Ovarian Stromal Cell-Conditioned Media, but Not Co-Culture, Improves Survival in Feline Follicles
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ovarian Stromal Cell Isolation and Culture
2.2. Cryopreservation, Recovery, and Co-Culture of Ovarian Stromal Cells
2.3. Follicle Isolation and Encapsulation
2.4. Co-Culture and CM Treatment
2.5. RNA Isolation, cDNA Production, and qRT-PCR Analysis
2.6. Oocyte Maturation
2.7. Statistical Analysis
3. Results
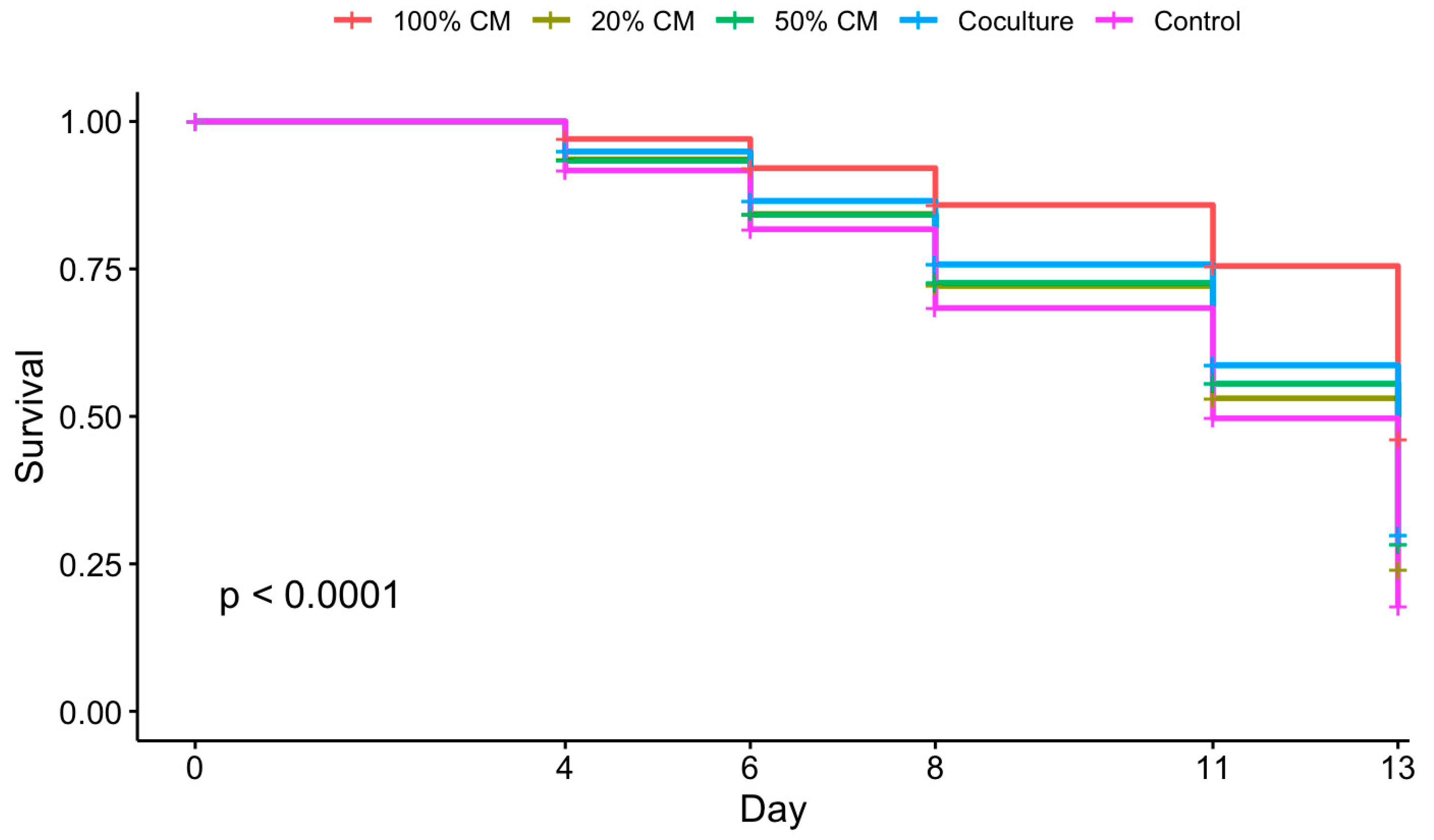
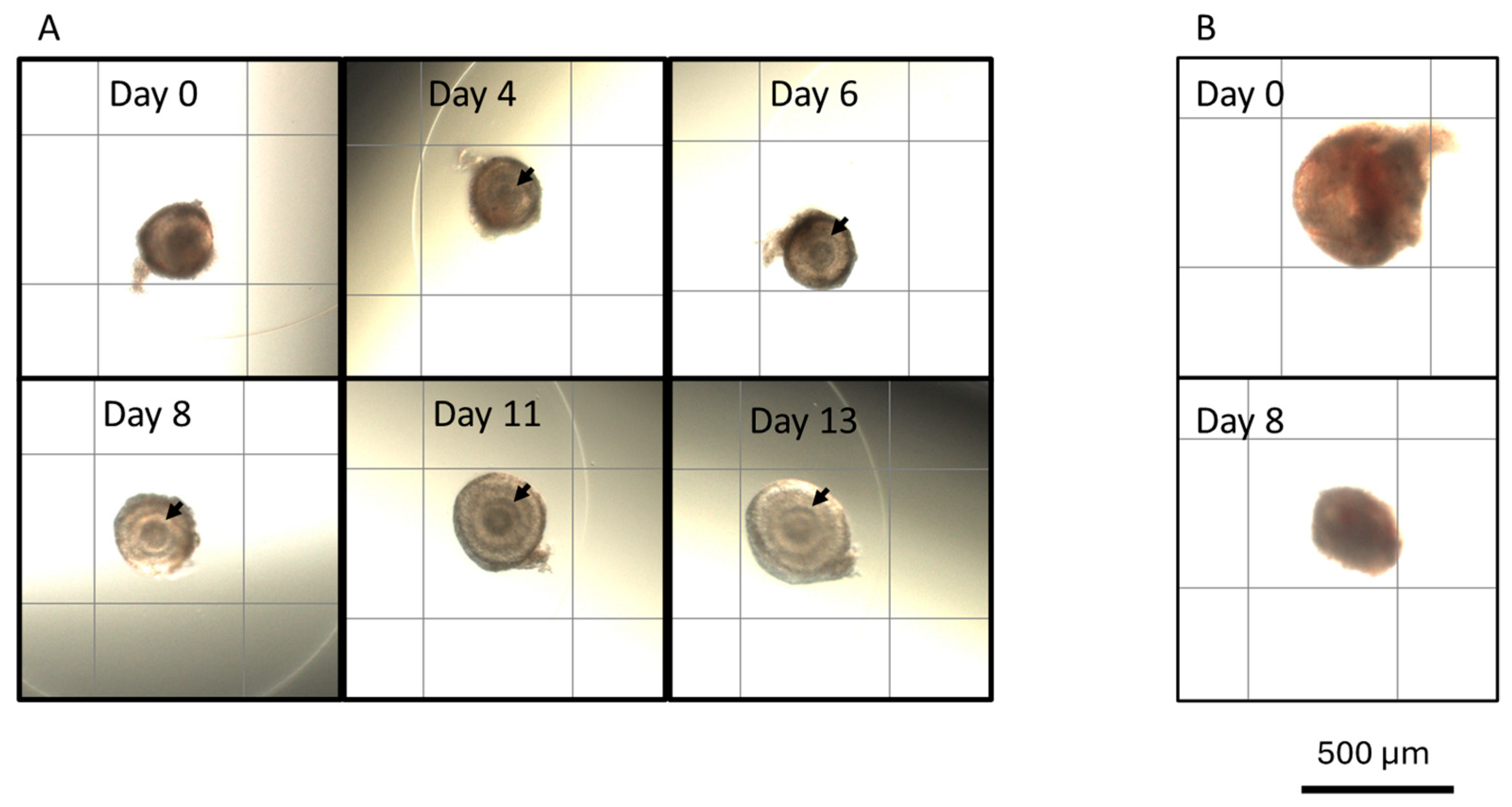
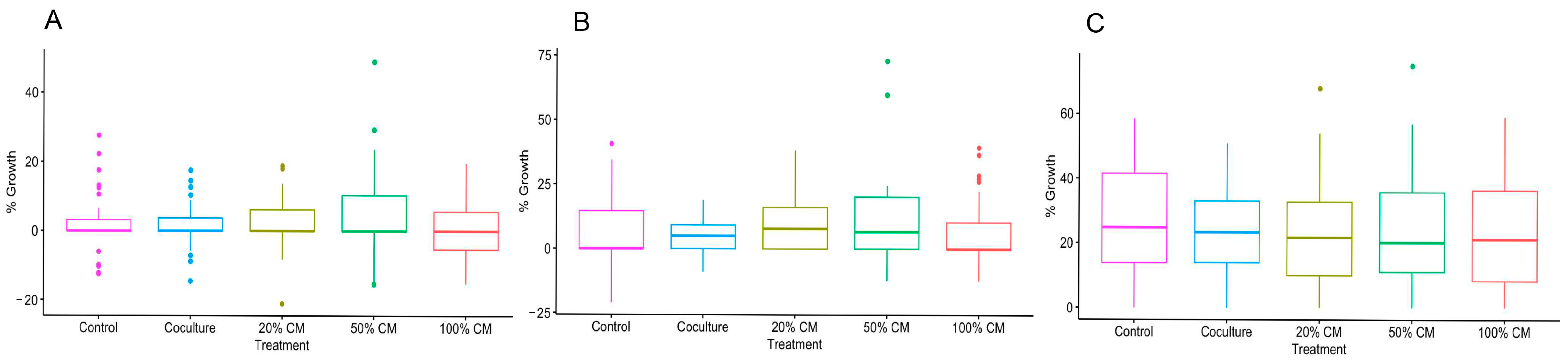
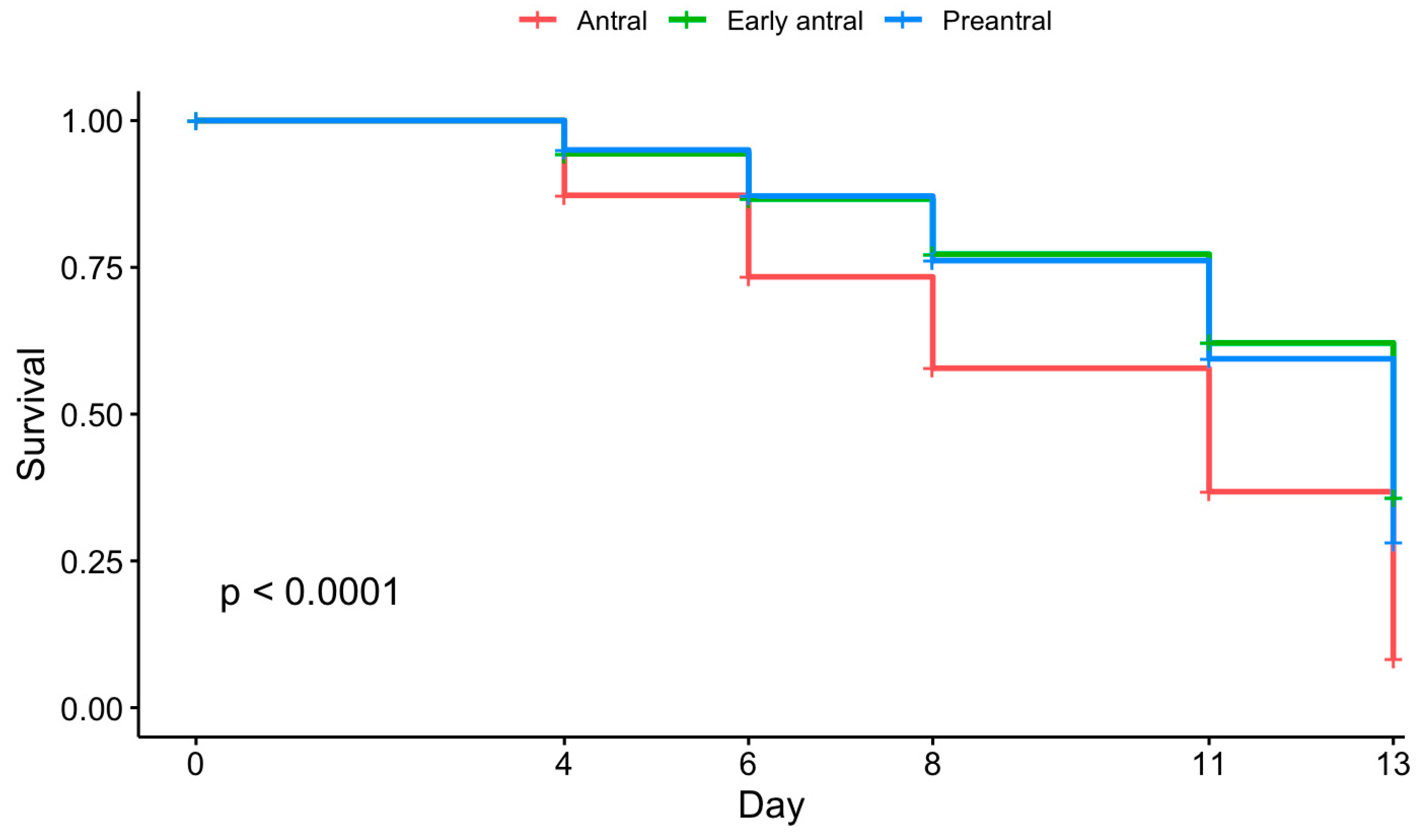
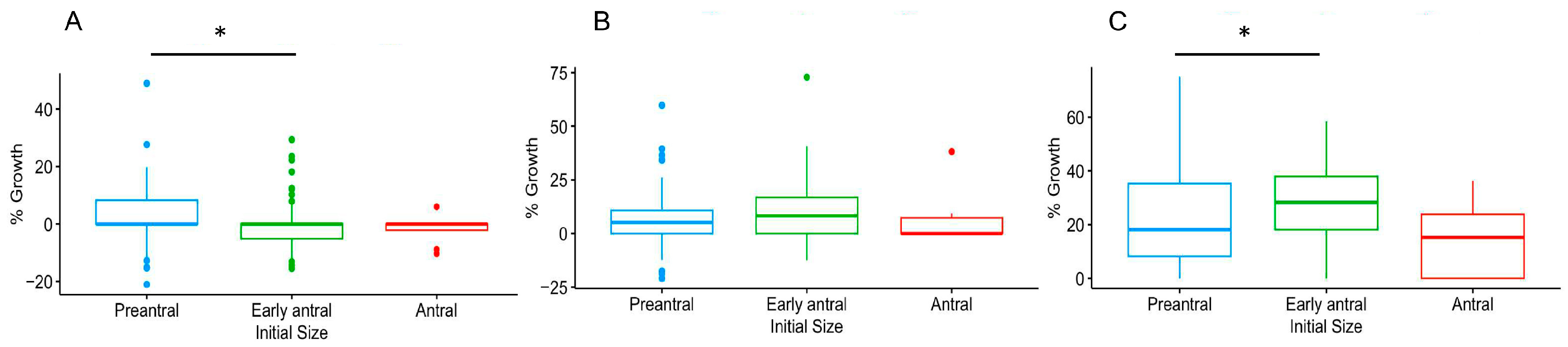
3.1. Culture Conditions and Follicular Stage Effects on Follicle Survival and Growth
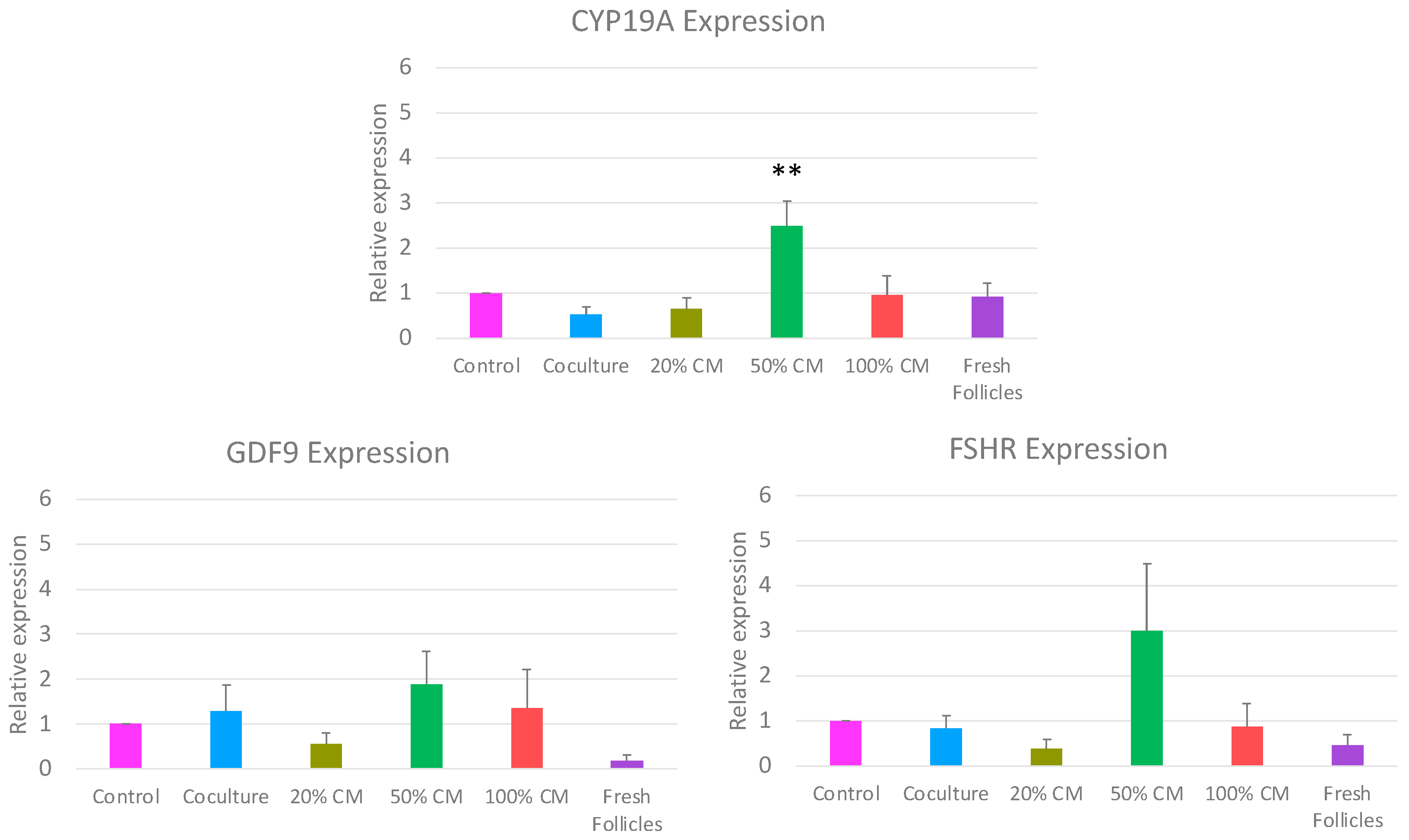
3.2. Impact of In Vitro Culture Conditions on Gene Expression
3.3. Impact of In Vitro Culture Conditions on Oocyte Maturation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senger, P.L. Pathways to Pregnancy & Parturition, 1st ed.; Current Conception, Inc.: Redmond, OR, USA, 1999. [Google Scholar]
