Seasonal Dynamics of Red Imported Fire Ant (Solenopsis invicta) Colony Structures Across Camellia oleifera Plantations and Fishponds in South China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
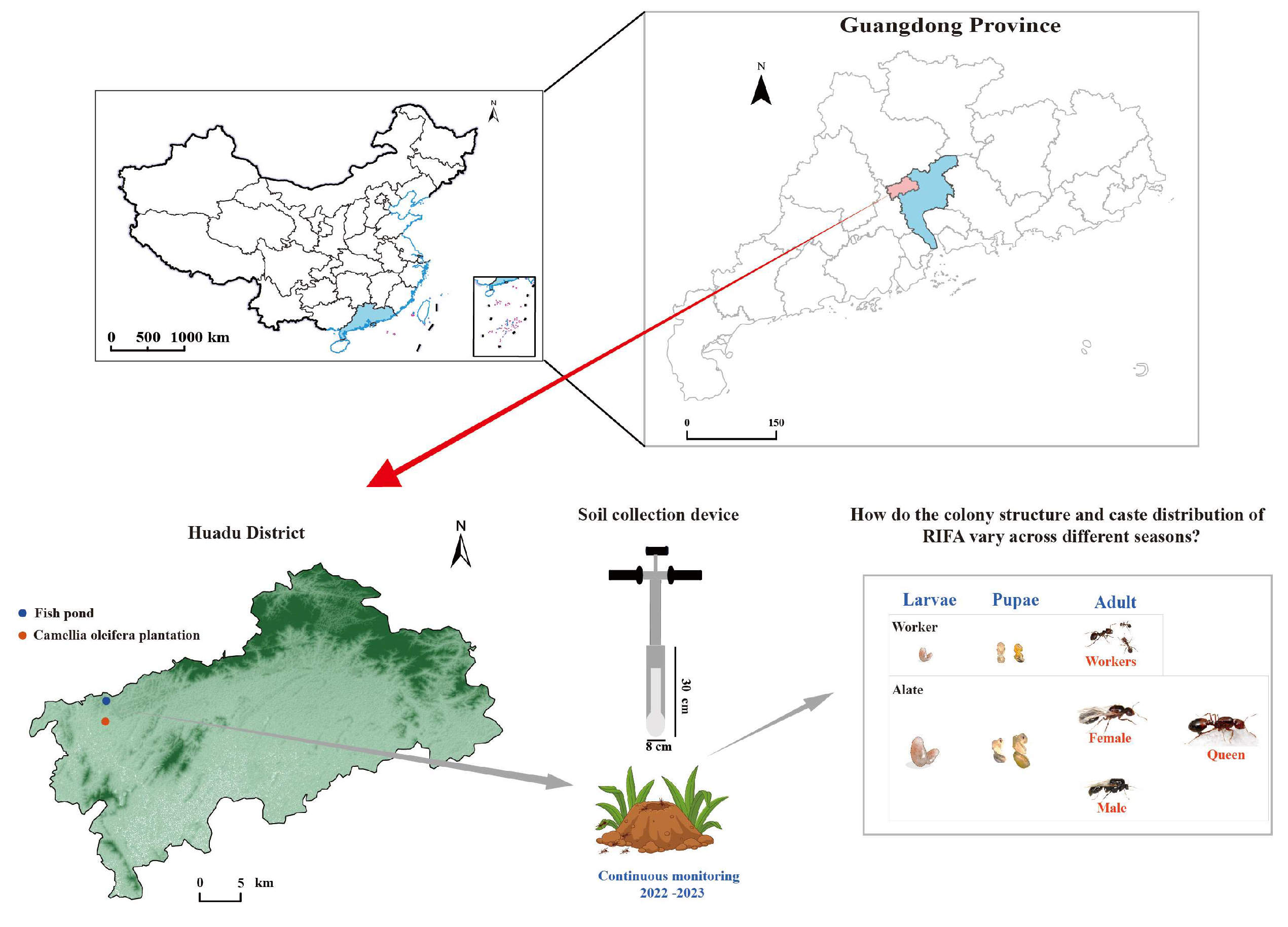
2.1. Study Area and Habitats
2.2. Sampling and Measurement
2.3. Colony Separation, Caste Identification, and Biomass Measurement
2.4. Source of Meteorological Data
2.5. Statistical Analysis
3. Results
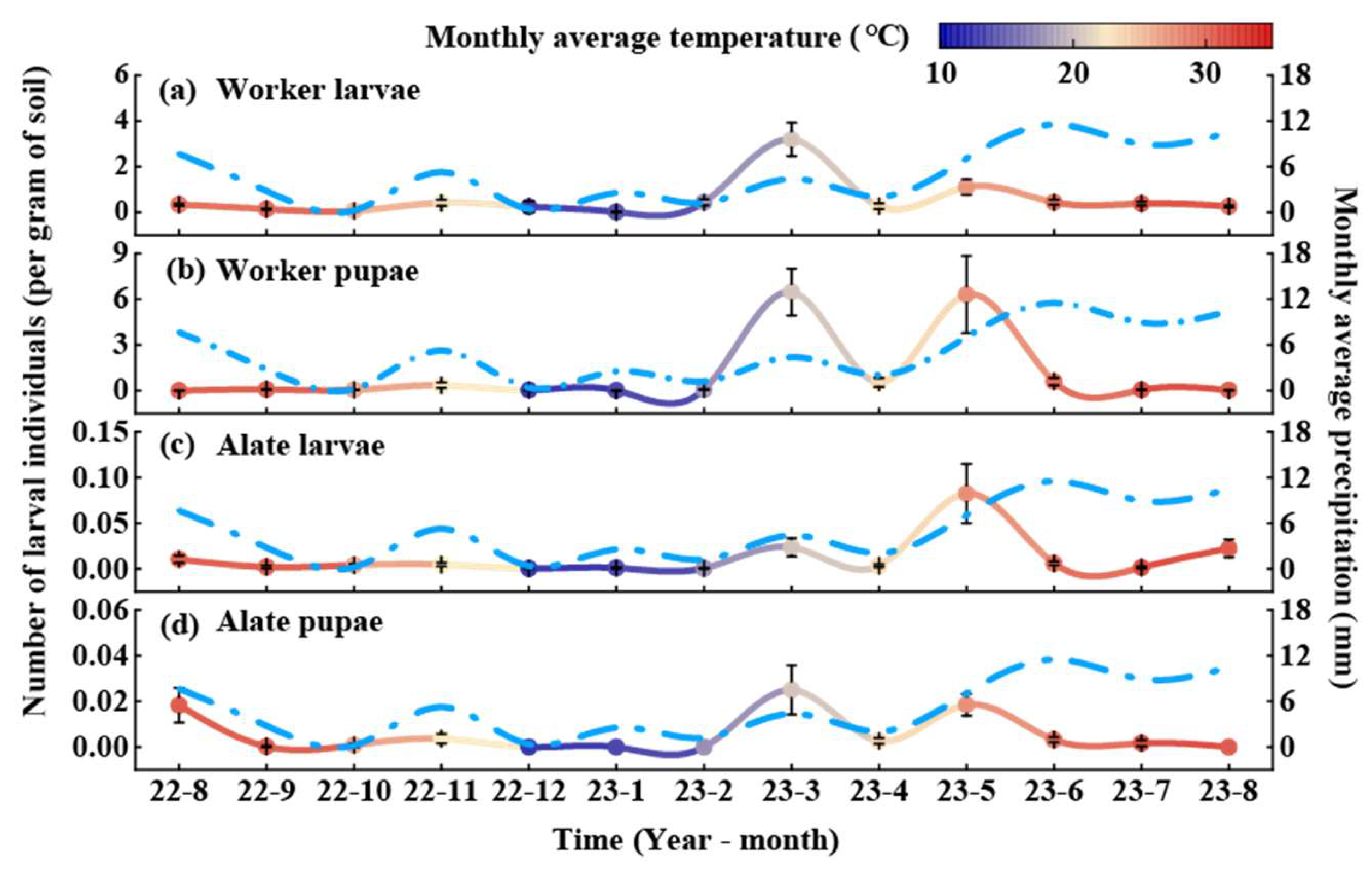
3.1. Dynamic Pattern of RIFA Colony Structure in C. oleifera Habitat
3.2. Dynamic Patterns of RIFA Colony Structure in Fishpond Habitat
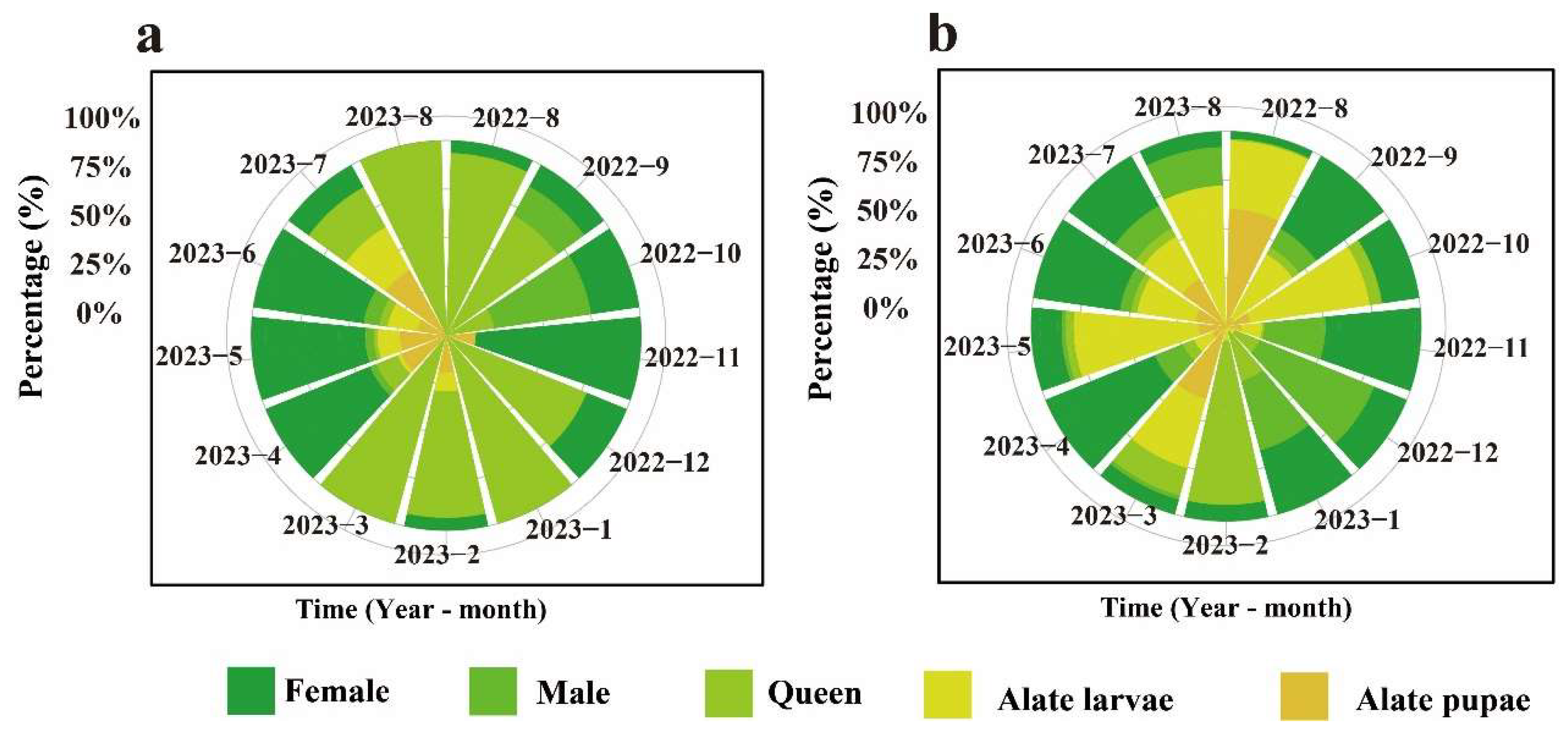
3.3. Dynamic Changes in the Proportion of Each RIFA Caste Stage
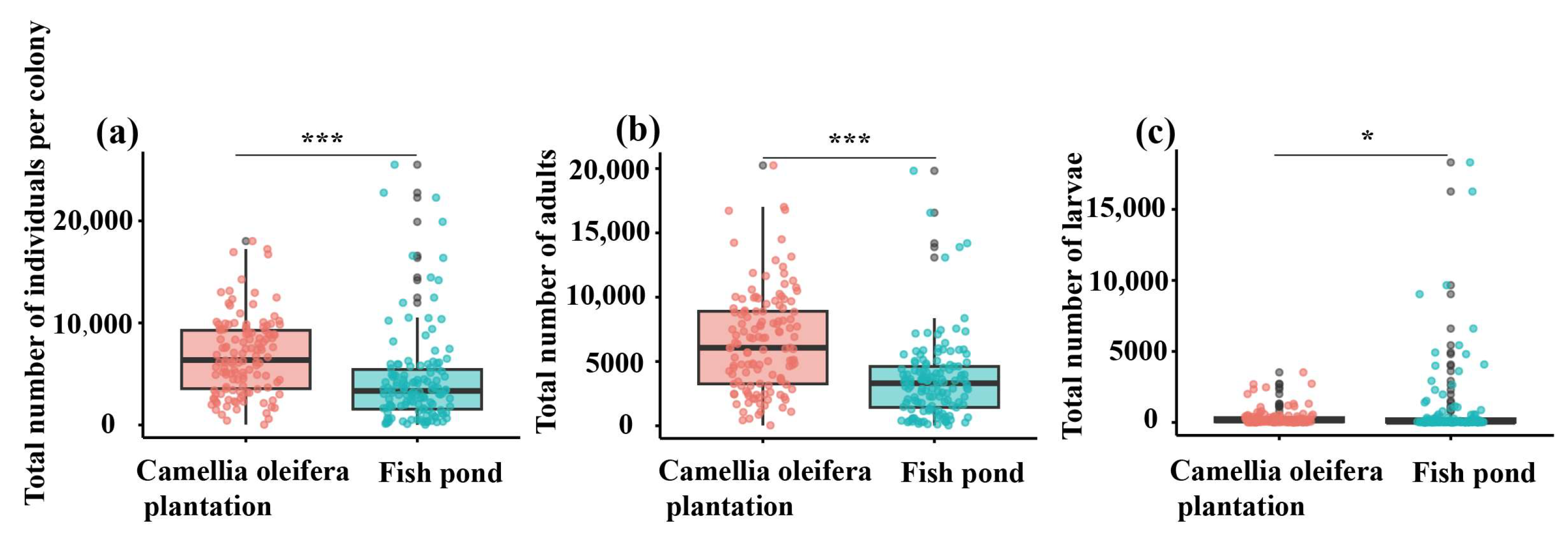
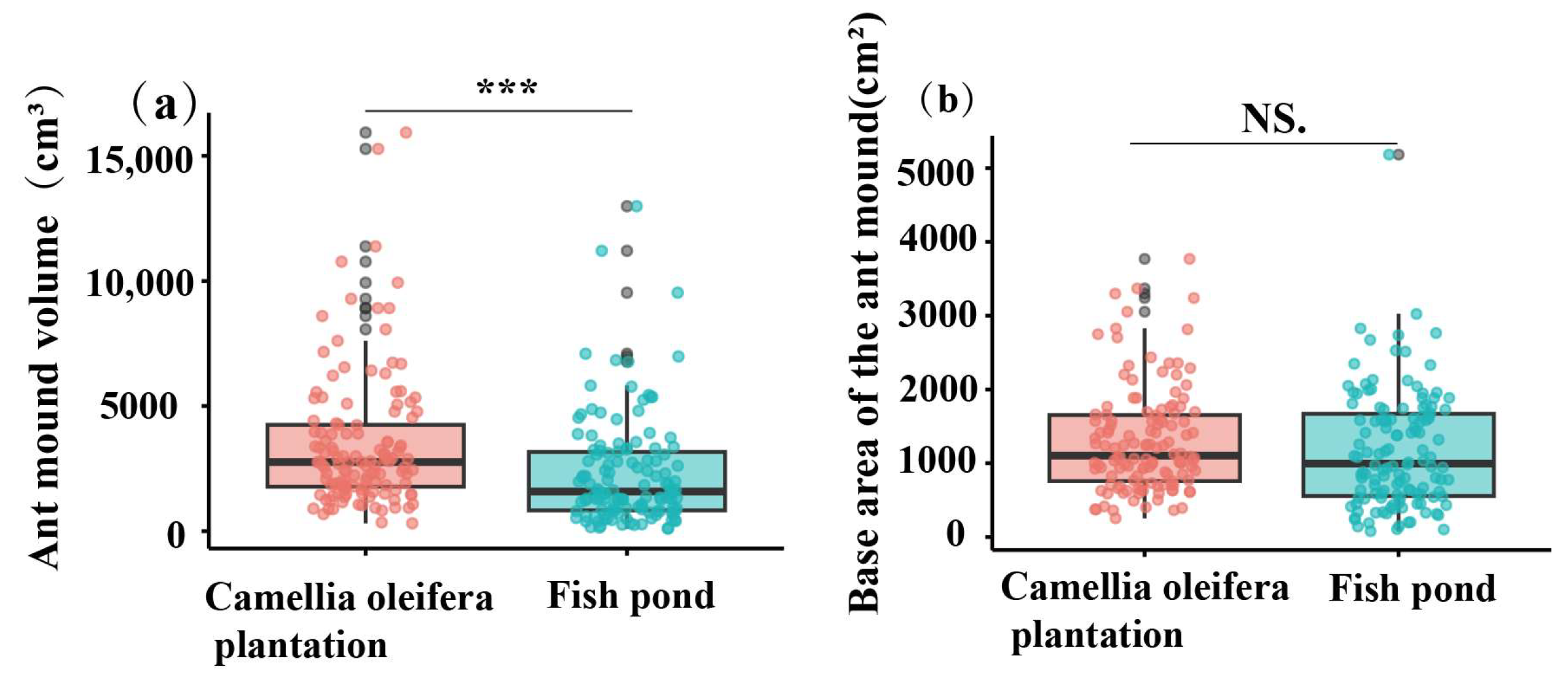
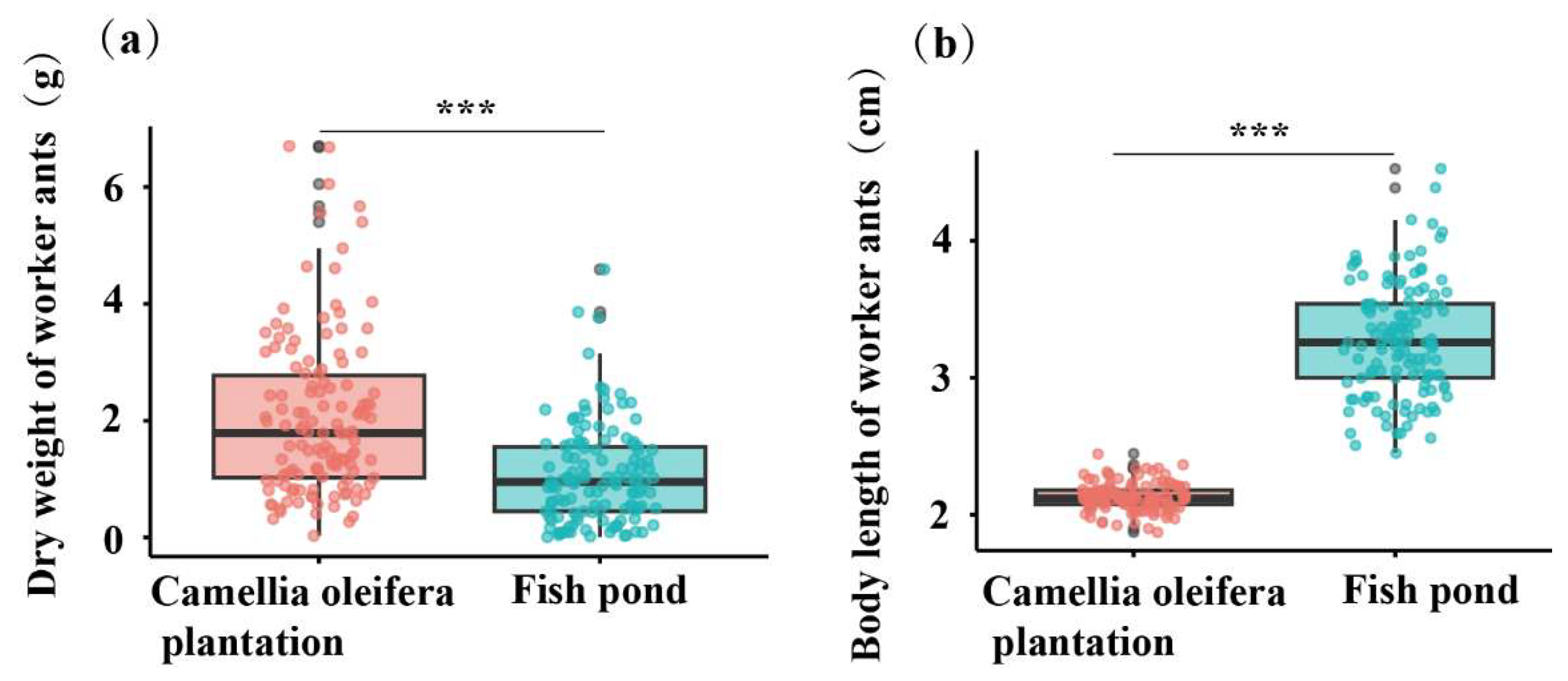
3.4. Differences in Colony Size, Nest Size, and Worker Characteristics of RIFA in Different Habitats
3.5. Correlation Matrix Between Colony Size and Ant Nest Size
3.6. Correlation Analysis Between Meteorological Factors and Ant Nest Structure in Different Habitats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Porter, S.D.; Bhatkar, A.; Mulder, R.; Vinson, S.B.; Clair, D.J. Distribution and density of polygyne fire ants (Hymenoptera: Formicidae) in Texas. J. Econ. Entomol. 1991, 84, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.D.; Savignano, D.A. Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology 1990, 71, 2095–2106. [Google Scholar] [CrossRef]
- Markin, G.; Dillier, J.; Collins, H. Growth and development of colonies of the red imported fire ant, Solenopsis invicta. Ann. Entomol. Soc. Am. 1973, 66, 803–808. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Li, N.D.; Liang, G.W.; Zeng, L. Spatial correlation of active mounds locative distribution of Solenopsis invicta Buren polygyne populations. J. Appl. Ecol. 2007, 18, 140–144. [Google Scholar]
- Vinson, S.B. Invasion of the red imported fire ant (Hymenoptera: Formicidae): Spread, biology, and impact. Am. Entomol. 1997, 43, 23–39. [Google Scholar] [CrossRef]
- Meer, R.K.V.; Morel, L.; Lofgren, C.S. A comparison of queen oviposition rates from monogyne and polygyne fire ant, Solenopsis invicta, colonies. Physiol. Entomol. 1992, 17, 384–390. [Google Scholar] [CrossRef]
- Cassill, D.L.; Tschinkel, W.R. Division of labour and specification of castes in the red imported fire ant Solenopsis invicta Buren. Anim. Behav. 1999, 29, 410–420. [Google Scholar]
- Chen, H.T.; Luo, L.Z.; Xiong, H.L. The outing activity of worker ants and nuptial flight of alates in the red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae) in Guangzhou area, Southern China. Acta Entomol. Sin. 2010, 53, 449–456. [Google Scholar] [CrossRef]
