Effect of Pre-Freezing 18 °C Holding Time on Post-Thaw Motility and Morphometry of Cryopreserved Boar Epididymal Sperm
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Boar Epididymal Semen Retrieval
2.2. Boar Epididymal Semen Dilution
2.3. Preparation of Boar Epididymal Semen Freezing Extender
2.4. Boar Epididymal Semen Cryopreservation and Thawing Process
2.5. Evaluations of Boar Epididymal Sperm Motility Traits
2.6. Semen Staining Preparation for Boar Epididymal Sperm Morphometry
2.7. Analysis of Boar Epididymal Sperm Morphometry
2.8. Statistical Analysis
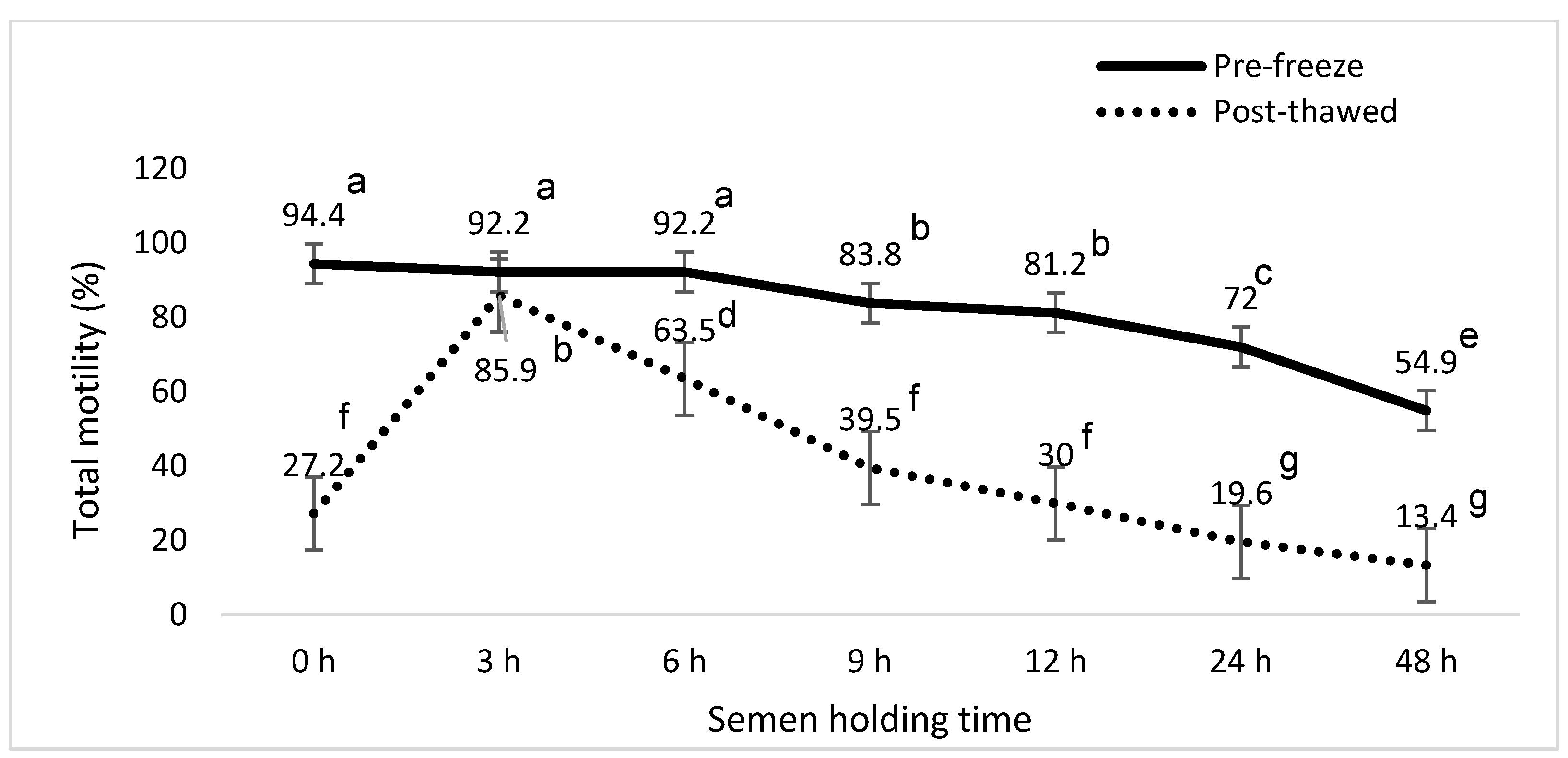
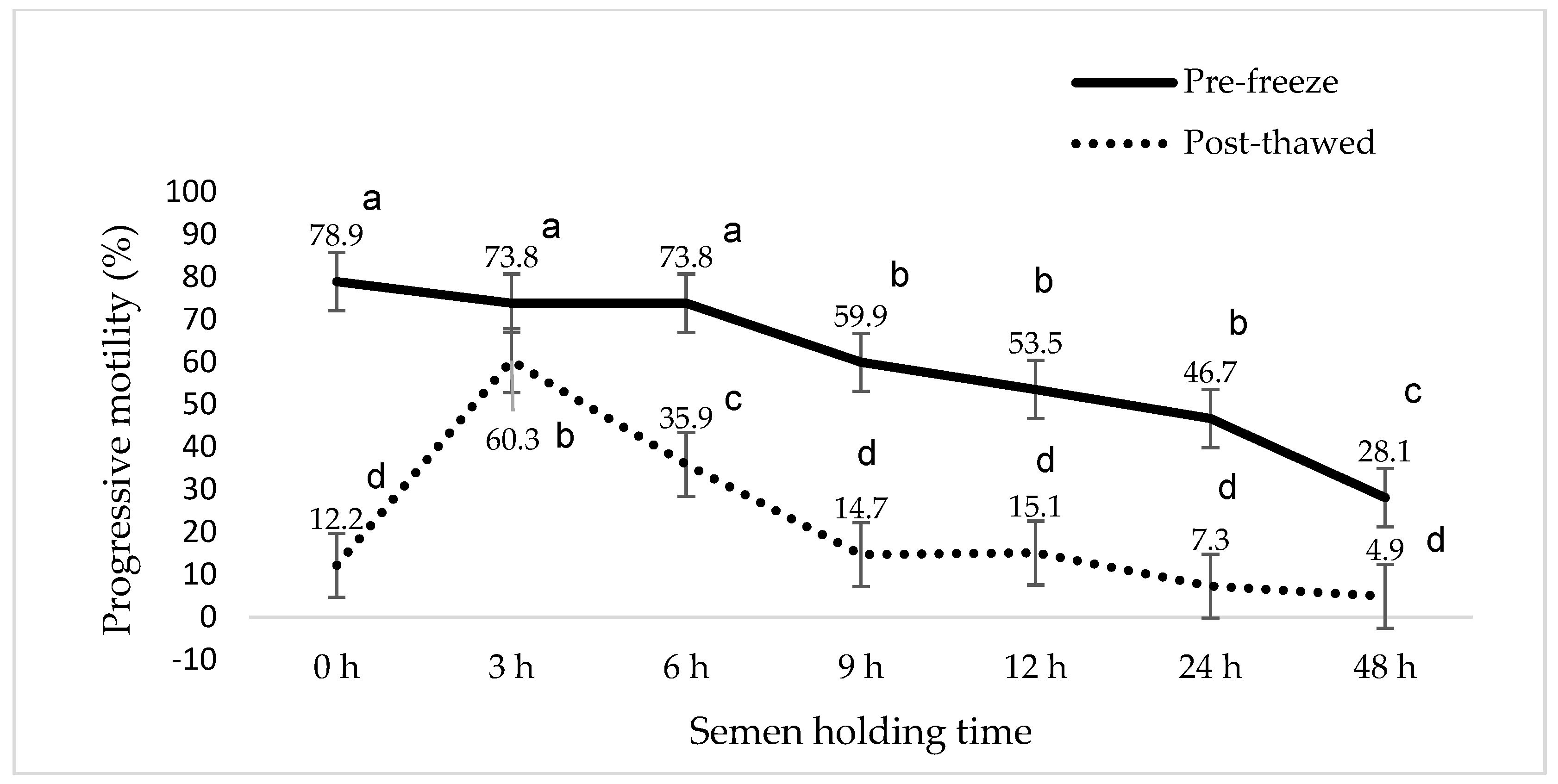
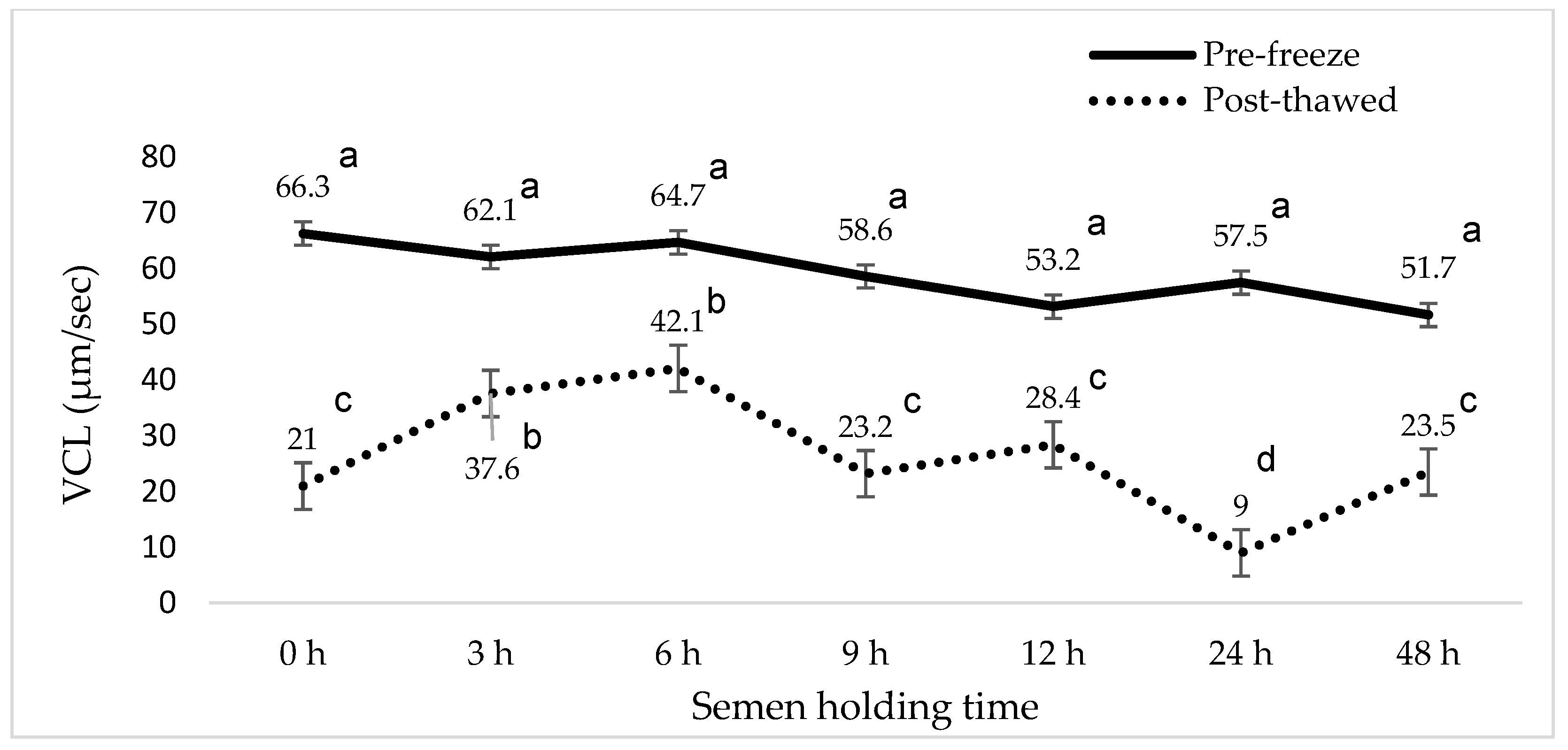
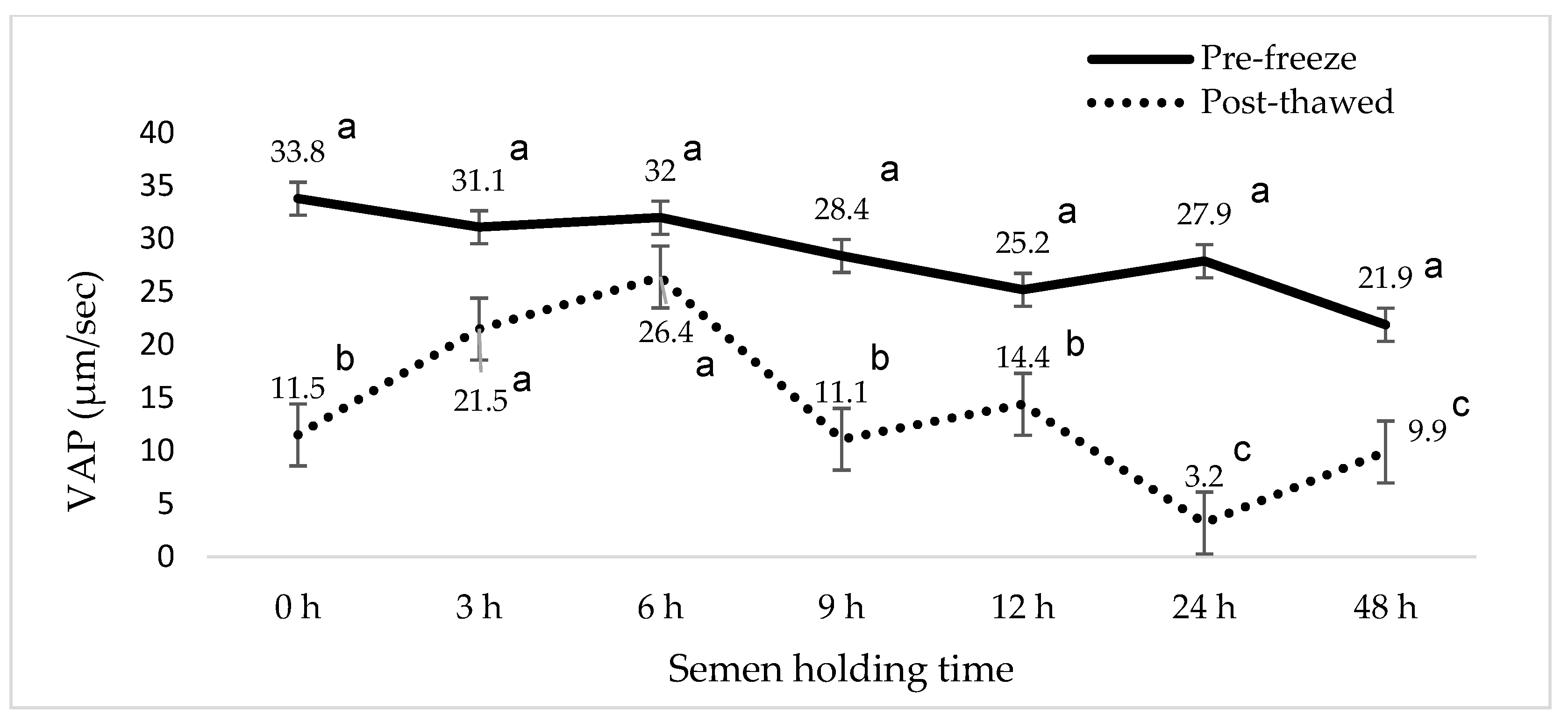
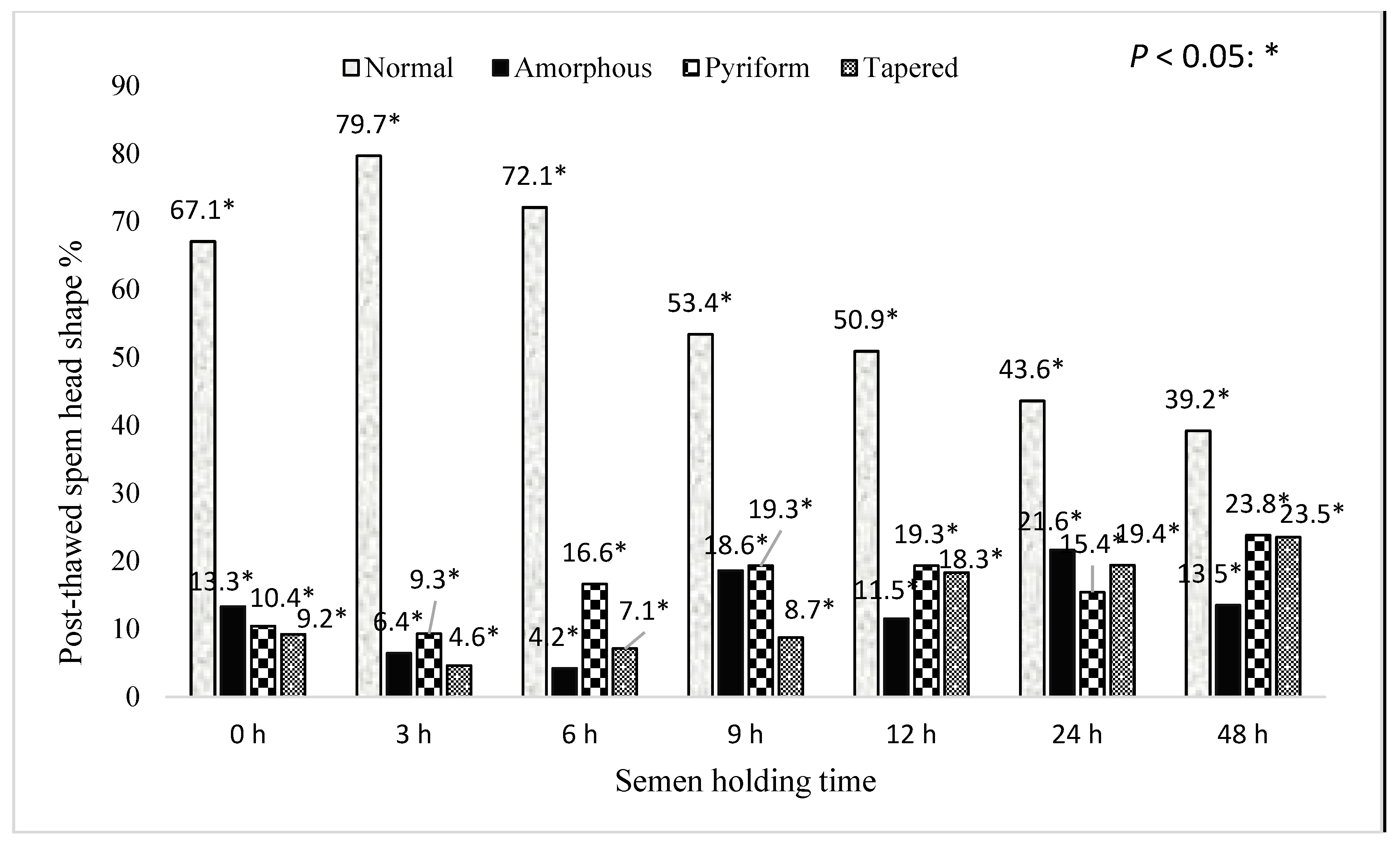
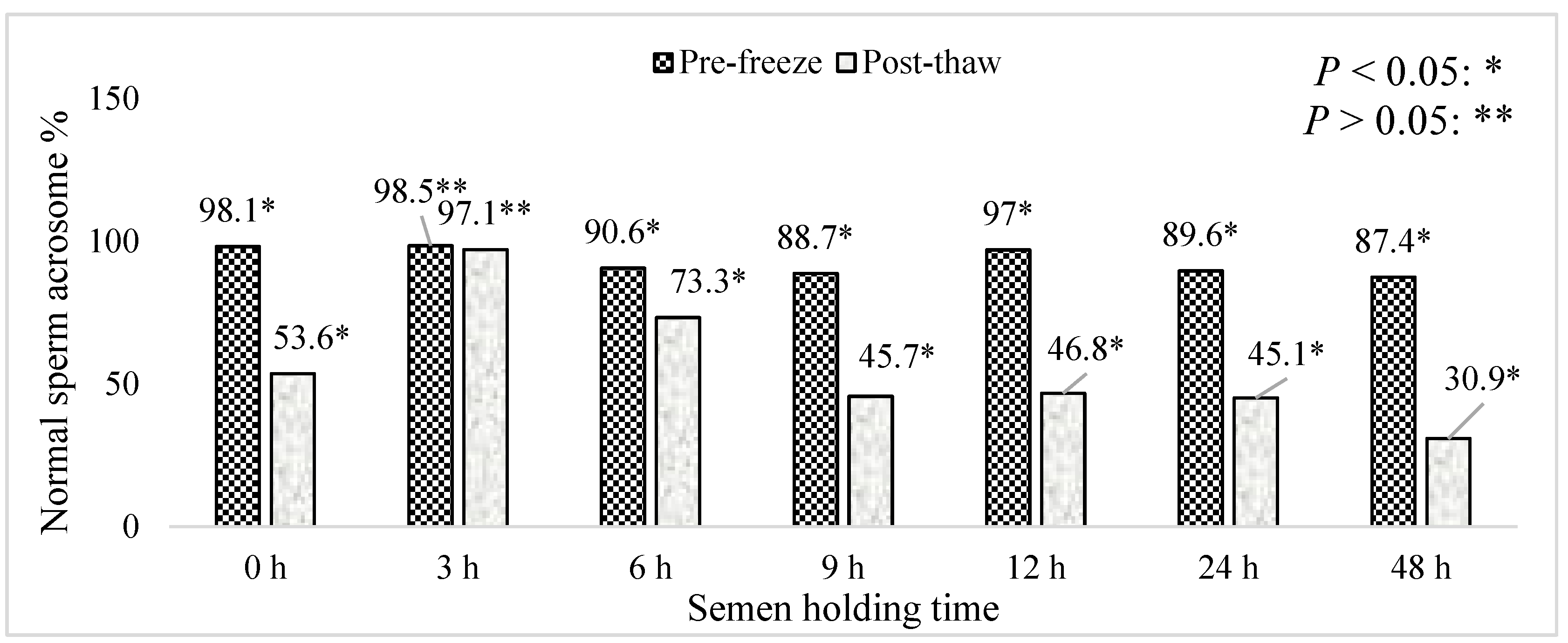
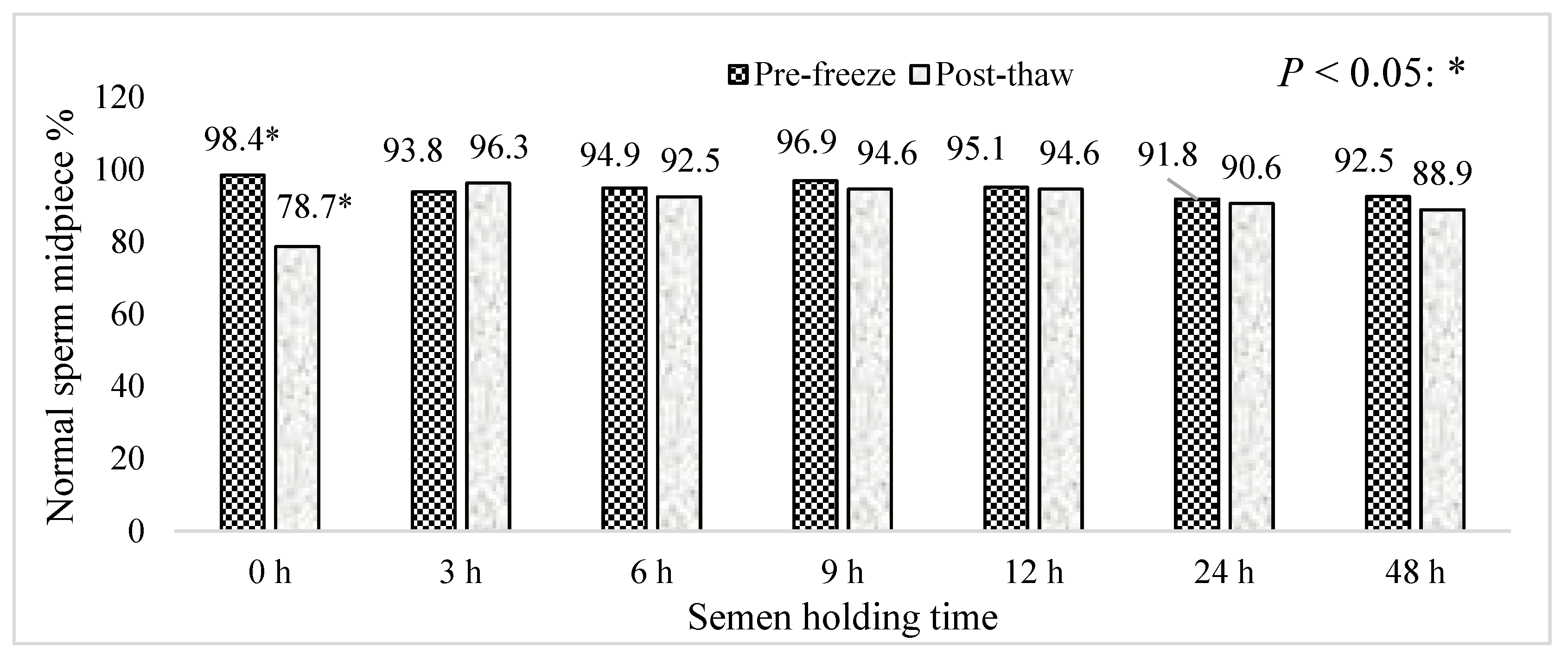
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heise, A.; Thompson, P.N.; Gerber, D. Influence of seminal plasma on fresh and post-thaw parameters of stallion epididymal spermatozoa. Anim. Reprod. Sci. 2011, 123, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.L.A.; Villaverde, A.I.S.B.; Lima, A.F.M.; Steagall, P.V.M.; Ferreira, J.C.P.; Taconeli, C.A.; Lopes, M.D. Impact of 24-h cooling prior to freezing on the survival of domestic cat (Felis catus) epididymal sperm. Reprod. Domest. Anim. 2009, 44, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Bolaji, U.F.O.; Ajasa, A.A.; Ahmed, R.O.; Bello, S.F.; Ositanwosu, O.E. Cattle Conservation in the 21st Century: A Mini Review. Open J. Anim. Sci. 2021, 11, 304. [Google Scholar] [CrossRef]