- Woodruff, T.K. Preserving fertility during cancer treatment. Nat. Med. 2009, 15, 1124–1125. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.E.; Kumar, T.R.; Duncan, F.E. In vitro ovarian follicle growth: A comprehensive analysis of key protocol variables. Biol. Reprod. 2020, 103, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.F. Research in nondomestic species: Experiences in reproductive physiology research for conservation of endangered felids. ILAR J. 2003, 44, 307–316. [Google Scholar] [CrossRef]
- Songsasen, N.; Thongkittidilok, C.; Yamamizu, K.; Wildt, D.E.; Comizzoli, P. Shrot-term hypertonic exposure enhances in vitro follicle growth and meiotic competence of enclosed oocytes while modestly affecting mRNA expression of aquaporin and stroidogenic genes in the domestic cat model. Theriogenology 2017, 90, 228–236. [Google Scholar] [CrossRef]
- Songsasen, N.; Comizzoli, P.; Nagashima, J.; Fujihara, M.; Wildt, D.E. The domestic dog and cat as models for understanding the regulation of ovarian follicle development in vitro. Reprod. Domest. Anim. 2012, 47 (Suppl. 6), 13–18. [Google Scholar] [CrossRef] [PubMed]
- Green, L.J.; Shikanov, A. In vitro culture methods of preantral follicles. Theriogenology 2016, 86, 229–238. [Google Scholar] [CrossRef]
