Neurobehavioral Comorbidities in Canine Idiopathic Epilepsy: New Insights into Cognitive and Emotional Domains
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Procedures
2.3. Statistical Analysis
3. Results
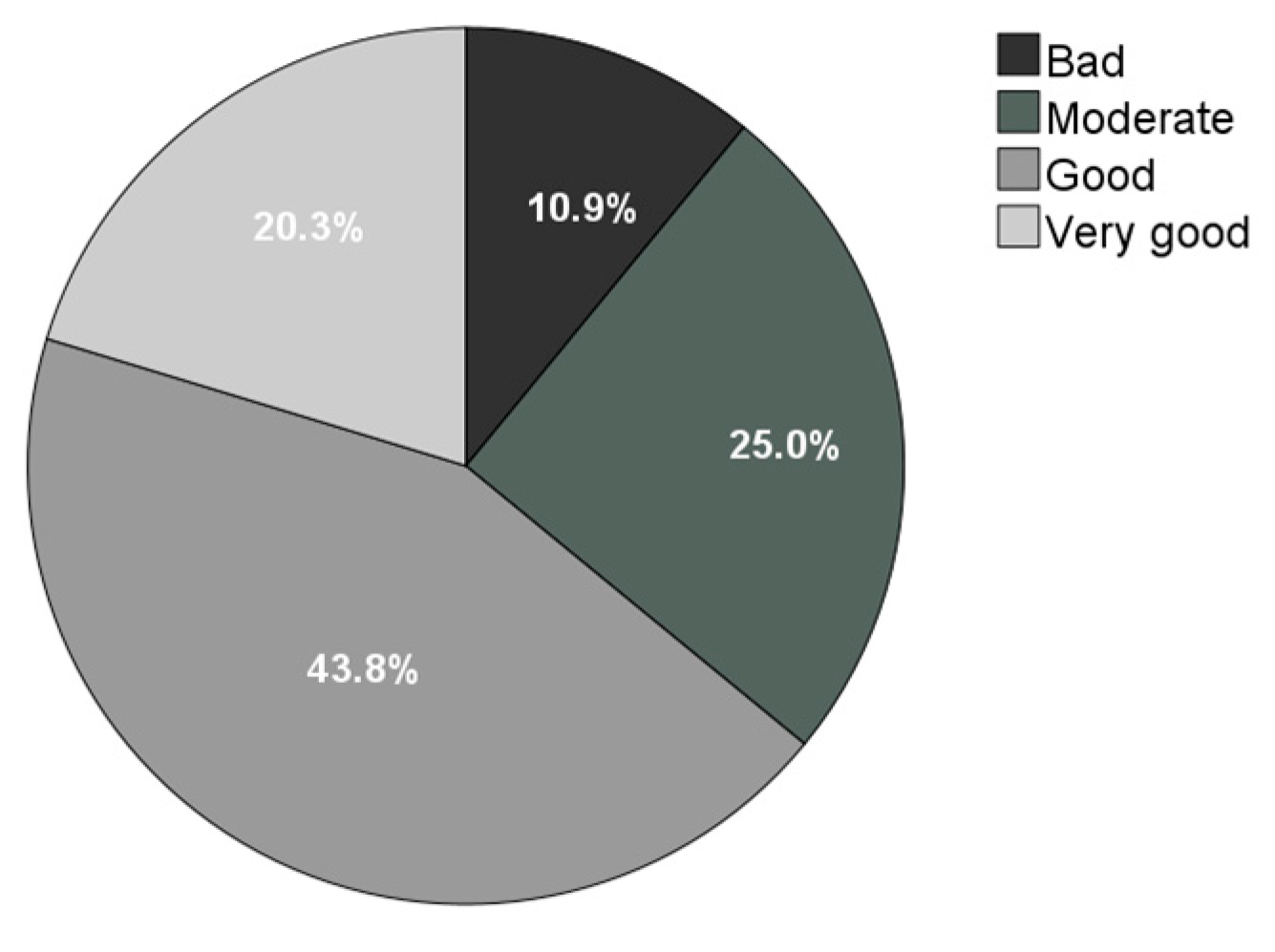
3.1. Demographics and Clinical Data
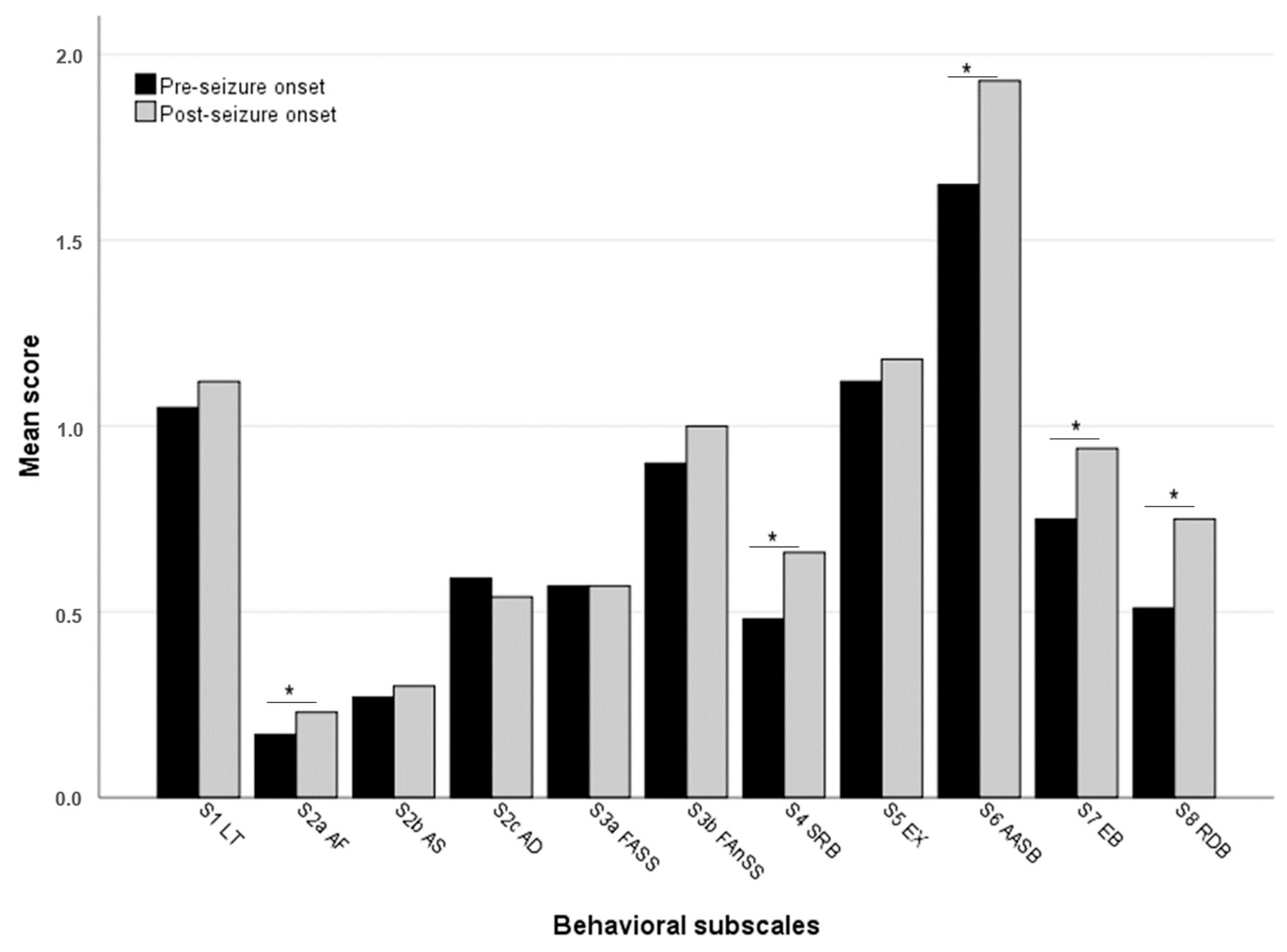
3.2. Changes in Questionnaire Scores After Seizure Onset
3.3. Differences in Questionnaire Scores According to Demographic Variables
3.4. Differences in Questionnaire Scores According to Seizure-Related Variables
3.5. Differences in Questionnaire Scores According to Treatment-Related Variables
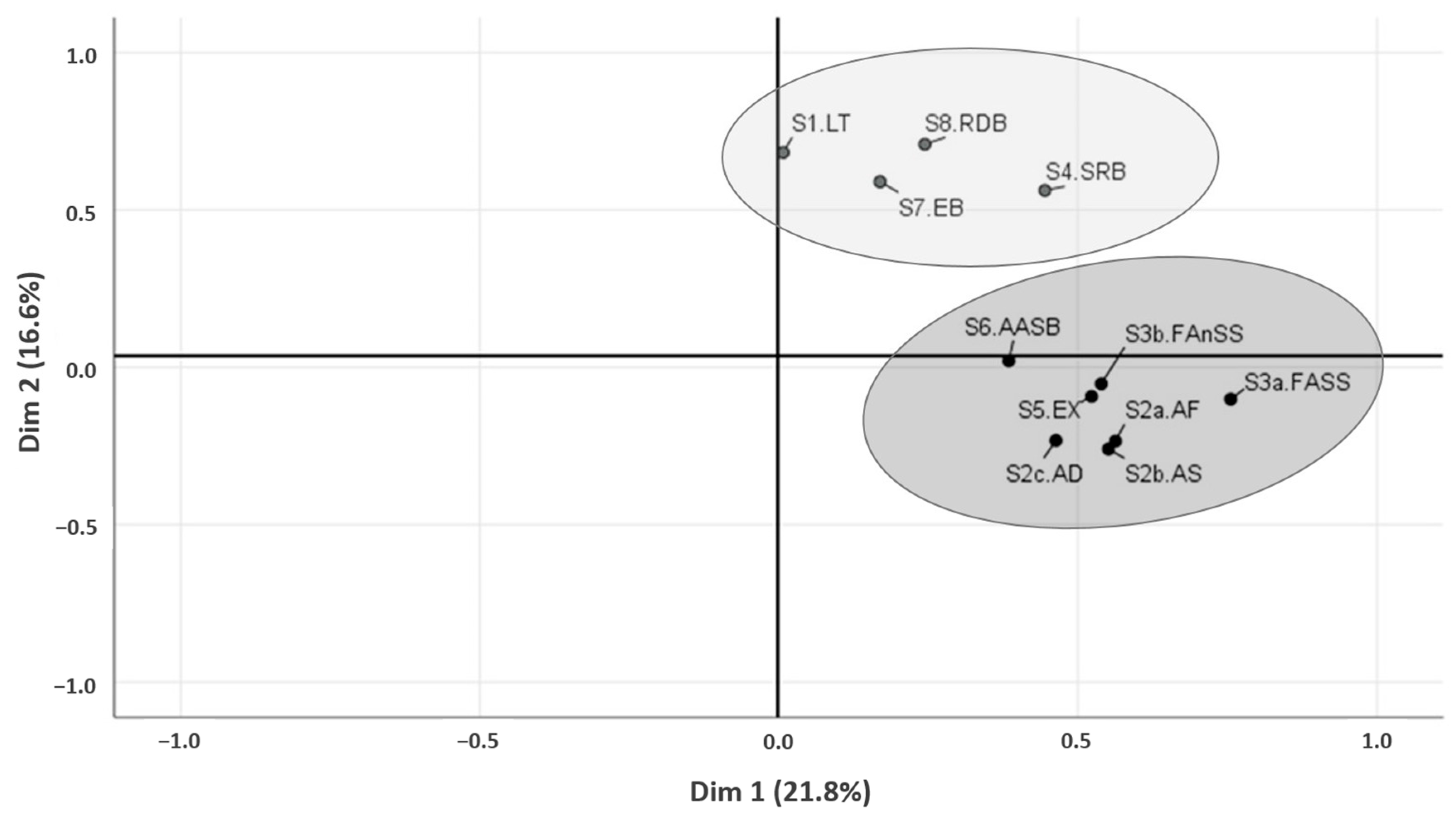
3.6. Principal Component Analysis and Clustering of the Behavior Questionnaire Subscales
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hermann, B.P.; Struck, A.F.; Busch, R.M.; Reyes, A.; Kaestner, E.; McDonald, C.R. Neurobehavioural comorbidities of epilepsy: Towards a network-based precision taxonomy. Nat. Rev. Neurol. 2021, 17, 731–746. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.S.; Rafati, A.; Ottman, R.; Christensen, J.; Kanner, A.M.; Jetté, N.; Newton, C.R. Psychiatric comorbidities in persons with epilepsy compared with persons without epilepsy: A systematic review and meta-analysis. JAMA Neurol. 2025, 82, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Stafstrom, C.E. Epilepsy and its neurobehavioral comorbidities: Insights gained from animal models. Epilepsia 2023, 64, 54–91. [Google Scholar] [CrossRef]
- Peek, S.I.; Meller, S.; Twele, F.; Packer, R.M.A.; Volk, H.A. Epilepsy is more than a simple seizure disorder: Parallels between human and canine cognitive and behavioural comorbidities. Vet. J. 2024, 303, 106060. [Google Scholar] [CrossRef]
- Bonavita, V.; De Simone, R. Towards a definition of comorbidity in the light of clinical complexity. Neurol. Sci. 2008, 29, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.K.; Caplan, R. Behavioral and psychiatric comorbidities in pediatric epilepsy: Toward an integrative model. Epilepsia 2007, 48, 1639–1651. [Google Scholar] [CrossRef]
- Watanangura, A.; Meller, S.; Suchodolski, J.S.; Pilla, R.; Khattab, M.R.; Loderstedt, S.; Becker, L.F.; Bathen-Nöthen, A.; Mazzuoli-Weber, G.; Volk, H.A. The effect of phenobarbital treatment on behavioral comorbidities and on the composition and function of the fecal microbiome in dogs with idiopathic epilepsy. Front. Vet. Sci. 2022, 9, 933905. [Google Scholar] [CrossRef]
- Lu, E.; Pyatka, N.; Burant, C.J.; Sajatovic, M. Systematic literature review of psychiatric comorbidities in adults with epilepsy. J. Clin. Neurol. 2021, 17, 176–186. [Google Scholar] [CrossRef]
- Dunn, D.W.; Besag, F.; Caplan, R.; Aldenkamp, A.; Gobbi, G.; Sillanpää, M. Psychiatric and behavioural disorders in children with epilepsy (ILAE Task Force Report): Anxiety, depression and childhood epilepsy. Epileptic Disord. 2016, 18, 24–30. [Google Scholar] [CrossRef]