- Bertol, M.A. Cryopreservation of epididymal sperm. In Cryopreservation in Eukaryotes; Marco-Jiménez, F., Akdemir, H., Eds.; Intechopen: London, UK, 2016; pp. 121–135. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.J.; Kim, Y.J. Changes in sperm membrane and ROS following cryopreservation of liquid boar semen stored at 15 °C. Anim. Reprod. Sci. 2011, 124, 118–124. [Google Scholar] [CrossRef]
- Khan, I.M.; Cao, Z.; Liu, H.; Khan, A.; Rahman, S.U.; Khan, M.Z.; Sathanawongs, A.; Zhang, Y. Impact of cryopreservation on spermatozoa freeze-thawed traits and relevance omics to assess sperm cryo-tolerance in farm animals. Front. Vet. Sci 2011, 8, 609180. [Google Scholar] [CrossRef]
- Casas, I.; Althouse, G.C. The protective effect of a 17 °C holding time on boar sperm plasma membrane fluidity after exposure to 5 °C. Cryobiology 2013, 66, 69–75. [Google Scholar] [CrossRef]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M.C. Storage of boar semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef]
- Yeste, M.; Estrada, E.; Rivera del Alamo, M.M.; Bonet, S.; Rigau, T.; Rodriguez-Gil, J.E. The increase in phosphorylation levels of serine residues of protein HSP70 during holding time at 17 °C is concomitant with a higher cryotolerance of boar spermatozoa. PLoS ONE 2014, 9, e90887. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Impact of storage prior to cryopreservation on plasma membrane function and fertility of boar sperm. Theriogenology 2005, 63, 396–410. [Google Scholar] [CrossRef]
