Potential Use of Tropical and Subtropical Fruits By-Products in Pig Diet: In Vitro Two-Step Evaluation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Locations of the Study
2.2. Samples Collection
2.3. Chemical Composition
2.4. Experimental Design and In Vitro Two-Step Digestibility Evaluation
2.5. Data Processing and Statistical Analysis
3. Results
3.1. Chemical Composition
3.2. In Vitro Digestibility
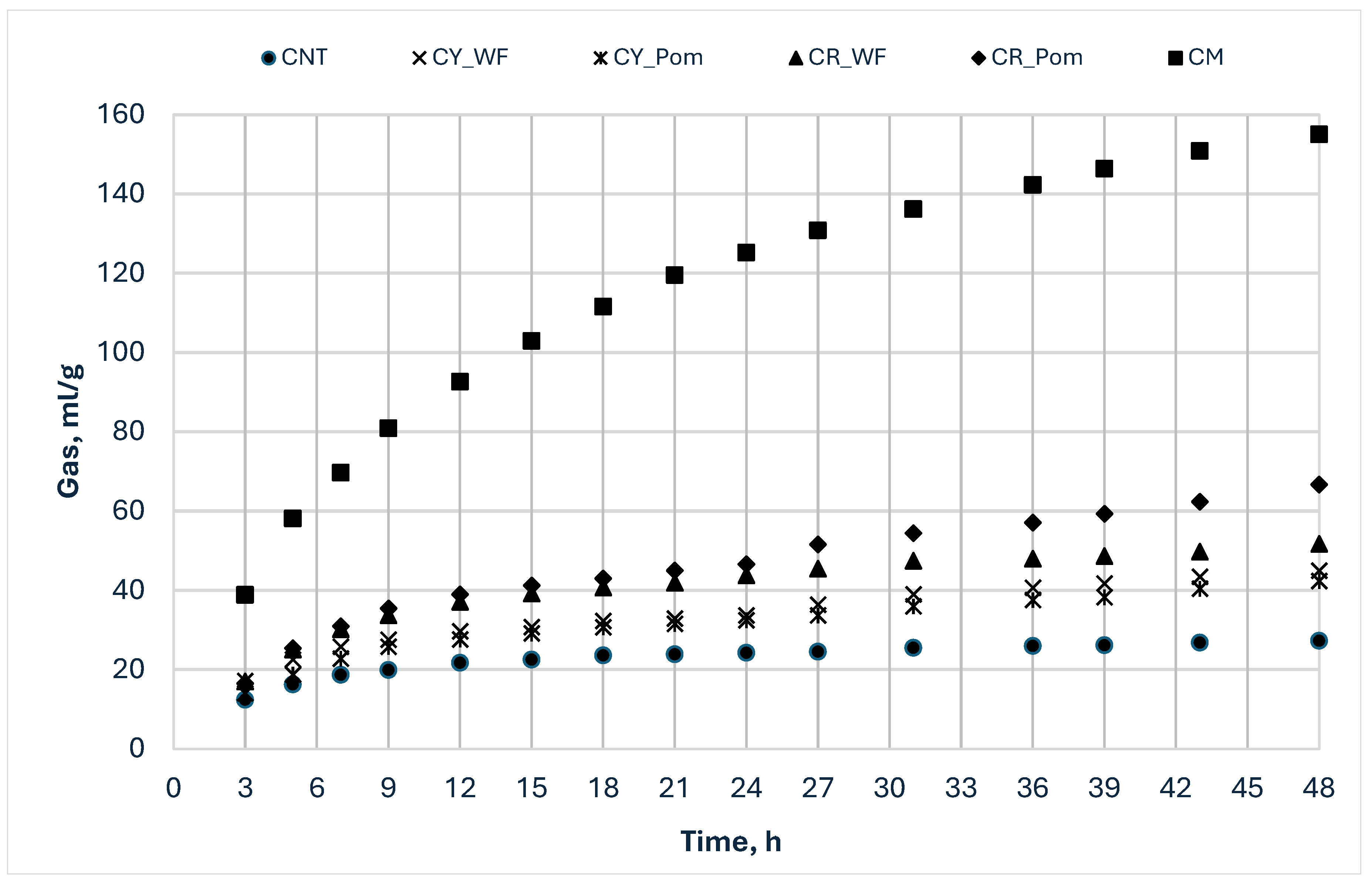
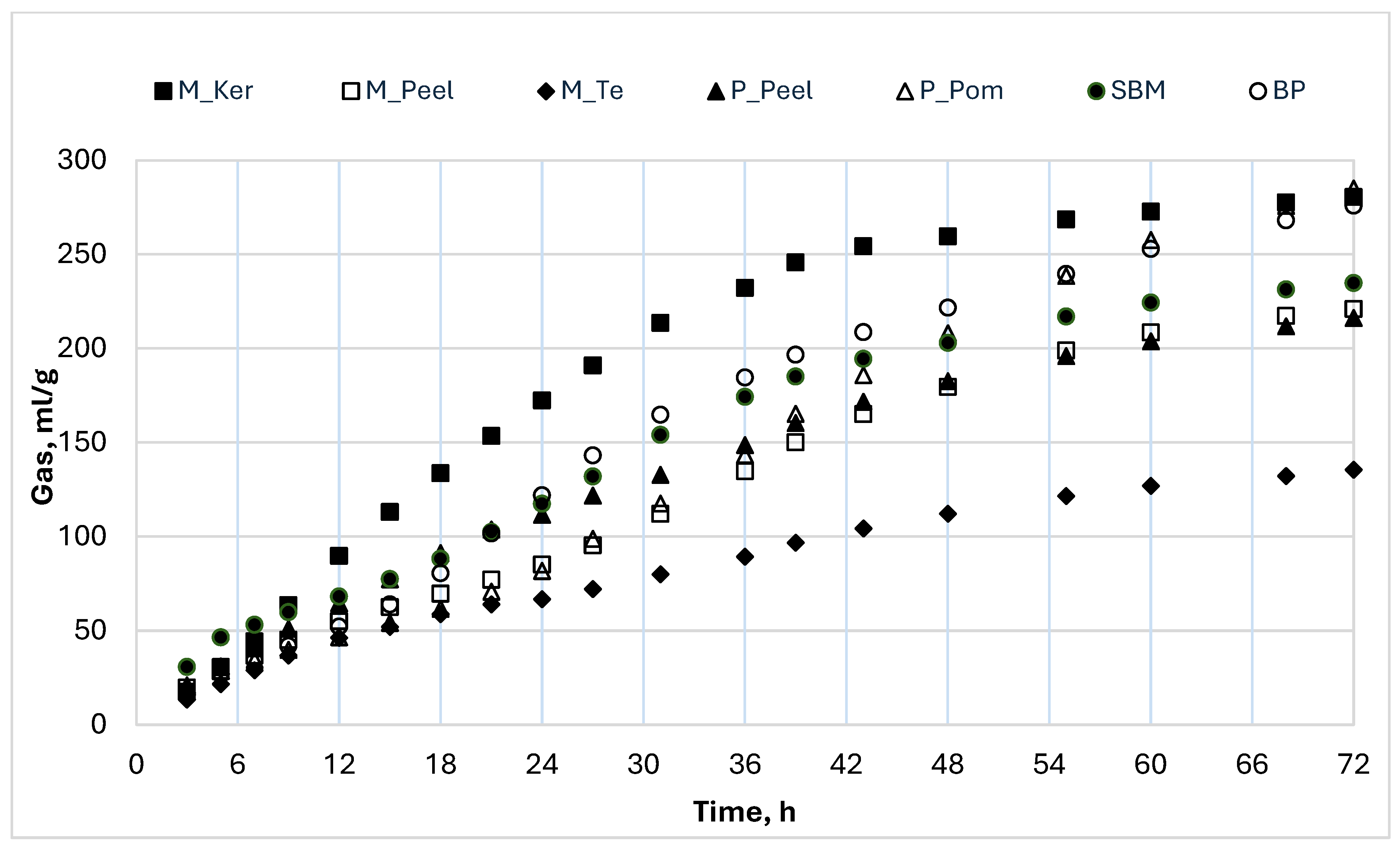
3.3. In Vitro Fermentation Characteristics
3.4. In Vitro Fermentation Kinetics
4. Discussion
4.1. Use of Pineapple By-Products in Pig Nutrition
4.2. Use of Cashew By-Products in Pig Nutrition
4.3. Use of Mango By-Products in Pig Nutrition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porter, S.D.; Reay, D.S.; Higgins, P.; Bomberg, E. A half-century of production-phase greenhouse gas emissions from food loss & waste in the global food supply chain. Sci. Total Environ. 2016, 571, 721–729. [Google Scholar]
