Potential Biological Impacts of Microplastics and Nanoplastics on Farm Animals: Global Perspectives with Insights from Bangladesh
Simple Summary
Abstract
1. Introduction
2. Plastic Use and Waste Management Practices in Bangladesh
3. Routes of MP and NP Infiltration into Farm Animals
4. Types of Plastic Particles in the Poultry and Livestock Sectors
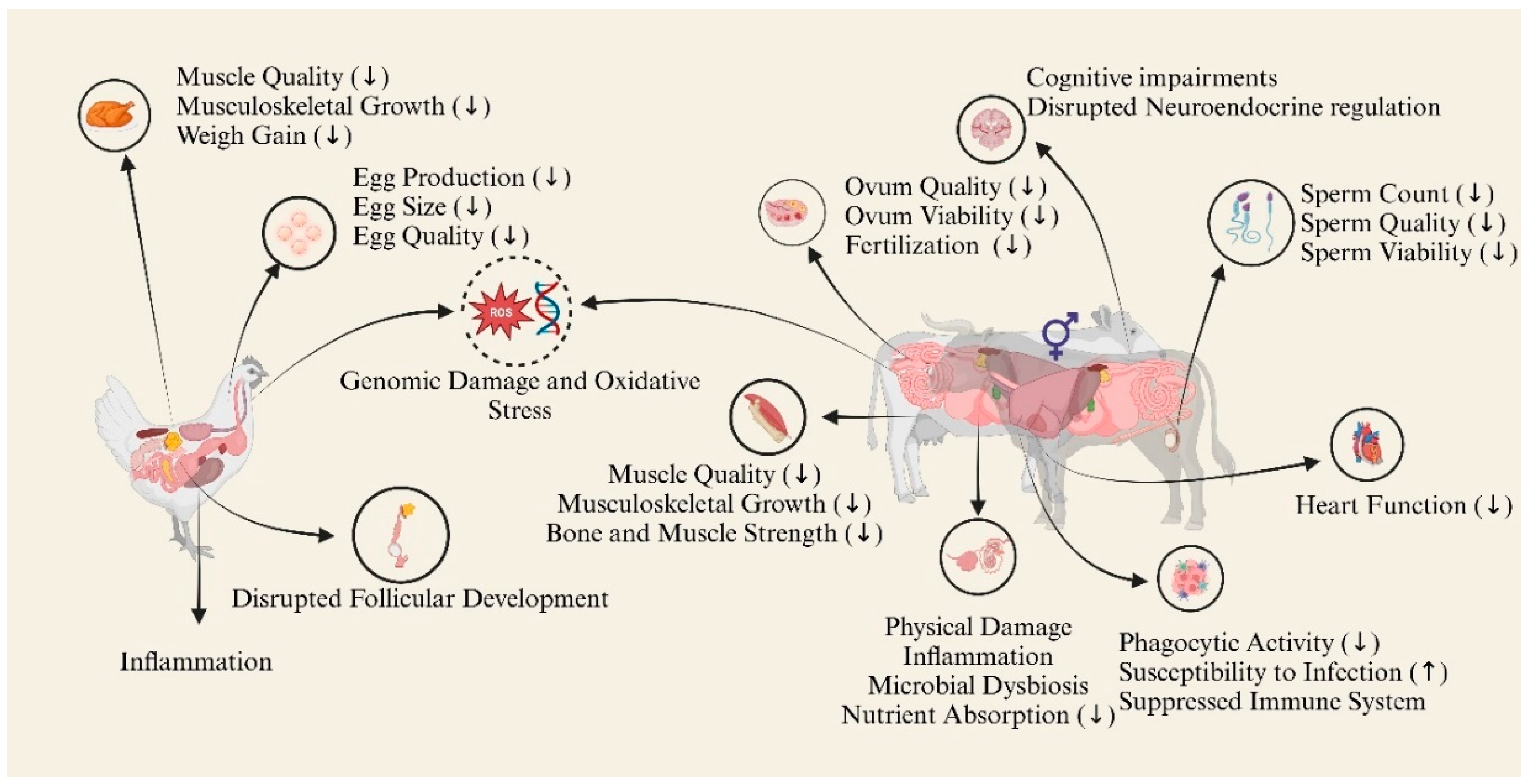
5. Potential Effects of MPs and NPs on Farm Animals
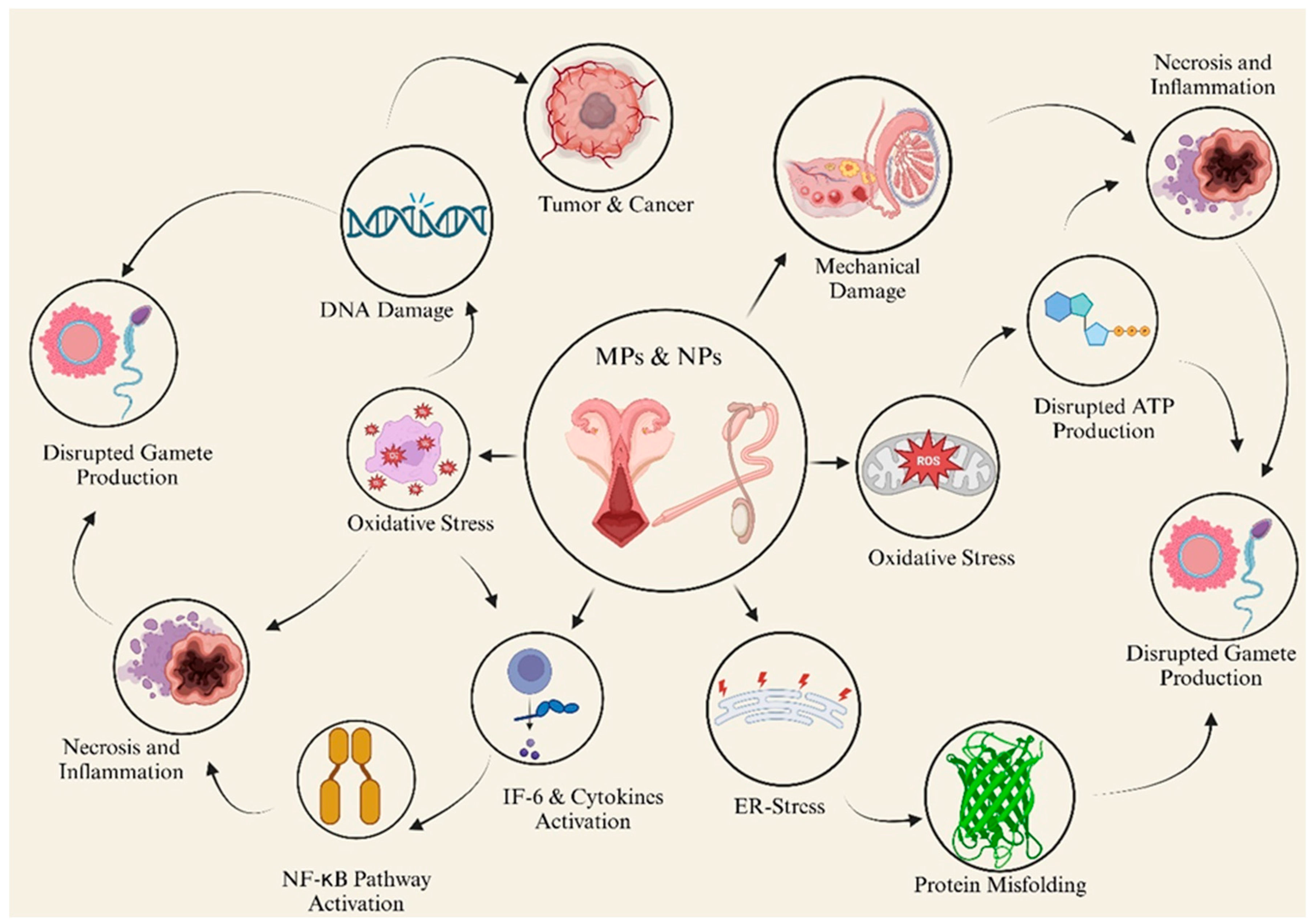
5.1. Adverse Effects on the Reproductive System
5.2. Nervous and Immune Systems
5.3. Adverse Effects on the Digestive System and Metabolism
5.4. Genomic Effects
6. Potential Risks to Human Health and Food Safety
7. Research Gaps and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, E.W. Livestock Issue. Brief. Funct. Genom. 2010, 9, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.P.; Wint, G.R.W.; Conchedda, G.; Van Boeckel, T.P.; Ercoli, V.; Palamara, E.; Cinardi, G.; D’Aietti, L.; Hay, S.I.; Gilbert, M. Mapping the Global Distribution of Livestock. PLoS ONE 2014, 9, e96084. [Google Scholar] [CrossRef] [PubMed]
- Font-i-Furnols, M. Meat Consumption, Sustainability and Alternatives: An Overview of Motives and Barriers. Foods 2023, 12, 2144. [Google Scholar] [CrossRef]
- DLS Livestock Economy at a Glance (2023–2024). Available online: https://dls.portal.gov.bd/sites/default/files/files/dls.portal.gov.bd/page/ee5f4621_fa3a_40ac_8bd9_898fb8ee4700/2024-08-13-10-26-93cb11d540e3f853de9848587fa3c81e.pdf (accessed on 25 April 2025).