- Lofgren, C.S.; Banks, W.A.; Glancey, B.M. Biology and control of imported fire ants. Annu. Rev. Entomol. 1975, 20, 1–30. [Google Scholar] [CrossRef]
- Tschinkel, W.R. Colony growth and the ontogeny of worker polymorphism in the fire ant, Solenopsis invicta. Behav. Ecol. Sociobiol. 1988, 22, 103–115. [Google Scholar] [CrossRef]
- Ross, K.; Keller, L. Experimental conversion of colony social organization by manipulation of worker genotype composition in fire ants (Solenopsis invicta). Behav. Ecol. Sociobiol. 2002, 51, 287–295. [Google Scholar]
- Saddoris, K.; Fritz, A.H.; Fritz, G. Evidence of selective mating and triploidy among two social forms of Solenopsis invicta (Hymenoptera: Formicidae). Fla. Entomol. 2016, 99, 566–568. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.J.; Zeng, L.; Lu, Y.Y. Impact of the red imported fire ant Solenopsis invicta Buren on biodiversity in South China: A review. J. Integr. Agric. 2019, 18, 788–796. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, Y.; Zeng, L. Spatial distribution characteristics of newly invaded Solenopsis invicta nests in newly invaded areas. Guangdong Agric. Sci. 2010, 37, 16–19. [Google Scholar] [CrossRef]
- Xu, Y.J.; Lu, Y.Y.; Zeng, L. Colony structure and dynamic of the fire ant Solenopsis invicta Buren in several habitats. J. Plant Prot. 2009, 36, 575–576. [Google Scholar]
- Zhang, H.M.; Wang, Y.; Yin, Y.Q.; Zhao, X.Q.; Li, X.Y.; Liu, Y.; Shen, A.D.; Chen, F.S. Community structure and dynamic of Solenopsis invicta in central Yunnan Province. Chin. Agric. Sci. Bull. 2023, 39, 112–118. [Google Scholar]
- Vatanparast, M.; Puckett, R.T.; Choi, D.S.; Park, Y. Comparison of gene expression in the red imported fire ant (Solenopsis invicta) under different temperature conditions. Sci. Rep. 2021, 11, 16476. [Google Scholar] [CrossRef]
- Lytle, A.; Costa, J.; Warren, R. Invasion and high-elevation acclimation of the red imported fire ant, Solenopsis invicta, in the southern Blue Ridge Escarpment region of North America. PLoS ONE 2020, 15, e0232264. [Google Scholar] [CrossRef]
- Li, J.; Heinz, K. Genetic variation in desiccation resistance and adaptability of the red imported fire ant (Hymenoptera: Formicidae) to arid regions. Ann. Entomol. Soc. Am. 1998, 91, 726–729. [Google Scholar] [CrossRef]
- Xu, Y.; Jun, H.; Yongyue, L.; Ling, Z.; Guangwen, L. Observation of nuptial flights of the red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae) in Mainland China. Sociobiology 2009, 54, 831–840. [Google Scholar]
