Ergothioneine Improves the Quality of Boar Sperm During In Vitro Liquid Preservation by Regulating Mitochondrial Respiratory Chain
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Semen Collection and Treatment
2.3. Experimental Design
2.4. Measurement of Sperm Motility and Kinetic Parameters
2.5. Measurement of Sperm Mitochondrial Membrane Potential
2.6. Measurement of Sperm Plasma Membrane Integrity
2.7. Measurement of Sperm Acrosome Integrity
2.8. Measurement of Sperm Total Antioxidant Capacity Activity, H2O2, and MDA Levels
2.9. Measurement of Sperm ROS Levels
2.10. Statistical Analysis
3. Results
3.1. Effects of Different Concentrations of EGT on Boar Sperm Motility and Kinetic Parameters
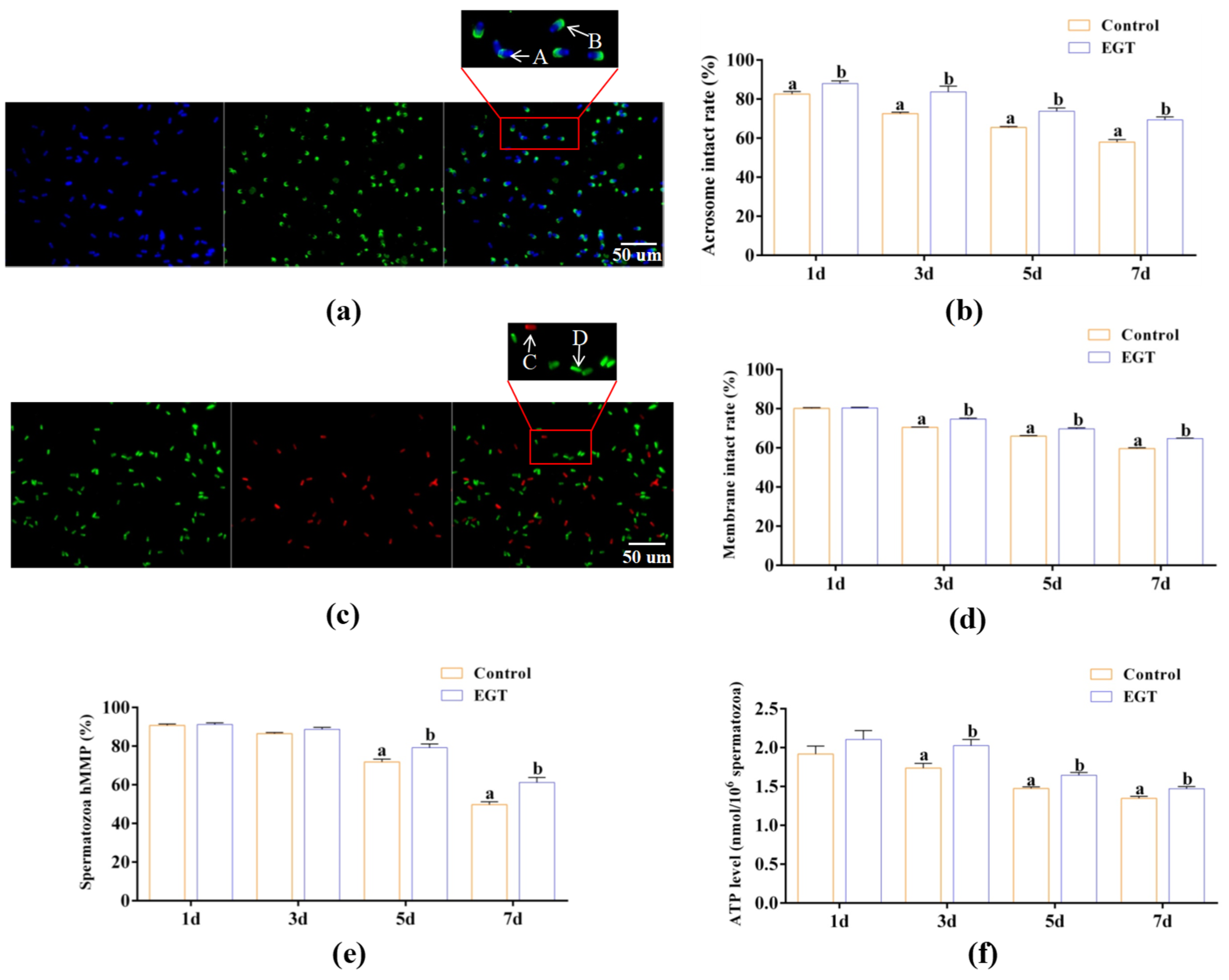
3.2. Effects of EGT on Sperm Acrosome and Plasma Membrane Integrity, MMP, and ATP Level
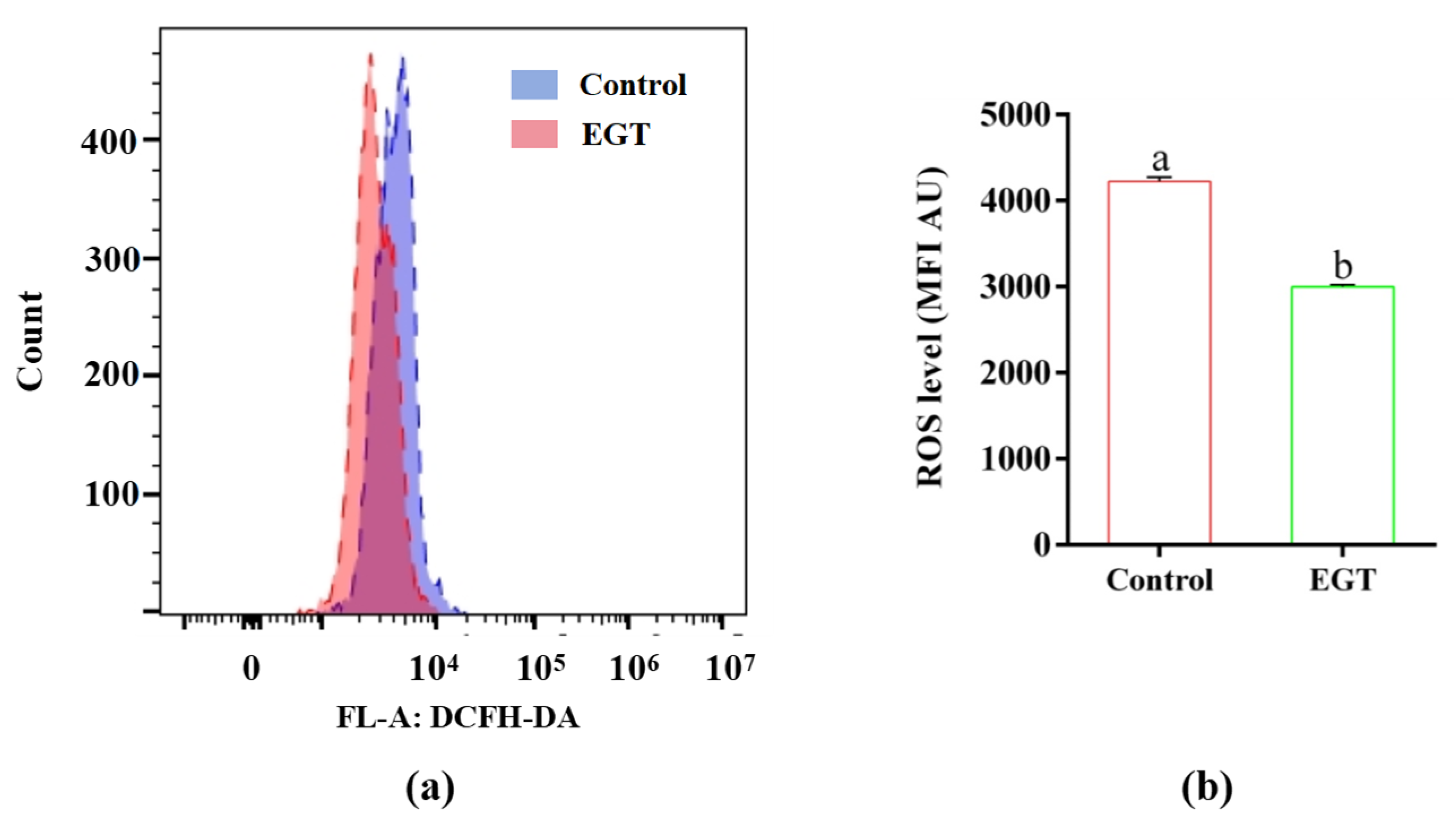
3.3. Effects of EGT on Boar Sperm T-AOC Activity and H2O2 Levels
3.4. Effects of ROT on Boar Sperm Motility and Kinetic Parameters
3.5. EGT Improved the Motility and Kinetic Parameters in ROT-Treated Sperm
3.6. EGT Improved the Acrosome and Plasma Membrane Integrity, MMP, and ATP Levels in ROT-Treated Sperm
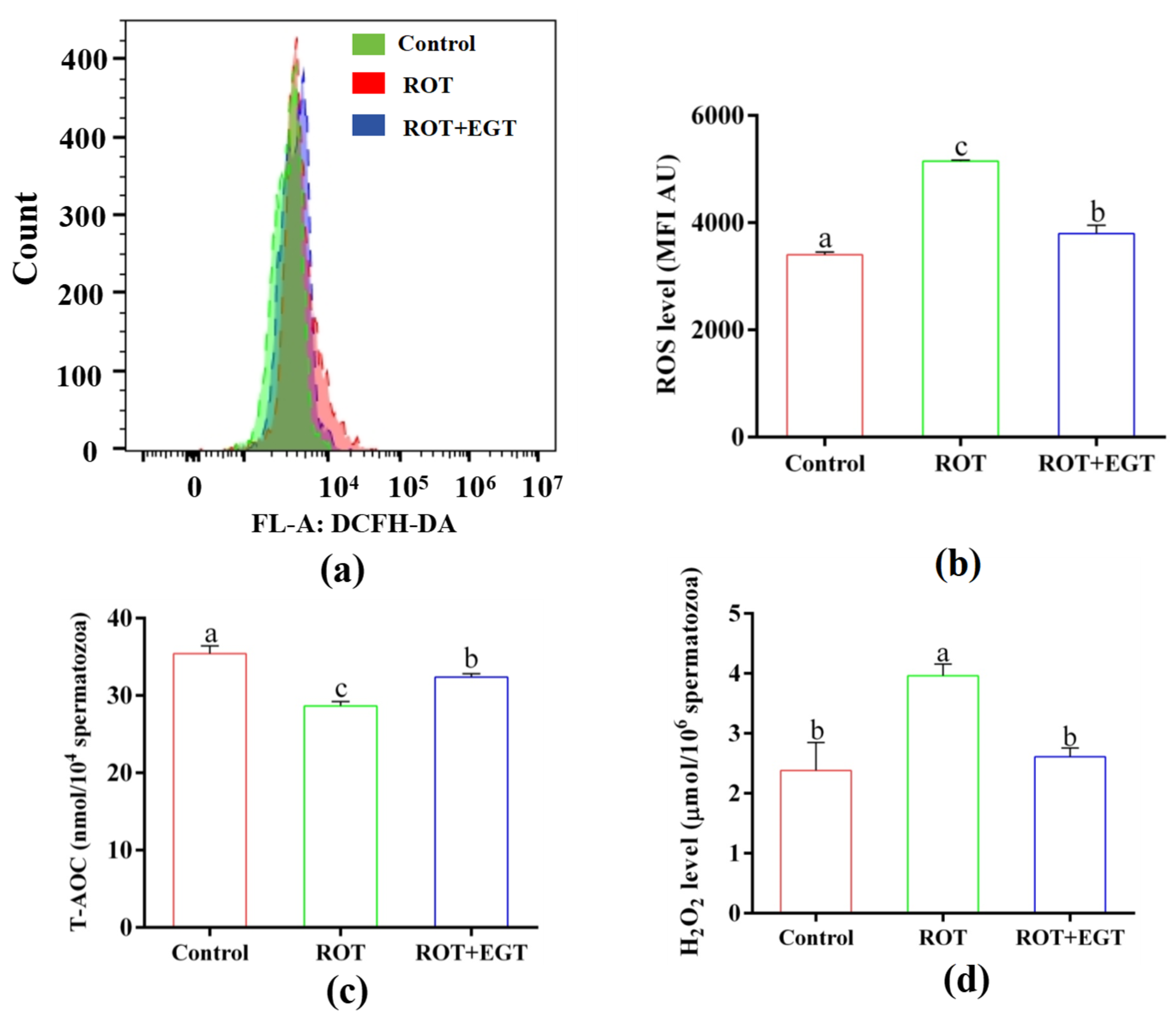
3.7. EGT Improved the Antioxidant Capability in ROT-Treated Sperm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EGT | Ergothioneine |
| MMP | Mitochondrial membrane potential |
| ATP | Adenosine triphosphate |
| MT-ND1 | NADH dehydrogenase 1 |
| ROT | Rotenone |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| GSH | Glutathione |
| MDA | Malondialdehyde |
| MCEAC | Management Committee of the Experimental Animal Center |
| CASA | Computer-assisted sperm analysis |
| VAP | Average path velocity |
| VSL | Average straight-line velocity |
| VCL | Average curvilinear velocity |
| BCF | Beat-cross frequency |
| STR | Straightness of path |
| PI | Propidium iodide |
| FITC-PNA | Fluorescein peanut agglutinin isothiocyanate |
| PBS | Phosphate-buffered saline |
| H2O2 | Hydrogen peroxide |
| BSA | Bovine Serum Albumin |
References
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M. Storage of boar semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef] [PubMed]