- Rahman, S.; Das, G.C. Effect of COVID-19 on the Livestock Sector in Bangladesh and Recommendations. J. Agric. Food Res. 2021, 4, 100128. [Google Scholar] [CrossRef]
- Melchor-Martínez, E.M.; Macías-Garbett, R.; Alvarado-Ramírez, L.; Araújo, R.G.; Sosa-Hernández, J.E.; Ramírez-Gamboa, D.; Parra-Arroyo, L.; Alvarez, A.G.; Monteverde, R.P.B.; Cazares, K.A.S.; et al. Towards a Circular Economy of Plastics: An Evaluation of the Systematic Transition to a New Generation of Bioplastics. Polymers 2022, 14, 1203. [Google Scholar] [CrossRef]
- Hoang, T.C. Plastic Pollution: Where Are We Regarding Research and Risk Assessment in Support of Management and Regulation? Integr. Environ. Assess. Manag. 2022, 18, 851–852. [Google Scholar] [CrossRef]
- Ragossnig, A.M.; Agamuthu, P. Plastic Waste: Challenges and Opportunities. Waste Manag. Res. J. A Sustain. Circ. Econ. 2021, 39, 629–630. [Google Scholar] [CrossRef]
- Andrady, A.L. Weathering and Fragmentation of Plastic Debris in the Ocean Environment. Mar. Pollut. Bull. 2022, 180, 113761. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, H.; Xiang, H.; Fan, J.; Jiang, H. The Surface Degradation and Release of Microplastics from Plastic Films Studied by UV Radiation and Mechanical Abrasion. Sci. Total Environ. 2022, 838, 156369. [Google Scholar] [CrossRef]
- Okeke, E.S.; Chukwudozie, K.I.; Addey, C.I.; Okoro, J.O.; Chidike Ezeorba, T.P.; Atakpa, E.O.; Okoye, C.O.; Nwuche, C.O. Micro and Nanoplastics Ravaging Our Agroecosystem: A Review of Occurrence, Fate, Ecological Impacts, Detection, Remediation, and Prospects. Heliyon 2023, 9, e13296. [Google Scholar] [CrossRef]
- Jannat, M.; Akter, S.; Ehsan, M. Conversion of Waste Polypropylene Plastic into Fuel. In AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2019; p. 120009. [Google Scholar]
- Islam, R.; Ruponti, S.A.; Rakib, A.; Nguyen, H.Q.; Mourshed, M. Current Scenario and Challenges of Plastic Pollution in Bangladesh: A Focus on Farmlands and Terrestrial Ecosystems. Front. Environ. Sci. Eng. 2023, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Rahman, M.A.; Ahmed Chowdhury, M.; Kumar Mohonta, S. Plastic Pollution in Bangladesh: A Review on Current Status Emphasizing the Impacts on Environment and Public Health. Environ. Eng. Res. 2020, 26, 200535. [Google Scholar] [CrossRef]
- Islam, S.; Karim, R.; Islam, T.; Oishi, H.T.; Tasnim, Z.; Das, H.; Kabir, A.H.M.E.; Sekine, M. Abundance, Characteristics, and Ecological Risks of Microplastics in the Riverbed Sediments around Dhaka City. Sci. Total Environ. 2023, 877, 162866. [Google Scholar] [CrossRef] [PubMed]
- Sadia, M.M.; Ishika, A.N.; Miazi, R.; Yasmin, F.; Shahrukh, S.; Sultana, G.N.N.; Hossain, M.E. Microplastic Pollution in Two Urban Rivers and an Associated Water Treatment Plant in Bangladesh. ISEE Conf. Abstr. 2022, 2022. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Knez, E.; Grembecka, M. Food and Human Safety: The Impact of Microplastics. Crit. Rev. Food Sci. Nutr. 2024, 64, 3502–3521. [Google Scholar] [CrossRef]
- Sheriff, I.; Yusoff, M.S.; Manan, T.S.B.A.; Koroma, M. Microplastics in Manure: Sources, Analytical Methods, Toxicodynamic, and Toxicokinetic Endpoints in Livestock and Poultry. Environ. Adv. 2023, 12, 100372. [Google Scholar] [CrossRef]