- Zhou, A.M.; Wang, Z.; Zeng, L.; Lu, Y.Y. Dynamics of red imported fire ant Solenopsis invicta Buren in banana plantations. J. Environ. Entomol. 2011, 33, 466–470. [Google Scholar]
- Li, N.D. Population Dynamics of Red Fire Ants in Newly Invaded Areas. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2006. [Google Scholar]
- Wang, F.; Wang, L.; Li, X.; Zeng, L.; Lu, Y.; Wu, S.; Zhu, J. Regulations for the Monitoring of Red Imported Fire Ant Epidemics GB/T 23626-2009; China Standards Press: Beijing, China, 2009. [Google Scholar]
- Song, Y.B.; Chen, M.; Wu, J.R.; Hong, J.X.; Ouyang, T.; Liang, Y.L.; Liang, M.R.; Lu, Y.Y. Impact on ant communities by chemical pesticides applied in controlling the red imported fire ant (Solenopsis invicta Buren) in the field. Insects 2024, 15, 876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Wang, Y.; Chen, Z.L.; Chen, A.D.; Li, X.Y.; Yin, Y.Q.; Zhao, X.; Xu, X.; Chen, F.; Yu, S. Population characteristics of Solenopsis invicta in Kunming. J. Biosaf. 2019, 28, 29–33. [Google Scholar]
- Ning, D.; Yang, F.; Xiao, Q.; Ran, H.; Xu, Y. A simple and efficient method for preventing ant escape (Hymenoptera: Formicidae). Myrmecol. News 2019, 29, 57–65. [Google Scholar]
- Mlot, N.J.; Tovey, C.A.; Hu, D.L. Fire ants self-assemble into waterproof rafts to survive floods. Proc. Natl. Acad. Sci. USA 2011, 108, 7669–7673. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Lofgren, C.S.; Jouvenaz, D.P.; Stringer, C.E.; Bishop, P.M.; Williams, D.F.; Wojcik, D.P.; Glancey, B.M. Techniques for Collecting, Rearing, and Handling Imported Fire Ants; Advances in Agricultural Technology, Science and Education Administration, United States Department of Agriculture: Washington, DC, USA, 1981; No. AAT-S-21. [Google Scholar]
- Wickham, H. Data analysis. In ggplot2: Elegant Graphics for Data Analysis; Springer International Publishing: Cham, Switzerland, 2016; pp. 189–201. [Google Scholar]
- Zhang, B. Batch difference analysis of two independent sample data based on the R language. New Discov. 2024, 1–7. [Google Scholar] [CrossRef]
- Sedgwick, P. Spearman’s rank correlation coefficient. BMJ 2014, 349, g5507. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Penick, C.A.; Tschinkel, W.R. Thermoregulatory brood transport in the fire ant, Solenopsis invicta. Insectes Sociaux 2008, 55, 176–182. [Google Scholar] [CrossRef]
- Lafferty, S.K. Invasion and high-elevation acclimation of the red imported fire ant, Solenopsis invicta. PLoS ONE 2018, 15, e0236453. [Google Scholar]
- Porter, S.D. Impact of temperature on colony growth and developmental rates of the ant, Solenopsis invicta. J. Insect Physiol. 1988, 34, 1127–1133. [Google Scholar] [CrossRef]
