Estradiol Reverses Ovariectomy-Induced Disruption of Hypothalamic Gene Expression and Behavior via Modulation of Gonadotropin Releasing Hormone and Calcium Signaling Pathways
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
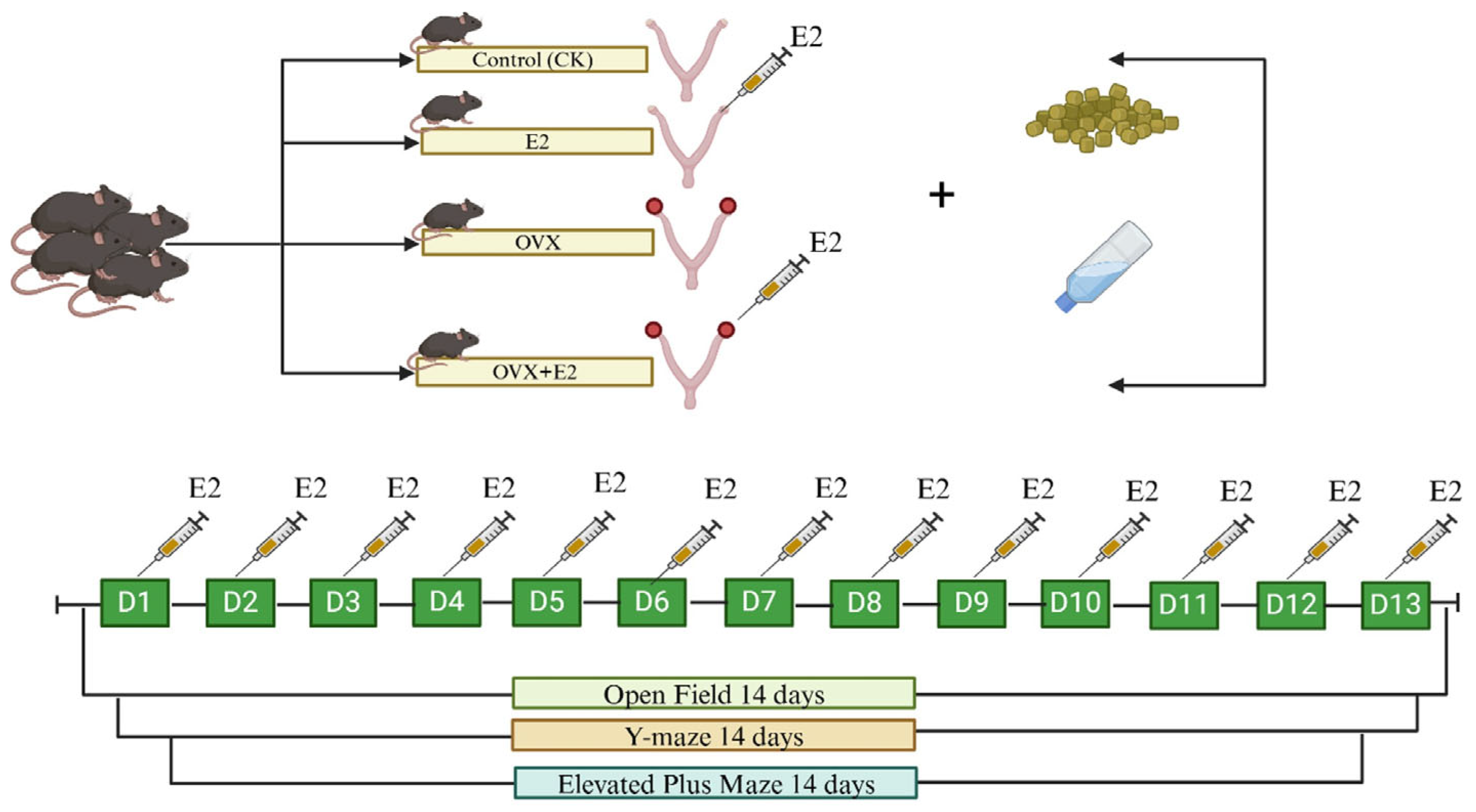
2.1. Animals and Treatments
2.2. Vaginal Smear
2.3. Serum Hormone Concentration Test
2.4. Behavioral Tests
2.5. Open Field Test (OFT)
2.6. Y-Maze
2.7. Elevated Plus Maze (EPM)
2.8. Sample Collection and RNA Sequencing
2.9. Identification of Differentially Expressed Genes
2.10. Analysis of the Important Pathways and the Functions of Hypothalamus Tissue
2.11. RT-qPCR Verification
2.12. Statistical Analysis
3. Results
3.1. Development of a Model Menopause and Vaginal Smear
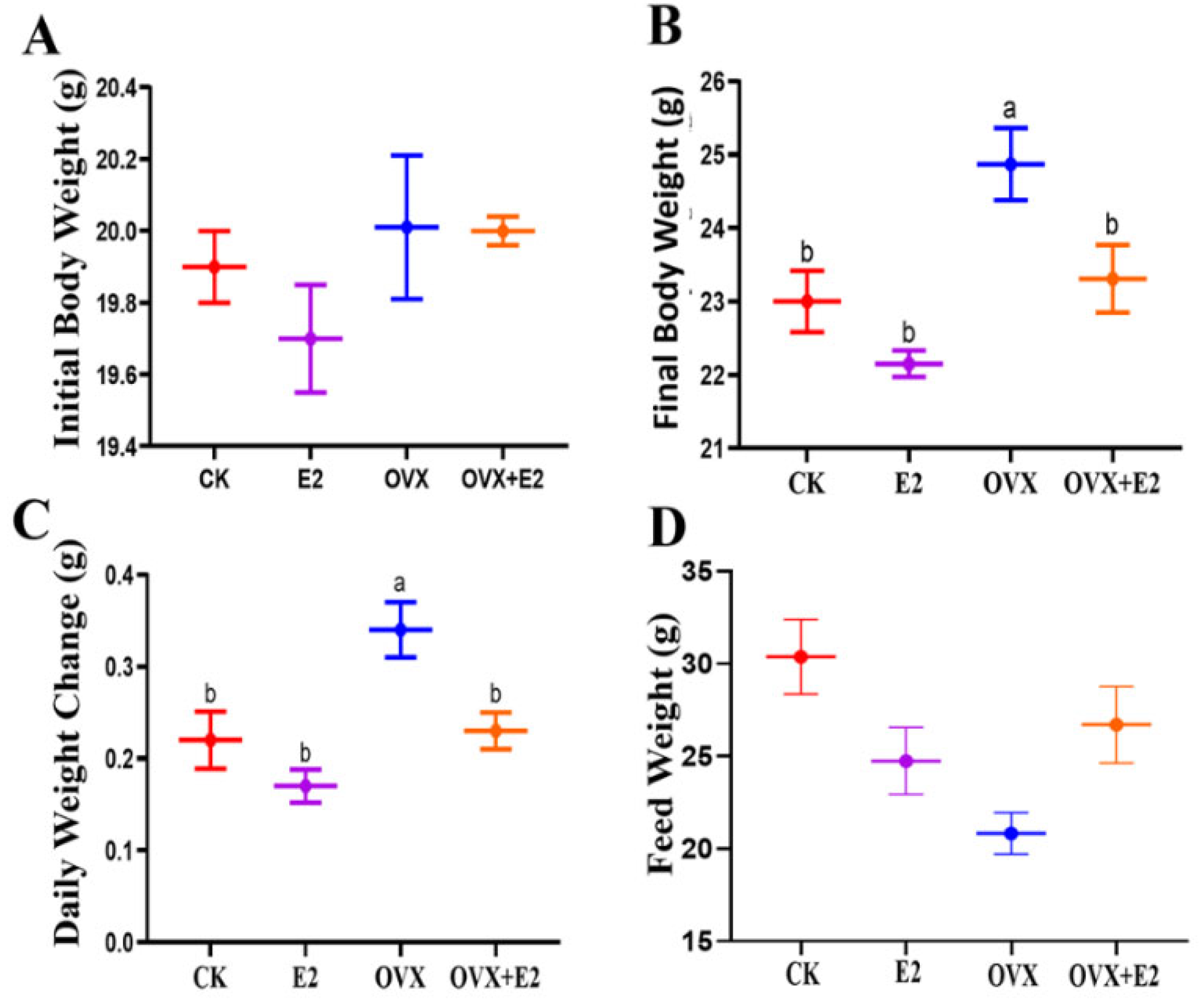
3.2. Consequence of Ovariectomy and 17β-Estradiol Replacement on Body Weight and Food Intake
3.3. Ovariectomy and 17β-Estradiol Replacement and Their Interactive Impact on Mice Anxiety
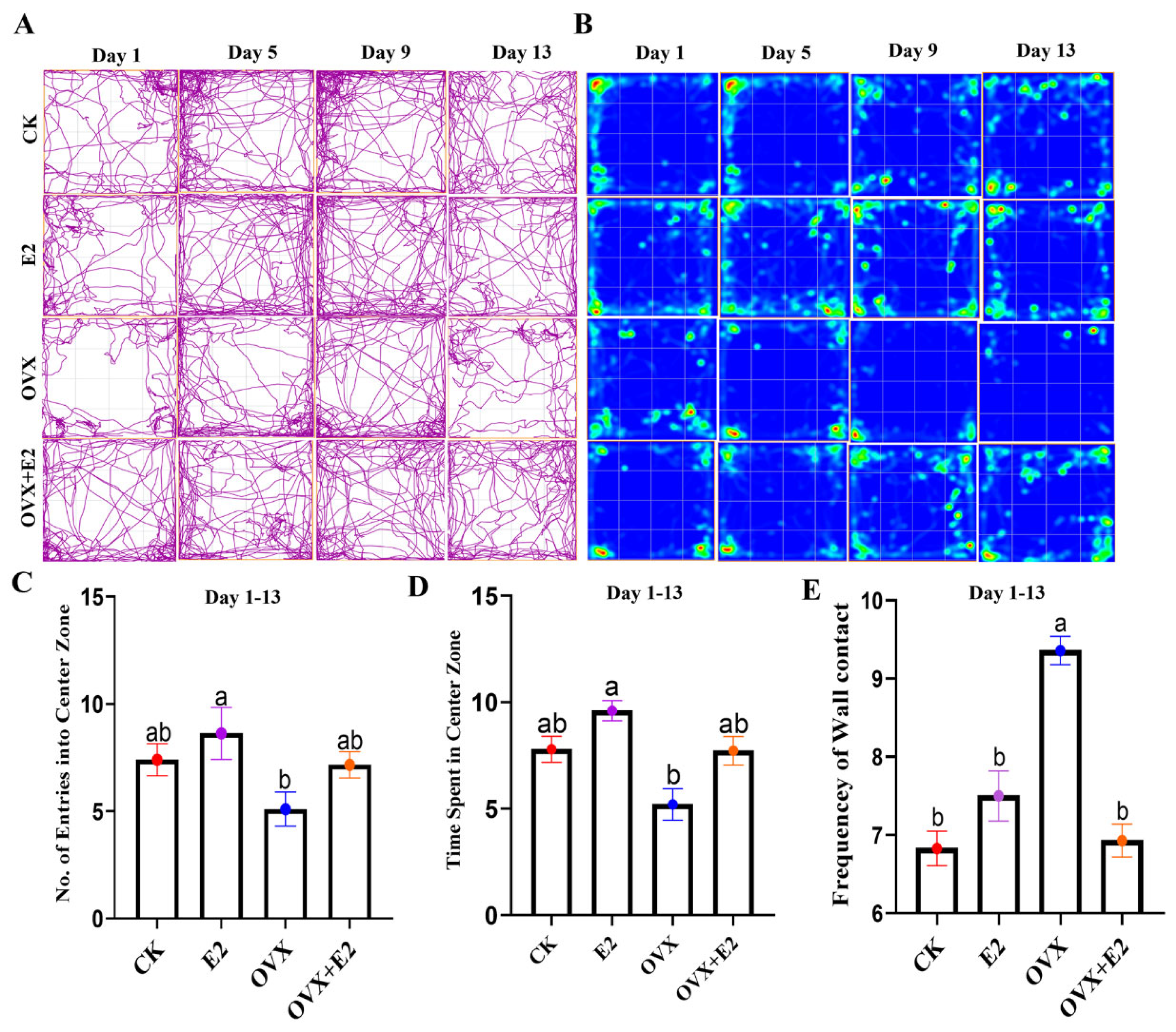
3.4. Result of the Open Field Test (OFT)
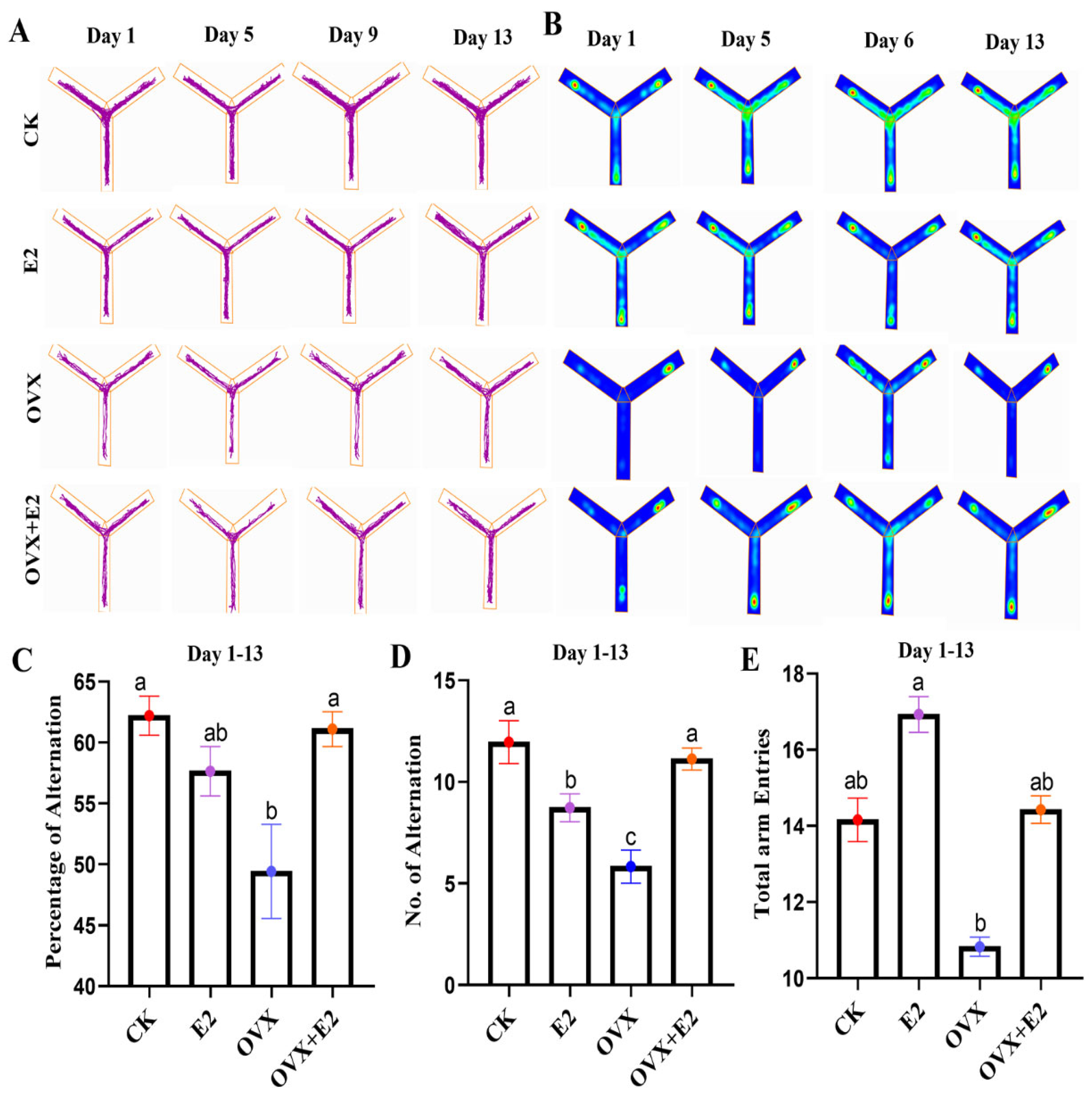
3.5. Result of the Y-Maze
3.6. Result of the Elevated Plus Maze (EPM)
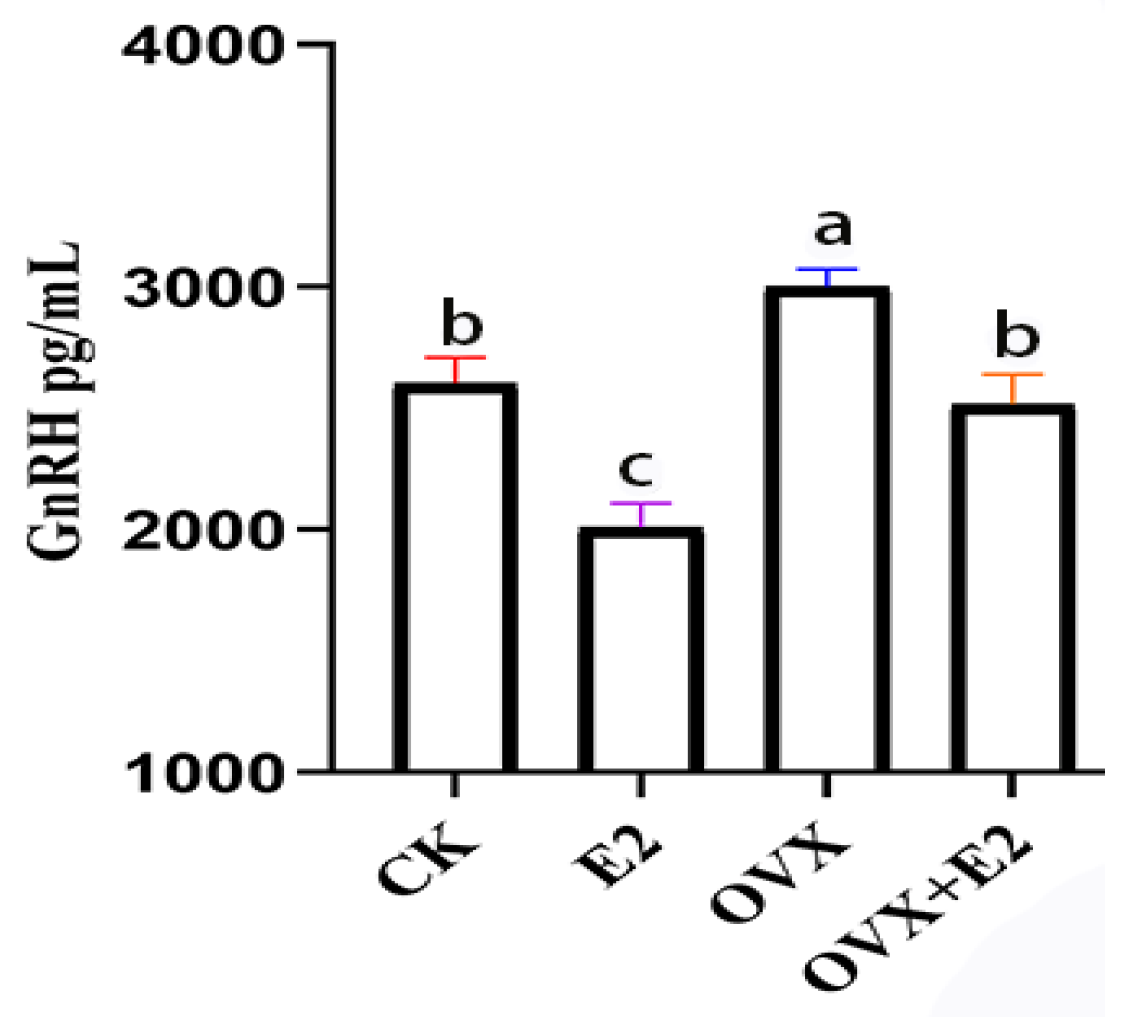
3.7. Implication of Ovariectomy and 17β-Estradiol Substitution on GnRH of the Hypothalamus Tissue of Mice
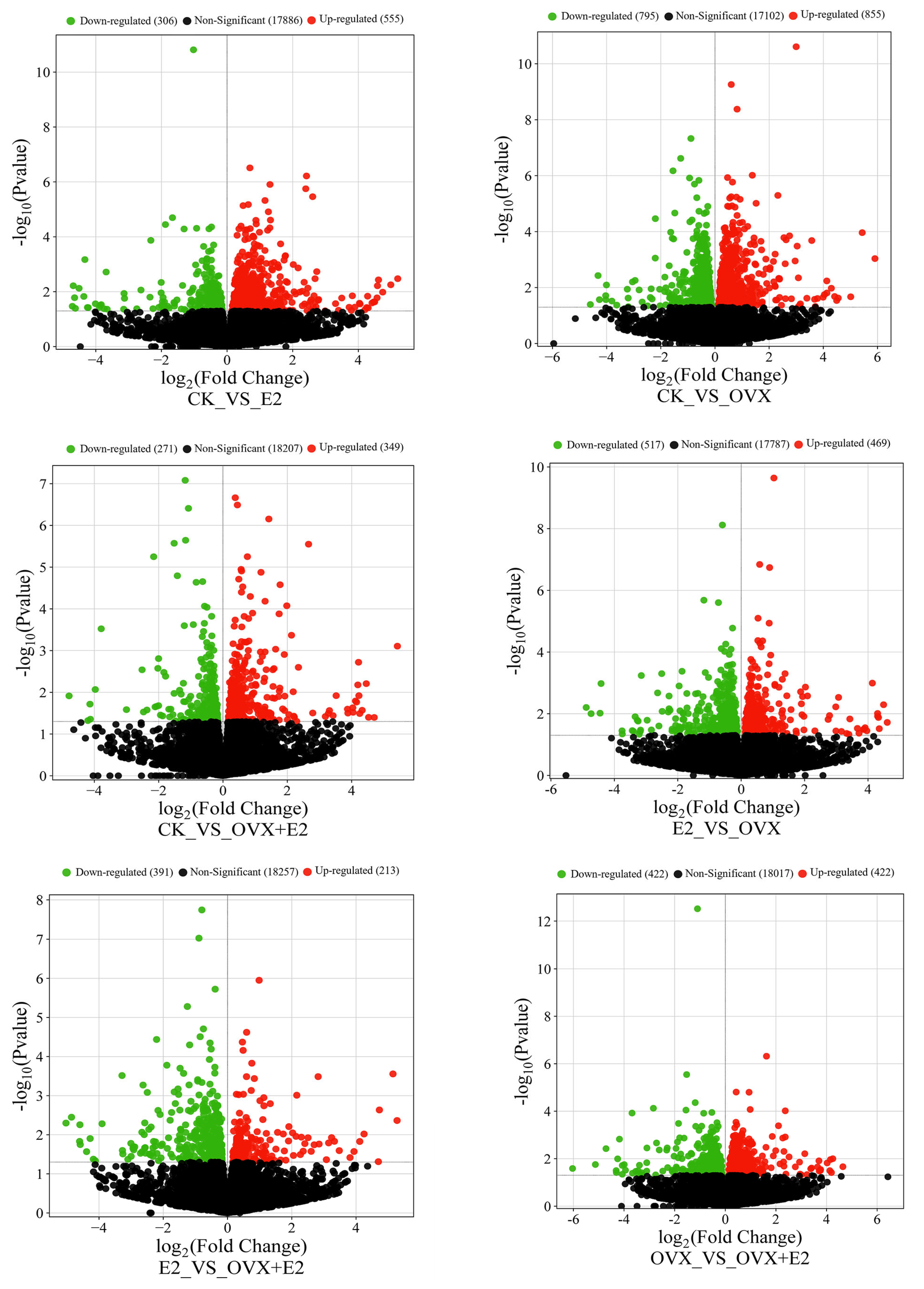
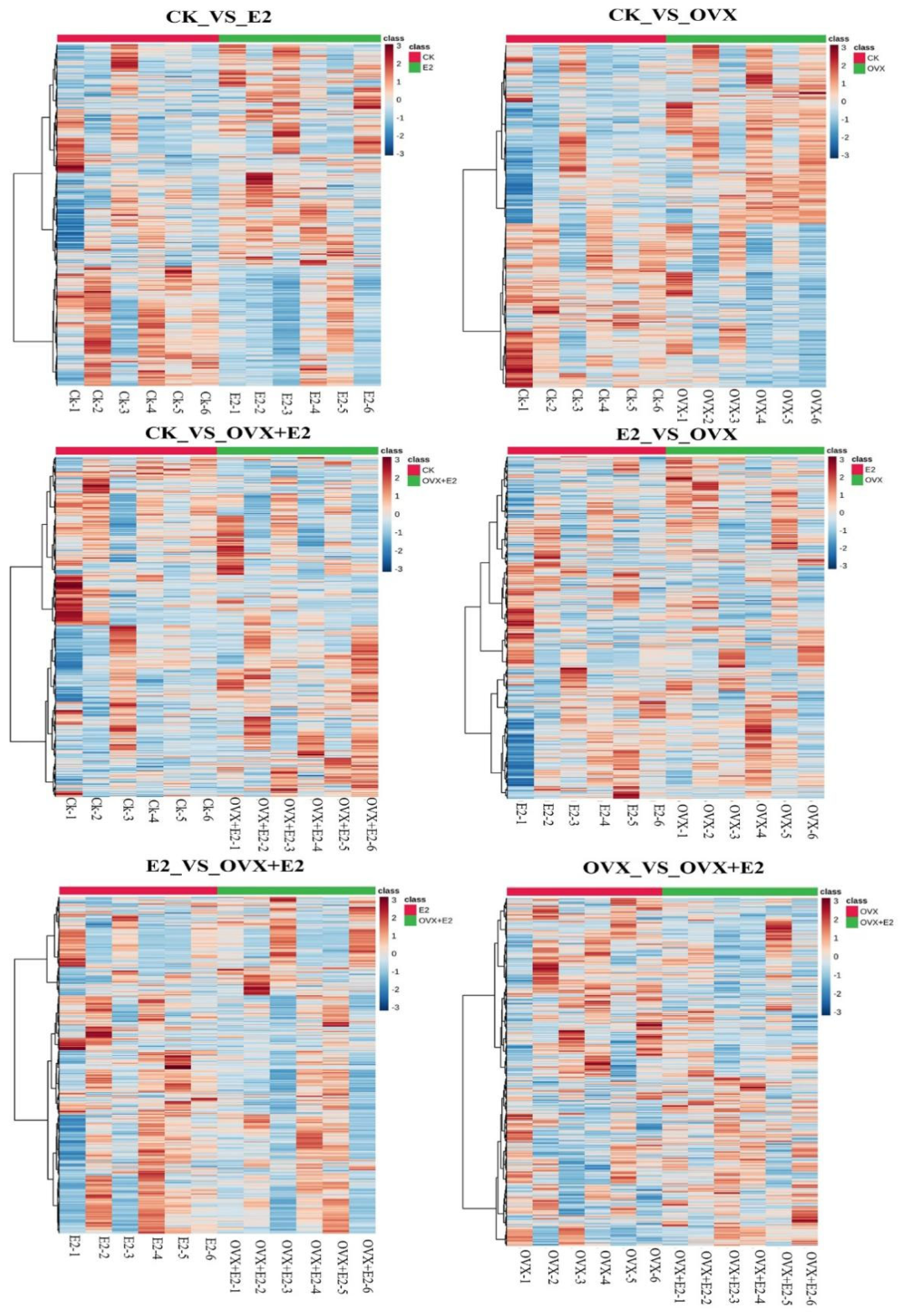
3.8. Effect on Gene Expression Profiles of Mouse Hypothalamic Tissues Following Ovariectomy and 17β-Estradiol Replacement
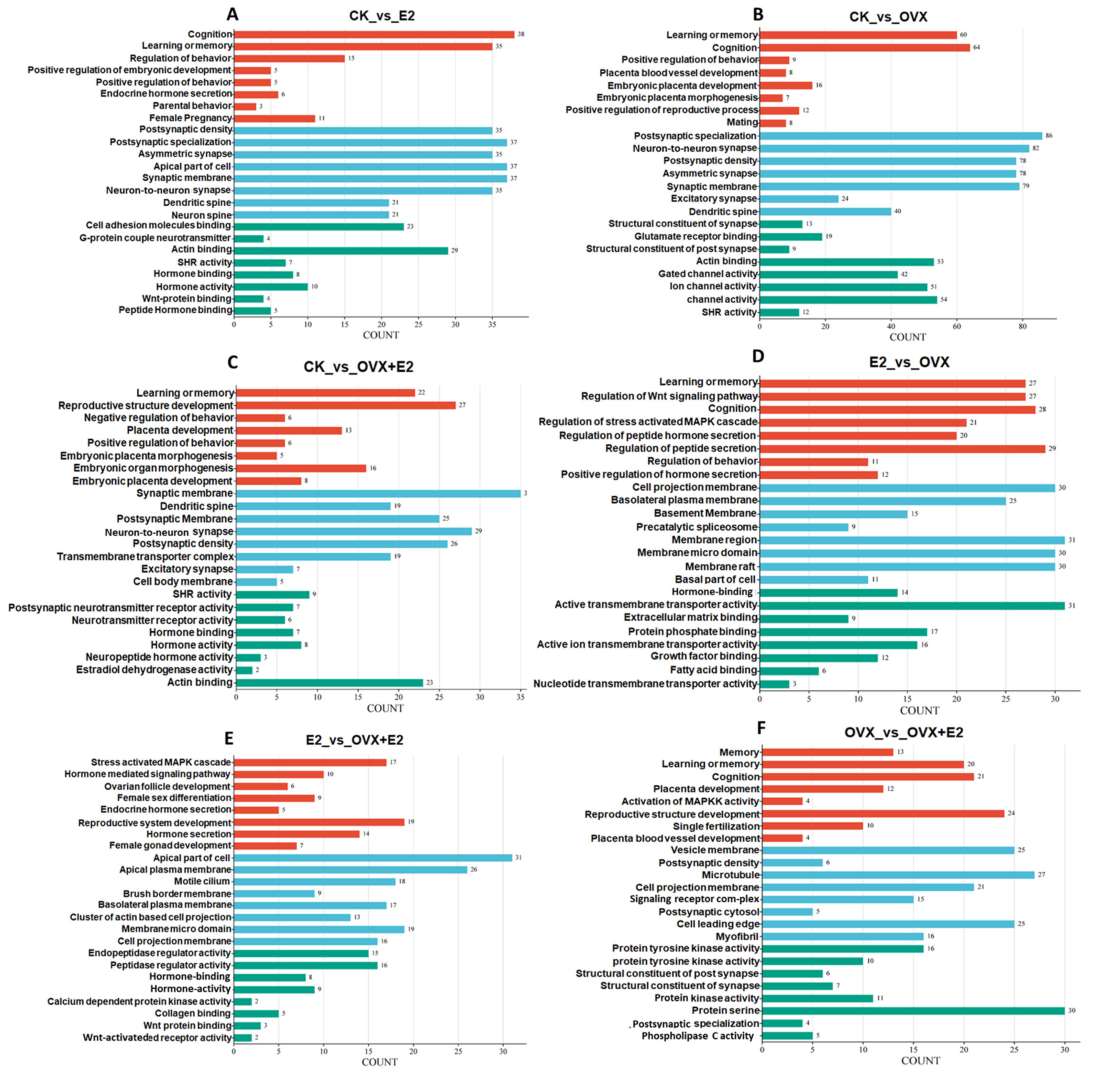
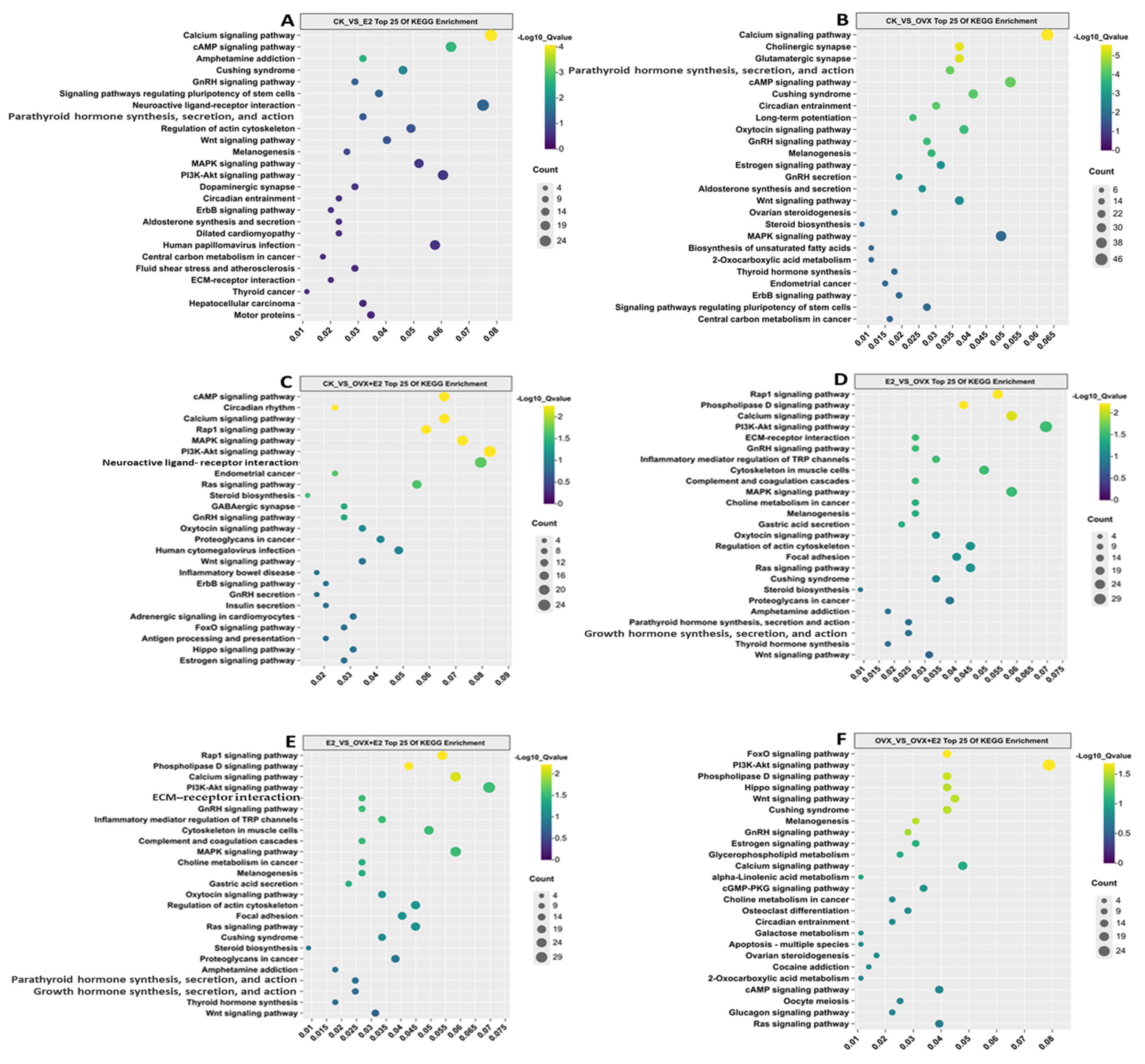
3.9. KEGG Enrichment Analysis of DEGs
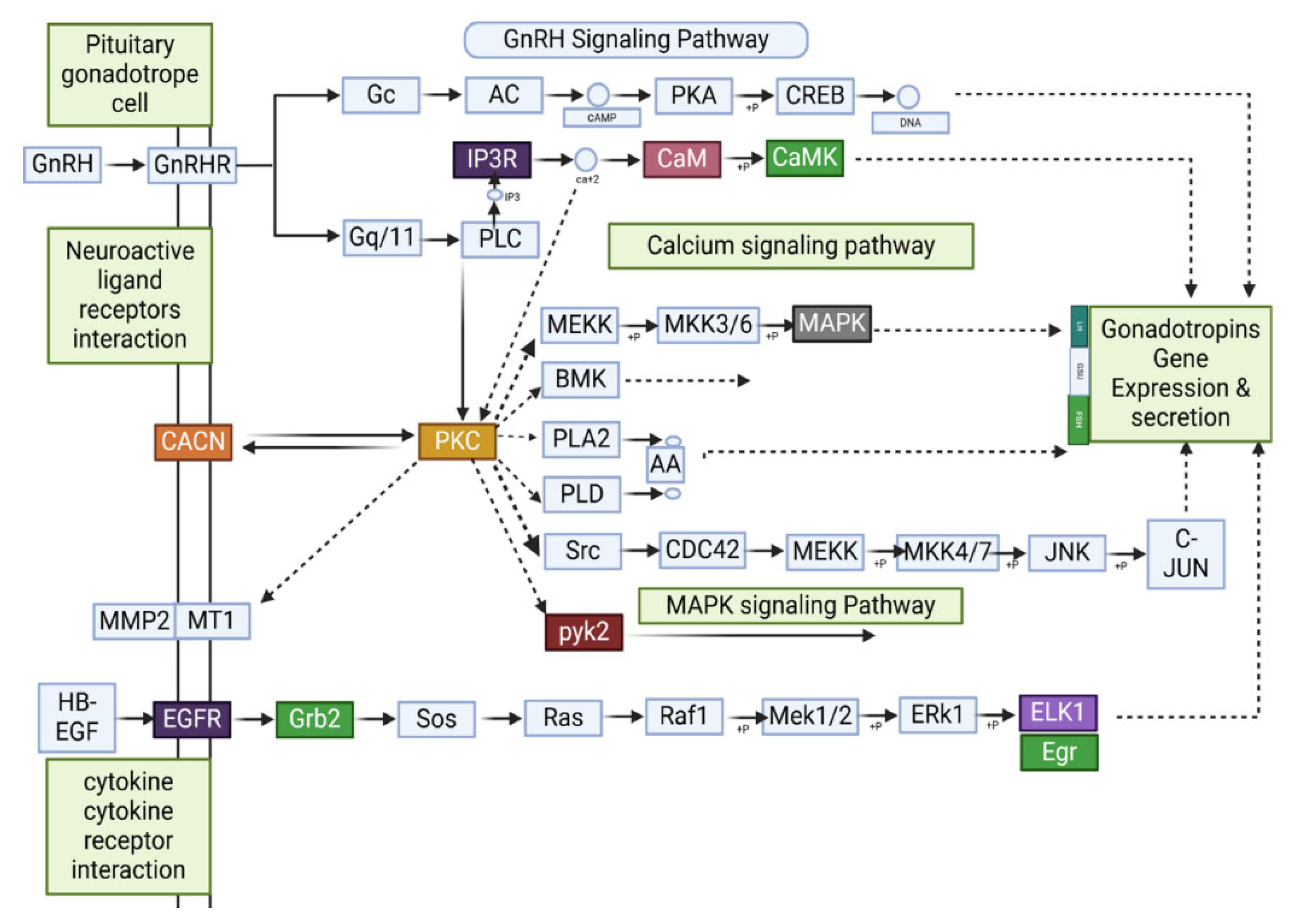
3.10. Impact of E2 and Ovariectomy on the GnRH Signaling Pathway in Mice Hypothalamus: Implications for Reproductive Function
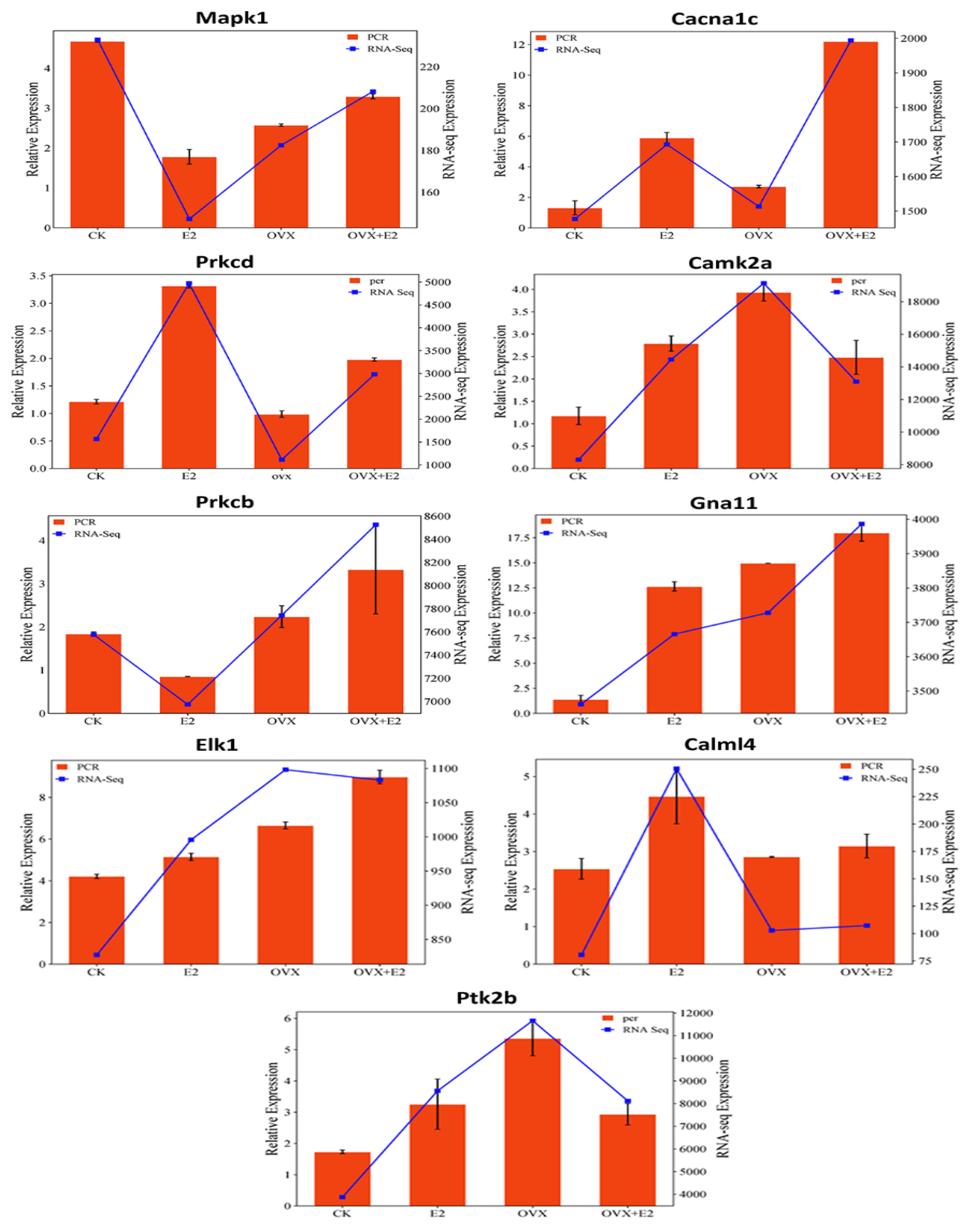
3.11. qPCR Validation
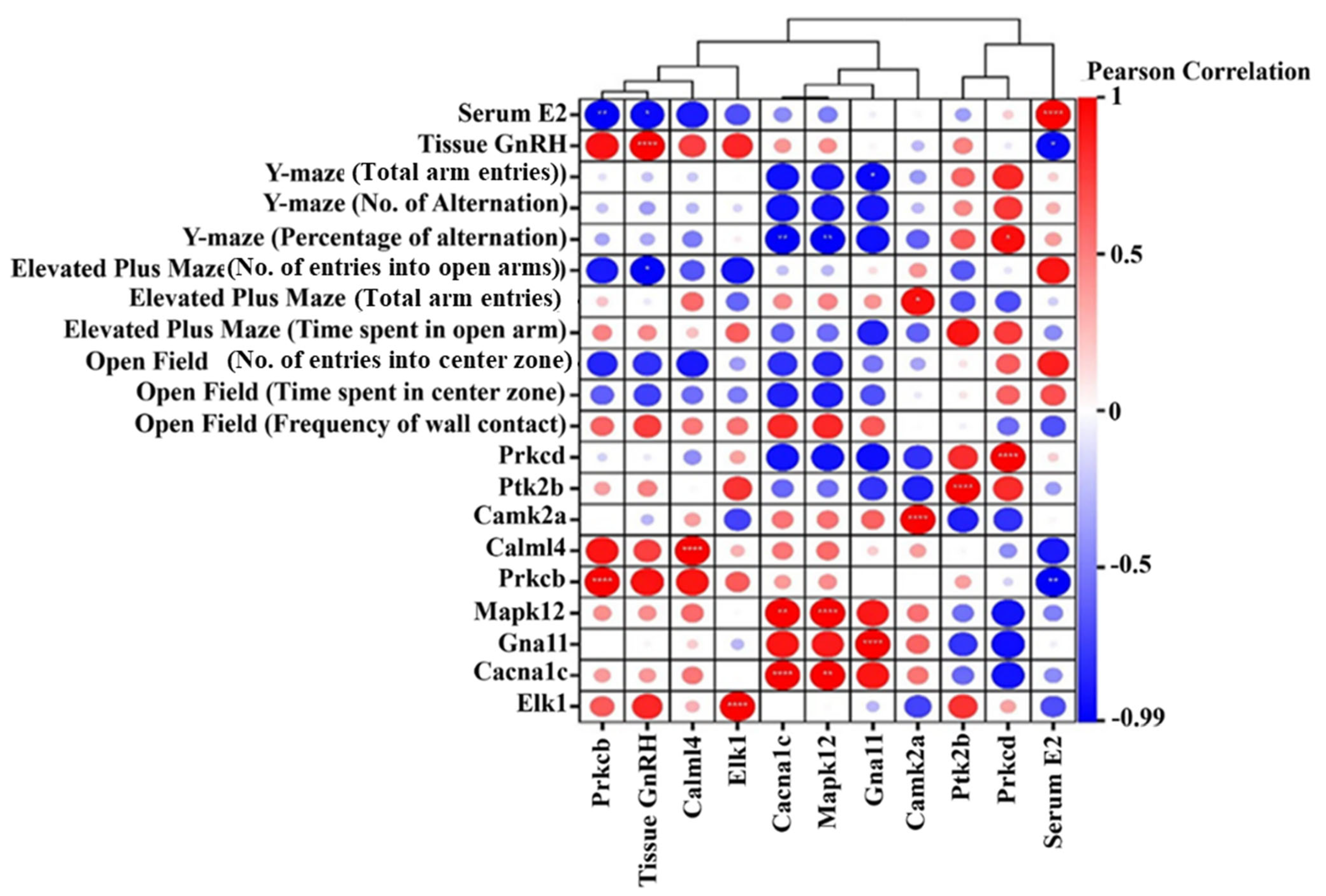
3.12. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Mei, L.; Osakada, T.; Lin, D. Hypothalamic control of innate social behaviors. Science 2023, 382, 399–404. [Google Scholar] [CrossRef]
- Scott, C.J.; Rose, J.L.; Gunn, A.J.; McGrath, B.M. Kisspeptin and the regulation of the reproductive axis in domestic animals. J. Endocrinol. 2018, 240, R1–R16. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, G.D. The hypothalamus. In The Pituitary; Elsevier: Amsterdam, The Netherlands, 2011; pp. 303–341. [Google Scholar] [CrossRef]