- Wasilewska, K.; Zasiadczyk, Ł.; Fraser, L.; Mogielnicka-Brzozowska, M.; Kordan, W. The benefits of cooling boar semen in long-term extenders prior to cryopreservation on sperm quality characteristics. Reprod. Domest. Anim 2016, 51, 781–788. [Google Scholar] [CrossRef]
- Batista, M.; Santana, M.; Alamo, D.; González, F.; Niño, T.; Cabrera, F.; Gracia, A. Effects of incubation temperature and semen pooling on the viability of fresh, chilled and freeze-thawed canine semen samples. Reprod. Domest. Anim. 2012, 47, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.; Batista, M.; Alamo, D.; González, F.; Niño, T.; Cabrera, F.; Gracia, A. Influence of cool storage before freezing on the quality of frozen–thawed semen samples in dogs. Reprod. Domest. Anim. 2013, 48, 165–170. [Google Scholar] [CrossRef]
- Torres, M.A.; Monteiro, M.S.; Passarelli, M.S.; Papa, F.O.; Dell’Aqua, J.A., Jr.; Alvarenga, M.A.; Martins, S.M.; de Andrade, A.F. The ideal holding time for boar semen is 24 h at 17 °C prior to short-cryopreservation protocols. Cryobiology 2019, 86, 58–64. [Google Scholar] [CrossRef]
- Czubaszek, M.; Andraszek, K.; Banaszewska, D.; Walczak-Jędrzejowska, R. The effect of the staining technique on morphological and morphometric parameters of boar sperm. PLoS ONE 2019, 14, e0214243. [Google Scholar] [CrossRef]
- Peña, F.J.; Saravia, F.; García-Herreros, M.; Núñezmartínez, I.; Tapia, J.A.; Johannisson, A.; Wallgren, M.; Rodríguez-Martínez, H. Identification of sperm morphometric subpopulations in two different portions of the boar ejaculate and its relation to postthaw quality. J. Androl. 2005, 26, 716–723. [Google Scholar] [CrossRef]