- Taylor, J.; Kolamunnage-Dona, R.; Marson, A.G.; Smith, P.E.; Aldenkamp, A.P.; Baker, G.A. Patients with epilepsy: Cognitively compromised before the start of antiepileptic drug treatment? Epilepsia 2010, 51, 48–56. [Google Scholar] [CrossRef]
- Kanner, A.M.; Helmstaedter, C.; Sadat-Hossieny, Z.; Meador, K. Cognitive disorders in epilepsy I: Clinical experience, real-world evidence and recommendations. Seizure 2020, 83, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Shihab, N.; Bowen, J.; Volk, H.A. Behavioral changes in dogs associated with the development of idiopathic epilepsy. Epilepsy Behav. 2011, 21, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Levitin, H.; Hague, D.W.; Ballantyne, K.C.; Selmic, L.E. Behavioral changes in dogs with idiopathic epilepsy compared to other medical populations. Front. Vet. Sci. 2019, 6, 396. [Google Scholar] [CrossRef] [PubMed]
- Watson, F.; Packer, R.M.A.; Rusbridge, C.; Volk, H.A. Behavioural changes in dogs with idiopathic epilepsy. Vet. Rec. 2020, 186, 93. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, T.S.; Metsähonkala, L.; Bergamasco, L.; Viitmaa, R.; Syrjä, P.; Lohi, H.; Snellman, M.; Jeserevics, J.; Cizinauskas, S. Benign familial juvenile epilepsy in Lagotto Romagnolo dogs. J. Vet. Intern. Med. 2007, 21, 464–471. [Google Scholar] [CrossRef]
- Packer, R.M.; Law, T.H.; Davies, E.; Zanghi, B.; Pan, Y.; Volk, H.A. Effects of a ketogenic diet on ADHD-like behavior in dogs with idiopathic epilepsy. Epilepsy Behav. 2016, 55, 62–68. [Google Scholar] [CrossRef]
- Packer, R.M.A.; McGreevy, P.D.; Pergande, A.; Volk, H.A. Negative effects of epilepsy and antiepileptic drugs on the trainability of dogs with naturally occurring idiopathic epilepsy. Appl. Anim. Behav. Sci. 2018, 200, 106–113. [Google Scholar] [CrossRef]
- Winter, J.; Packer, R.M.A.; Volk, H.A. Preliminary assessment of cognitive impairments in canine idiopathic epilepsy. Vet. Rec. 2018, 182, 633. [Google Scholar] [CrossRef]
- Packer, R.M.A.; McGreevy, P.D.; Salvin, H.E.; Valenzuela, M.J.; Chaplin, C.M.; Volk, H.A. Cognitive dysfunction in naturally occurring canine idiopathic epilepsy. PLoS ONE 2018, 13, e0192182. [Google Scholar] [CrossRef]
- Erath, J.R.; Nessler, J.N.; Riese, F.; Hünerfauth, E.; Rohn, K.; Tipold, A. Behavioral changes under levetiracetam treatment in dogs. Front. Vet. Sci. 2020, 7, 169. [Google Scholar] [CrossRef]
- Kolstad, E.; Bjørk, M.; Gilhus, N.E.; Alfstad, K.; Clench-Aas, J.; Lossius, M. Young people with epilepsy have an increased risk of eating disorder and poor quality diet. Epilepsia Open 2017, 3, 40–45. [Google Scholar] [CrossRef]
