Post-Reproductive Lifespan in Honey Bee Workers with Varying Life Expectancies
Simple Summary
Abstract
1. Introduction
2. Methods
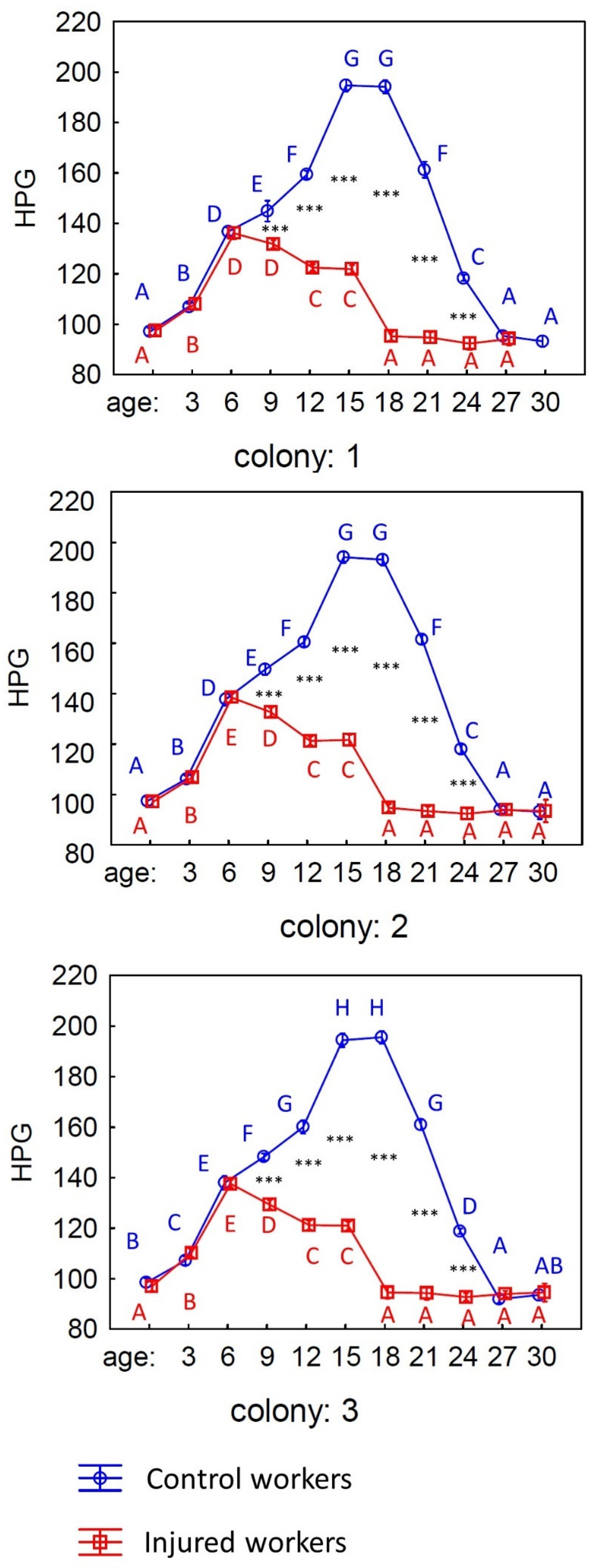
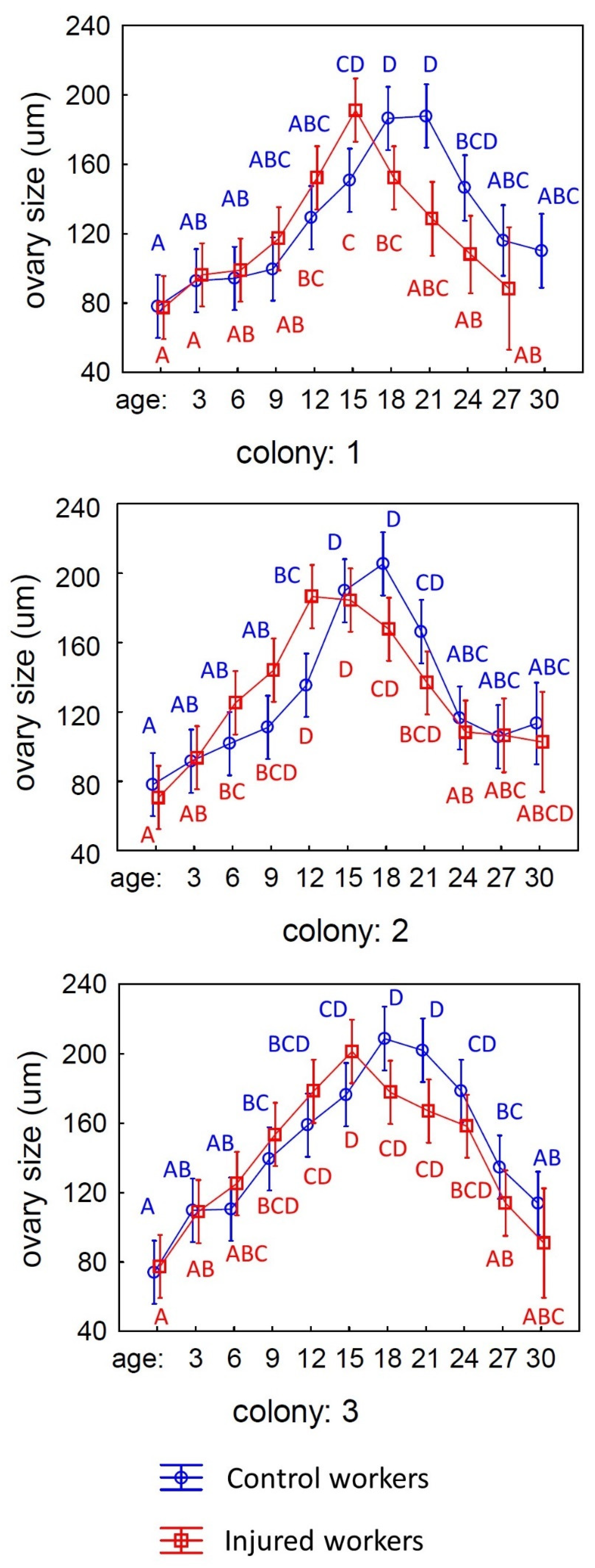
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shanley, D.P.; Kirkwood, T.B.L. Evolution of the Human Menopause. Bioessays 2001, 23, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.N.; Ellis, S.; Lahdenperä, M.; Croft, D.P.; Lummaa, V. Menopause Has Not Evolved as a General Trait in Mammals: A Response to ‘Do Mammals Have Menopause?’. BioRxiv 2024. [Google Scholar] [CrossRef]
- Croft, D.P.; Brent, L.J.N.; Franks, D.W.; Cant, M.A. The Evolution of Prolonged Life after Reproduction. Trends Ecol. Evol. 2015, 30, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Lahdenperä, M.; Lummaa, V.; Helle, S.; Tremblay, M.; Russell, A.F. Fitness Benefits of Prolonged Post-Reproductive Lifespan in Women. Nature 2004, 428, 178–181. [Google Scholar] [CrossRef]
- Walker, M.L.; Herndon, J.G. Menopause in Nonhuman Primates? Biol. Reprod. 2008, 79, 398–406. [Google Scholar] [CrossRef]
- Ellis, S.; Franks, D.W.; Nattrass, S.; Cant, M.A.; Bradley, D.L.; Giles, D.; Balcomb, K.C.; Croft, D.P. Postreproductive Lifespans Are Rare in Mammals. Ecol. Evol. 2018, 8, 2482–2494. [Google Scholar] [CrossRef]
- Reznick, D.; Bryant, M.; Holmes, D. The Evolution of Senescence and Post-Reproductive Lifespan in Guppies (Poecilia Reticulata). PLoS Biol. 2005, 4, e7. [Google Scholar] [CrossRef]
- Bellino, F.L.; Wise, P.M. Nonhuman Primate Models of Menopause Workshop1. Biol. Reprod. 2003, 68, 10–18. [Google Scholar] [CrossRef]
- de Jesús Rovirosa-Hernández, M.; González, M.H.; Guevara-Pérez, M.Á.; García-Orduña, F.; de los Ángeles Aguilar-Tirado, A.; Puga-Olguín, A.; Vásquez-Domínguez, B.P. Menopause in Nonhuman Primates: A Comparative Study with Humans. In A Multidisciplinary Look at Menopause; IntechOpen: London, UK, 2017; Volume 2017, p. 25. [Google Scholar]
- Uematsu, K.; Kutsukake, M.; Fukatsu, T.; Shimada, M.; Shibao, H. Altruistic Colony Defense by Menopausal Female Insects. Curr. Biol. 2010, 20, 1182–1186. [Google Scholar] [CrossRef]
- Tsuji, K.; Kikuta, N.; Kikuchi, T. Determination of the Cost of Workers Reproduction via Diminished Life Span in the Ant Diacamma Sp.: Life Span and Cost of Wirkers Reproduction in Ants. Evolution 2012, 66, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.D. The Genetical Evolution of Social Behaviour. I. J. Theor. Biol. 1964, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.D. The Genetical Evolution of Social Behaviour. II. J. Theor. Biol. 1964, 7, 17–52. [Google Scholar] [CrossRef]