- García-Herreros, M.; Barón, F.J.; Aparicio, I.M.; Santos, A.J.; García-Marín, L.J.; Gil, M.C. Morphometric changes in boar spermatozoa induced by cryopreservation. Int. J. Androl. 2008, 31, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Talluri, T.R.; Jhamb, D.; Paul, N.; Mehta, S.C.; Singh, J.; Dedar, R.K.; Legha, R.A.; Pal, Y. Cryopreservation and freezability of epididymal and ejaculated stallion spermatozoa. Indian J. Anim. Sci. 2023, 93, 691–696. [Google Scholar] [CrossRef]
- Finelli, R.; Leisegang, K.; Tumallapalli, S.; Henkel, R.; Agarwal, A. The validity and reliability of computer-aided semen analyzers in performing semen analysis: A systematic review. Transl. Androl. Urol. 2021, 10, 3069–3079. [Google Scholar] [CrossRef] [PubMed]
- van der Horst, G.; Maree, L.; du Plessis, S.S. Present perspectives of CASA applications in diverse mammalian spermatozoa. Reprod. Fertil. Dev. 2018, 30, 875–888. [Google Scholar] [CrossRef]
- Yániz, J.L.; Soler, C.; Santolaria, P. Computer assisted sperm morphometry in mammals: A review. Anim. Reprod. Sci. 2015, 156, 1–12. [Google Scholar] [CrossRef]
- van der Horst, G. Status of sperm functionality assessment in wildlife species: From fish to primates. Animals 2021, 11, 1491. [Google Scholar] [CrossRef] [PubMed]
- Thema, M.A.; Sithole, A.; Mphaphathi, M.L.; Ledwaba, M.R.; Sebopela, M.D.; Mkhize, N.R. Improving cryosurvivability of boar epididymal sperm quality using various chicken egg yolk concentration for freezing. Cryobiology 2024, 117, 105079. [Google Scholar] [CrossRef]