- Swanson, L.W. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000, 886, 113–164. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat. Rev. Endocrinol. 2016, 12, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C. Gonadotropin-releasing hormone neurons: Multiple inputs, multiple outputs. Endocrinology 2004, 145, 4016–4017. [Google Scholar] [CrossRef]
- Constantin, S. Targeting KNDy neurons to control GnRH pulses. Curr. Opin. Pharmacol. 2022, 67, 102316. [Google Scholar] [CrossRef]
- Ali, E.S.; Mangold, C.; Peiris, A.N. Estriol: Emerging clinical benefits. Menopause 2017, 24, 1081–1085. [Google Scholar] [CrossRef]
- Findlay, J.K.; Liew, S.H.; Simpson, E.R.; Korach, K.S. Estrogen signaling in the regulation of female reproductive functions. Handb. Exp. Pharmacol. 2010, 198, 29–35. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Guo, Y.J.; Wang, K.Y.; Chen, L.M.; Jiang, P. Neuroprotective effects of vitamin D and 17ss-estradiol against ovariectomy-induced neuroinflammation and depressive-like state: Role of the AMPK/NF-kappaB pathway. Int. Immunopharmacol. 2020, 86, 106734. [Google Scholar] [CrossRef]
- Parkin, R.A.; Murray, A.J. The therapeutic potential of irisin to mitigate the risk of metabolic syndrome in postmenopausal women. Front. Reprod. Health 2024, 6, 1355922. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, J.; Zhu, S.; He, M.; Ma, S.; Jia, Q.; Sun, Q.; Song, L.; Wang, Y.; Duan, L. Berberine ameliorates ovariectomy-induced anxiety-like behaviors by enrichment in equol generating gut microbiota. Pharmacol. Res. 2021, 165, 105439. [Google Scholar] [CrossRef] [PubMed]
- Flores, V.A.; Pal, L.; Manson, J.E. Hormone Therapy in Menopause: Concepts, Controversies, and Approach to Treatment. Endocr. Rev. 2021, 42, 720–752. [Google Scholar] [CrossRef]
- Hashemzadeh, M.; Haseefa, F.; Peyton, L.; Park, S.; Movahed, M.R. The effects of estrogen and hormone replacement therapy on platelet activity: A review. Am. J. Blood Res. 2022, 12, 33–42. [Google Scholar]
- He, J.; Zhang, J.; Wang, Y.; Liu, W.; Gou, K.; Liu, Z.; Cui, S. MiR-7 Mediates the Zearalenone Signaling Pathway Regulating FSH Synthesis and Secretion by Targeting FOS in Female Pigs. Endocrinology 2018, 159, 2993–3006. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, Q.; Zhou, C.; Jiang, H.; Sun, Y.; Wang, H.; Luo, X.; Wang, Z.; Zhang, J.; Wang, K.; et al. Transcriptomic changes in the hypothalamus of ovariectomized mice: Data from RNA-seq analysis. Ann. Anat. 2022, 241, 151886. [Google Scholar] [CrossRef] [PubMed]
- Pechenino, A.S.; Frick, K.M. The effects of acute 17beta-estradiol treatment on gene expression in the young female mouse hippocampus. Neurobiol. Learn. Mem. 2009, 91, 315–322. [Google Scholar] [CrossRef]
