Dietary Supplementation with Whole-Fat or Defatted Antarctic Krill Powder Improves the Growth Performance, Body Coloration, and Immune Capability of Red–White Koi Carp (Cyprinus carpio var. koi)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Feeding Management
2.2. Experimental Feed
2.3. Sample Collection
2.4. Index Measurement
2.4.1. Growth Index Measurement
2.4.2. Determination of the Chroma Value
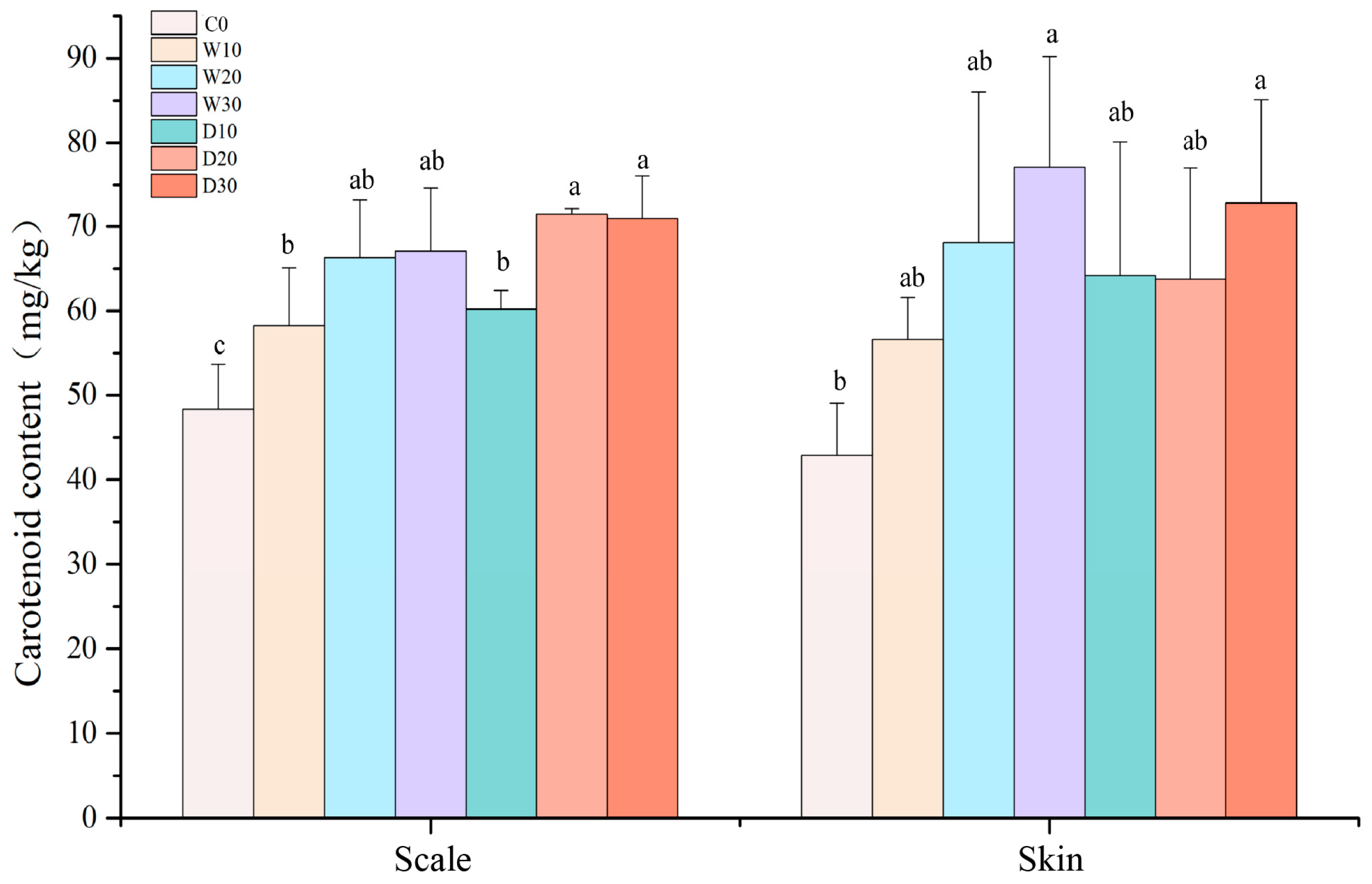
2.4.3. Determination of Carotenoid Content
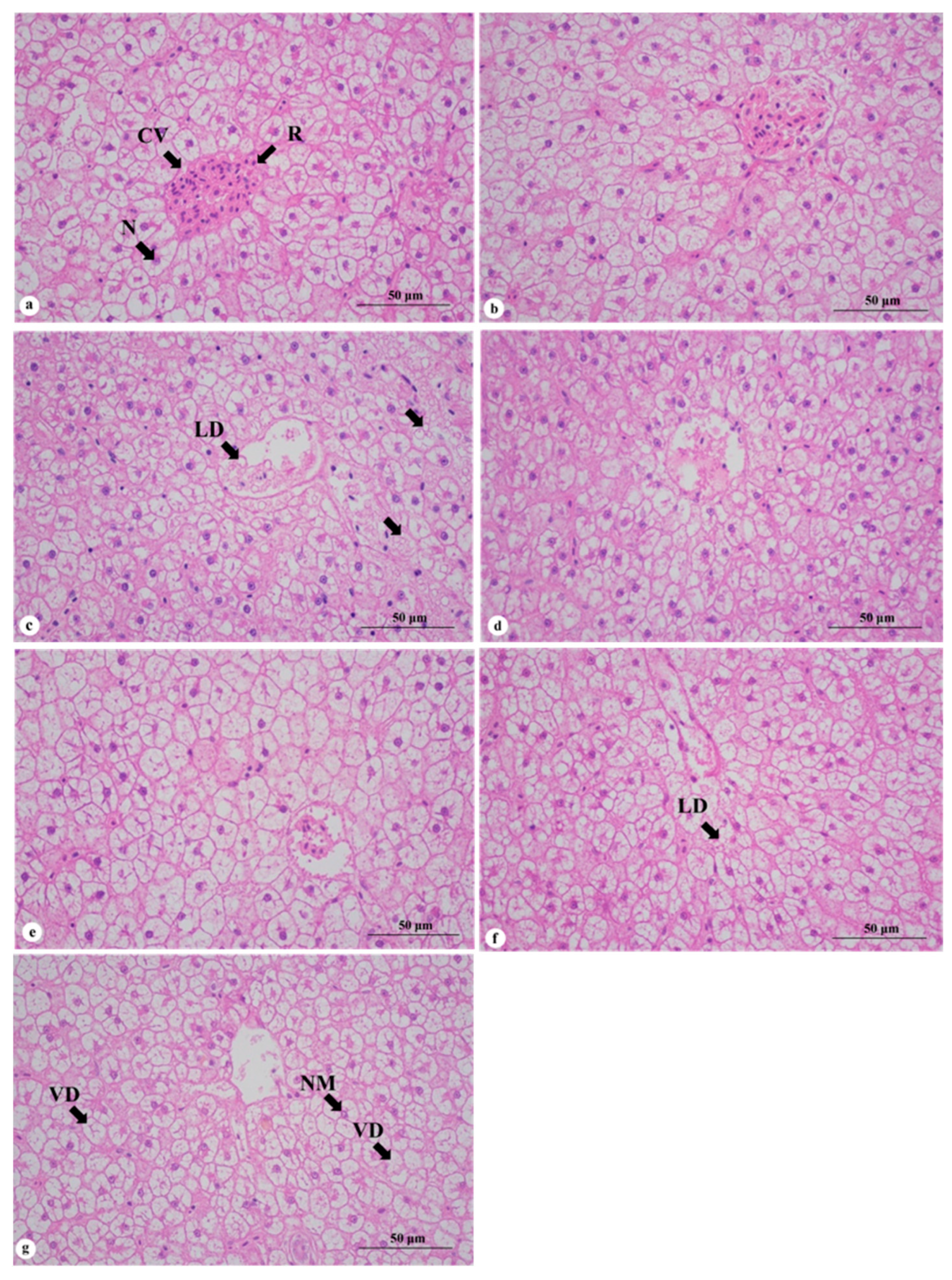
2.4.4. Preparation of Hematoxylin–Eosin (HE)-Stained Tissue Sections
2.4.5. Determination of Liver Biochemical Indexes
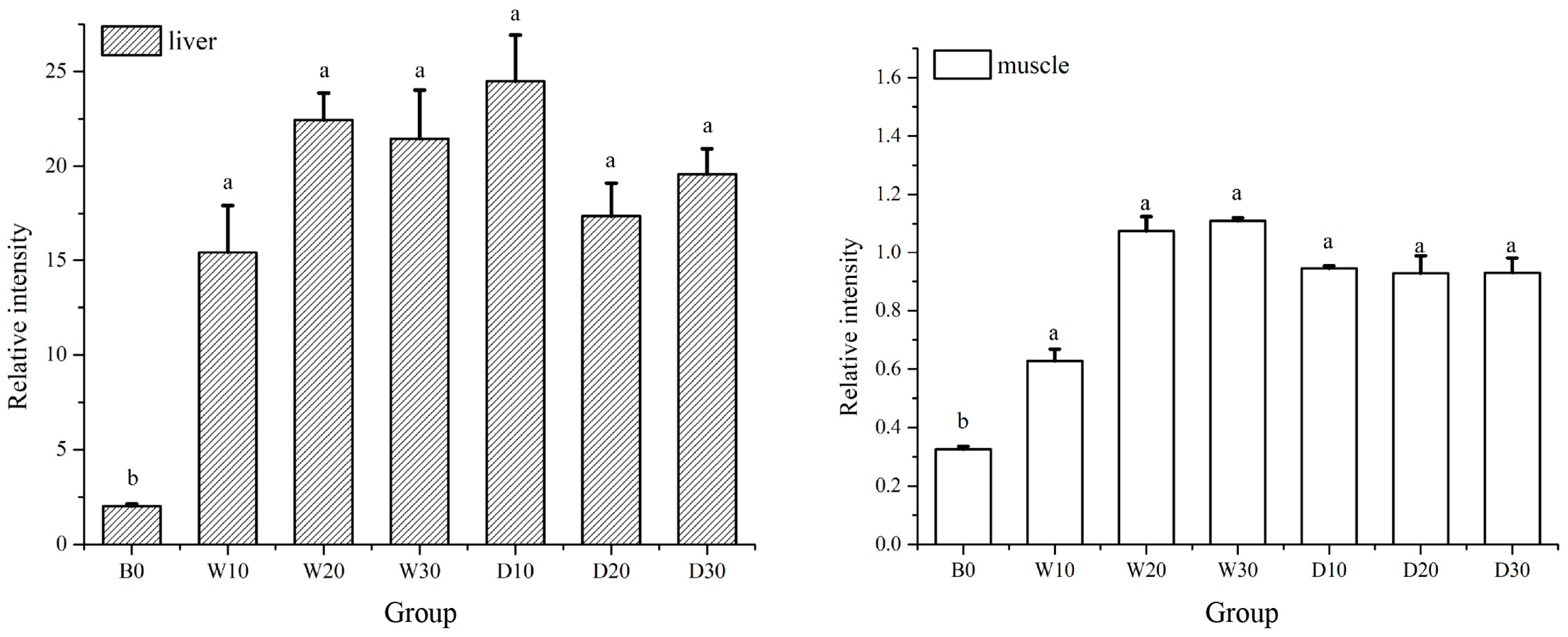
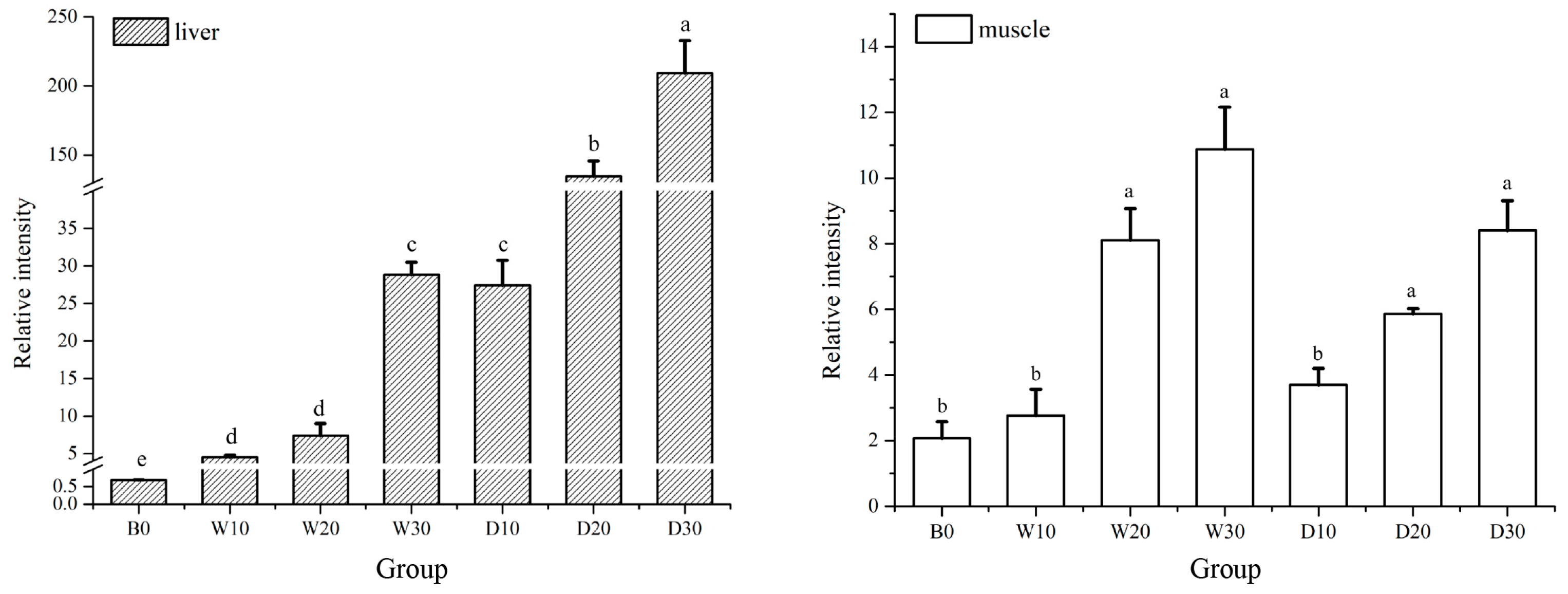
2.4.6. Fluorescence qRT-PCR Assay of IGF-1, LPL, and TYR
2.5. Data Processing and Analysis
3. Results
3.1. Effects of Replacing Fish Meal with Whole-Fat or Defatted Antarctic Krill Meal on the Growth of Red–White Koi Carp
3.2. Effects of Replacing Fish Meal with Whole-Fat or Defatted Antarctic Krill Powder on Body Color of Red–White Koi Carp
3.3. Effects of Replacing Fish Meal with Whole-Fat or Defatted Antarctic Krill Powder on Liver of Red–White Koi Carp
3.4. Effects of Substituting Fish Meal with Whole-Fat or Defatted Antarctic Krill Meal on Non-Specific Immune Indexes of Red–White Koi Carp
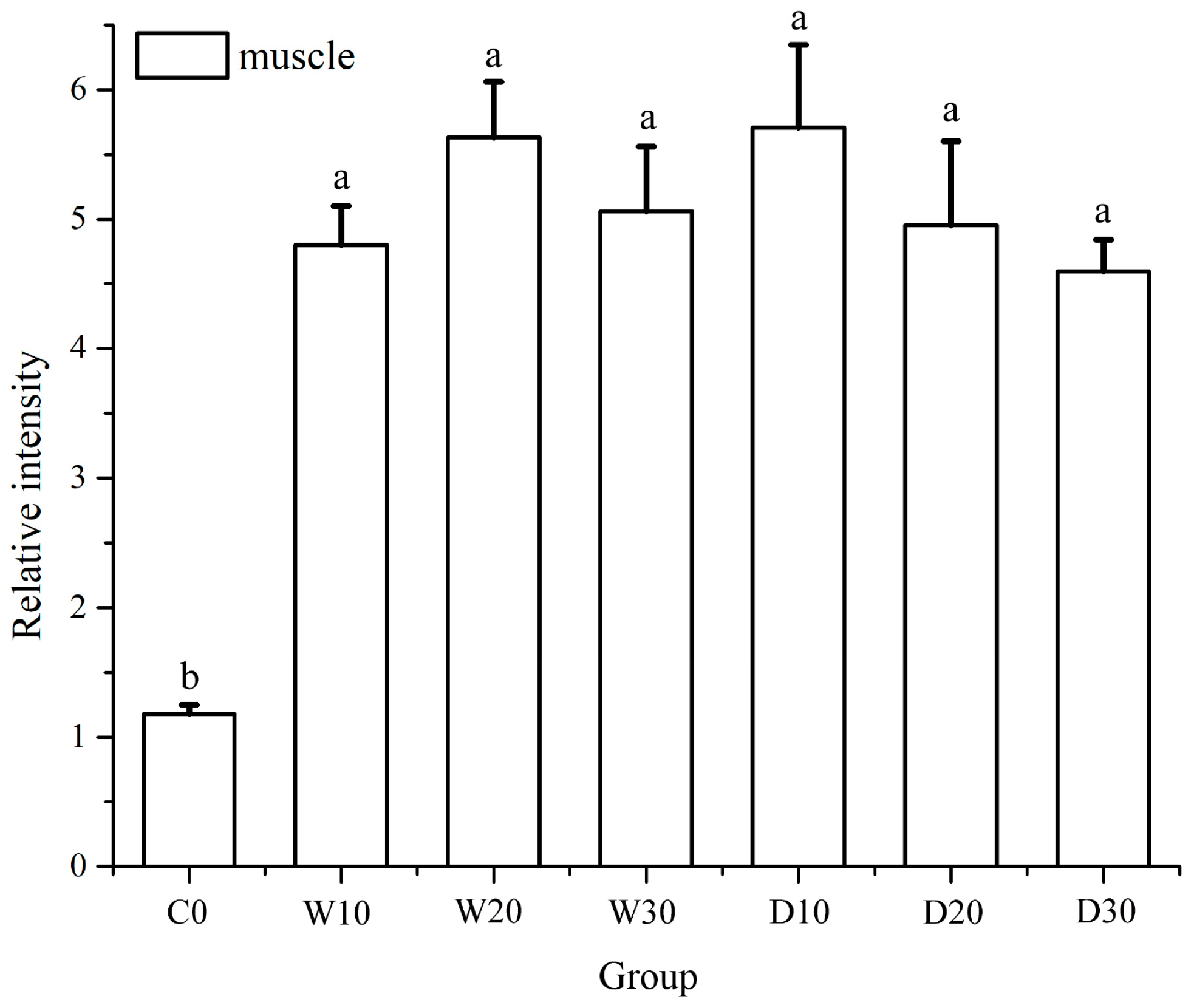
3.5. Effects of Replacing Fish Meal with Whole-Fat or Defatted Antarctic Krill Meal on the Expression Levels of IGF-1, LPL and TYR in the Liver and Muscle Tissue of Red–White Koi Carp
4. Discussion
4.1. Effects of Replacing Fish Meal with Whole-Fat or Defatted Antarctic Krill Powder on Growth Performance of Red–White Koi Carp
4.2. Effect of Replacing Fish Meal with Whole-Fat or Defatted Antarctic Krill Powder on Body Color of Red–White Koi Carp
4.3. Effect of Replacing Fish Meal with Whole-Fat or Defatted Antarctic Krill Meal on Liver of Red–White Koi Carp
4.4. Effect of Substituting Fish Meal with Whole-Fat or Defatted Antarctic Krill Meal on Non-Specific Immune Indexes of Red–White Koi Carp
4.5. Effects of Replacing Fish Meal with Whole-Fat or Defatted Antarctic Krill Powder on the Expression of IGF-1, LPL and TYR in the Liver and Muscle Tissue of Red–White Koi Carp
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, P.; Jiang, Z.Q.; Han, Y.Z.; Zhang, Z.M.; Shi, H.Y.; Yu, L.N. Effects of dietary fat level on body color, growth and some physiological and biochemical indexes of red-white Koi carp. J. Tianjin Agric. Coll. 2011, 18, 23–31. [Google Scholar]