- Lopez Rodriguez, A.; Van Soom, A.; Arsenakis, I.; Maes, D. Boar management and semen handling factors affect the quality of boar extended semen. Porc. Health Manag. 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Berean, D.; Blaga-Petrean, A.; Bogdan, I.; Bogdan, S.; Tamas-Krumpe, O.M.; Cimpean, R.; Pall, E.; Nap, M.E.; Bogdan, L.M. Effect of L-arginine and eugenol on ram semen kinematic parameters and post thawed fertility rate after trans-cervical artificial insemination. Indian. J. Anim. Res. 2023, 57, 552–557. [Google Scholar] [CrossRef]
- Tahara, E.B.; Navarete, F.D.T.; Kowaltowski, A.J. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free. Radic. Biol. Med. 2009, 46, 1283–1297. [Google Scholar] [CrossRef]
- Upadhyay, V.R.; Ramesh, V.; Dewry, R.K.; Yadav, D.K.; Ponraj, P. Bimodal interplay of reactive oxygen and nitrogen species in physiology and pathophysiology of bovine sperm function. Theriogenology 2022, 187, 82–94. [Google Scholar] [CrossRef]
- Yeoman, R.R.; Jones, W.D.; Rizk, B.M. Evidence for nitric oxide regulation of hamster sperm hyperactivation. J. Androl. 1998, 19, 58–64. [Google Scholar] [CrossRef]
- Herrero, M.B.; Lamirande, E.; Gagnon, C. Nitric Oxide is a Signaling Molecule in Spermatozoa. Curr. Pharm. Des. 2003, 9, 419–425. [Google Scholar] [CrossRef]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M. Oxidative stress, DNA damage and apoptosis in male infertility: A clinical approach. BJU Int. 2005, 95, 503–507. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Fan, X.; Lv, Y.; Zeng, W.; Longevity, C. Resveratrol Improves Boar Sperm Quality via 5′AMP-Activated Protein Kinase Activation during Cryopreservation. Oxid. Med. Cell. Longev. 2019, 2019, 5921503. [Google Scholar] [CrossRef]
- Iakovlev, T.E. Effect of antibiotics on the quantity of sperm of bulls and boars. Veterinariia 1970, 46, 103–104. [Google Scholar] [PubMed]
- Li, D.; Zhang, W.; Tian, X.; He, Y.; Xiao, Z.; Zhao, X.; Lin, F.; Renrang, D.; Gongshe, Y.; Taiyong, Y. Hydroxytyrosol effectively improves the quality of pig sperm at 17 degrees C. Theriogenology 2022, 177, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Koppers, A.J.; De, I.G.N.; Finnie, J.M.; McLaughlin, E.A.; John, A.R. Significance of Mitochondrial Reactive Oxygen Species in the Generation of Oxidative Stress in Spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J. Bioactive metabolites: The double-edged sword in your food. Cell 2022, 185, 4469–4471. [Google Scholar] [CrossRef]