- Gresack, J.E.; Frick, K.M. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol. Biochem. Behav. 2006, 84, 112–119. [Google Scholar] [CrossRef]
- Rhodes, M.E.; Balestreire, E.M.; Czambel, R.K.; Rubin, R.T. Estrous cycle influences on sexual diergism of HPA axis responses to cholinergic stimulation in rats. Brain Res. Bull. 2002, 59, 217–225. [Google Scholar] [CrossRef]
- Sollozo-Dupont, I.; Estrada-Camarena, E.; Carro-Juarez, M.; Lopez-Rubalcava, C. GABAA/benzodiazepine receptor complex mediates the anxiolytic-like effect of Montanoa tomentosa. J. Ethnopharmacol. 2015, 162, 278–286. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Tian, Y.; Li, Y.; Han, J.; Tai, F.; Jia, R. Social environment enrichment alleviates anxiety-like behavior in mice: Involvement of the dopamine system. Behav. Brain Res. 2024, 456, 114687. [Google Scholar] [CrossRef]
- Dietrich, M.O.; Zimmer, M.R.; Bober, J.; Horvath, T.L. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell 2015, 160, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Matar, M.A.; Joseph, Z. Animal models of post-traumatic stress disorder. Curr. Protoc. Neurosci. 2013, 64, 9–45. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.M.N.; Costa Junior, D.C.; da Silva, D.M.A.; Alves, A.C.B.; Chaves, R.C.; Reboucas, M.O.; Valentim, J.T.; de Oliveira, A.A.; Sales, I.S.L.; Nicolau, L.A.D.; et al. Long-term administration of omeprazole in mice: A study of behavior, inflammatory, and oxidative stress alterations with focus on central nervous system. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 6165–6175. [Google Scholar] [CrossRef]
- Themann, A.; Rodriguez, M.; Reyes-Arce, J.; Iniguez, S.D. Chlordiazepoxide reduces anxiety-like behavior in the adolescent mouse elevated plus maze: A pharmacological validation study. Pharmacol. Biochem. Behav. 2024, 242, 173819. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, Z.; Xia, J.; Cui, Z.; Li, L.; Qi, Z.; Liu, W. Gene expression profile associated with Asmt knockout-induced depression-like behaviors and exercise effects in mouse hypothalamus. Biosci. Rep. 2022, 42, BSR20220800. [Google Scholar] [CrossRef]
- Xu, H.; Qing, T.; Shen, Y.; Huang, J.; Liu, Y.; Li, J.; Zhen, T.; Xing, K.; Zhu, S.; Luo, M. RNA-seq analyses the effect of high-salt diet in hypertension. Gene 2018, 677, 245–250. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, C.; Chen, J.; Xu, Q.; Liu, S.; Chao, X.; Yang, H.; Wang, T.; Muhammad, A.; Schinckel, A.P.; et al. Characterization and analysis of transcriptomes of multiple tissues from estrus and diestrus in pigs. Int. J. Biol. Macromol. 2024, 256, 128324. [Google Scholar] [CrossRef]