- Winston, M.L. The Biology of the Honeybee; Harvard University Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Dixon, L.; Kuster, R.; Rueppell, O. Reproduction, Social Behavior, and Aging Trajectories in Honeybee Workers. Age 2014, 36, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Kraus, F.B.; Neumann, P.; Van Praagh, J.; Moritz, R.F.A. Sperm Limitation and the Evolution of Extreme Polyandry in Honeybees (Apis mellifera L.). Behav. Ecol. Sociobiol. 2004, 55, 494–501. [Google Scholar] [CrossRef]
- Schlüns, H.; Koeniger, G.; Koeniger, N.; Moritz, R.F.A. Sperm Utilization Pattern in the Honeybee (Apis mellifera). Behav. Ecol. Sociobiol. 2004, 56, 458–463. [Google Scholar] [CrossRef]
- Heidinger, I.; Meixner, M.; Berg, S.; Büchler, R. Observation of the Mating Behavior of Honey Bee (Apis mellifera L.) Queens Using Radio-Frequency Identification (RFID): Factors Influencing the Duration and Frequency of Nuptial Flights. Insects 2014, 5, 513–527. [Google Scholar] [CrossRef]
- Gençer, H.V.; Firatli, Ç. Reproductive and Morphological Comparisons of Drones Reared in Queenright and Laying Worker Colonies. J. Apic. Res. 2005, 44, 163–167. [Google Scholar] [CrossRef]
- Berg, S.; Koeniger, N.; Koeniger, G.; Fuchs, S. Body Size and Reproductive Success of Drones (Apis mellifera L). Apidologie 1997, 28, 449–460. [Google Scholar] [CrossRef]
- Oldroyd, B.P.; Beekman, M. Effects of Selection for Honey Bee Worker Reproduction on Foraging Traits. PLoS Biol. 2008, 6, e56. [Google Scholar] [CrossRef]
- Robinson, G.E. Regulation of Division of Labor in Insect Societies. Annu. Rev. Entomol. 1992, 37, 637–665. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.R.; Reeve, H.K.; Smith, M.L. Promiscuous Honey Bee Queens Increase Colony Productivity by Suppressing Worker Selfishness. Curr. Biol. 2012, 22, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- van Zweden, J.S.; Cardoen, D.; Wenseleers, T. Social Evolution: When Promiscuity Breeds Cooperation. Curr. Biol. 2012, 22, R922–R924. [Google Scholar] [CrossRef] [PubMed]
- Naeger, N.L.; Peso, M.; Even, N.; Barron, A.B.; Robinson, G.E. Altruistic Behavior by Egg-Laying Worker Honeybees. Curr. Biol. 2013, 23, 1574–1578. [Google Scholar] [CrossRef]
- Robinson, G.E.; Page, R.E.; Fondrk, M.K. Intracolonial Behavioral Variation in Worker Oviposition, Oophagy, and Larval Care in Queenless Honey Bee Colonies. Behav. Ecol. Sociobiol. 1990, 26, 315–323. [Google Scholar] [CrossRef]