- Thema, M.A.; Mphaphathi, M.L.; Ledwaba, M.R.; Nedambale, T.L. Sperm cryopreservation in Windsnyer boars; principles, technique, and updated outcomes. Anim. Reprod. 2023, 20, e20220100. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Roca, J.; Alvarez-Rodriguez, M.; Martinez-Serrano, C.A. How does the boar epididymis regulate the emission of fertile spermatozoa? Anim. Reprod. Sci. 2022, 246, 106829. [Google Scholar] [CrossRef]
- Rath, D.; Niemann, H. In vitro fertilization of porcine oocytes with fresh and frozen-thawed ejaculated or frozen-thawed epididymal semen obtained from identical boars. Theriogenology 1997, 47, 785–793. [Google Scholar] [CrossRef]
- Okazaki, T.; Akiyosh, T.; Kan, M.; Mori, M.; Teshima, H.; Shimada, M. Artificial insemination with seminal plasma improves the reproductive performance of frozen-thawed boar epididymal spermatozoa. J. Androl. 2012, 33, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B. Cryopreservation of Boar Semen: Studies on Sperm Viability In Vitro and Fertility. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2000. No. 91. [Google Scholar]
- Tomás, C.; Gómez-Fernández, J.; Gómez-Izquierdo, E.; de Mercado, E. Effect of the holding time at 15 °C prior to cryopreservation, the thawing rate and the post-thaw incubation temperature on the boar sperm quality after cryopreservation. Anim. Reprod. Sci. 2014, 144, 115–121. [Google Scholar] [CrossRef]