- Zhou, K.; Yang, H.; Chen, R.; Wang, W.; Qu, Z. Causal relationship among obesity and body fat distribution and epilepsy subtypes. Front. Neurol. 2022, 13, 984824. [Google Scholar] [CrossRef]
- McElroy, S.L.; Guerdjikova, A.I.; Martens, B.; Keck, P.E., Jr.; Pope, H.G.; Hudson, J.I. Role of antiepileptic drugs in the management of eating disorders. CNS Drugs 2009, 23, 139–156. [Google Scholar] [CrossRef]
- Bhatti, S.F.; De Risio, L.; Muñana, K.; Penderis, J.; Stein, V.M.; Tipold, A.; Berendt, M.; Farquhar, R.G.; Fischer, A.; Long, S.; et al. International Veterinary Epilepsy Task Force consensus proposal: Medical treatment of canine epilepsy in Europe. BMC Vet. Res. 2015, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Luño, I.; Muniesa, A.; Palacio, J.; García-Belenguer, S.; Rosado, B. Detection of owner-perceived emotional eating in companion dogs: A regression modelling approach. Vet. Rec. 2021, 189, e63. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, S.L.; Blackwell, E.J.; Wetz, K.E.; Packer, R.M.A. Owner reported management of interictal anxiety behaviours in canine epilepsy. Vet. Rec. 2022, 190, e1321. [Google Scholar] [CrossRef] [PubMed]
- De Risio, L.; Bhatti, S.; Muñana, K.; Penderis, J.; Stein, V.; Tipold, A.; Berendt, M.; Farquhar, R.; Fischer, A.; Long, S.; et al. International Veterinary Epilepsy Task Force consensus proposal: Diagnostic approach to epilepsy in dogs. BMC Vet. Res. 2015, 11, 148. [Google Scholar] [CrossRef]
- Hsu, Y.; Serpell, J.A. Development and validation of a questionnaire for measuring behavior and temperament traits in pet dogs. J. Am. Vet. Med. Assoc. 2003, 223, 1293–1300. [Google Scholar] [CrossRef]
- Watson, F.; Rusbridge, C.; Packer, R.M.A.; Casey, R.A.; Heath, S.; Volk, H.A. A review of treatment options for behavioural manifestations of clinical anxiety as a comorbidity in dogs with idiopathic epilepsy. Vet. J. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Pandolfi, M.; Scaia, M.F.; Fernandez, M.P. Sexual dimorphism in aggression: Sex-specific fighting strategies across species. Front. Behav. Neurosci. 2021, 15, 659615. [Google Scholar] [CrossRef]
- Feltes, E.S.M.; Stull, J.W.; Herron, M.E.; Haug, L.I. Characteristics of intrahousehold interdog aggression and dog and pair factors associated with a poor outcome. J. Am. Vet. Med. Assoc. 2020, 256, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, M.; Houpt, K.A. Signalment factors, comorbidity, and trends in behavior diagnoses in dogs: 1644 cases (1991–2001). J. Am. Vet. Med. Assoc. 2006, 229, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, S.L.; Volk, H.A.; Asher, L.; Daley, M.; Packer, R.M.A. Investigating the potential for seizure prediction in dogs with idiopathic epilepsy: Owner-reported prodromal changes and seizure triggers. Vet. Rec. 2020, 187, 152. [Google Scholar] [CrossRef] [PubMed]