- Nikodemus, D.; Lazic, D.; Bach, M.; Bauer, T.; Pfeiffer, C.; Wiltzer, L.; Lain, E.; Schömig, E.; Gründemann, D. Paramount levels of ergothioneine transporter SLC22A4 mRNA in boar seminal vesicles and cross-species analysis of ergothioneine and glutathione in seminal plasma. J. Physiol. Pharmacol. 2011, 62, 411–419. [Google Scholar]
- Salama, S.A.; Omar, H.A. Modulating NF-κB, MAPK, and PI3K/AKT signaling by ergothioneine attenuates iron overload-induced hepatocellular injury in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22729. [Google Scholar] [CrossRef]
- Ishimoto, T.; Masuo, Y.; Kato, Y.; Nakamichi, N. Ergothioneine-induced neuronal differentiation is mediated through activation of S6K1 and neurotrophin 4/5-TrkB signaling in murine neural stem cells. Cell. Signal. 2019, 53, 269–280. [Google Scholar] [CrossRef]
- Li, J.; Dong, Y.; Wang, H.; Zhang, Q.; Guo, Q.; Li, Y. The Cryoprotectant Effects of Safffower Polysaccharides on the Quality of Frozen-Thawed Boar Sperm. Animals 2025, 15, 843. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, S.; Zhang, Q.; Zhang, H.; Li, T.; Wang, Q.; Guo, M.; Feng, H.; Song, Y.; et al. Methylprednisolone improves the quality of liquid preserved boar spermatozoa in vitro and reduces polymorphonuclear neutrophil chemotaxis and phagocytosis. Front. Vet. Sci. 2023, 10, 1177873. [Google Scholar] [CrossRef]
- Zhu, Z.; Umehara, T.; Okazaki, T.; Goto, M.; Fujita, Y.; Hoque, S.A.M.; Kawai, T.; Zeng, W.; Shimada, M. Gene Expression and Protein Synthesis in Mitochondria Enhance the Duration of High-Speed Linear Motility in Boar Sperm. Front. Physiol. 2019, 10, 252. [Google Scholar] [CrossRef]
- Nadaf, S.M.; Ramesh, V.; Mech, M.; Haider Khan, M.; Ahmed, F.A.; Ponraj, P.; Mitra, A. Comparative ejaculatory response, fresh and frozen semen quality and fertility to artificial vagina vs electroejaculation method of semen collection in mithun (Bos frontalis) bulls. Andrologia 2022, 54, e14330. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Quan, F.; Chen, D.; Zhang, B.; Zhang, Y. Alterations in mitochondrial function and spermatozoal motility in goat spermatozoa following incubation with a human lysozyme plasmid. Anim. Reprod. Sci. 2010, 121, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.T.; Wang, J.R.; Sun, L.Z.; Jin, X.H.; Shi, X.Y.; Lin, J.Y.; Yue, S.L.; Zhou, J.B. Effects of astaxanthin on plasma membrane function and fertility of boar sperm during cryopreservation. Theriogenology 2021, 164, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Aboagla, M.E.; Maeda, T. Arbutin’s suppression of cryodamage in goat sperm and its mechanism of cryoprotection. Theriogenology 2011, 76, 538–546. [Google Scholar] [CrossRef]
- Zhu, Z.; Kawai, T.; Umehara, T.; Hoque, S.A.M.; Zeng, W.; Shimada, M. Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radic. Biol. Med. 2019, 141, 159–171. [Google Scholar] [CrossRef]
- Rodríguez, A.L.; Rijsselaere, T.; Vyt, P.; Soom, A.V.; Maes, D. Effect of Dilution Temperature on Boar Semen Quality. Reprod. Domest. Anim. 2012, 47, e63–e66. [Google Scholar] [CrossRef]
- Pavaneli, A.P.P.; Passarelli, M.D.S.; Freitas, D.; Vieira, F.; Ravagnani, G.M.; Torres, M.A.; Martins, S.M.M.K.; Yeste, M.; Andrade, A.F.C.D. Removal of seminal plasma prior to liquid storage of boar spermatozoa: A practice that can improve their fertilizing ability. Theriogenology 2019, 125, 79–86. [Google Scholar] [CrossRef]
- Lee, S.; Iwasaki, Y.; Shikina, S.; Yoshizaki, G. Generation of functional eggs and sperm from cryopreserved whole testes. Proc. Natl. Acad. Sci. USA 2013, 110, 1640–1645. [Google Scholar] [CrossRef]
- Karunakaran, M.; Chakurkar, E.B.; Ratnakaran, U.; Naik, P.K.; Mondal, M.; Mondal, A.; Singh, N.P. Characteristics of boar semen preserved at liquid state. J. Appl. Anim. Res. 2016, 45, 217–220. [Google Scholar] [CrossRef]
- Lewandowska, E.; Wesierski, D.; Mazur-Milecka, M.; Liss, J.; Jezierska, A. Ensembling noisy segmentation masks of blurred sperm images. Comput. Biol. Med. 2023, 166, 107520. [Google Scholar] [CrossRef]
- Van de Hoek, M.; Rickard, J.P.; de Graaf, S.P. Motility Assessment of Ram Spermatozoa. Biology 2022, 11, 1715. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Shibahara, H.; Obara, H.; Suzuki, T.; Takamizawa, S.; Yamaguchi, C.; Tsunoda, H.; Sato, I. Relationships between Sperm Motility Characteristics Assessed by the Computer-Aided Sperm Analysis (CASA) and Fertilization Rates in Vitro. J. Assist. Reprod. Genet. 