- Beekman, M.; Calis, J.N.M.; Boot, W.J. Parasitic Honeybees Get Royal Treatment. Nature 2000, 404, 723. [Google Scholar] [CrossRef]
- Makert, G.R.; Paxton, R.J.; Hartfelder, K. Ovariole Number—A Predictor of Differential Reproductive Success among Worker Subfamilies in Queenless Honeybee (Apis mellifera L.) Colonies. Behav. Ecol. Sociobiol. 2006, 60, 815–825. [Google Scholar] [CrossRef]
- Ratnieks, F.L.W. Egg-Laying, Egg-Removal, and Ovary Development by Workers in Queenright Honey Bee Colonies. Behav. Ecol. Sociobiol. 1993, 32, 191–198. [Google Scholar] [CrossRef]
- Kuszewska, K.; Woloszczuk, A.; Woyciechowski, M. Reproductive Cessation and Post-Reproductive Lifespan in Honeybee Workers. Biology 2024, 13, 287. [Google Scholar] [CrossRef]
- Diaz Brinton, R. Minireview: Translational Animal Models of Human Menopause: Challenges and Emerging Opportunities. Endocrinology 2012, 153, 3571–3578. [Google Scholar] [CrossRef]
- Kuszewska, K.; Woyciechowski, M. Reversion in Honeybee, Apis Mellifera, Workers with Different Life Expectancies. Animal Behaviour. 2013, 85, 247–253. [Google Scholar] [CrossRef]
- Moroń, D.; Lenda, M.; Skórka, P.; Woyciechowski, M. Short-Lived Ants Take Greater Risks during Food Collection. Am. Nat. 2012, 180, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, T.; Takeuchi, H.; Kubo, T. Laying Workers in Queenless Honeybee (Apis mellifera L.) Colonies Have Physiological States Similar to That of Nurse Bees but Opposite That of Foragers. J. Insect Physiol. 2008, 54, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Csondes, A.; Fondrk, M.K.; Page, R.E. Complex Social Behaviour Derived from Maternal Reproductive Traits. Nature 2006, 439, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Woyciechowski, M.; Kuszewska, K. Swarming Generates Rebel Workers in Honeybees. Curr. Biol. 2012, 22, 707–711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larsen, A.; Reynaldi, F.J.; Guzmán-Novoa, E. Bases Del Sistema Inmune de La Abeja Melífera (Apis mellifera). Revisión. Rev. Mex. Cienc. Pecu. 2019, 10, 705–728. [Google Scholar] [CrossRef]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.-L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune Pathways and Defence Mechanisms in Honey Bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic Stress in the Honeybee Apis Mellifera from Nosema Ceranae Infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Neukirch, A. Dependence of the Life Span of the Honeybee (Apis meilifica) upon Flight Performance and Energy Consumption. J. Comp. Physiol. 1982, 146, 35–40. [Google Scholar] [CrossRef]