- Micah, A.D.; Wen, B.; Yusuf, A.; Onimisi, M.M.; Adeyemi, S.O.; Gao, J.; Chen, Z. Effects of dietary astaxanthin on chromatic, biochemical, and histological characteristics in juvenile blood parrotfish (Vieja melanurus ♀ × Amphilophus citrinellus ♂). Aquacult Int 2024, 32, 5977–5996. [Google Scholar] [CrossRef]
- Sun, L.J.; Wu, L.Y.; Bai, D.I.; Zhu, G.X. Effect of staxanthin on parrot blood body color, growth and nonspecific immune indexes. J. North. Agric. 2016, 44, 91–95. [Google Scholar]
- Lin, S.J.; Hossain, A.; Shahidi, F. Dietary lipid and astaxanthin contents affect the pigmentation of Arctic charr (Salvelinus alpinus). Food Prod. Process. Nutr. 2024, 6, 77. [Google Scholar] [CrossRef]
- Ai, C.X.; Tao, Q.Y. Fishmeal substitution—Technical countermeasures for the development of aquatic compound feed under high fishmeal prices. Feed Ind. 2013, 34, 1–7. [Google Scholar]
- Zhou, Q.C.; Mai, K.S.; Liu, Y.J.; Tan, B.P. Advances in animal and plant protein sources in place of fish meal. J. Fish. China 2005, 29, 404–410. [Google Scholar] [CrossRef]
- Yoshitomi, B.; Aoki, M.; Oshima, S.; Hata, K. Evaluation of krill (Euphausia superba) meal as a partial replacement for fish meal in rainbow trout (Oncorhynchus mykiss) diets. Aquaculture 2006, 261, 440–446. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhao, L.; Guo, X.H.; Zhang, X.M. Nutritional Characteristics of Antarctic Krill Meal and Its Application in Aquatic Feed. Chin. J. Anim. Nutr. 2021, 33, 6601–6611. [Google Scholar]
- Zhang, Y.L.; Luo, K.; Kong, J.; Liang, M.Q.; Luan, S.; Chen, Q.; Cao, B.X. A comparative study on growth and gonadal development of Litopenaeus vannamei broodstock fed different formulated feeds. J. Fish. Sci. China 2017, 24, 306–316. [Google Scholar] [CrossRef]
- Liu, Z.D.; Chen, X.Z.; Huang, H.L.; Jiang, H.; Feng, C.L.; Gao, L.J.; Huang, Y.Q. Analysis and evaluation of nutrient composition of Antarctic krill powder. J. Chin. Mar. Drugs 2012, 31, 43–48. [Google Scholar] [CrossRef]
- Li, N. Study on Extraction, Properties and Preservation Application of Astaxanthin from Euphausia Superba; Shanghai Ocean University: Shanghai, China, 2021. [Google Scholar]
- Yue, Y. Analysis on the Nutritional Components, Storage Property and Safty Evaluation of Antarctic Krill Meal; Shanghai Ocean University: Shanghai, China, 2013. [Google Scholar]