- Tipold, A.; Keefe, T.J.; Löscher, W.; Rundfeldt, C.; de Vries, F. Clinical efficacy and safety of imepitoin in comparison with phenobarbital for the control of idiopathic epilepsy in dogs. J. Vet. Pharmacol. Ther. 2015, 38, 160–168. [Google Scholar] [CrossRef]
- Landsberg, G.; Hunthausen, W.; Ackerman, L. The effects of aging on behavior in senior pets. In Handbook of Behavior Problems of the Dog and Cat, 3rd ed.; Elsevier Saunders: Philadelphia, PA, USA, 2012; pp. 211–235. [Google Scholar]
- Polin, C.; Gellé, T.; Auditeau, E.; Adou, C.; Clément, J.P.; Calvet, B. Repetitive behaviors in Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2023, 96, 483–497. [Google Scholar] [CrossRef]
- Rosado, B.; González-Martínez, A.; Pesini, P.; García-Belenguer, S.; Palacio, J.; Villegas, A.; Suárez, M.L.; Santamarina, G.; Sarasa, M. Effect of age and severity of cognitive dysfunction on spontaneous activity in pet dogs—Part 1: Locomotor and exploratory behaviour. Vet. J. 2012, 194, 189–195. [Google Scholar] [CrossRef]
- Wang, P.; Wang, W.P.; Zhang, S.; Wang, H.X.; Lou, Y.; Fan, Y.H. Impaired spatial learning related with decreased expression of calcium/calmodulin-dependent protein kinase IIα and cAMP-response element binding protein in the pentylenetetrazol-kindled rats. Brain Res. 2008, 1238, 108–117. [Google Scholar] [CrossRef]
- Vasilev, D.S.; Tumanova, N.L.; Kim, K.K.; Lavrentyeva, V.V.; Lukomskaya, N.Y.; Zhuravin, I.A.; Magazanik, L.G.; Zaitsev, A.V. Transient morphological alterations in the hippocampus after pentylenetetrazole-induced seizures in rats. Neurochem. Res. 2018, 43, 1671–1682. [Google Scholar] [CrossRef]
- Power, K.N.; Gramstad, A.; Gilhus, N.E.; Hufthammer, K.O.; Engelsen, B.A. Cognitive function after status epilepticus versus after multiple generalized tonic-clonic seizures. Epilepsy Res. 2018, 140, 39–45. [Google Scholar] [CrossRef]
- Mariajoseph, F.P.; Sagar, P.; Muthusamy, S.; Amukotuwa, S.; Seneviratne, U. Seizure-induced reversible MRI abnormalities in status epilepticus: A systematic review. Seizure 2021, 92, 166–173. [Google Scholar] [CrossRef]
- Bosque Varela, P.; Machegger, L.; Oellerer, A.; Steinbacher, J.; McCoy, M.; Pfaff, J.; Trinka, E.; Kuchukhidze, G. Imaging of status epilepticus: Making the invisible visible. A prospective study on 206 patients. Epilepsy Behav. 2023, 141, 109130. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.S. A psychobiological framework for defining discrete emotions in animals. Appl. Anim. Behav. Sci. 2025, 286, 106595. [Google Scholar] [CrossRef]
- Lindquist, K.A.; Wager, T.D.; Kober, H.; Bliss-Moreau, E.; Barrett, L.F. The brain basis of emotion: A meta-analytic review. Behav. Brain Sci. 2012, 35, 121–143. [Google Scholar] [CrossRef]
- Malezieux, M.; Klein, A.S.; Gogolla, N. Neural circuits for emotion. Annu. Rev. Neurosci. 2023, 46, 211–231. [Google Scholar] [CrossRef]