- Tschinkel, W.R. The Fire Ants; Harvard University Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Chen, X.; Jinag, S.; Huang, S.; Meng, Z.; Liu, J.; Yue, D. Nuptial flights of Solenopsis invicta in Shenzhen. Chin. Bull. Entomol. 2012, 49, 1293–1297. [Google Scholar]
- Tschinkel, W. Sociometry and Sociogenesis of Colonies of the Fire Ant Solenopsis invicta During One Annual Cycle. Ecol. Monogr. 1993, 63, 425–457. [Google Scholar] [CrossRef]
- Petralia, R.; Vinson, S.B. Developmental Morphology of Larvae and Eggs of the Imported Fire Ant, Solenopsis invicta. Ann. Entomol. Soc. Am. 1979, 72, 472–484. [Google Scholar] [CrossRef]
- Tschinkel, W.R. Social control of egg-laying rate in queens of the fire ant, Solenopsis invicta. Physiol. Entomol. 1988, 13, 297–310. [Google Scholar] [CrossRef]
- Song, J.; Zhang, H.; Li, M.; Han, W.; Yin, Y.; Lei, J. Prediction of spatiotemporal invasive risk of the red imported fire ant Solenopsis invicta (Hymenoptera: Formicidae) in China. Insects 2021, 12, 874. [Google Scholar] [CrossRef]
- Privman, E.; Cohen, P.; Cohanim, A.B.; Riba-Grognuz, O.; Shoemaker, D.; Keller, L. Positive selection on sociobiological traits in invasive fire ants. Mol. Ecol. 2018, 27, 3116–3130. [Google Scholar] [CrossRef]
- Wanchoo, A.; Zhang, W.; Ortiz-Urquiza, A.; Boswell, J.; Xia, Y.; Keyhani, N. Red imported fire ant (Solenopsis invicta) chemosensory proteins are expressed in tissue-, developmental-, and caste-specific patterns. Front. Physiol. 2020, 11, 585883. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Liu, R.; Sun, Y.; Xiao, J.; Gao, L.; Gao, X.; Wang, H. Impacts of changing climate on the distribution of Solenopsis invicta Buren in Mainland China: Exposed urban population distribution and suitable habitat change. Ecol. Indic. 2022, 142, 108944. [Google Scholar] [CrossRef]
- Ometto, L.; Shoemaker, D.; Ross, K.; Keller, L. Evolution of gene expression in fire ants: The effects of developmental stage, caste, and species. Mol. Biol. Evol. 2011, 28, 1381–1392. [Google Scholar] [CrossRef]
- Zhang, H.M.; Yin, Y.Q.; Wang, Y.; Zhao, X.Q.; Li, X.Y.; Chen, A.D.; Chen, F.S.; Liu, Y. Investigation on the occurrence and damage of Solenopsis invicta in different seasons in Yiliang County, Yunnan Province. Plant Quar. 2023, 37, 58–61. [Google Scholar]
- Cassill, D.; Tschinkel, W.; Vinson, S. Nest complexity, group size, and brood rearing in the fire ant, Solenopsis invicta. Insectes Sociaux 2002, 49, 158–163. [Google Scholar] [CrossRef]