- More, P.K.; Singh, S.; Shikha, S. Waste utilization of fruits and vegetables-A review. South Asian J. Food Technol. Environ. 2018, 4, 605–615. [Google Scholar]
- Kiatti, D.d.; Vastolo, A.; Koura, B.I.; Vitaglione, P.; Cutrignelli, M.I.; Calabrò, S. The Chemical Characteristics and In Vitro Degradability of Pineapple By-Products as Potential Feed for Ruminants. Animals 2023, 13, 3238. [Google Scholar] [CrossRef]
- Kiatti, D.d.; Koura, B.I.; Vastolo, A.; Chiacchio, M.F.; Vitaglione, P.; Dossa, L.H.; Cutrignelli, M.I.; Calabrò, S. Sustainable ruminant nutrition in West Africa by in vitro characterization of cashew apple by-products. Heliyon 2024, 10, e37737. [Google Scholar] [CrossRef]
- Agbangba, C.E. Réponses Agronomiques de l’ananas (Ananas Comosus) à La Fertilisation Minérale Au Bénin: Croissance, Rendement et Qualité Du Fruit. Ph.D. Thesis, Université Cheikh Anta Diop de Dakar (UCAD), Dakar, Senegal, 2016. [Google Scholar]
- Vieira, I.M.M.; Santos, B.L.P.; Santos, C.V.M.; Ruzene, D.S.; Silva, D.P. Valorization of PineappleWaste: A Review on How the Fruit’s Potential Can Reduce Residue Generation. Bioenergy Res. 2022, 15, 924–934. [Google Scholar] [CrossRef]
- Sodjinou, M.K.B.; Assouma-Imorou, A.; Olounlade, A.O. Technical Efficiency of Pineapple Production and Challenges in Southern Benin. Afr. J. Agric. Res. 2022, 18, 522–534. [Google Scholar]
- Das, I.; Arora, A. Post-harvest processing technology for cashew apple—A review. J. Food Eng. 2017, 194, 87–98. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations (FAO). Available online: https://www.fao.org/faostat/en/#home (accessed on 13 November 2022).