2001, 18, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Clarke, G.N.; Baker, H.W. Relationship between Sperm Motility Assessed with the Hamilton-Thorn Motility Analyzer and Fertilization Rates in Vitro. J. Androl. 1991, 12, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Muvhali, P.T.; Bonato, M.; Malecki, I.A.; Cloete, S.W.P. Mass Sperm Motility Is Correlated to Sperm Motility as Measured by Computer-Aided Sperm Analysis (CASA) Technology in Farmed Ostriches. Animals 2022, 12, 1104. [Google Scholar] [CrossRef]
- Bae, J.T.; Lee, C.H.; Lee, G.S.; Kim, J.H.; Hong, J.T. Glycation inhibitory and antioxidative activities of ergothioneine. J. Soc. Cosmet. Sci. Korea 2019, 45, 151–159. [Google Scholar]
- Ko, H.J.; Kim, J.; Ahn, M.; Kim, J.H.; Lee, G.S.; Shin, T. Ergothioneine alleviates senescence of fibroblasts induced by UVB damage of keratinocytes via activation of the Nrf2/HO-1 pathway and HSP70 in keratinocytes. Exp. Cell Res. 2021, 400, 112516. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.S.; Yang, C.; Sit, A.S.M.; Kwan, Y.W.; Lee, S.M.Y.; Hoi, M.P.M.; Chan, S.W.; Hausman, M.; Vanhoutte, P.M.; Leung, G.P.H. Uptake and protective effects of ergothioneine in human endothelial cells. J. Pharmacol. Exp. Ther. 2014, 350, 691–700. [Google Scholar] [CrossRef]
- Behof, W.J.; Whitmore, C.A.; Haynes, J.R.; Rosenberg, A.J.; Tantawy, M.N.; Peterson, T.E.; Harrison, F.E.; Beelman, R.B.; Pham, W. A novel antioxidant ergothioneine PET radioligand for in vivo imaging applications. Sci. Rep. 2021, 11, 18450. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Spencer, J.P.; Mahmood, N. Protection against oxidative damage and cell death by the natural antioxidant ergothioneine. Food. Chem. Toxicol. 1999, 37, 1043–1053. [Google Scholar] [CrossRef]
- Olaciregui, M.; Luño, V.; Domingo, P.; González, N.; Gil, L. In vitro developmental ability of ovine oocytes following intracytoplasmic injection with freeze-dried spermatozoa. Sci. Rep. 2017, 7, 1096. [Google Scholar] [CrossRef]
- Berger, T.; Anderson, D.L.; Penedo, M.C.T. Porcine sperm fertilizing potential in relationship to sperm functional capacities. Anim. Reprod. Sci. 1996, 44, 231–239. [Google Scholar] [CrossRef]