- Markin, G.P.; Dillier, J.H. The seasonal life cycle of the imported fire ant, Solenopsis saevissima richteri, on the Gulf Coast of Mississippi. Ann. Entomol. Soc. Am. 1971, 64, 562–565. [Google Scholar] [CrossRef]
- Kelly, L.; Sellers, J. Abundance and distribution of the invasive ant, Solenopsis invicta (Hymenoptera: Formicidae), in cypress savannas of North Carolina. J. Entomol. Sci. 2014, 107, 1072–1080. [Google Scholar] [CrossRef]
- Chen, S.; Ding, F.; Hao, M.; Jiang, D. Mapping the potential global distribution of red imported fire ant (Solenopsis invicta Buren) based on a machine learning method. Sustainability 2020, 12, 10182. [Google Scholar] [CrossRef]
- Schmid, R.B.; Lundgren, J. Red imported fire ant, Solenopsis invicta (Buren) (Hymenoptera: Formicidae), abundance and arthropod community diversity affected by pasture management. Fla. Entomol. 2022, 105, 53–57. [Google Scholar] [CrossRef]
- Wang, J. Driving forces of urban construction land expansion in the mainland of China. China Land Sci. 2009, 26, 1323–1332. [Google Scholar]
- Ma, C.; Zhao, S.; Yan, S.; Wang, Y.; Lin, Q.; Fang, Y.; Liu, H.; Wu, P. Analysis on new occurrence records of red imported fire ant (Solenopsis invicta Buren) in China in 2021. Chin. J. Invasive Species 2023, 15, 87–95. [Google Scholar] [CrossRef]
- Shi, L.; Liu, F.; Peng, L. Impact of Red Imported Fire Ant Nest-Building on Soil Properties and Bacterial Communities in Different Habitats. Soil Biol. Biochem. 2023, 79, 2026. [Google Scholar] [CrossRef]
- Seagraves, M.; Mcpherson, R. Residual susceptibility of the red imported fire ant (Hymenoptera: Formicidae) to four agricultural insecticides. J. Econ. Entomol. 2003, 96, 645–648. [Google Scholar] [CrossRef]
- Li, H. Preliminary study on the control effects of ten pesticides to red imported fire ant. Guangdong Agric. Sci. 2007, 34, 57–59. [Google Scholar]
- Siddiqui, J.A.; Luo, Y.; Sheikh, U.; Bamisile, B.S.; Khan, M.M.; Imran, M.; Hafeez, M.; Ghani, M.I.; Lei, N.; Xu, Y. Transcriptome analysis reveals differential effects of beta-cypermethrin and fipronil insecticides on detoxification mechanisms in Solenopsis invicta. Front. Physiol. 2022, 13, 1018731. [Google Scholar] [CrossRef] [PubMed]
- Overton, K.J. Mating Flight Initiation and Nutritional Status of Solenopsis invicta (Hymenoptera: Formicidae) Alates Infected with Thelohania solenopsae (Microsporida: Thelohaniidae). Master’s Thesis, Texas A&M University, College Station, TX, USA, 2005, unpublished. [Google Scholar]
- Callcott, A.; Porter, S.; Weeks, R.D.; Graham, L.C.; Johnson, S.; Gilbert, L. Fire ant decapitating fly cooperative release programs (1994–2008): Two Pseudacteon species. J. Insect Sci. 2011, 11, 119. [Google Scholar] [CrossRef]