- Da Silva, J.; De Brito, E.S.; Ferreira, S.R.S. Biorefinery of cashew by-products: Recovery of value-added compounds. Food Bioprocess Technol. 2023, 16, 944–960. [Google Scholar] [CrossRef]
- Muchiri, D.R.; Mahungu, S.M.; Gituanja, S.N. Studies on mango (Mangifera indica, L.) kernel fat of some Kenyan varieties in Meru. J. Am. Oil Chem. Soc. 2012, 89, 1567–1575. [Google Scholar] [CrossRef]
- Ravani, A.; Joshi, D. Mango and it’s by product utilization–a review. Trends Post Harvest Technol. 2013, 1, 55–67. [Google Scholar]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Bio-processing of agro-byproducts to animal feed. Crit. Rev. Biotechnol. 2012, 32, 382–400. [Google Scholar] [CrossRef]
- Timbilfou, K.; Grace, K.M.; Etienne, S.; Drissa, B.; Isidor, O.Z.M.; Vianey, T. Influence of Sorbent Type on Drying Efficiency, Production Costs and Nutritional Values of Mango By-Products Feeds for Livestock. Food Nutr. Sci. 2023, 14, 1221–1231. [Google Scholar] [CrossRef]
- Sanon, H.O.; Kanwe, A.B.; Millogo, A.; Ledin, I. Chemical composition, digestibility, and voluntary feed intake of mango residues by sheep. Trop. Anim. Health Prod. 2013, 45, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.D.; Mintert, J.R.; Anderson, J.D.; Anderson, D.P. Feed grains and livestock: Impacts on meat supplies and prices. Choices 2008, 23, 11–15. [Google Scholar]
- Pinotti, L.; Tretola, M.; Luciano, A.; Ottoboni, M. Feed design applying circular economy principles: The case of former food products. In Book of Abstracts of the 70th Annual Meeting of the European Federation of Animal Science; Wageningen Academic: Wageningen, The Netherlands, 2019; p. 247. [Google Scholar]
- Mourad, M. Recycling, recovering and preventing food waste: Competing solutions for food systems sustainability in the United States and France. J. Clean. Prod. 2016, 126, 461–477. [Google Scholar] [CrossRef]
- Ravindran, V.; Blair, R. Feed resources for poultry production in Asia and the Pacific. II. Plant protein sources. World’s Poult. Sci. J. 1992, 48, 205–231. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I. A review on the use of agro-industrial CO-products in animals’ diets. Ital. J. Anim. Sci. 2022, 21, 577–594. [Google Scholar] [CrossRef]
- Kiendrebeogo, T.; Logtene, Y.M.; Kabore-Zoungrana, C.Y. Effets de rations a base de dechets de mangue sur les performances ponderales et la qualite de la carcasse de porcs Korhogo en croissance au Burkina Faso. J. Appl. Biosci. 2019, 129, 13039–13049. [Google Scholar] [CrossRef]
- Barry, D.; Kiendrebeogo, T.; Sere, M.; Combari, A.; Logtene, Y.M.; Kabore-Zoungrana, C.Y. Effects of Mango Wastes-Based Diets on the Growing Parameters of Laying Hens and Biometric Parameters of the Eggs. Open Access Libr. J. 2019, 6, e5868. [Google Scholar] [CrossRef]
- Milogo, X.D.; Kiendrebeogo, T.; Ouedraogo, I.Z.; Koara, H.; Sawadogo, D.P.; Bougouma-Yameogo, V.M.C. Influence of Mango Feed-Based Diets on the Production and Economic Profitability of Montbeliarde Cows’ Milk at the End of Lactation in Burkina Faso. J. Anim. Sci. 2022, 12, 239–250. [Google Scholar] [CrossRef]
- INSAE. Cahier Des Villages et Quartiers de Ville Département de l ’Atlantique: Ministère Chargé du Plan, de La Prospective et du Développement; INSAE: Cotonou, Bénin, 2004; pp. 1–34. [Google Scholar]