- Bilal, M.; Taj, M.; Ul Hassan, H.; Yaqub, A.; Shah, M.; Sohail, M.; Rafiq, N.; Atique, U.; Abbas, M.; Sultana, S.; et al. First Report on Microplastics Quantification in Poultry Chicken and Potential Human Health Risks in Pakistan. Toxics 2023, 11, 612. [Google Scholar] [CrossRef]
- Dong, X.; Liu, X.; Hou, Q.; Wang, Z. From Natural Environment to Animal Tissues: A Review of Microplastics (Nanoplastics) Translocation and Hazards Studies. Sci. Total Environ. 2023, 855, 158686. [Google Scholar] [CrossRef]
- Ramachandraiah, K.; Ameer, K.; Jiang, G.; Hong, G.-P. Micro- and Nanoplastic Contamination in Livestock Production: Entry Pathways, Potential Effects and Analytical Challenges. Sci. Total Environ. 2022, 844, 157234. [Google Scholar] [CrossRef]
- Urli, S.; Corte Pause, F.; Crociati, M.; Baufeld, A.; Monaci, M.; Stradaioli, G. Impact of Microplastics and Nanoplastics on Livestock Health: An Emerging Risk for Reproductive Efficiency. Animals 2023, 13, 1132. [Google Scholar] [CrossRef]
- Grechi, N.; Franko, R.; Rajaraman, R.; Stöckl, J.B.; Trapphoff, T.; Dieterle, S.; Fröhlich, T.; Noonan, M.J.; de Ferraz, A.M.M.M. Microplastics Are Present in Women’s and Cows’ Follicular Fluid and Polystyrene Microplastics Compromise Bovine Oocyte Function in Vitro. bioRxiv 2022. [Google Scholar] [CrossRef]
- Muhib, M.I.; Rahman, M.M. Microplastics Contamination in Fish Feeds: Characterization and Potential Exposure Risk Assessment for Cultivated Fish of Bangladesh. Heliyon 2023, 9, e19789. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, G.W.; Koldewey, H.J.; Duncan, E.; Napper, I.E.; Niloy, M.D.N.H.; Nelms, S.E.; Sarker, S.; Bhola, S.; Nishat, B. Plastic Pollution in Aquatic Systems in Bangladesh: A Review of Current Knowledge. Sci. Total Environ. 2021, 761, 143285. [Google Scholar] [CrossRef] [PubMed]
- Kawsar, M.D.A.; Munny, F.J.; Saif, U.M.; Harun-Al-Rashid, A.; Rahman, M.D.A.; Barman, S.K.; Adikari, D.; Alam, M.D.T.; Kunda, M.; Pandit, D. Unveiling the Microplastic Crisis: Insights into Bangladesh’s Aquatic Ecosystems—Origins, Impact, and Solutions. J. Hazard. Mater. Adv. 2024, 14, 100430. [Google Scholar] [CrossRef]
- Khan, H.M.S.; Setu, S. Microplastic Ingestion by Fishes from Jamuna River, Bangladesh. Environ. Nat. Resour. J. 2022, 20, 157–167. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sobhan, F.; Uddin, M.N.; Sharifuzzaman, S.M.; Chowdhury, S.R.; Sarker, S.; Chowdhury, M.S.N. Microplastics in Fishes from the Northern Bay of Bengal. Sci. Total Environ. 2019, 690, 821–830. [Google Scholar] [CrossRef]
- Sultana, N.; Tista, R.R.; Islam, M.S.; Begum, M.; Islam, S.; Naser, M.N. Microplastics in Freshwater Wild and Farmed Fish Species of Bangladesh. Environ. Sci. Pollut. Res. 2023, 30, 72009–72025. [Google Scholar] [CrossRef]
- Islam, A.; Karmakar, P.; Hoque, M.M.; Roy, S.; Hassan, R. Appraisal of Public Awareness Regarding Plastic Waste in the Tangail Municipality, Bangladesh. Int. J. Environ. Stud. 2021, 78, 900–913. [Google Scholar] [CrossRef]