- Kinnear, H.M.; Tomaszewski, C.E.; Chang, A.L.; Moravek, M.B.; Xu, M.; Padmanabhan, V.; Shikanov, A. The ovarian stroma as a new frontier. Reproduction 2020, 16, R25–R39. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, L.; Tang, W.; Xiong, J.; Chen, D.; Dai, Y.; Wu, C.; Wei, S.; Dai, J.; Wu, M.; et al. Ovarian microenvironment: Challenges and opportunities in protecting against chemotherapy-associated ovarian damage. Hum. Reprod. Update 2024, 30, 614–647. [Google Scholar] [CrossRef]
- Grubliauskaitė, M.; Vlieghe, H.; Moghassemi, S.; Dadashzadeh, A.; Camboni, A.; Gudlevičienė, Z.; Amorim, C.A. Influence of ovarian stromal cells on human ovarian follicle growth in a 3D environment. Hum. Reprod. Open 2024, 2024, hoad052. [Google Scholar] [CrossRef]
- Green, L.J.; Zhou, H.; Padmanabhan, V.; Shikanov, A. adipose-derived stem cells promote survival, growth, and maturation of early-stage murine follicles. Stem Cell Res. Ther. 2019, 10, 102. [Google Scholar] [CrossRef]
- Tingen, C.M.; Kiesewetter, S.E.; Jozefik, J.; Thomas, C.; Tagler, D.; Shea, L.; Woodruff, T.K. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction 2011, 141, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Tagler, D.; Makanji, Y.; Tu, T.; Bernabé, B.P.; Lee, R.; Zhu, J.; Kniazeva, E.; Hornick, J.E.; Woodruff, T.K.; Shea, L.D. Promoting extracellular matrix remodeling via ascorbic acid enhances the survival of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol. Bioeng. 2014, 111, 1417–1429. [Google Scholar] [CrossRef]
- Kedem, A.; Aelion-Brauer, A.; Guo, P.; Wen, D.; Ding, B.-S.; Lis, R.; Cheng, D.; Sandler, V.M.; Rafii, S.; Rosenwaks, Z. Activated ovarian endothelial cells promote early follicular development and survival. J. Ovarian Res. 2017, 10, 64. [Google Scholar] [CrossRef]
- Hong, H.-E.; Kim, O.-H.; Kwak, B.J.; Choi, H.J.; Kim, K.-H.; Ahn, J.; Kim, S.-J. Antioxidant action of hypoxic conditioned media from adipose-derived stem cells in the hepatic injury of expressing higher reactive oxygen species. Ann. Surg. Treat. Res. 2019, 97, 159–167. [Google Scholar] [CrossRef]
- Ra, K.; Park, S.C.; Lee, B.C. Female reproductive aging and oxidative stress: Mesenchymal stem cell conditioned medium as a promising antioxidant. Int. J. Mol. Sci. 2023, 24, 5053. [Google Scholar] [CrossRef] [PubMed]