- Harden, C.L.; Maroof, D.A.; Nikolov, B.; Fowler, K.; Sperling, M.; Liporace, J.; Pennell, P.; Labar, D.; Herzog, A. The effect of seizure severity on quality of life in epilepsy. Epilepsy Behav. 2007, 11, 208–211. [Google Scholar] [CrossRef]
- Van Hezik-Wester, V.; de Groot, S.; Kanters, T.; Versteegh, M.; Wagner, L.; Ardesch, J.; Brouwer, W.; van Exel, J. Burden of illness in people with medically refractory epilepsy who suffer from daily to weekly seizures: 12-month follow-up of participants in the EPISODE study. Front. Neurol. 2022, 13, 1012486. [Google Scholar] [CrossRef]
- Wessmann, A.; Volk, H.A.; Parkin, T.; Ortega, M.; Anderson, T.J. Evaluation of quality of life in dogs with idiopathic epilepsy. J. Vet. Intern. Med. 2014, 28, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.M.; Volk, H.A. Epilepsy beyond seizures: A review of the impact of epilepsy and its comorbidities on health-related quality of life in dogs. Vet. Rec. 2015, 177, 306–315. [Google Scholar] [CrossRef]
- Kähn, C.; Meyerhoff, N.; Meller, S.; Nessler, J.N.; Volk, H.A.; Charalambous, M. The postictal phase in canine idiopathic epilepsy: Semiology, management, and impact on the quality of life from the owners’ perspective. Animals 2023, 14, 103. [Google Scholar] [CrossRef]
- Sajobi, T.T.; Wang, M.; Ferro, M.A.; Brobbey, A.; Goodwin, S.; Speechley, K.N.; Wiebe, S. Multivariate trajectories across multiple domains of health-related quality of life in children with new-onset epilepsy. Epilepsy Behav. 2017, 75, 72–78. [Google Scholar] [CrossRef]
- Packer, R.M.A.; Hobbs, S.L.; Blackwell, E.J. Behavioral interventions as an adjunctive treatment for canine epilepsy: A missing part of the epilepsy management toolkit? Front. Vet. Sci. 2019, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Watanangura, A.; Meller, S.; Farhat, N.; Suchodolski, J.S.; Pilla, R.; Khattab, M.R.; Lopes, B.C.; Bathen-Nöthen, A.; Fischer, A.; Busch-Hahn, K.; et al. Behavioral comorbidities treatment by fecal microbiota transplantation in canine epilepsy: A pilot study of a novel therapeutic approach. Front. Vet. Sci. 2024, 11, 1385469. [Google Scholar] [CrossRef] [PubMed]
- Vas, J.; Topál, J.; Péch, É.; Miklósi, Á. Measuring attention deficit and activity in dogs: A new application and validation of a human ADHD questionnaire. Appl. Anim. Behav. Sci. 2007, 103, 105–117. [Google Scholar] [CrossRef]
- Salvin, H.E.; McGreevy, P.D.; Sachdev, P.S.; Valenzuela, M.J. The canine cognitive dysfunction rating scale (CCDR): A data-driven and ecologically relevant assessment tool. Vet. J. 2011, 188, 331–336. [Google Scholar] [CrossRef]
- Madari, A.; Farbakova, J.; Katina, S.; Smolek, T.; Novak, P.; Weissova, T.; Novak, M.; Zilka, N. Assessment of severity and progression of canine cognitive dysfunction syndrome using the CAnine DEmentia Scale (CADES). Appl. Anim. Behav. Sci. 2015, 171, 138–145. [Google Scholar] [CrossRef]