- Gnanglè, C.P.; Glèlè Kakaï, R.; Assogbadjo, A.E.; Vodounnon, S.; Yabi, J.A.; Sokpon, N. Tendances Climatiques Passées, Modelisation, Perception et Adaptations Locale au Bénin. Climatologie 2011, 8, 27–40. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005; Volume 12. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Non-Starch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Boisen, S.; Fernández, J.A. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim. Feed Sci. Technol. 1997, 68, 277–286. [Google Scholar] [CrossRef]
- Musco, N.; Cutrignelli, M.I.; Calabrò, S.; Tudisco, R.; Infascelli, F.; Grazioli, R.; Lo Presti, V.; Gresta, F.; Chiofalo, B. Comparison of nutritional and antinutritional traits among different species (Lupinus albus L., Lupinus luteus L., Lupinus angustifolius L.) and varieties of lupin seeds. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1227–1241. [Google Scholar] [CrossRef]
- EC Council. Regulation 882/2004 on Official controls performed to ensure verification of compliance with feed and food law, animal health and animal welfare rules. Off. J. Eur. Union 2004, L191/1, 1–52. [Google Scholar]
- Barmpatsalou, V.; Dubbelboer, I.R.; Rodler, A.; Jacobson, M.; Karlsson, E.; Pedersen, B.L.; Bergström, C.A. Physiological properties, composition and structural profiling of porcine gastrointestinal mucus. Eur. J. Pharm. Biopharm. 2021, 169, 156–167. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabró, S.; Liotta, L.; Musco, N.; Di Rosa, A.R.; Cutrignelli, M.I.; Chiofalo, B. In vitro fermentation and chemical characteristics of Mediterranean by-products for swine nutrition. Animals 2019, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.S.; Thomaz, M.C.; Castelini, F.R.; Alvarenga, P.V.A.; De Oliveira, J.A.; Ramos, G.F.; Ono, R.K.; Milani, N.C.; dos Santos Ruiz, U. Evaluation of pineapple byproduct at increasing levels in heavy finishing pigs feeding. Anim. Feed Sci. Technol. 2020, 269, 114664. [Google Scholar] [CrossRef]
- Choct, M.; Kocher, A. Non-starch carbohydrates: Digestion and its secondary effects in monogastrics. Proc. Nutr. Soc. Aust. 2000, 24, 31–38. [Google Scholar]
- Göhl, B. Les Aliments du Bétail Sous Les Tropiques; FAO, Division de Production et Santé Animale: Roma, Italy, 1982. [Google Scholar]
- Wadhwa, M.; Bakshi, M.P.S. Utilization of Fruit and Vegetable Wastes as Livestock Feed and as Substrates for Generation of Other Value-Added Products; RAP Publication: Bangkok, Thailand, 2013; Volume 4, p. 67. [Google Scholar]
- Trox, J.; Vadivel, V.; Vetter, W.; Stuetz, W.; Kammerer, D.R.; Carle, R.; Scherbaum, V.; Gola, U.; Nohr, D.; Biesalski, H.K. Catechin and epicatechin in testa and their association with bioactive compounds in kernels of cashew nut (Anacardium occidentale L.). Food Chem. 2011, 128, 1094–1099. [Google Scholar] [CrossRef]
- Poommarin, P.; Sulabo, R.C.; Sevilla, C.C. Feeding value of dried cashew nut testa in finishing pigs: Effects on nutrient digestibility and gut morphology. Songklanakarin J. Sci. Technol. 2018, 40, 1300–1305. [Google Scholar]
- Acero, L.H.; Lagan, C.G.; Padul, M.A.C. Growth performance of fattening hogs fed with fresh and dried cashew apple. Int. Proc. Chem. Biol. Environ. Eng. 2013, 51, 23–27. [Google Scholar]
- Farias, L.A.; Lopes, J.B.; de Figueirêdo, A.V.; de Noronha Albuquerque, D.M.; de Araújo Neto, A.A.; Ramos, L.D.S.N. Cashew pulp meals (Anacardium occidentale L.) for growing pig: Nutrient metabolism and performance. Ci. Anim. Bras. 2008, 51, 100–109. [Google Scholar]
- Heuzé, V.; Tran, G.; Archimède, H. Mango (Mangifera indica) Forage. Feedipedia. 2019. Available online: https://feedipedia.org/node/129 (accessed on 31 July 2019).