- Taghizabet, N.; Bahmanpour, S.; Zarei-fard, N.; Mohseni, G.; Aliakbari, F.; Dehghani, F. Effect of endometrial cell-conditioned medium and platelet-rich plasma on the developmental competence of mouse preantral follicles: An in vitro study. Clin. Exp. Reprod. Med. 2022, 49, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Yan, L.; Xin, Z.; Hao, J.; Liu, W.; Wang, S.; Liao, S.; Wang, H.; Yang, X. Protective effects of human umbilical cord mesenchymal stem cell-derived conditioned medium on ovarian damage. J. Mol. Cell Biol. 2020, 12, 372–385. [Google Scholar] [CrossRef]
- Soares, M.; Sahrari, K.; Chiti, M.C.; Amorim, C.A.; Ambroise, J.; Donnez, J.; Dolmans, M.-M. The best source of isolated stromal cells for the artificial ovary: Medulla or cortex, cryopreserved or fresh? Hum. Reprod. 2015, 30, 1589–1598. [Google Scholar] [CrossRef]
- Xu, M.; West-Farrell, E.R.; Stouffer, R.L.; Shea, L.D.; Woodruff, T.K.; Zelinski, M.B. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol. Reprod. 2009, 81, 587–594. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Chansaenroj, A.; Songsasen, N.; Chatdarong, K. Equine Chorionic Gonadotropin induces in vitro follicular growth from the multi-layered secondary developmental stage in cats. Theriogenology 2019, 123, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Thongkittidilok, C.; Singh, R.P.; Comizzoli, P.; Wildt, D.; Songsasen, N. Insulin promotes preantral follicle growth and antrum formation through temporal expression of genes regulating steroidogenesis and water transport in the cat. Reprod. Fertil. Dev. 2018, 30, 1369. [Google Scholar] [CrossRef]
- Barrett, S.L.; Albertini, D.F. Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol. Reprod. 2007, 76, 949–957. [Google Scholar] [CrossRef]
- Van Blerkom, J.; Runner, M.N. Mitochondrial reorganization during resumption of arrested meiosis in the mouse oocyte. Am. J. Anat. 1984, 171, 335–355. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Therneau, T.; Grambsch, P. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar]
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using “Ggplot2” 2021. Available online: https://cran.r-project.org/web/packages/survminer/survminer.pdf (accessed on 27 March 2024).