- Huang, Z.-Y.; Otis, G.W. Factors Determining Hypopharyngeal Gland Activity of Worker Honey Bees (Apis mellifera L.). Ins. Soc. 1989, 36, 264–276. [Google Scholar] [CrossRef]
- Huang, Z.-Y.; Robinson, G.E. Regulation of Honey Bee Division of Labor by Colony Age Demography. Behav. Ecol. Sociobiol. 1996, 39, 147–158. [Google Scholar] [CrossRef]
- Woyciechowski, M.; Kuszewska, K.; Pitorak, J.; Kierat, J. Honeybee Worker Larvae Perceive Queen Pheromones in Their Food. Apidologie 2017, 48, 144–149. [Google Scholar] [CrossRef]
- Ronai, I.; Allsopp, M.H.; Tan, K.; Dong, S.; Liu, X.; Vergoz, V.; Oldroyd, B.P. The Dynamic Association between Ovariole Loss and Sterility in Adult Honeybee Workers. Proc. R. Soc. B 2017, 284, 20162693. [Google Scholar] [CrossRef] [PubMed]
- Backx, A.G.; Guzmán-Novoa, E.; Thompson, G.J. Factors Affecting Ovary Activation in Honey Bee Workers: A Meta-Analysis. Insect. Soc. 2012, 59, 381–388. [Google Scholar] [CrossRef]
- Peso, M.; Niño, E.L.; Grozinger, C.M.; Barron, A.B. Effect of Honey Bee Queen Mating Condition on Worker Ovary Activation. Insect. Soc. 2013, 60, 123–133. [Google Scholar] [CrossRef]
- Hoover, S.E.R.; Keeling, C.I.; Winston, M.L.; Slessor, K.N. The Effect of Queen Pheromones on Worker Honey Bee Ovary Development. Naturwissenschaften 2003, 90, 477–480. [Google Scholar] [CrossRef]
- Human, H.; Nicolson, S.W.; Strauss, K.; Pirk, C.W.W.; Dietemann, V. Influence of Pollen Quality on Ovarian Development in Honeybee Workers (Apis mellifera scutellata). J. Insect Physiol. 2007, 53, 649–655. [Google Scholar] [CrossRef]
- Hoover, S.E.R.; Higo, H.A.; Winston, M.L. Worker Honey Bee Ovary Development: Seasonal Variation and the Influence of Larval and Adult Nutrition. J. Comp. Physiol. B 2006, 176, 55–63. [Google Scholar] [CrossRef]


| Effect (F/R) | SS | Degr. of Freedom | MS | Den.Syn. Error df | Den.Syn. Error MS | F | p | |
|---|---|---|---|---|---|---|---|---|
| Intercept | Fixed | 12,197,200 | 1 | 12,197,200 | 5.9568 | 1.11953 | 10,894,963 | 0.000000 |
| Colony | Random | 1 | 2 | 1 | 2.2938 | 10.67207 | 0 | 0.940422 |
| Age | Fixed | 405,213 | 10 | 40,521 | 20.3319 | 15.97009 | 2537 | 0.000000 |
| Treatment | Fixed | 172,080 | 1 | 172,080 | 2.3328 | 7.96339 | 21,609 | 0.000011 |
| Colony * age | Random | 320 | 20 | 16 | 19.3393 | 12.89786 | 1 | 0.319215 |
| Colony * treatment | Random | 15 | 2 | 8 | 20.6224 | 12.88767 | 1 | 0.557920 |
| Age * treatment | Fixed | 266,187 | 10 | 26,619 | 20.1321 | 12.89145 | 2065 | 0.000000 |