- Stathopoulou, K.M.; Georgakopoulos, S.; Tasoulis, S.; Plagianakos, V.P. Investigating the overlap of machine learning algorithms in the final results of RNA-seq analysis on gene expression estimation. Health Inf. Sci. Syst. 2024, 12, 14. [Google Scholar] [CrossRef]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef]
- Pouladvand, N.; Azarnia, M.; Zeinali, H.; Fathi, R.; Tavana, S. An overview of different methods to establish a murine premature ovarian failure model. Anim. Model. Exp. Med. 2024, 7, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Asarian, L.; Geary, N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1215–R1267. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.N.; Sukan-Karacagil, B.; Acar-Tek, N. Roles of citrus fruits on energy expenditure, body weight management, and metabolic biomarkers: A comprehensive review. Nutr. Rev. 2024, 82, 1292–1307. [Google Scholar] [CrossRef]
- Luine, V.N.; Richards, S.T.; Wu, V.Y.; Beck, K.D. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm. Behav. 1998, 34, 149–162. [Google Scholar] [CrossRef]
- Jiménez-Herrera, R.; Contreras, A.; Djebari, S.; Mulero-Franco, J.; Iborra-Lázaro, G.; Jeremic, D.; Navarro-López, J.; Jiménez-Díaz, L. Systematic characterization of a non-transgenic Aβ1–42 amyloidosis model: Synaptic plasticity and memory defcits in female and male mice. Biol. Sex Differ. 2023, 14, 59. [Google Scholar] [CrossRef]
- Daniel, J.M.; Hulst, J.L.; Berbling, J.L. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology 2006, 147, 607–614. [Google Scholar] [CrossRef]
- Bean, L.A.; Ianov, L.; Foster, T.C. Estrogen receptors, the hippocampus, and memory. Neuroscientist 2014, 20, 534–545. [Google Scholar] [CrossRef]
- Galea, L.A.; Wide, J.K.; Paine, T.A.; Holmes, M.M.; Ormerod, B.K.; Floresco, S.B. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav. Brain Res. 2001, 126, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Estrada, C.M.; Ghisays, V.; Nguyen, E.T.; Caldwell, J.L.; Streicher, J.; Solomon, M.B. Estrogen signaling in the medial amygdala decreases emotional stress responses and obesity in ovariectomized rats. Horm. Behav. 2018, 98, 33–44. [Google Scholar] [CrossRef]
- Djiogue, S.; Djiyou Djeuda, A.B.; Seke Etet, P.F.; Ketcha Wanda, G.J.M.; Djikem Tadah, R.N.; Njamen, D. Memory and exploratory behavior impairment in ovariectomized Wistar rats. Behav. Brain Funct. 2018, 14, 14. [Google Scholar] [CrossRef]