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means 2023. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 27 March 2024).
- Topman, G.; Sharabani-Yosef, O.; Gefen, A. A method for quick, low-cost automated confluency measurements. Microsc. Microanal. 2011, 17, 915–922. [Google Scholar] [CrossRef]
- Park, A.; Oh, H.J.; Ji, K.; Choi, E.M.; Kim, D.; Kim, E.; Kim, M.K. Effect of passage number of conditioned medium collected from equine amniotic fluid mesenchymal stem cells: Porcine oocyte maturation and embryo development. Int. J. Mol. Sci. 2022, 23, 6569. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- de Groot, M.; Schuurs, T.A.; Leuvenink, H.G.; van Schilfgaarde, R. Macrophage overgrowth Affects neighboring nonovergrown encapsulated islets. J. Surg. Res. 2003, 115, 235–241. [Google Scholar] [CrossRef]
- Harris, S.E.; Adriaens, I.; Leese, H.J.; Gosden, R.G.; Picton, H.M. Carbohydrate metabolism by murine ovarian follicles and oocytes grown in vitro. Reproduction 2007, 134, 415–424. [Google Scholar] [CrossRef]
- Herrick, J.R.; Rajput, S.; Pasquariello, R.; Ermisch, A.; Santiquet, N.; Schoolcraft, W.B.; Krisher, R.L. Developmental and molecular response of bovine embryos to reduced nutrients in vitro. Reprod. Fertil. 2020, 1, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Krisher, R.L.; Heuberger, A.L.; Paczkowski, M.; Stevens, J.; Pospisil, C.; Prather, R.S.; Sturmey, R.G.; Herrick, J.R.; Schoolcraft, W.B. Applying metabolomic analyses to the practice of embryology: Physiology, development and assisted reproductive technology. Reprod. Fertil. Dev. 2015, 27, 602. [Google Scholar] [CrossRef] [PubMed]
- Ermisch, A.F.; Herrick, J.R.; Pasquariello, R.; Dyer, M.C.; Lyons, S.M.; Broeckling, C.D.; Rajput, S.K.; Schoolcraft, W.B.; Krisher, R.L. A novel culture medium with reduced nutrient concentrations supports the development and viability of mouse embryos. Sci. Rep. 2020, 10, 9263. [Google Scholar] [CrossRef] [PubMed]
- Santos, É.C.D.; Fonseca Junior, A.M.D.; Lima, C.B.D.; Ispada, J.; Silva, J.V.A.D.; Milazzotto, M.P. Less is more: Reduced nutrient concentration during in vitro culture improves embryo production rates and morphophysiology of bovine embryos. Theriogenology 2021, 173, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, K.; Okuno, K.; Kuroda, R.; Shanas, N.; Cicalese, S.M.; Eguchi, K.; Elliott, K.J.; Kawai, T.; Corbett, C.B.; Peluzzo, A.M.; et al. Glucose consumption of vascular cell types in culture: Toward optimization of experimental conditions. Am. J. Physiol.-Cell Physiol. 2022, 322, C73–C85. [Google Scholar] [CrossRef]
- Choi, J.K.; Agarwal, P.; He, X. In vitro culture of early secondary preantral follicles in hanging drop of ovarian cell-conditioned medium to obtain MII oocytes from outbred deer mice. Tissue Eng. Part A 2013, 19, 2626–2637. [Google Scholar] [CrossRef]
- Vanacker, J.; Amorim, C.A. Alginate: A Versatile Biomaterial to encapsulate isolated ovarian follicles. Ann. Biomed. Eng. 2017, 45, 1633–1649. [Google Scholar] [CrossRef]