- Li, X.; Xu, G.; Jiang, G.; Zhou, S. Effects of fish meal replaced by Antarctic krill meal on growth performance, feed utilization and body color of Juvenile Ocellaris Clownfish. J. Qingdao Agric. Univ. (Nat. Sci.) 2019, 36, 213–217. [Google Scholar]
- Wei, J.L. Application Effects of Krill Meal in Feeds for Juvenile Starry Flounder (Platichthys stellatus) and Pearl Gentian Grouper (♀Epinephelus fuscoguttatus×♂ Epinephelus lanceolatu); Shanghai Ocean University: Shanghai, China, 2015. [Google Scholar]
- Kong, F.H.; Liang, M.Q.; Wu, L.; Zheng, K.K.; Wang, X.X.; Chang, Q.; Gong, R.Z. Effects of different levels of Antarctic krill powder on growth, non-specific immunity and fluorine residues in diets of turbot Scophthalmus. Prog. Fish. Sci. 2012, 33, 54–60. [Google Scholar]
- Gaber, M.M.A. The Effect of Different Levels of Krill Meal Supplementation of Soybean-based Diets on Feed Intake, Digestibility, and Chemical Composition of Juvenile Nile Tilapia Oreochromis niloticus, L. J World Aquacult Soc 2010, 36, 346–353. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.J.; Huang, Y.; Shao, Q.J. Effects of defat antarctic krill meal on growing performance and feed utilization of Acanthopagrus schlegelii though replacement fish meal by two plant based proteins. J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2015, 36, 52–57. [Google Scholar] [CrossRef]
- Lee, R.H.C. Aquaculture feed and seafood quality. Bull. Fish. Res. Agency 2010, 31, 43–50. [Google Scholar]
- Wu, G.Q.; Wang, X.J.; Mou, X.D.; Liu, C.; Liu, Y.; Yang, Y.X.; Hu, Y.C.; Song, H.M. Effects of dietary lipid level on astaxanthin absorbtion, growth performance and serum biochemical indices of Vieja synspila♀ × Amphilophus citrinellus ♂. Mar. Fish. 2022, 44, 67–77. [Google Scholar] [CrossRef]
- Yang, H.; Mu, X.; Luo, D.; Hu, Y.; Song, H.; Liu, C.; Luo, J. Sodium taurocholate, a novel effective feed-additive for promoting absorption and pigmentation of astaxanthin in blood parrot (Cichlasoma synspilum ♀×Cichlasoma citrinellum ♂). Aquaculture 2012, 350–353, 42–45. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data using Real-Time Quantitative PCR. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, J.; Chen, S.; Chang, Q.; Wang, Z.; Zhao, J.; Liu, C.; Hu, J. Effects of dietary skimmed krill meal on the body composition and digestive ability of juvenile spotted halibut (Verasper variegatus). Mar. Sci. 2017, 41, 38–45. [Google Scholar]