- Walf, A.A.; Paris, J.J.; Frye, C.A. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology 2009, 34, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Huang, G.D.; Xue, Y.X.; Yang, M.; Jia, X.J. Role of estrogen in sex differences in memory, emotion and neuropsychiatric disorders. Mol. Biol. Rep. 2024, 51, 415. [Google Scholar] [CrossRef] [PubMed]
- Samy, D.M.; Mostafa, D.K.; Saleh, S.R.; Hassaan, P.S.; Zeitoun, T.M.; Ammar, G.A.G.; Elsokkary, N.H. Carnosic Acid Mitigates Depression-Like Behavior in Ovariectomized Mice via Activation of Nrf2/HO-1 Pathway. Mol. Neurobiol. 2023, 60, 610–628. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, H.; Bao, Q.; Wang, Y.; Lu, J.; Ni, X. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav. Immun. 2016, 56, 175–186. [Google Scholar] [CrossRef]
- Rodriguez-Landa, J.F.; Cueto-Escobedo, J.; Puga-Olguin, A.; Rivadeneyra-Dominguez, E.; Bernal-Morales, B.; Herrera-Huerta, E.V.; Santos-Torres, A. The Phytoestrogen Genistein Produces Similar Effects as 17beta-Estradiol on Anxiety-Like Behavior in Rats at 12 Weeks after Ovariectomy. Biomed. Res. Int. 2017, 2017, 9073816. [Google Scholar] [CrossRef]
- Hassanein, E.M.; Szelenyi, Z.; Szenci, O. Gonadotropin-Releasing Hormone (GnRH) and Its Agonists in Bovine Reproduction I: Structure, Biosynthesis, Physiological Effects, and Its Role in Estrous Synchronization. Animals 2024, 14, 1473. [Google Scholar] [CrossRef]
- Madeo, F.; Bauer, M.A.; Carmona-Gutierrez, D.; Kroemer, G. Spermidine: A physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy 2019, 15, 165–168. [Google Scholar] [CrossRef]
- Wang, T.A.; Teo, C.F.; Akerblom, M.; Chen, C.; Tynan-La Fontaine, M.; Greiner, V.J.; Diaz, A.; McManus, M.T.; Jan, Y.N.; Jan, L.Y. Thermoregulation via Temperature-Dependent PGD(2) Production in Mouse Preoptic Area. Neuron 2019, 103, 309–322. [Google Scholar] [CrossRef]
- Pollard, K.J.; Wartman, H.D.; Daniel, J.M. Previous estradiol treatment in ovariectomized mice provides lasting enhancement of memory and brain estrogen receptor activity. Horm. Behav. 2018, 102, 76–84. [Google Scholar] [CrossRef]
- Lundholm, L.; Putnik, M.; Otsuki, M.; Andersson, S.; Ohlsson, C.; Gustafsson, J.A.; Dahlman-Wright, K. Effects of estrogen on gene expression profiles in mouse hypothalamus and white adipose tissue: Target genes include glutathione peroxidase 3 and cell death-inducing DNA fragmentation factor, alpha-subunit-like effector A. J. Endocrinol. 2008, 196, 547–557. [Google Scholar] [CrossRef]