- Shikanov, A.; Xu, M.; Woodruff, T.K.; Shea, L.D. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials 2009, 30, 5476–5485. [Google Scholar] [CrossRef]
- Johnston, L.A.; O’Brien, S.J.; Wildt, D.E. In vitro maturation and fertilization of domestic cat follicular oocytes. Gamete Res. 1989, 24, 343–356. [Google Scholar] [CrossRef]
- Uchikura, K.; Nagano, M.; Hishinuma, M. The effect of ovarian status and follicular diameter on maturational ability of domestic cat oocytes. J. Vet. Med. Sci. 2011, 73, 561–566. [Google Scholar] [CrossRef]
- Chen, S.A.; Besman, M.J.; Sparkes, R.S.; Zollman, S.; Klisak, I.; Mohandas, T.; Hall, P.F.; Shively, J.E. Human aromatase: cDNA cloning, southern blot analysis, and assignment of the gene to chromosome 15. DNA (Mary Ann Liebert Inc.) 1988, 7, 27–38. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Pant, D.; Bischoff, S.; Gavin, W.; Melican, D.; Keefer, C. Expression of pluripotency-determining factors Oct-4 and NANOG in pre-implantation goat embryos. Reprod. Fertil. Dev. 2004, 17, 203. [Google Scholar] [CrossRef]
| Gene ID | Forward Sequence (5′ to 3′) | Melting Temperature | Reverse Sequence (5′ to 3′) | Product Length (nt) | Accession Number | Source |
|---|---|---|---|---|---|---|
| ACTG | ATC CAC GAG ACC ACC TTC | 54 °C | CAC CGT GTT AGC GTA GAG | 75 | XM_023244304.2 | This study |
| FSHR | GGA TCT TTG CTT TCA TGG TC | 51.6 °C | AAC ATA GAG CTG TGA CAA GG | 113 | NM_001048014.1 | This study |
| GDF9 | CAT CCG TGG ACC TGC TAT TT | 54.8 °C | CCA GGT TGC ACA CAC ATT TC | 129 | NM_001165900.1 | [23] |
| CYP19A | CAA TCC TGC TGC TCA CTG | 53.6 °C | CCA TGC AAT AGC CAG GAC | 84 | GU306147.1 | [24] |
| Treatment | Total Oocytes | GV (%) | GVBD (%) | MII (%) | D (%) |
|---|---|---|---|---|---|
| Control | 43 | 9.3 | 46.5 | 0 | 41.9 |
| Co-culture | 46 | 6.5 | 47.8 | 0 | 39.1 |
| 100% CM | 43 | 14 | 53.5 | 0 | 25.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marks, B.; Nagashima, J.B.; Keefer, C.L.; Songsasen, N. Ovarian Stromal Cell-Conditioned Media, but Not Co-Culture, Improves Survival in Feline Follicles. Animals 2025, 15, 1539. https://doi.org/10.3390/ani15111539
Marks B, Nagashima JB, Keefer CL, Songsasen N. Ovarian Stromal Cell-Conditioned Media, but Not Co-Culture, Improves Survival in Feline Follicles. Animals. 2025; 15(11):1539. https://doi.org/10.3390/ani15111539
Chicago/Turabian StyleMarks, Batsheva, Jennifer Beth Nagashima, Carol L. Keefer, and Nucharin Songsasen. 2025. "Ovarian Stromal Cell-Conditioned Media, but Not Co-Culture, Improves Survival in Feline Follicles" Animals 15, no. 11: 1539. https://doi.org/10.3390/ani15111539
APA StyleMarks, B., Nagashima, J. B., Keefer, C. L., & Songsasen, N. (2025). Ovarian Stromal Cell-Conditioned Media, but Not Co-Culture, Improves Survival in Feline Follicles. Animals, 15(11), 1539. https://doi.org/10.3390/ani15111539