- Briano, J.; Calcaterra, L.; Varone, L. Fire ants (Solenopsis spp.) and their natural enemies in southern South America. Psyche J. Entomol. 2012, 2012, 1–19. [Google Scholar] [CrossRef]
- Ascunce, M.; Kassu, G.; Bouwma, A.; Reed, D.L.; Briano, J.; Oi, D.L.; Shoemaker, D.H. Prevalence and genetic diversity of a microsporidian parasite in the black imported fire ant and its social parasitic ant (Formicidae: Myrmicinae: Solenopsis) in Buenos Aires Province, Argentina. Insects 2023, 14, 901. [Google Scholar] [CrossRef]
- Varone, L.; Heraty, J.; Calcaterra, L. Distribution, abundance and persistence of Orasema species (Hym: Eucharitidae) parasitic on fire ants in South America. Biol. Control 2010, 55, 72–78. [Google Scholar] [CrossRef]
- Vargo, E. Sex investment ratios in monogyne and polygyne populations of the fire ant Solenopsis invicta. J. Evol. Biol. 1996, 9, 783–789. [Google Scholar] [CrossRef]
- Deheer, C.; Tschinkel, W.R. The success of alternative reproductive tactics in monogyne populations of the ant Solenopsis invicta: Significance for transitions in social organization. Behav. Ecol. 1998, 9, 130–135. [Google Scholar] [CrossRef]
- Cohen, P.; Privman, E. The social supergene dates back to the speciation time of two Solenopsis fire ant species. Sci. Rep. 2020, 10, 11538. [Google Scholar] [CrossRef]
- Hettesheimer, D.R.; Zeng, H.; Hunt, B.G.; Ross, K.G. Biased social chromosome transmission in males of the fire ant Solenopsis invicta. G3 Genes Genomes Genet. 2024, 15, jkae289. [Google Scholar] [CrossRef]
- Shoemaker, D.; Ross, K. Effects of social organization on gene flow in the fire ant Solenopsis invicta. Nature 1996, 383, 613–616. [Google Scholar] [CrossRef]
- Macom, T.; Porter, S. Comparison of polygyne and monogyne red imported fire ant (Hymenoptera: Formicidae) population densities. Ann. Entomol. Soc. Am. 1996, 89, 535–543. [Google Scholar] [CrossRef]
- Vargo, E.; Porter, S. Colony reproduction by budding in the polygyne form of Solenopsis invicta (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 1989, 82, 307–313. [Google Scholar] [CrossRef]
- Liang, G. Colony structure of Solenopsis invicta in winter in South China. J. South China Agric. Univ. 2007, 28, 16–18. [Google Scholar]
- Tschinkel, W.; Adams, E.; Macom, T. Territory area and colony size in the fire ant Solenopsis invicta. J. Anim. Ecol. 1995, 64, 473–480. [Google Scholar] [CrossRef]
- Showler, A.; Knaus, R.; Reagan, T.E. Foraging territoriality of the imported fire ant, Solenopsis invicta Buren, in sugarcane as determined by neutron activation analysis. Insectes Sociaux 1989, 36, 235–239. [Google Scholar] [CrossRef]