- Rao, D.S.; Ravi, A.; Yedukondalu, R. Inclusion of dried mango (Mangifera indica) peels in finisher rations of pigs on their performance. Indian J. Anim. Nutr. 2003, 20, 120–123. [Google Scholar]
| Items | DM | Ash | NDF | ADF | ADL | EE | CP | NSC |
|---|---|---|---|---|---|---|---|---|
| % | % DM | |||||||
| P_Peel | 14.51 | 5.95 | 35.02 | 17.22 | 1.83 | 0.47 | 7.77 | 44.31 |
| P_Pom | 17.63 | 3.91 | 20.32 | 9.82 | 1.70 | 0.27 | 9.18 | 50.20 |
| CNT | 91.42 | 2.25 | 22.51 | 17.60 | 6.79 | 26.42 | 11.52 | 35.63 |
| CY_WF | 13.00 | 3.00 | 16.13 | 15.90 | 7.67 | 2.19 | 7.54 | 61.32 |
| CY_Pom | 21.62 | 2.33 | 33.82 | 28.51 | 11.63 | 4.73 | 10.21 | 43.24 |
| CR_WF | 13.35 | 3.54 | 19.75 | 18.42 | 9.69 | 1.51 | 6.21 | 59.61 |
| CR_Pom | 27.22 | 2.16 | 33.53 | 31.80 | 11.50 | 3.17 | 7.73 | 47.60 |
| M_Peel | 22.27 | 4.45 | 12.43 | 11.32 | 1.52 | 1.46 | 2.51 | 75.54 |
| M_Ker | 42.12 | 2.40 | 10.90 | 4.18 | 0.14 | 11.23 | 4.69 | 68.70 |
| M_T | 52.04 | 3.82 | 25.32 | 24.13 | 8.68 | 3.66 | 3.97 | 59.93 |
| CM | 86.71 | 1.48 | 7.66 | 2.83 | 2.40 | 1.72 | 7.65 | 68.25 |
| SBM | 89.32 | 7.77 | 14.91 | 5.07 | 0.40 | 0.99 | 51.23 | 14.66 |
| BP | 24.34 | 1.50 | 47.15 | 23.83 | 2.70 | 0.83 | 9.32 | 33.02 |
| Items | DMDe | DMDm | DMDt |
|---|---|---|---|
| Incubation at 48 h | |||
| CNT | 18.8 e | 12.0 d | 28.5 e |
| CY_WF | 60.0 b | 24.3 b | 69.7 b |
| CY_Pom | 30.0 d | 24.7 b | 47.3 c |
| CR_WF | 61.4 b | 20.4 c | 69.3 b |
| CR_Pom | 38.0 c | 10.6 d | 44.6 d |
| CM | 67.3 a | 64.1 a | 88.3 a |
| p-value | <0.0001 | <0.0001 | <0.0001 |
| MSE | 2.742 | 0.530 | 0.837 |
| Incubation at 72 h | |||
| P_Peel | 50.3 b | 59.0 e | 79.6 d |
| P_Pom | 68.3 a | 68.8 cd | 90.1 b |
| M_Peel | 48.4 b | 70.1 c | 84.6 c |
| M_Ker | 10.0 c | 67.4 d | 70.7 e |
| M_T | 11.8 c | 24.7 f | 33.6 f |
| SBM | 65.2 a | 83.2 a | 94.2 a |
| BP | 64.0 a | 78.0 b | 92.1 b |
| p-value | <0.0001 | <0.0001 | <0.0001 |
| MSE | 8.07 | 0.907 | 0.752 |
| Items | pH | OMCV | Ace | Pro | Iso-But | But | Iso-Val | Val | SCFA | BCFA |
|---|---|---|---|---|---|---|---|---|---|---|
| ml/g | mmol/g | |||||||||
| Incubation at 48 h | ||||||||||
| CNT | 6.25 AB | 27.31 D | 11.62 D | 3.04 E | 0.22 D | 2.55 B | 0.54 D | 0.51 D | 18.52 D | 4.12 D |
| CY_WF | 6.25 AB | 45.05 C | 20.44 B | 7.23 BC | 1.34 B | 3.85 B | 2.50 A | 1.04 AC | 36.44 BC | 10.63 AB |
| CY_Pom | 6.24 B | 42.03 C | 17.43 C | 6.20 CD | 0.97 C | 3.40 B | 2.11 A | 1.74 B | 31.73 BC | 9.66 B |