- Tang, B.; Hu, S.; Ouyang, Q.; Wu, T.; Lu, Y.; Hu, J.; Hu, B.; Li, L.; Wang, J. Comparative transcriptome analysis identifies crucial candidate genes and pathways in the hypothalamic-pituitary-gonadal axis during external genitalia development of male geese. BMC Genom. 2022, 23, 136. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.T.; Yamanaka, K.; Miyamoto, Y.; Waki, H.; Gouraud, S.S.S. Estradiol-dependent gene expression profile in the amygdala of young ovariectomized spontaneously hypertensive rats. Physiol. Genom. 2022, 54, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.J.; Ronnekleiv, O.K. Minireview: Neural signaling of estradiol in the hypothalamus. Mol. Endocrinol. 2015, 29, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Constantin, S.; Piet, R.; Iremonger, K.; Hwa Yeo, S.; Clarkson, J.; Porteous, R.; Herbison, A.E. GnRH neuron firing and response to GABA in vitro depend on acute brain slice thickness and orientation. Endocrinology 2012, 153, 3758–3769. [Google Scholar] [CrossRef]
- Herbison, A.E. A simple model of estrous cycle negative and positive feedback regulation of GnRH secretion. Front. Neuroendocrinol. 2020, 57, 100837. [Google Scholar] [CrossRef]
- Cheong, R.Y.; Kwakowsky, A.; Barad, Z.; Porteous, R.; Herbison, A.E.; Abraham, I.M. Estradiol acts directly and indirectly on multiple signaling pathways to phosphorylate cAMP-response element binding protein in GnRH neurons. Endocrinology 2012, 153, 3792–3803. [Google Scholar] [CrossRef]
- Melamed, P.; Savulescu, D.; Lim, S.; Wijeweera, A.; Luo, Z.; Luo, M.; Pnueli, L. Gonadotrophin-releasing hormone signalling downstream of calmodulin. J. Neuroendocrinol. 2012, 24, 1463–1475. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, H.; Zhu, M.; Xie, Y.; Nan, Y. MicroRNA-200c Koyunda MAPK8 Gen Düzenleyici Foliküler Gelişim Mekanizmasına Aracılık Eder. Kafkas Univ. Vet. Fak. Derg. 2021, 27, 279–284. [Google Scholar] [CrossRef]
- Aggarwal, A.; Sharma, N.; Khera, A.; Sandhir, R.; Rishi, V. Quercetin alleviates cognitive decline in ovariectomized mice by potentially modulating histone acetylation homeostasis. J. Nutr. Biochem. 2020, 84, 108439. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y.; Li, N.; Zhang, Y.; Zheng, Q.; Sun, M.; Tang, J.; Xu, Z. Melatonin in Reproductive Medicine: A Promising Therapeutic Target? Curr. Med. Chem. 2023, 30, 3090–3118. [Google Scholar] [CrossRef]