- Hermo, L.; Barin, K.; Oko, R. Developmental expression of immobilin in the rat epididymis. Anat. Rec. 1994, 240, 86–103. [Google Scholar] [CrossRef]
- Monteiro, G.A.; Papa, F.O.; Zahn, F.S.; Dellaqua, J.A., Jr.; Melo, C.M.; Maziero, R.R.D.; Avanzi, B.R.; Alvarenga, M.A.; Guasti, P.N. Cryopreservation and fertility of ejaculated and epididymal stallion sperm. Anim. Reprod. Sci. 2011, 127, 197–2011. [Google Scholar] [CrossRef]
- Patrizio, P. Cryopreservation of epididymal sperm. Mol. Cell. Endocrinol. 2000, 169, 491. [Google Scholar] [CrossRef]
- Ledwaba, M.R.; Mphaphathi, M.L.; Thema, M.A.; Pilane, C.M.; Nedambale, T.L. Investigation of the efficacy of dithiothreitol and glutathione on in vitro fertilization of cryopreserved Large White boar semen. Animals 2022, 12, 1137. [Google Scholar] [CrossRef] [PubMed]
- Prinosilová, P.; Sedlackova, M.; Kopecká, V.; Hlavicová, J. Boar sperm head membrane damage during cryopreservation evaluated by electron microscopy. Res. Pig Breed. 2012, 6, 58–61. [Google Scholar]




| Composition | Fraction A |
|---|---|
| Glucose | 37.0 |
| Sodium citrate | 6.0 |
| Ethylene diaminuteetraacetic acid | 1.25 |
| Sodium bicarbonate | 1.25 |
| Gentamycin | - |
| Penicillin | 1.10 |
| Streptomycin | 1.10 |
| Potassium chloride | 0.75 |
| Holding time | |||||||
|---|---|---|---|---|---|---|---|
| 0 h | 3 h | 6 h | 9 h | 12 h | 24 h | 48 h | |
| Pre-freeze sperm head trait | |||||||
| Length (µm) | 9.1 ± 0.8 a | 9.0 ± 0.5 a | 8.9 ± 0.8 b | 8.8 ± 0.6 b | 8.9 ± 0.5 b | 8.9 ± 0.7 b | 8.7 ± 0.6 c |
| Width (µm) | 4.5 ± 0.5 b | 4.7 ± 0.3 a | 4.7 ± 0.4 a | 4.7 ± 0.3 a | 4.8 ± 0.3 a | 4.6 ± 0.4 b | 4.7 ± 0.3 a |