| Colony *age * treatment | Random | 245 | 19 | 13 | 852.0000 | 12.58334 | 1 | 0.428249 |
| Error | 10,721 | 852 | 13 |
| Effect (F/R) | SS | Degr. of Freedom | MS | Den.Syn. Error df | Den.Syn. Error MS | F | p | |
|---|---|---|---|---|---|---|---|---|
| Intercept | Fixed | 15242.16 | 1 | 15242.16 | 2.1431 | 0.961790 | 15847.70 | 0.000035 |
| Colony | Random | 1.93 | 2 | 0.97 | 0.0000 | |||
| Age | Fixed | 3.34 | 10 | 0.33 | 22.3264 | 0.245854 | 1.36 | 0.261033 |
| Treatment | Fixed | 0.73 | 1 | 0.73 | 3.9368 | 0.144161 | 5.05 | 0.088933 |
| Colony * age | Random | 4.70 | 20 | 0.24 | 19.7836 | 0.371684 | 0.63 | 0.842550 |
| Colony * treatment | Random | 0.22 | 2 | 0.11 | 22.8221 | 0.386314 | 0.28 | 0.759051 |
| Age * treatment | Fixed | 3.13 | 10 | 0.31 | 21.6474 | 0.380891 | 0.82 | 0.612494 |
| Colony * age * treatment | Random | 6.98 | 19 | 0.37 | 852.0000 | 0.823350 | 0.45 | 0.980575 |
| Error | 701.49 | 852 | 0.82 |
| Effect (F/R) | SS | Degr. of Freedom | MS | Den.Syn. Error df | Den.Syn. Error MS | F | p | |
|---|---|---|---|---|---|---|---|---|
| Intercept | Fixed | 14,152,702 | 1 | 14,152,702 | 2.0059 | 33000.62 | 428.8617 | 0.002291 |
| Colony | Random | 68,482 | 2 | 34,241 | 5.8845 | 3270.95 | 10.4682 | 0.011528 |
| Age | Fixed | 1,089,478 | 10 | 108,948 | 20.2087 | 2873.23 | 37.9182 | 0.000000 |
| Treatment | Fixed | 3178 | 1 | 3178 | 2.1594 | 1637.41 | 1.9411 | 0.289633 |
| Colony * age | Random | 57,784 | 20 | 2889 | 19.3800 | 1187.67 | 2.4327 | 0.028045 |
| Colony * treatment | Random | 3307 | 2 | 1654 | 20.8205 | 1191.17 | 1.3882 | 0.271678 |
| Age * treatment | Fixed | 123,825 | 10 | 12,382 | 20.2694 | 1189.87 | 10.4066 | 0.000006 |
| Colony * age * treatment | Random | 22,547 | 19 | 1187 | 852.0000 | 1295.69 | 0.9159 | 0.562966 |
| Error | 1,103,930 | 852 | 1296 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuszewska, K. Post-Reproductive Lifespan in Honey Bee Workers with Varying Life Expectancies. Animals 2025, 15, 1402. https://doi.org/10.3390/ani15101402
Kuszewska K. Post-Reproductive Lifespan in Honey Bee Workers with Varying Life Expectancies. Animals. 2025; 15(10):1402. https://doi.org/10.3390/ani15101402
Chicago/Turabian StyleKuszewska, Karolina. 2025. "Post-Reproductive Lifespan in Honey Bee Workers with Varying Life Expectancies" Animals 15, no. 10: 1402. https://doi.org/10.3390/ani15101402
APA StyleKuszewska, K. (2025). Post-Reproductive Lifespan in Honey Bee Workers with Varying Life Expectancies. Animals, 15(10), 1402. https://doi.org/10.3390/ani15101402