- Kousoulaki, K.; Ronnestad, I.; Olsen, H.J.; Rathore, R.; Campbell, P.; Nordrum, S.; Berge, R.K.; MjoS, S.A.; Kalananthan, T.; Albrektsen, S. Krill hydrolysate free amino acids responsible for feed intake stimulation in Atlantic salmon (Salmo salar). Aquacult. Nutr. 2013, 19, 47–61. [Google Scholar] [CrossRef]
- He, J.; Wang, P.; Feng, J.; Liu, Y.; Dang, H.; Deng, R. Effects of replacing fish meal with corn gluten meal on the growth, serum biochemical indices and liver histology of large yellow croaker larimichthys crocea. Acta Hydrobiol. Sin. 2017, 41, 506–515. [Google Scholar] [CrossRef]
- Bunea, R.; Farrah, K.E.; Deutsch, L. Evaluation of the effects of Neptune Krill Oil on the clinical course of hyperlipidemia. Altern. Med. Rev. 2004, 9, 420–428. [Google Scholar]
- Nogueira, N.; Canada, P.; Caboz, J.; Andrade, C.; Cordeiro, N. Effect of different levels of synthetic astaxanthin on growth, skin color and lipid metabolism of commercial sized red porgy (Pagrus pagrus). Anim. Feed Sci. Technol. 2021, 276, 114916. [Google Scholar] [CrossRef]
- Karlsen, R.; Suontama, J.; Olsen, R.E. Effect of Antarctic krillmeal on quality of farmed Atlantic cod (Gadus morhua L.). Aquac. Res. 2010, 37, 1676–1684. [Google Scholar] [CrossRef]
- Fujita, T.; Satake, M.; Hikichi, S.; Takeda, M.; Shimeno, S.; Kuwabara, H.; Miki, W.; Yamaguchi, K.; Konosu, S. Pigmentation of Cultured Yellowtail with Krill Oil. Bull. Jpn. Soc. Sci. Fish. 1983, 49, 1595–1600. [Google Scholar] [CrossRef]
- OuYang, J.; Zhao, X.Y.; Ma, T.T. Astaxanthin content, structure and antioxidant activity in the processing of Antarctic krill (Euphausia superba) meal. J. Aquat. Sci. 2022, 46, 420–429. [Google Scholar] [CrossRef]
- Burri, L.; Berge, K.; Chumkam, S.; Jintasataporn, O. Effects of different feed inclusion levels of krill meal on growth and hepatopancreas morphology of Litopenaeus vannamei. Aquac. Res. 2020, 51, 4819–4823. [Google Scholar] [CrossRef]
- Liu, L.Z.; Liu, Y.; Qin, G.C.; Wei, C.; Li, Y.M.; Cui, L.; Tian, X.L. Evaluation of regulatory capacity of three lactic acid bacteria on the growth performance, non-specific immunity, and intestinal microbiota of the sea cucumber Apostichopus japonicus. Aquaculture 2024, 579, 740156. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, S.Q.; Cai, Y.; Shi, H.Z.; Zhou, Y.C.; Zhang, D.D.; Guo, W.L.; Wang, S.F. Effects of Five Prebiotics on Growth, Antioxidant Capacity, Non-Specific Immunity, Stress Resistance, and Disease Resistance of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Animals 2023, 13, 754. [Google Scholar] [CrossRef]