- Hobbs, S.L.; Law, T.H.; Volk, H.A.; Younis, C.; Casey, R.A.; Packer, R.M.A. Impact of canine epilepsy on judgement and attention biases. Sci. Rep. 2020, 10, 17719. [Google Scholar] [CrossRef]
| Variable | Categories | n (%) |
|---|---|---|
| Age at first seizure | <6 months | 1 (1.4%) |
| 6 months–6 years | 51 (72.9%) | |
| >6 years | 18 (25.7%) | |
| Interictal period length | >6 months | 27 (38.6%) |
| <6 months–>1 month | 18 (25.7%) | |
| <1 month–>1 week | 22 (31.4%) | |
| <1 week | 3 (4.3%) | |
| Seizure type | Generalized (primary or secondary) | 66 (94.3%) |
| Focal | 4 (5.7%) | |
| Cluster/status epilepticus | Yes | 46 (65.7%) |
| No | 24 (34.3%) | |
| Response to treatment | Drug-naïve (untreated epilepsy) | 12 (17.1%) |
| Drug-sensitive epilepsy (DSE) | 29 (41.4%) | |
| Drug-refractory epilepsy (DRE) | 29 (37.5%) |
| Section | Item (Abbreviated Question) | Mean (±SD) Before | Mean (±SD) After | p-Value 1 |
|---|---|---|---|---|
| 1. Learning and trainability | 1.1. When off leash, returns immediately when called | 1.8 (±1.0) | 1.9 (±0.9) | NS |
| 1.2. Obeys the “sit” command immediately | 1.9 (±1.2) | 1.9 (±1.1) | NS | |
| 1.3. Learns new tricks easily | 1.8 (±1.0) | 1.6 (±1.0) | 0.053 | |
| 1.4. Eliminate in inappropriate areas (not alone at home) | 0.6 (±0.9) | 0.8 (±0.9) | 0.065 (trend) | |
| 2. Aggression | 2a. Aggression toward family members | |||
| 2.1. When verbally corrected/punished | 0.2 (±0.6) | 0.2 (±0.6) | NS | |
| 2.2. When toys, bones or others are taken away | 0.3 (±0.5) | 0.3 (±0.5) | NS | |
| 2.3. When approached directly while eating | 0.1 (±0.5) | 0.2 (±0.6) | NS | |
| 2.4. When approached directly while resting | 0.07 (±0.3) | 0.1 (±0.4) | 0.025 | |
| 2b. Aggression toward strangers | ||||
| 2.5. When approached directly by an adult on leash | 0.2 (±0.5) | 0.3 (±0.6) | NS | |
| 2.6. When approached directly by a child on leash | 0.2 (±0.6) | 0.2 (±0.6) | NS | |
| 2.7. When unfamiliar people try to touch/pet | 0.3 (±0.6) | 0.3 (±0.7) | NS | |
| 2.8. Toward unfamiliar people visiting home | 0.3 (±0.6) | 0.4 (±0.6) | NS | |
| 2c. Aggression toward other dogs | ||||
| 2.9. When approached by unfamiliar male dog on leash | 0.7 (±0.9) | 0.6 (±0.85) | NS | |
| 2.10. When approached by unfamiliar female dog on leash | 0.5 (±0.8) | 0.4 (±0.7) | NS | |
| 3. Fear and anxiety | 3a. Fear and anxiety to social stimuli | |||
| 3.1. When approached by unfamiliar adult | 0.6 (±0.9) | 0.5 (±0.8) | NS | |
| 3.2. When approached by unfamiliar child | 0.5 (±0.8) | 0.4 (±0.75) | NS | |
| 3.3. When unfamiliar people try to touch/pet | 0.5 (±0.9) | 0.5 (±0.9) | NS | |
| 3.4. When approached by unfamiliar dog | 0.6 (±09) | 0.6 (±0.8) | NS | |