- Wang, S.J.; Li, X.F.; Jiang, L.S.; Dai, L.Y. Estrogen stimulates leptin receptor expression in ATDC5 cells via the estrogen receptor and extracellular signal-regulated kinase pathways. J. Endocrinol. 2012, 213, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Jara-Medina, K.; Lillo, L.; Lagunas, C.; Cabello-Guzman, G.; Valenzuela-Melgarejo, F.J. Identification of Vascular Genes Differentially Expressed in the Brain of Patients with Alzheimer’s Disease. Curr. Vasc. Pharmacol. 2024, 22, 404–416. [Google Scholar] [CrossRef]
- Navarro, V.M.; Gottsch, M.L.; Chavkin, C.; Okamura, H.; Clifton, D.K.; Steiner, R.A. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 2009, 29, 11859–11866. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, K.; Kajihara, S.; Umatani, C.; Nakajo, M.; Kanda, S.; Oka, Y. Estrogen upregulates the firing activity of hypothalamic gonadotropin-releasing hormone (GnRH1) neurons in the evening in female medaka. J. Neuroendocrinol. 2022, 34, e13101. [Google Scholar] [CrossRef]
- Zhai, M.; Cao, S.; Liang, H.; Xie, Y.; Zhao, Z. A New Function of the DRD1 Gene: GnRH Secretion Regulation in Sheep Hypothalamic Neurons. Genes 2025, 16, 273. [Google Scholar] [CrossRef]
- Halet, G. PKC signaling at fertilization in mammalian eggs. Biochim. Biophys. Acta 2004, 1742, 185–189. [Google Scholar] [CrossRef]
- Ely, H.A.; Mellon, P.L.; Coss, D. GnRH induces the c-Fos gene via phosphorylation of SRF by the calcium/calmodulin kinase II pathway. Mol. Endocrinol. 2011, 25, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Dobkin-Bekman, M.; Ben-Navi, L.R.; Shterntal, B.; Sviridonov, L.; Przedecki, F.; Naidich-Exler, M.; Brodie, C.; Seger, R.; Naor, Z. Differential role of PKC isoforms in GnRH and phorbol 12-myristate 13-acetate activation of extracellular signal-regulated kinase and Jun N-terminal kinase. Endocrinology 2010, 151, 4894–4907. [Google Scholar] [CrossRef]
- Mugami, S.; Kravchook, S.; Navi, L.R.-B.; Seger, R.; Naor, Z. Differential roles of PKC isoforms (PKCs) and Ca2+ in GnRH and phorbol 12-myristate 13-acetate (PMA) stimulation of p38MAPK phosphorylation in immortalized gonadotrope cells. Mol. Cell. Endocrinol. 2017, 439, 141–154. [Google Scholar] [CrossRef]
- Tan, S.; Zhou, Y.; Zhao, H.; Wu, J.; Yu, H.; Yang, Y.; Yang, Y.; Zhao, H.; Li, H. Comprehensive transcriptome analysis of hypothalamus reveals genes associated with disorders of sex development in pigs. J. Steroid Biochem. Mol. Biol. 2021, 210, 105875. [Google Scholar] [CrossRef]
