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Strum, J.M. Fine structure of the dermal luminescent organs, photophores, in the fish, Porichthys notatus. Anat. Rec. 1969, 164, 433–461. [Google Scholar] [CrossRef]
- Lawry, J.V. Dioptric modifications of the scales overlying the photophores of the lantern fish, Tarletonbeania crenularis (Myctophidae). J. Anat. 1973, 114, 55. [Google Scholar]
- Cavallaro, M.; Ammendolia, G.; Andaloro, F.; Battaglia, P. First record of the mesopelagic fish Diaphus dumerilii (Bleeker, 1856) in the Mediterranean Sea. Mar. Biodivers. 2017, 47, 585–588. [Google Scholar] [CrossRef]
- Bassot, J.M. Données Histochimiques et Cytologiques sur les Photophores du Téléostéen Maurolicus Pennanti; Masson: Issy-les-Moulineaux, France, 1960. [Google Scholar]
- Mallefet, J.; Duchatelet, L.; Hermans, C.; Baguet, F. Luminescence control of Stomiidae photophores. Acta Histochem. 2019, 121, 7–15. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallaro, M.; Pansera, L.; Mhalhel, K.; Abbate, F.; Levanti, M.; Guerrera, M.C.; Montalbano, G.; Briglia, M.; Aragona, M.; Laurà, R. The Skin Photophores of Chauliodus sloani Bloch & Schneider, 1801 (Pisces: Stomiidae): A Morphological, Ultrastructural and Immunohistochemical Study. Animals 2025, 15, 1738. https://doi.org/10.3390/ani15121738
Cavallaro M, Pansera L, Mhalhel K, Abbate F, Levanti M, Guerrera MC, Montalbano G, Briglia M, Aragona M, Laurà R. The Skin Photophores of Chauliodus sloani Bloch & Schneider, 1801 (Pisces: Stomiidae): A Morphological, Ultrastructural and Immunohistochemical Study. Animals. 2025; 15(12):1738. https://doi.org/10.3390/ani15121738
Chicago/Turabian StyleCavallaro, Mauro, Lidia Pansera, Kamel Mhalhel, Francesco Abbate, Maria Levanti, Maria Cristina Guerrera, Giuseppe Montalbano, Marilena Briglia, Marialuisa Aragona, and Rosaria Laurà. 2025. "The Skin Photophores of Chauliodus sloani Bloch & Schneider, 1801 (Pisces: Stomiidae): A Morphological, Ultrastructural and Immunohistochemical Study" Animals 15, no. 12: 1738. https://doi.org/10.3390/ani15121738
APA StyleCavallaro, M., Pansera, L., Mhalhel, K., Abbate, F., Levanti, M., Guerrera, M. C., Montalbano, G., Briglia, M., Aragona, M., & Laurà, R. (2025). The Skin Photophores of Chauliodus sloani Bloch & Schneider, 1801 (Pisces: Stomiidae): A Morphological, Ultrastructural and Immunohistochemical Study. Animals, 15(12), 1738. https://doi.org/10.3390/ani15121738