| Area (µm2) | 36.6 ± 5.7 a | 34.8 ± 3.2 b | 34.1 ± 4.0 b | 33.3 ± 3.3 c | 34.6 ± 2.9 b | 33.0 ± 3.9 c | 33.0 ± 3.6 c |
| Perimeter (µm) | 21.4 ± 2.6 b | 23.7 ± 1.2 a | 23.3 ± 1.8 a | 23.0 ± 1.3 a | 23.5 ± 1.1 a | 23.1 ± 1.7 a | 23.1 ± 1.3 a |
| Pre-freeze sperm midpiece trait | |||||||
| Width (µm) | 1.1 ± 0.3 c | 2.8 ± 1.1 a | 2.7 ± 1.2 a | 2.2 ± 1.1 b | 2.6 ± 1.0 a | 2.4 ± 1.2 b | 2.6 ± 1.3 a |
| Pre-freeze sperm shape indices | |||||||
| Ellipticity | 2.0 ± 0.4 | 1.9 ± 0.1 | 1.9 ± 0.1 | 1.9 ± 0.1 | 1.9 ± 0.1 | 1.9 ± 0.1 | 1.9 ± 0.1 |
| Elongation | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Regularity | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 |
| Roughness | 1.0 ± 0.3 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 |
| Holding time | |||||||
|---|---|---|---|---|---|---|---|
| 0 h | 3 h | 6 h | 9 h | 12 h | 24 h | 48 h | |
| Post-thawed sperm head trait | |||||||
| Length (µm) | 8.4 ± 0.7 c | 9.1 ± 0.9 a | 8.7 ± 0.7 b | 8.7 ± 0.8 b | 8.7 ± 0.4 b | 8.7 ± 0.6 b | 8.8 ± 0.9 b |
| Width (µm) | 4.4 ± 0.4 b | 4.5 ± 0.4 a | 4.6 ± 0.4 a | 4.6 ± 0.5 a | 4.6 ± 0.3 a | 4.5 ± 0.4 a | 4.5 ± 0.7 a |
| Area (µm2) | 29.9 ± 3.3 c | 38.2 ± 6.9 a | 32.3 ± 3.9 b | 32.4 ± 4.2 b | 32.3 ± 2.7 b | 31.7 ± 3.8 b | 32.9 ± 6.2 b |
| Perimeter (µm) | 21.9 ± 1.8 a | 20.1 ± 1.9 b | 22.8 ± 1.8 a | 22.9 ± 1.9 a | 22.9 ± 1.0 a | 22.7 ± 1.6 a | 23.1 ± 2.6 a |
| Post-thawed sperm midpiece trait | |||||||
| Width (µm) | 1.3 ± 0.5 a | 1.0 ± 0.3 a | 1.0 ± 0.4 a | 1.0 ± 0.3 a | 1.0 ± 0.2 a | 1.1 ± 0.4 a | 1.1 ± 0.5 a |