- Ferdous, Z.; Datta, A.; Anwar, M. Plastic Mulch and Indigenous Microorganism Effects on Yield and Yield Components of Cauliflower and Tomato in Inland and Coastal Regions of Bangladesh. J. Crop Improv. 2017, 31, 261–279. [Google Scholar] [CrossRef]
- Rummana, S.; Amin, A.K.M.R.; Faruk, G.M. Effect of Irrigation and Mulch Materials on Growth and Yield of Wheat. Bangladesh Agron. J. 2018, 21, 71–76. [Google Scholar] [CrossRef][Green Version]
- North, E.J.; Halden, R.U. Plastics and Environmental Health: The Road Ahead. Rev. Environ. Health 2013, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Bhuiyan, A.M.; Gayen, T.K. Rise in Single-Use Plastic Pollution Amid COVID-19 Pandemic: Bangladesh Perspective. J. Agric. Food Environ. 2021, 2, 80–84. [Google Scholar] [CrossRef]
- Hamid, M.D.A.; Ahmed, S.T.; Parvez, M.; Patwary, S. Scope, Opportunity and Challenges of Polyester Staple Fiber (PSF) Production in Bangladesh. J. Knowl. Learn. Sci. Technol. 2023, 2, 106–119. [Google Scholar] [CrossRef]
- Shaibur, M.R.; Sarwar, S.; Hossain, M.S.; Ambade, B.; Chakraborty, T.K.; Ahmed, F.F. Plastic Waste Production and Management in Jashore Municipality and Its Surrounding Areas, Bangladesh: An Overview. Phys. Chem. Earth Parts A/B/C 2024, 134, 103580. [Google Scholar] [CrossRef]
- Siddique, S.; Roy, B.B.; Zaman, S.; Khan, A.; Al Alam, M.A.; Chowdhury, R.B.; Biswas, K.F.; Hossain, M.M.; Murakami, S.; Sujauddin, M. Discerning the Circularity of the Plastic Industry in Bangladesh through the Lens of Material Flow Analysis. Sustain. Prod. Consum. 2022, 33, 700–715. [Google Scholar] [CrossRef]
- Jerin, D.T.; Sara, H.H.; Radia, M.A.; Hema, P.S.; Hasan, S.; Urme, S.A.; Audia, C.; Hasan, M.T.; Quayyum, Z. An Overview of Progress towards Implementation of Solid Waste Management Policies in Dhaka, Bangladesh. Heliyon 2022, 8, e08918. [Google Scholar] [CrossRef]
- Ziani, K.; Ioniță-Mîndrican, C.B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- Maganti, S.S.; Akkina, R.C. Detection and Characterisation of Microplastics in Animal Feed. Online J. Anim. Feed. Res. 2023, 13, 348–356. [Google Scholar] [CrossRef]
- Jovanović, B. Ingestion of Microplastics by Fish and Its Potential Consequences from a Physical Perspective. Integr. Environ. Assess. Manag. 2017, 13, 510–515. [Google Scholar] [CrossRef]
- Corte Pause, F.; Urli, S.; Crociati, M.; Stradaioli, G.; Baufeld, A. Connecting the Dots: Livestock Animals as Missing Links in the Chain of Microplastic Contamination and Human Health. Animals 2024, 14, 350. [Google Scholar] [CrossRef]
- Khaleduzzaman, A.B.M.; Salim, H.M. Development of Local Calibrations for the Nutritional Evaluation of Fish Meal and Meat & Bone Meal by Using Near-Infrared Reflectance Spectroscopy. J. Appl. Anim. Res. 2020, 48, 257–263. [Google Scholar] [CrossRef]
- Beriot, N.; Peek, J.; Zornoza, R.; Geissen, V.; Huerta Lwanga, E. Low Density-Microplastics Detected in Sheep Faeces and Soil: A Case Study from the Intensive Vegetable Farming in Southeast Spain. Sci. Total Environ. 2021, 755, 142653. [Google Scholar] [CrossRef] [PubMed]
- Walkinshaw, C.; Tolhurst, T.J.; Lindeque, P.K.; Thompson, R.; Cole, M. Detection and Characterisation of Microplastics and Microfibres in Fishmeal and Soybean Meal. Mar. Pollut. Bull. 2022, 185, 114189. [Google Scholar] [CrossRef]