| 3.5. When groomed/bathed by household member | 0.7 (±0.9) | 0.8 (±0.85) | NS | |
| 3b. Fear and anxiety to non-social stimuli | ||||
| 3.6. In response to sudden/loud noises | 1.0 (±1.0) | 1.1 (±1.0) | NS | |
| 3.7. In response to strange/unfamiliar objects | 0.6 (±0.8) | 0.6 (±0.8) | NS | |
| 3.8. During thunderstorms, fireworks or similar | 1.1 (±1.2) | 1.2 (±1.2) | NS | |
| 4. Separation-related behaviors | 4.1. Restlessness, agitation, pacing | 0.6 (±0.8) | 0.9 (±1.0) | 0.020 |
| 4.2. Whining, barking and/or howling | 0.6 (±0.8) | 0.8 (±1.0) | 0.022 | |
| 4.3. Chewing/scratching at house elements. | 0.3 (±0.6) | 0.4 (±0.8) | NS | |
| 4.4. House-soiling | 0.3 (±0.7) | 0.4 (±0.7) | 0.072 (trend) | |
| 5. Excitability | 5.1. When playing with household members | 1.2 (±0.9) | 1.2 (±0.8) | NS |
| 5.2. When doorbell rings | 1.4 (±1.1) | 1.6 (±1.1) | NS | |
| 5.3. Just before being taken for a walk | 1.4 (±1.0) | 1.5 (±1.0) | NS | |
| 5.4. General excitation (no specific context) | 0.3 (±0.6) | 0.4 (±0.6) | NS | |
| 6. Attachment and attention-seeking behaviors | 6.1. Tends to follow owners at home | 1.7 (±0.9) | 2.1 (±0.9) | 0.008 |
| 6.2. Tends to sit close to/in contact with owners | 1.8 (±0.9) | 2.0 (±0.85) | 0.005 | |
| 6.3. Tends to nudge, nuzzle or paw for attention | 1.5 (±1.0) | 1.7 (±1.0) | 0.027 | |
| 7. Eating behavior | 7.1. Steals food | 0.9 (±0.9) | 1.0 (±1.0) | 0.017 |
| 7.2. Pica | 0.6 (±0.7) | 0.75 (±0.95) | 0.070 (trend) | |
| 7.3. Emotional eating | 0.8 (±0.8) | 1.1 (±1.0) | 0.007 | |
| 7.4. Amount of food intake * | 0.9 (±0.5) | 1.2 (±0.6) | 0.012 | |
| 7.5. Voracity * | 1.1 (±0.7) | 1.3 (±0.8) | 0.007 | |
| 8. Repetitive and demented behaviors | 8.1. Repetitive behaviors (fly snapping, shadow chasing, circling, tail chasing…) | 0.6 (±0.8) | 0.8 (±1.0) | 0.066 (trend) |
| 8.2. Demented behaviors (staring into space, aimless wandering, disorientation…) | 0.4 (±0.75) | 0.7 (±1.0) | 0.002 |
| Section/Item (Abbreviated) | Sex | Mean (±SD) | p Value 1 |
|---|---|---|---|
| S2/2.10. Female dog aggression | Male | 0.2 (±0.5) | 0.001 |
| Female | 0.7 (±0.8) | ||
| S3/3.6. Noise fear/anxiety | Male | 0.9 (±0.9) | 0.041 |
| Female | 1.4 (±1.1) |
| Section/Item (Abbreviated) | Age at First Seizure | Mean (±SD) | p Value 1 |
|---|---|---|---|
| S1/1.2. Obeying to “sit” | ≤6 years | 2.1 (±1.1) | |
| >6 years | 1.4 (±1.1) | 0.027 | |
| S1/1.3. Learning new tricks | ≤6 years | 1.75 (±1.0) | |
| >6 years | 1.0 (±0.9) | 0.005 | |
| S3/3.5. Fear of grooming/bathing | ≤6 years | 0.7 (±0.9) | |
| >6 years | 1.1 (±0.6) | 0.018 | |
| S8/8.1. Repetitive behaviors | ≤6 years | 0.6 (±0.8) | |
| >6 years | 1.3 (±1.0) | 0.005 |
| Section/Item (Abbreviated) | Seizure Frequency | Mean (±SD) | p Value 1 |
|---|---|---|---|
| S3/3.5. Fear of grooming/bathing | >6 months <6 months–>1 month | 0.6 (±0.7) a 0.6 (±0.8) | |