- Wei, Y.T.; Chen, H.; Jia, M.X.; Zhou, H.H.; Zhang, Y.J. Effects of dietary Antarctic krill Euphausia superba meal on growth performance and muscle quality of triploid rainbow trout Oncorhynchus mykiss farmed in sea water. Aquaculture 2019, 509, 72–84. [Google Scholar] [CrossRef]
- Trenzado, C.E.; Morales, A.E.; Palma, J.M.; Higuera, M.D.L. Blood antioxidant defenses and hematological adjustments in crowded/uncrowded rainbow trout (Oncorhynchus mykiss) fed on diets with different levels of antioxidant vitamins and HUFA. Comp. Biochem. Physiol. Part C 2009, 149, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Fattman, C.L.; Schaefer, L.M.; Oury, T.D. Extracellular Superoxide dismutase in biology and medicine. Free Radic. Biol. Med. 2003, 35, 236–256. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.H.; Nguyen, H.T.; Le, B.T.N.; Tran, P.H.; Kim, O.T.P. Characterization of single nucleotide polymorphism in IGF1 and IGF1R genes associated with growth traits in striped catfish (Pangasianodon hypophthalmus Sauvage, 1878). Aquaculture 2021, 538, 736542. [Google Scholar] [CrossRef]
- Weber, G.M. Effects of IGF1 and IGF2 on In Vitro Ovarian Follicle Maturation in Rainbow Trout, Oncorhynchus mykiss. Fishes 2023, 8, 367. [Google Scholar] [CrossRef]
- Zhan, Q.P.; Tian, Y.Y.; Dai, Y.F.; Li, Y.Q.; Li, Y.Y.; Liu, Y.X.; Xue, C.H.; Wang, J.F. Antarctic krill oil promotes longitudinal bone growth in adolescent male mice. Food Biosci. 2019, 28, 170–176. [Google Scholar] [CrossRef]
- Zhang, L.L.; Guo, B.; Liang, M.Q.; Xu, H.G.; Wei, Y.L. Dietary krill hydrolysates affect the expression of growth-related genes in juvenile turbot (Scophthalmus maximus L.). Aquacult. Nutr. 2019, 25, 406–413. [Google Scholar] [CrossRef]
- Chen, Q.L.; Luo, Z.; Pan, Y.X.; Zheng, J.L.; Zhu, Q.L.; Sun, L.D.; Zhuo, M.Q.; Hu, W. Differential induction of enzymes and genes involved in lipid metabolism in liver and visceral adipose tissue of juvenile yellow catfish Pelteobagrus fulvidraco exposed to copper. Aquat. Toxicol. 2013, 136–137, 72–78. [Google Scholar] [CrossRef]
- Yu, H.R.; Wang, J.Q.; Liu, D.W. Effect of Lipid Levels on the Growth Performance and Hepatic Lipid Deposition in the Post-Larval Coho Salmon (Oncorhynchus kisutch). Am. J. Biochem. Biotechnol. 2021, 17, 208–216. [Google Scholar] [CrossRef]