| CR_WF | 6.32 A | 52.06 BC | 17.44 C | 5.34 D | 1.23 B | 3.09 B | 2.25 A | 1.28 C | 30.60 C | 11.45 A |
| CR_Pom | 6.20 B | 58.05 B | 20.72 B | 7.54 B | 0.96 C | 3.91 B | 1.54 | 1.96 B | 36.73 B | 6.84 C |
| CM | 5.75 C | 142 A | 38.51 A | 13.13 A | 1.90 C | 26.0 A | 2.47 A | 5.86 | 87.92 A | 4.98 D |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| MSE | 0.003 | 28.52 | 2.569 | 0.387 | 0.015 | 1.273 | 0.05 | 0.033 | 11.02 | 0.467 |
| Incubation at 72 h | ||||||||||
| P_Peel | 5.90 B | 226 C | 59.42 AB | 17.81 BC | 1.57 | 15.02 B | 2.20 | 2.58 | 98.52 | 3.84 C |
| P_Pom | 5.63 A | 285 A | 64.81 A | 26.74 A | 1.73 | 12.45 B | 2.34 | 3.02 | 111.00 | 2.67 D |
| M_Peel | 5.75 C | 221 C | 55.10 A | 20.13 BC | 1.65 | 9.02 B | 2.33 | 2.39 | 90.64 | 4.38 B |
| M_Ker | 5.70 CD | 281 A | 43.74 CD | 26.66 A | 1.58 | 25.72 A | 2.26 | 2.13 | 102.01 | 3.73 C |
| M_T | 6.12 A | 136 D | 32.02 D | 12.84 C | 1.45 | 12.75 AB | 2.89 | 2.99 | 69.92 | 6.23 A |
| SBM | 5.84B C | 253 B | 49.63 BC | 28.14 A | 1.87 | 13.48 B | 2.36 | 3.09 | 98.43 | 4.30 B |
| BP | 5.61 D | 276 A | 60.30 AB | 24.22 AB | 1.43 | 16.83 AB | 2.10 | 2.86 | 108.14 | 3.29 CD |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.147 | <0.0001 |
| MSE | 0.007 | 47.37 | 46.19 | 22.56 | 0.100 | 33.10 | 0.223 | 0.143 | 290 | 0.061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiatti, D.d.; Serrapica, F.; Musco, N.; Di Palo, R.; Calabrò, S. Potential Use of Tropical and Subtropical Fruits By-Products in Pig Diet: In Vitro Two-Step Evaluation. Animals 2025, 15, 1454. https://doi.org/10.3390/ani15101454
Kiatti Dd, Serrapica F, Musco N, Di Palo R, Calabrò S. Potential Use of Tropical and Subtropical Fruits By-Products in Pig Diet: In Vitro Two-Step Evaluation. Animals. 2025; 15(10):1454. https://doi.org/10.3390/ani15101454
Chicago/Turabian StyleKiatti, Dieu donné, Francesco Serrapica, Nadia Musco, Rossella Di Palo, and Serena Calabrò. 2025. "Potential Use of Tropical and Subtropical Fruits By-Products in Pig Diet: In Vitro Two-Step Evaluation" Animals 15, no. 10: 1454. https://doi.org/10.3390/ani15101454
APA StyleKiatti, D. d., Serrapica, F., Musco, N., Di Palo, R., & Calabrò, S. (2025). Potential Use of Tropical and Subtropical Fruits By-Products in Pig Diet: In Vitro Two-Step Evaluation. Animals, 15(10), 1454. https://doi.org/10.3390/ani15101454