| <1 month–>1 week <1 week | 1.2 (±1.0) b 0.7 (±0.6) | 0.034 | |
| S4/4.1. Restlessness (separation-related) | >6 months <6 months–>1 month | 0.5 (±0.8) a 0.9 (±0.9) | |
| <1 month–>1 week <1 week | 1.4 (±1.0) b 1.0 (±1.0) | 0.017 |
| Section/Item (Abbreviated) | Cluster or SE | Mean (±SD) | p Value 1 |
|---|---|---|---|
| S1/1.3. Learning new tricks | Yes | 1.4 (±1.0) | |
| No | 1.9 (±1.0) | 0.027 | |
| S1/1.4. House-soiling | Yes | 1.0 (±1.0) | |
| No | 0.3 (±0.6) | 0.003 | |
| S8/8.2. Demented behaviors | Yes | 0.9 (±1.1) | |
| No | 0.3 (±0.7) | 0.009 |
| Section/Item (Abbreviated) | Medication Status | Mean (±SD) | p Value 1 |
|---|---|---|---|
| S2/2.2. Resource-related aggression | Drug-naïve | 0.4 (±0.7) | 0.015 |
| Phenobarbital | 0.1 (±0.35) a | ||
| Other ASMs | 0.6 (±0.6) b | ||
| S2/2.4. Resting-related aggression | Drug-naïve | 0.1 (±0.3) | 0.015 |
| Phenobarbital | 0.05 (±0.2) a | ||
| Other ASMs | 0.4 (±0.7) b | ||
| S2/2.5. Adult-directed aggression | Drug-naïve | 0.2 (±0.6) | 0.033 |
| Phenobarbital | 0.2 (±0.5) a | ||
| Other ASMs | 0.5 (±0.6) b | ||
| S6/6.2. Sit in contact with owner | Drug-naïve | 2.6 (±0.7) a | 0.044 |
| Phenobarbital | 1.9 (±0.8) b | ||
| Other ASMs | 2.1 (±1.0) | ||
| S7/7.5. Voracity * | Drug-naïve | 1.6 (±0.8) a | 0.009 |
| Phenobarbital | 1.5 (±0.7) a | ||
| Other ASMs | 0.8 (±0.8) b |
| Section/Item (Abbreviated) | Treatment Response | Mean (±SD) | p Value 1 |
|---|---|---|---|
| S1/1.4. House-soiling | DRE | 1.0 (±1.0) | |
| non-DRE | 0.6 (±0.8) | 0.045 | |
| S4/4.1. Restlessness (separation-related) | DRE | 1.3 (±1.0) | |
| non-DRE | 0.6 (±0.9) | 0.001 | |
| S4/4.4. House-soiling (separation-related) | DRE | 0.7 (±0.9) | |
| non-DRE | 0.3 (±0.5) | 0.052 | |
| S7/7.2. Pica | DRE | 1.1 (±1.1) | |
| non-DRE | 0.5 (±0.8) | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belén, R.; Jorge, P.; Carolina, M.; Sylvia, G.-B. Neurobehavioral Comorbidities in Canine Idiopathic Epilepsy: New Insights into Cognitive and Emotional Domains. Animals 2025, 15, 1592. https://doi.org/10.3390/ani15111592
Belén R, Jorge P, Carolina M, Sylvia G-B. Neurobehavioral Comorbidities in Canine Idiopathic Epilepsy: New Insights into Cognitive and Emotional Domains. Animals. 2025; 15(11):1592. https://doi.org/10.3390/ani15111592
Chicago/Turabian StyleBelén, Rosado, Palacio Jorge, Menchaca Carolina, and García-Belenguer Sylvia. 2025. "Neurobehavioral Comorbidities in Canine Idiopathic Epilepsy: New Insights into Cognitive and Emotional Domains" Animals 15, no. 11: 1592. https://doi.org/10.3390/ani15111592
APA StyleBelén, R., Jorge, P., Carolina, M., & Sylvia, G.-B. (2025). Neurobehavioral Comorbidities in Canine Idiopathic Epilepsy: New Insights into Cognitive and Emotional Domains. Animals, 15(11), 1592. https://doi.org/10.3390/ani15111592