- Liang, X.F.; Li, Y.Q.; Li, G.S.; Bai, J.J.; Lao, H.H.; Hiromi, O.; Oku, H.; Ogata, H.Y. Cis-acting element and in vivo regulation of lipoprotein ligase gene of red sea vream pagrosomus magor. J. Trop. Oceanogr. 2004, 23, 7. [Google Scholar]
- Cheng, H.L.; Sun, S.P.; Peng, Y.X.; Shi, X.Y.; Shen, X.; Meng, X.P.; Dong, Z.G. cDNA sequence and tissues expression analysis of lipoprotein lipase from common carp (Cyprinus carpio Var. Jian). Mol. Biol. Rep. 2010, 37, 2665–2673. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.S.A.J. Molecular characterization of gilthead sea bream (Sparus aurata) lipoprotein lipase. Transcriptional regulation by season and nutritional condition in skeletal muscle and fat storage tissues. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2005, 142, 224–232. [Google Scholar] [CrossRef]
- Albalat, A.; Sánchez-Gurmaches, J.; Gutiérrez, J.; Navarro, I. Regulation of lipoprotein lipase activity in rainbow trout (Oncorhynchus mykiss) tissues. Gen. Comp. Endocr. 2006, 146, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine Omega-3 Phospholipids: Metabolism and Biological Activities. Int. J. Mol. Sci. 2012, 13, 15401–15419. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.A.; Duchateau, G.S.M.J.; Patterson, A.B.; Mitchell, E.S.; van Bruggen, P.; Koek, J.H.; Melville, S.; Verkade, H.J. Age dependent incorporation of 14C-DHA into rat brain and body tissues after dosing various 14C-DHA-esters. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 89–96. [Google Scholar] [CrossRef]
- Singh, A.P.; Nüsslein-Volhard, C. Zebrafish Stripes as a Model for Vertebrate Colour Pattern Formation. Curr. Biol. 2015, 25, 81–92. [Google Scholar] [CrossRef]
- Hidehito, I.; Yoshitaka, B.; Akihiko, K.; Hiroshi, H. Expression of the tyrosinase-encoding gene in a colorless melanophore mutant of the medaka fish, Oryzias latipes. Gene 1994, 150, 319–324. [Google Scholar] [CrossRef]
- Jiang, Y.L. Early Body Color Variation and Cloning, Expression Analysis of TYR Gene in Amphilophus Citrinellus; Shanghai Ocean University: Shanghai, China, 2016. [Google Scholar]
| Ingredient (g/kg) | Group | ||||||
|---|---|---|---|---|---|---|---|
| C0 | W10 | W20 | W30 | D10 | D20 | D30 | |
| Wheat flour | 220.00 | 220.00 | 220.00 | 220.00 | 220.00 | 220.00 | 220.00 |