- Cassill, D.L.; Tschinkel, W. Regulation of diet in the fire ant, Solenopsis invicta. J. Insect Behav. 1999, 12, 307–328. [Google Scholar] [CrossRef]
- Feldhaar, H. Ant nutritional ecology: Linking the nutritional niche plasticity on individual and colony-level to community ecology. Curr. Opin. Insect Sci. 2014, 5, 25–30. [Google Scholar] [CrossRef]
- Zhou, Y.; Lei, Y.; Lu, L.; He, Y. Temperature- and food-dependent foraging gene expression in foragers of the red imported fire ant Solenopsis invicta. Physiol. Entomol. 2020, 45, 1–6. [Google Scholar] [CrossRef]
- Malone, M.; Ivanov, K.; Taylor, S.V.; Schürch, R. Fast range expansion of the red imported fire ant in Virginia and prediction of future spread in the United States. Ecosphere 2023, 14, e4652. [Google Scholar] [CrossRef]
- Onatayo, E. Integrating AI with Remote Sensing for Real-Time Disaster Monitoring and Rapid Response. Int. J. Res. Publ. Rev. 2025, 6, 613–622. [Google Scholar] [CrossRef]
- Duan, Y.; Li, X.; Wang, L.; Yu, T.; Liu, C.; Cao, S. Research and Development of a New Remote Control RIFA Trap. Int. J. New Dev. Eng. Soc. 2021, 5, 207–212. [Google Scholar]
- VanExel, K.; Sherchan, S.; Liu, S. Optimizing Deep Learning Models for Climate-Related Natural Disaster Detection from UAV Images and Remote Sensing Data. J. Imaging 2025, 11, 32. [Google Scholar] [CrossRef]
- Zhang, N.; Chai, X.; Li, N.; Zhang, J.; Sun, T. Applicability of UAV-based Optical Imagery and Classification Algorithms for Detecting Pine Wilt Disease at Different Infection Stages. GISci. Remote Sens. 2023, 60, 47–62. [Google Scholar] [CrossRef]
- Darracq, A.K.; Hromada, S.J.; Neighbors, L.; Smith, L.; Conner, L.; McCleery, R. Reducing Populations of an Invasive Ant Influences Survival, Growth, and Diet of Southern Toads (Anaxyrus terrestris). J. Herpetol. 2022, 56, 84–91. [Google Scholar] [CrossRef]
- Morehart, M.A.; Gitzen, R.A.; Terhune, T.M.; Lepczyk, C.; Sisson, D.C. Changes in native small mammal populations with removal of invasive ant. J. Mammal. 2021, 102, 1318–1330. [Google Scholar] [CrossRef]
- Wang, X.; Qin, Y.; Xu, Y.; Feng, X.C.; Zhao, S.; Lu, Y.; Li, Z. Surveillance and invasive risk of the red imported fire ant, Solenopsis invicta Buren in China. Pest Manag. Sci. 2022, 78, 4478–4487. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Wen, T.; Liu, Y.-H.; Huang, R.-N.; Liu, H.K.-H. Elucidating how the red imported fire ant (Solenopsis invicta) diffused spatiotemporally among different landscapes in north Taiwan, 2008–2015. Ecol. Evol. 2021, 11, 18604–18614. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Yoon, S.; Lee, J.; Seo, C. Predicting Habitat Changes and Vulnerability of Climate-sensitive Insects under SSP Scenarios. GEO DATA 2024, 5, 57–68. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, H.; Liu, L.; Lyu, W.; Xu, L.; Zhang, Z.; Han, Y. Hypervolume Niche Dynamics and Global Invasion Risk of Phenacoccus solenopsis under Climate Change. Insects 2024, 15, 250. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Hung, S.; Lin, X.; Huang, R.-N. Herbal plants as alternatives for the management of the red imported fire ant, Solenopsis invicta. J. Appl. Entomol. 2021, 146, 975–989. [Google Scholar] [CrossRef]
- Roge, G.N. Pheromones as Component of Integrated Pest Management. J. Insect Sci. 2021, 21, 234–242. [Google Scholar]
- Menalled, F.; Landis, D.; Dyer, L. Research and Extension Supporting Ecologically Based IPM Systems. J. Crop Improv. 2004, 11, 153–174. [Google Scholar] [CrossRef]
- Shimoji, H.; Suwabe, M.; Kikuchi, T.; Ohnishi, H.; Tanaka, H.; Kawara, K.; Hidaka, Y.; Enoki, T.; Tsuji, K. Resilience of native ant community against invasion of exotic ants after anthropogenic disturbances of forest habitats. Ecol. Evol. 2022, 12, e9073. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, D.; Wang, Y.; Shen, Z.; Li, B.; Shu, Z.; Chu, C. Tree species richness suppresses red imported fire ant invasion in a subtropical plantation forest. J. Appl. Ecol. 2024, 61, 132–144. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Hong, J.; Song, Y.; Yue, K.; Chen, M.; Wu, J.; Ou, Y.; Liang, M.; Lu, Y. Seasonal Dynamics of Red Imported Fire Ant (Solenopsis invicta) Colony Structures Across Camellia oleifera Plantations and Fishponds in South China. Animals 2025, 15, 1483. https://doi.org/10.3390/ani15101483
Liang Y, Hong J, Song Y, Yue K, Chen M, Wu J, Ou Y, Liang M, Lu Y. Seasonal Dynamics of Red Imported Fire Ant (Solenopsis invicta) Colony Structures Across Camellia oleifera Plantations and Fishponds in South China. Animals. 2025; 15(10):1483. https://doi.org/10.3390/ani15101483
Chicago/Turabian StyleLiang, Yuling, Jingxin Hong, Yunbo Song, Kuo Yue, Meng Chen, Jiarui Wu, Yangting Ou, Mingrong Liang, and Yongyue Lu. 2025. "Seasonal Dynamics of Red Imported Fire Ant (Solenopsis invicta) Colony Structures Across Camellia oleifera Plantations and Fishponds in South China" Animals 15, no. 10: 1483. https://doi.org/10.3390/ani15101483
APA StyleLiang, Y., Hong, J., Song, Y., Yue, K., Chen, M., Wu, J., Ou, Y., Liang, M., & Lu, Y. (2025). Seasonal Dynamics of Red Imported Fire Ant (Solenopsis invicta) Colony Structures Across Camellia oleifera Plantations and Fishponds in South China. Animals, 15(10), 1483. https://doi.org/10.3390/ani15101483