| Post-thawed sperm shape indices | |||||||
| Ellipticity | 2.0 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.8 | 1.9 ± 0.2 | 1.9 ± 0.3 | 2.0 ± 0.6 |
| Elongation | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.1 |
| Regularity | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 |
| Roughness | 1.2 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.0 | 0.8 ± 0.1 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thema, M.A.; Mkhize, N.R.; Sebopela, M.D.; Ledwaba, M.R.; Mphaphathi, M.L. Effect of Pre-Freezing 18 °C Holding Time on Post-Thaw Motility and Morphometry of Cryopreserved Boar Epididymal Sperm. Animals 2025, 15, 1691. https://doi.org/10.3390/ani15121691
Thema MA, Mkhize NR, Sebopela MD, Ledwaba MR, Mphaphathi ML. Effect of Pre-Freezing 18 °C Holding Time on Post-Thaw Motility and Morphometry of Cryopreserved Boar Epididymal Sperm. Animals. 2025; 15(12):1691. https://doi.org/10.3390/ani15121691
Chicago/Turabian StyleThema, Mamonene Angelinah, Ntuthuko Raphael Mkhize, Maleke Dimpho Sebopela, Mahlatsana Ramaesela Ledwaba, and Masindi Lottus Mphaphathi. 2025. "Effect of Pre-Freezing 18 °C Holding Time on Post-Thaw Motility and Morphometry of Cryopreserved Boar Epididymal Sperm" Animals 15, no. 12: 1691. https://doi.org/10.3390/ani15121691
APA StyleThema, M. A., Mkhize, N. R., Sebopela, M. D., Ledwaba, M. R., & Mphaphathi, M. L. (2025). Effect of Pre-Freezing 18 °C Holding Time on Post-Thaw Motility and Morphometry of Cryopreserved Boar Epididymal Sperm. Animals, 15(12), 1691. https://doi.org/10.3390/ani15121691