| Imported fish meal | 500.00 | 400.00 | 300.00 | 200.00 | 400.00 | 300.00 | 200.00 |
| Soy Protein | 110.00 | 110.00 | 110.00 | 110.00 | 110.00 | 110.00 | 110.00 |
| Whole-fat Antarctic krill meal | 0.00 | 100.00 | 200.00 | 300.00 | 0.00 | 0.00 | 0.00 |
| Defatted Antarctic krill meal | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 | 200.00 | 300.00 |
| Yeast | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 |
| Seaweed meal | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Soybean oil | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 |
| Ca (H2PO4)2 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Choline chloride | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Amino acid 1 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Vitamin premix 2 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Mineral premix 3 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| Nutrient levels (%) | |||||||
| Crude protein | 48.19 | 48.09 | 47.99 | 47.69 | 48.60 | 49.00 | 49.20 |
| Crude fat | 8.75 | 8.70 | 8.65 | 8.85 | 8.20 | 7.65 | 7.35 |
| Primers | Primer Sequences (5′→3′) |
|---|---|
| β-actin F | TGCAAAGCCGGATTCGCTGG |
| β-actin R | AGTTGGTGACAATACCGTGC |
| IGF-1 F | GCAGTGTACCATGCGCTGTCT |
| IGF-1 R | GAAATAAAAGCCCCTGTCTCCA |
| TYR F | GCCCGTCCTCGGTGTTCTCC |
| TYR R | GGTTTGGGTCGTGGTTTCCT |
| LPL F | CGATTGARCCARCGAGTG |
| LPL R | CTTGTTGCARCGRTTCTT |
| Group | Item | |||||||
|---|---|---|---|---|---|---|---|---|
| Initial Weight/g | Final Weight/g | SR/% | SGR (%d) | WGR/% | HSI/% | VSI/% | CF (g/cm3) | |
| C0 | 13.87 ± 0.65 a | 61.23 ± 12.46 a | 100.00 ± 0.00 | 2.44 ± 0.24 b | 335.88 ± 62.58 b | 2.78 ± 0.53 a | 8.79 ± 0.85 a | 3.41 ± 0.18 a |
| W10 | 13.63 ± 0.58 a | 65.33 ± 8.80 a | 100.00 ± 0.00 | 2.57 ± 0.27 a | 372.74 ± 74.49 a | 3.08 ± 0.46 a | 9.18 ± 0.44 a | 3.43 ± 0.32 a |
| W20 | 13.98 ± 1.42 a | 75.48 ± 8.17 a | 100.00 ± 0.00 | 2.79 ± 0.18 a | 435.23 ± 55.64 a | 2.23 ± 0.11 d | 8.27 ± 0.74 b | 3.07 ± 0.19 c |
| W30 | 14.03 ± 1.27 a | 74.99 ± 6.57 a | 100.00 ± 0.00 | 2.77 ± 0.23 a | 430.20 ± 73.10 a | 2.41 ± 0.28 c | 5.68 ± 0.35 c | 3.07 ± 0.17 c |
| D10 | 13.04 ± 1.19 a | 70.36 ± 9.64 a | 100.00 ± 0.00 | 2.79 ± 0.18 a | 435.12 ± 58.15 a | 3.18 ± 0.49 a | 6.31 ± 0.68 c | 3.18 ± 0.22 b |
| D20 | 13.11 ± 1.47 a | 66.17 ± 6.46 a | 100.00 ± 0.00 | 2.67 ± 0.23 a | 399.46 ± 66.97 a | 2.85 ± 0.38 a | 5.86 ± 0.50 c | 3.24 ± 0.27 b |