- Sutkeviciene, N.; Riskeviciene, V.; Januskauskas, A.; Zilinskas, H.; Andersson, M. Assessment of sperm quality traits in relation to fertility in boar semen. Acta. Vet. Scand. 2009, 51, 53. [Google Scholar] [CrossRef]
- Mannowetz, N.; Wandernoth, P.M.; Wennemuth, G. Glucose is a pH-Dependent Motor for Sperm Beat Frequency during Early Activation. PLoS ONE 2012, 7, e41030. [Google Scholar] [CrossRef]
- Gnaiger, E. Bioenergetics at low oxygen: Dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir. Physiol. 2001, 128, 277–297. [Google Scholar] [CrossRef]
- Ko, E.Y.; Sabanegh, E.S.; Agarwal, A. Male infertility testing: Reactive oxygen species and antioxidant capacity. Fertil. Steril. 2014, 102, 1518–1527. [Google Scholar] [CrossRef]
- Graziewicz, M.A.; Day, B.J.; Copeland, W.C. The mitochondrial DNA polymerase as a target of oxidative damage. Nucleic. Acids. Res. 2002, 30, 2817–2824. [Google Scholar] [CrossRef]
- Tan, E.C.T.; Janssen, A.J.M.; Roestenberg, P.; Heuvel, L.P.V.D.; Rodenburg, R.J.T. Mitochondrial dysfunction in muscle tissue of complex regional pain syndrome type I patients. Eur. J. Pain. 2011, 15, 708–715. [Google Scholar]
- Xie, X.; Le, L.; Fan, Y.; Lv, L.; Zhang, J. Autophagy is induced through the ROS-TP53-DRAM1 pathway in response to mitochondrial protein synthesis inhibition. Autophagy 2012, 8, 1071–1084. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, W.; Yang, Q.; Zhao, H.; Zhang, W.; Adetunji, A.O.; Hoque, S.A.M.; Kou, X.; Min, L. Pyrroloquinoline Quinone Improves Ram Sperm Quality through Its Antioxidative Ability during Storage at 4 °C. Antioxidants. 2024, 13, 104. [Google Scholar] [CrossRef]
- Heo, G.; Sun, M.H.; Jiang, W.J.; Li, X.H.; Lee, S.H.; Guo, J.; Zhou, D.J.; Cui, X.S. Rotenone causes mitochondrial dysfunction and prevents maturation in porcine oocytes. PLoS ONE 2022, 17, e0277477. [Google Scholar] [CrossRef]
- Plaza Dávila, M.; Bucci, D.; Galeati, G.; Peña, F.J.; Mari, G.; Giaretta, E.; Tamanini, C.; Spinaci, M. Epigallocatechin-3-Gallate (EGCG) Reduces Rotenone Effect on Stallion Sperm-Zona Pellucida Heterologous Binding. Reprod. Domest. Anim. 2015, 50, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.J.; Ueno, M.; Yanagimachi, R. Inhibition of hamster sperm acrosome reaction and fertilization by oligomycin, antimycin A, and rotenone. J. Exp. Zool. 1977, 199, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Mollier, R.T.; Kumar, D.; Katiyar, R.; Chamuah, J.K.; Kumar, S.; Chaudhary, J.K.; Deori, S.; Kalita, H.; Mishra, V.K. Temporal effect of flaxseed oil in boar’s diet on semen quality, antioxidant status and in-vivo fertility under hot humid sub-tropical condition. Sci. Rep. 2024, 14, 21694. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Groups (EGT) | Storage Time (Day) | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | ||
| Total motility/(%) | 0 mM | 88.06 ± 1.57 | 75.88 ± 1.23 a | 62.27 ± 1.06 a | 49.27 ± 1.74 a | 36.28 ± 0.36 a |
| 0.15 mM | 88.06 ± 1.57 | 78.45 ± 0.54 ab | 68.43 ± 0.71 b | 54.73 ± 0.68 b | 40.24 ± 1.39 b | |
| 0.3 mM | 88.06 ± 1.57 | 82.07 ± 0.60 b | 73.58 ± 1.07 b | 59.31 ± 0.66 c | 48.58 ± 0.75 c | |
| 0.6 mM | 88.06 ± 1.57 | 72.25 ± 1.72 a | 60.78 ± 0.86 a | 49.24 ± 1.65 a | 34.73 ± 1.45 a | |
| Progressive Motility/(%) | 0 mM | 78.87 ± 2.58 | 61.19 ± 1.23 ab | 44.23 ± 1.91 a | 35.21 ± 1.38 a | 23.22 ± 0.52 a |
| 0.15 mM | 78.87 ± 2.58 | 62.31 ± 0.54 a | 55.02 ± 0.86 b | 39.18 ± 0.55 b | 24.97 ± 0.65 ab | |
| 0.3 mM | 78.87 ± 2.58 | 68.21 ± 0.60 c | 57.67 ± 0.62 b | 45.01 ± 1.05 c | 26.44 ± 0.25 b | |
| 0.6 mM | 78.87 ± 2.58 | 59.06 ± 1.72 b | 44.72 ± 0.70 a | 39.77 ± 1.15 b | 25.98 ± 1.08 ab | |
| VAP/(μm/s) | 0 mM | 50.61 ± 2.10 | 34.59 ± 0.50 a | 31.34 ± 0.63 a | 16.47 ± 1.38 a | 18.35 ± 0.36 a |
| 0.15 mM | 50.61 ± 2.10 | 34.02 ± 0.33 a | 32.68 ± 1.54 a | 18.24 ± 0.55 a | 18.96 ± 0.39 a | |
| 0.3 mM | 50.61 ± 2.10 | 42.65 ± 0.78 b | 39.88 ± 1.60 b | 30.58 ± 1.05 b | 25.44 ± 0.95 b | |
| 0.6 mM | 50.61 ± 2.10 | 35.27 ± 0.65 a | 31.73 ± 1.13 a | 29.64 ± 1.15 b | 22.58 ± 1.55 b | |
| VSL/(μm/s) | 0 mM | 44.87 ± 1.15 | 36.91 ± 0.86 a | 32.34 ± 0.51 a | 13.51 ± 0.45 a | 12.74 ± 0.41 a |
| 0.15 mM | 44.87 ± 1.15 | 37.87 ± 0.40 a | 34.21 ± 0.92 a | 16.26 ± 0.77 b | 15.84 ± 0.77 b | |
| 0.3 mM | 44.87 ± 1.15 | 41.71 ± 0.78 b | 38.80 ± 0.30 b | 28.74 ± 0.40 d | 23.82 ± 1.06 c | |
| 0.6 mM | 44.87 ± 1.15 | 38.91 ± 0.66 a | 33.90 ± 0.53 a | 20.49 ± 0.49 c | 16.97 ± 0.53 b | |
| VCL/(μm/s) | 0 mM | 68.42 ± 1.66 | 51.12 ± 1.72 a | 36.24 ± 1.16 a | 21.40 ± 0.83 a | 14.95 ± 1.21 a |
| 0.15 mM | 68.42 ± 1.66 | 48.45 ± 1.64 a | 43.41 ± 0.83 a | 25.97 ± 1.10 a | 13.10 ± 1.23 a | |