- Wu, R.T.; Cai, Y.F.; Chen, Y.X.; Yang, Y.W.; Xing, S.C.; Liao, X. Di Occurrence of Microplastic in Livestock and Poultry Manure in South China. Environ. Pollut. 2021, 277, 116790. [Google Scholar] [CrossRef]
- Prata, J.C.; Dias-Pereira, P. Microplastics in Terrestrial Domestic Animals and Human Health: Implications for Food Security and Food Safety and Their Role as Sentinels. Animals 2023, 13, 661. [Google Scholar] [CrossRef]
- Jahandari, A. Microplastics in the Urban Atmosphere: Sources, Occurrences, Distribution, and Potential Health Implications. J. Hazard. Mater. Adv. 2023, 12, 100346. [Google Scholar] [CrossRef]
- Leon, L.I.D.; Bautista, I.M.R.; Deza, A.G.M.; Kok, J.F.F.; Del Mundo, E.F.; VinceCruz-Abeledo, C.C. Microplastic Fragments from Poultry Entrails in Wet Markets from South Caloocan, Philippines. Res. Sq. 2022; in press. [Google Scholar]
- Lwanga, E.H.; Vega, J.M.; Quej, V.K.; Chi, J.D.L.A.; del Cid, L.S.; Chi, C.; Segura, G.E.; Gertsen, H.; Salánki, T.; van der Ploeg, M.; et al. Field Evidence for Transfer of Plastic Debris along a Terrestrial Food Chain. Sci. Rep. 2017, 7, 14071. [Google Scholar] [CrossRef]
- Susanti, R.; Yuniastuti, A.; Fibriana, F. The Evidence of Microplastic Contamination in Central Javanese Local Ducks from Intensive Animal Husbandry. Water Air Soil. Pollut. 2021, 232, 178. [Google Scholar] [CrossRef]
- Hou, L.; Wang, D.; Yin, K.; Zhang, Y.; Lu, H.; Guo, T.; Li, J.; Zhao, H.; Xing, M. Polystyrene Microplastics Induce Apoptosis in Chicken Testis via Crosstalk between NF-ΚB and Nrf2 Pathways. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 262, 109444. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Van Der Veen, I.; Van Mourik, L.M.; Van Velzen, M.J.M.; Groenewoud, Q.R.; Leslie, H.A. Final Plastic Particles in Livestock Feed, Milk, Meat and Blood, A Pilot Study. Environ. Health 2022. [Google Scholar]
- Bahrani, F.; Mohammadi, A.; Dobaradaran, S.; De-la-Torre, G.E.; Arfaeinia, H.; Ramavandi, B.; Saeedi, R.; Tekle-Röttering, A. Occurrence of Microplastics in Edible Tissues of Livestock (Cow and Sheep). Environ. Sci. Pollut. Res. 2024, 31, 22145–22157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics Contamination in Eggs: Detection, Occurrence and Status. Food Chem. 2022, 397, 133771. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene Microplastics Induced Female Reproductive Toxicity in Mice. J. Hazard. Mater. 2022, 424, 127629. [Google Scholar] [CrossRef]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to Polystyrene Microplastics Causes Reproductive Toxicity through Oxidative Stress and Activation of the P38 MAPK Signaling Pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef]
- Giannandrea, D.; Parolini, M.; Citro, V.; De Felice, B.; Pezzotta, A.; Abazari, N.; Platonova, N.; Sugni, M.; Chiu, M.; Villa, A.; et al. Nanoplastic Impact on Bone Microenvironment: A Snapshot from Murine Bone Cells. J. Hazard. Mater. 2024, 462, 132717. [Google Scholar] [CrossRef]