- Pawson, A.J.; McNeilly, A.S. The pituitary effects of GnRH. Anim. Reprod. Sci. 2005, 88, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, M.; Picart, C.; Gueorguieva, I.; Charbit, J.; Edouard, T.; Linglart, A.; Luton, D.; Chanson, P. The different forms of primary hyperparathyroidism at different ages of life: Childhood, pregnancy, lactation, old age. Proceedings of Annales d’Endocrinologie; p. 101696.
- Lin, J.; Li, Y.; Huang, Z.; Zhu, Y.; Li, L.; Yang, H.; Liang, X.; Qin, Y.; Zhou, J.; Xian, J.; et al. Rare correlation of somatic PRKACA mutations with pregnancy-associated aldosterone- and cortisol-producing adenomas: A case report and literature review. BMC Endocr. Disord. 2024, 24, 116. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Q.; Cui, B.; Cheng, D.; Lyu, H.; Jiang, L.G.; Zheng, K.G.; Liu, S.Z.; Pan, J.; Zhang, C.; Bai, J.; et al. Activated proline-rich tyrosine kinase 2 regulates meiotic spindle assembly in the mouse oocyte. J. Cell Biochem. 2018, 119, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Duan, C.; Zhang, S.; Liu, Y.; Zhang, Y. Prolactin Regulates Ovine Ovarian Granulosa Cell Apoptosis by Affecting the Expression of MAPK12 Gene. Int. J. Mol. Sci. 2023, 24, 10269. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, A.; Muhammad, M.; Chao, X.; Zhang, C.; Chen, J.; Yang, H.; Liu, S.; Ding, Y.; Wang, Z.; Bi, H.; et al. Estradiol Reverses Ovariectomy-Induced Disruption of Hypothalamic Gene Expression and Behavior via Modulation of Gonadotropin Releasing Hormone and Calcium Signaling Pathways. Animals 2025, 15, 1467. https://doi.org/10.3390/ani15101467
Muhammad A, Muhammad M, Chao X, Zhang C, Chen J, Yang H, Liu S, Ding Y, Wang Z, Bi H, et al. Estradiol Reverses Ovariectomy-Induced Disruption of Hypothalamic Gene Expression and Behavior via Modulation of Gonadotropin Releasing Hormone and Calcium Signaling Pathways. Animals. 2025; 15(10):1467. https://doi.org/10.3390/ani15101467
Chicago/Turabian StyleMuhammad, Asim, Mubashir Muhammad, Xiaohuan Chao, Chunlei Zhang, Jiahao Chen, Huan Yang, Shuhan Liu, Yuan Ding, Ziming Wang, Hongwei Bi, and et al. 2025. "Estradiol Reverses Ovariectomy-Induced Disruption of Hypothalamic Gene Expression and Behavior via Modulation of Gonadotropin Releasing Hormone and Calcium Signaling Pathways" Animals 15, no. 10: 1467. https://doi.org/10.3390/ani15101467
APA StyleMuhammad, A., Muhammad, M., Chao, X., Zhang, C., Chen, J., Yang, H., Liu, S., Ding, Y., Wang, Z., Bi, H., Guo, W., Fan, J., & Zhou, B. (2025). Estradiol Reverses Ovariectomy-Induced Disruption of Hypothalamic Gene Expression and Behavior via Modulation of Gonadotropin Releasing Hormone and Calcium Signaling Pathways. Animals, 15(10), 1467. https://doi.org/10.3390/ani15101467