| 0.3 mM | 68.42 ± 1.66 | 60.59 ± 1.48 b | 45.40 ± 1.69 b | 35.54 ± 1.32 b | 23.25 ± 1.10 b | |
| 0.6 mM | 68.42 ± 1.66 | 50.24 ± 1.56 a | 41.69 ± 1.49 a | 34.13 ± 0.93 b | 22.35 ± 1.58 b | |
| Group (ROT) | Total Motility (%) | Progressive Motility (%) | VAP (μm/s) | VSL(μm/s) | VCL (μm/s) | BCF(Hz) |
|---|---|---|---|---|---|---|
| 0 µM | 95.02 ± 0.36 a | 87.25 ± 0.54 a | 57.13 ± 1.11 a | 54.80 ± 2.01 a | 81.37 ± 1.58 ab | 44.65 ± 1.23 a |
| 0.1 µM | 94.33 ± 0.21 a | 89.08 ± 0.63 a | 59.58 ± 1.28 a | 46.51 ± 0.95 b | 84.86 ± 1.82 a | 46.53 ± 3.04 a |
| 1 µM | 91.94 ± 0.17 a | 80.94 ± 0.36 b | 54.55 ± 0.90 a | 48.17 ± 0.94 b | 77.19 ± 1.13 b | 31.96 ± 1.34 b |
| 10 µM | 82.24 ± 0.21 b | 61.96 ± 0.51 c | 42.88 ± 1.04 b | 39.31 ± 1.59 c | 61.07 ± 1.48 c | 29.31 ± 1.21 b |
| Groups | Total Motility (%) | Progressive Motility (%) | VAP /(μm/s) | VSL /(μm/s) | VCL /(μm/s) | BCF (Hz) |
|---|---|---|---|---|---|---|
| Control | 93.55 ± 0.22 a | 73.44 ± 1.59 a | 56.07 ± 0.98 a | 61.77 ± 1.30 a | 79.86 ± 1.39 a | 11.98 ± 0.51 a |
| ROT | 79.12 ± 0.29 c | 59.76 ± 1.67 b | 49.56 ± 0.86 b | 54.59 ± 1.04 b | 70.59 ± 1.22 b | 9.87 ± 0.42 b |
| EGT + ROT | 87.36 ± 0.28 b | 70.73 ± 0.67 a | 56.14 ± 1.05 a | 59.62 ± 0.64 a | 79.97 ± 1.49 a | 12.81 ± 0.44 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Liu, X.; Li, Y.; Cheng, Y.; Li, J. Ergothioneine Improves the Quality of Boar Sperm During In Vitro Liquid Preservation by Regulating Mitochondrial Respiratory Chain. Animals 2025, 15, 1450. https://doi.org/10.3390/ani15101450
Guo Q, Liu X, Li Y, Cheng Y, Li J. Ergothioneine Improves the Quality of Boar Sperm During In Vitro Liquid Preservation by Regulating Mitochondrial Respiratory Chain. Animals. 2025; 15(10):1450. https://doi.org/10.3390/ani15101450
Chicago/Turabian StyleGuo, Qing, Xue Liu, Yang Li, Ye Cheng, and Jingchun Li. 2025. "Ergothioneine Improves the Quality of Boar Sperm During In Vitro Liquid Preservation by Regulating Mitochondrial Respiratory Chain" Animals 15, no. 10: 1450. https://doi.org/10.3390/ani15101450
APA StyleGuo, Q., Liu, X., Li, Y., Cheng, Y., & Li, J. (2025). Ergothioneine Improves the Quality of Boar Sperm During In Vitro Liquid Preservation by Regulating Mitochondrial Respiratory Chain. Animals, 15(10), 1450. https://doi.org/10.3390/ani15101450