- Sun, R.; Xu, K.; Yu, L.; Pu, Y.; Xiong, F.; He, Y.; Huang, Q.; Tang, M.; Chen, M.; Yin, L.; et al. Preliminary Study on Impacts of Polystyrene Microplastics on the Hematological System and Gene Expression in Bone Marrow Cells of Mice. Ecotoxicol. Environ. Saf. 2021, 218, 112296. [Google Scholar] [CrossRef]
- Shengchen, W.; Jing, L.; Yujie, Y.; Yue, W.; Shiwen, X. Polystyrene Microplastics-Induced ROS Overproduction Disrupts the Skeletal Muscle Regeneration by Converting Myoblasts into Adipocytes. J. Hazard. Mater. 2021, 417, 125962. [Google Scholar] [CrossRef]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene Microplastics Induced Male Reproductive Toxicity in Mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef]
- Yan, W.; Li, Z.J.; Lin, Z.Y.; Ji, S.Q.; Tse, W.K.F.; Meng, Z.Q.; Liu, C.; Li, L. Microplastic Exposure Disturbs Sleep Structure, Reduces Lifespan, and Decreases Ovary Size in Drosophila Melanogaster. Zool. Res. 2024, 45, 805–820. [Google Scholar] [CrossRef]
- Okamura, T.; Hamaguchi, M.; Hasegawa, Y.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; et al. Oral Exposure to Polystyrene Microplastics of Mice on a Normal or High-Fat Diet and Intestinal and Metabolic Outcomes. Environ. Health Perspect. 2023, 131, 27006. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Dai, H.; Cheng, Y.; Wang, B.; Xu, J.; Zhang, Y.; Chen, Y.; Xu, F.; Ma, Q.; Lin, F.; et al. Oral Feeding of Nanoplastics Affects Brain Function of Mice by Inducing Macrophage IL-1 Signal in the Intestine. Cell Rep. 2023, 42, 112346. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of Polystyrene Microplastic on the Gut Barrier, Microbiota and Metabolism of Mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene Microplastics Affect the Distribution of Gut Microbiota and Inflammation Development in Mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Yin, K.; Wang, D.; Zhang, Y.; Lu, H.; Wang, Y.; Xing, M. Dose-Effect of Polystyrene Microplastics on Digestive Toxicity in Chickens (Gallus gallus): Multi-Omics Reveals Critical Role of Gut-Liver Axis. J. Adv. Res. 2023, 52, 3–18. [Google Scholar] [CrossRef]
- Sun, H.; Chen, N.; Yang, X.; Xia, Y.; Wu, D. Effects Induced by Polyethylene Microplastics Oral Exposure on Colon Mucin Release, Inflammation, Gut Microflora Composition and Metabolism in Mice. Ecotoxicol. Environ. Saf. 2021, 220, 112340. [Google Scholar] [CrossRef]
- Chang, X.; Li, Y.; Han, Y.; Fang, Y.; Xiang, H.; Zhao, Z.; Zhao, B.; Zhong, R. Polystyrene Exposure Induces Lamb Gastrointestinal Injury, Digestive Disorders and Inflammation, Decreasing Daily Gain, and Meat Quality. Ecotoxicol. Environ. Saf. 2024, 277, 116389. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, Z.; Xia, Y.; Cheng, S.; Gao, J.; Sun, W.; Jiang, X.; Zhang, J.; Mao, L.; Qin, X.; et al. Repression of Autophagy Leads to Acrosome Biogenesis Disruption Caused by a Sub-Chronic Oral Administration of Polystyrene Nanoparticles. Environ. Int. 2022, 163, 107220. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, L.; Han, L.; Wang, J.; Zhang, W.; Liu, Z.; Gao, A. Polystyrene Micro-/Nanoplastics Induced Hematopoietic Damages via the Crosstalk of Gut Microbiota, Metabolites, and Cytokines. Environ. Int. 2022, 161, 107131. [Google Scholar] [CrossRef]