| D30 | 13.50 ± 0.56 a | 79.68 ± 12.50 a | 100.00 ± 0.00 | 2.96 ± 0.04 a | 489.04 ± 14.16 a | 2.68 ± 0.25 b | 6.12 ± 0.64 c | 3.14 ± 0.21 b |
| Group | L* | a* | b* |
|---|---|---|---|
| C0 | 58.46 ± 4.97 | 6.42 ± 1.85 b | 25.10 ± 6.72 c |
| W10 | 58.90 ± 3.40 | 5.49 ± 1.79 b | 25.00 ± 4.87 c |
| W20 | 58.42 ± 3.78 | 7.98 ± 2.61 a | 29.05 ± 6.14 a |
| W30 | 56.45 ± 3.38 | 8.85 ± 2.67 a | 30.96 ± 6.15 a |
| D10 | 58.36 ± 3.28 | 5.94 ± 2.27 b | 24.25 ± 5.58 c |
| D20 | 58.91 ± 4.47 | 5.75 ± 2.45 b | 26.83 ± 5.71 b |
| D30 | 57.95 ± 8.40 | 7.75 ± 2.21 a | 27.50 ± 6.18 b |
| Group | Item | ||
|---|---|---|---|
| AKP (U/mg Protein) | LZM (U/mg Protein) | SOD (U/mg Protein) | |
| C0 | 10.03 ± 2.09 a | 41.07 ± 7.27 d | 1.79 ± 0.56 d |
| W10 | 10.53 ± 1.72 a | 58.89 ± 6.61 c | 8.29 ± 0.13 b |
| W20 | 11.42 ± 2.51 a | 55.13 ± 10.20 c | 4.27 ± 1.70 b |
| W30 | 13.06 ± 2.09 a | 63.91 ± 10.90 c | 2.76 ± 1.03 c |
| D10 | 12.27 ± 2.13 a | 62.05 ± 6.70 c | 31.02 ± 9.52 a |
| D20 | 7.98 ± 1.73 b | 84.78 ± 3.70 b | 11.30 ± 4.50 b |
| D30 | 7.21 ± 2.19 b | 120.83 ± 4.90 a | 9.50 ± 2.99 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Liang, Y.; Yang, Y.; Liu, C.; Liu, Y.; Mu, X.; Wang, X. Dietary Supplementation with Whole-Fat or Defatted Antarctic Krill Powder Improves the Growth Performance, Body Coloration, and Immune Capability of Red–White Koi Carp (Cyprinus carpio var. koi). Animals 2025, 15, 1561. https://doi.org/10.3390/ani15111561
Song H, Liang Y, Yang Y, Liu C, Liu Y, Mu X, Wang X. Dietary Supplementation with Whole-Fat or Defatted Antarctic Krill Powder Improves the Growth Performance, Body Coloration, and Immune Capability of Red–White Koi Carp (Cyprinus carpio var. koi). Animals. 2025; 15(11):1561. https://doi.org/10.3390/ani15111561
Chicago/Turabian StyleSong, Hongmei, Yixin Liang, Yexin Yang, Chao Liu, Yi Liu, Xidong Mu, and Xuejie Wang. 2025. "Dietary Supplementation with Whole-Fat or Defatted Antarctic Krill Powder Improves the Growth Performance, Body Coloration, and Immune Capability of Red–White Koi Carp (Cyprinus carpio var. koi)" Animals 15, no. 11: 1561. https://doi.org/10.3390/ani15111561
APA StyleSong, H., Liang, Y., Yang, Y., Liu, C., Liu, Y., Mu, X., & Wang, X. (2025). Dietary Supplementation with Whole-Fat or Defatted Antarctic Krill Powder Improves the Growth Performance, Body Coloration, and Immune Capability of Red–White Koi Carp (Cyprinus carpio var. koi). Animals, 15(11), 1561. https://doi.org/10.3390/ani15111561