- Jiang, L.; Ye, Y.; Han, Y.; Wang, Q.; Lu, H.; Li, J.; Qian, W.; Zeng, X.; Zhang, Z.; Zhao, Y.; et al. Microplastics Dampen the Self-Renewal of Hematopoietic Stem Cells by Disrupting the Gut Microbiota-Hypoxanthine-Wnt Axis. Cell Discov. 2024, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Han, J.S.; Park, E.J.; Seong, E.; Lee, G.H.; Kim, D.W.; Son, H.Y.; Han, H.Y.; Lee, B.S. Repeated-Oral Dose Toxicity of Polyethylene Microplastics and the Possible Implications on Reproduction and Development of the next Generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef]
- Zou, W.; Lu, S.; Wang, J.; Xu, Y.; Shahid, M.A.; Saleem, M.U.; Mehmood, K.; Li, K. Environmental Microplastic Exposure Changes Gut Microbiota in Chickens. Animals 2023, 13, 2503. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, G.; Peng, H.; Qi, L.; Zhang, D.; Nie, Q.; Zhang, X.; Luo, W. Microplastic Exposure Induces Muscle Growth but Reduces Meat Quality and Muscle Physiological Function in Chickens. Sci. Total Environ. 2023, 882, 163305. [Google Scholar] [CrossRef]
- Ilechukwu, I.; Ehigiator, B.E.; Ben, I.O.; Okonkwo, C.J.; Olorunfemi, O.S.; Modo, U.E.; Ilechukwu, C.E.; Ohagwa, N.J. Chronic Toxic Effects of Polystyrene Microplastics on Reproductive Parameters of Male Rats. Environ. Anal. Health Toxicol. 2022, 37, e2022015. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; An, Y.-J. Nanoplastic Ingestion Induces Behavioral Disorders in Terrestrial Snails: Trophic Transfer Effects via Vascular Plants. Environ. Sci. Nano 2020, 7, 975–983. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The Plastic Brain: Neurotoxicity of Micro- and Nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- Muhammad, A.; Zhou, X.; He, J.; Zhang, N.; Shen, X.; Sun, C.; Yan, B.; Shao, Y. Toxic Effects of Acute Exposure to Polystyrene Microplastics and Nanoplastics on the Model Insect, Silkworm Bombyx Mori. Environ. Pollut. 2021, 285, 117255. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene Microplastics Induce Gut Microbiota Dysbiosis and Hepatic Lipid Metabolism Disorder in Mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lu, L.; Zheng, M.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Polystyrene Microplastics Cause Tissue Damages, Sex-Specific Reproductive Disruption and Transgenerational Effects in Marine Medaka (Oryzias melastigma). Environ. Pollut. 2019, 254, 113024. [Google Scholar] [CrossRef]
- Huang, W.; Song, B.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y. Microplastics and Associated Contaminants in the Aquatic Environment: A Review on Their Ecotoxicological Effects, Trophic Transfer, and Potential Impacts to Human Health. J. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yan, Z.; Shen, R.; Huang, Y.; Ren, H.; Zhang, Y. Enhanced Reproductive Toxicities Induced by Phthalates Contaminated Microplastics in Male Mice (Mus musculus). J. Hazard. Mater. 2021, 406, 124644. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability 2020, 12, 5514. [Google Scholar] [CrossRef]
- Millican, J.M.; Agarwal, S. Plastic Pollution: A Material Problem? Macromolecules 2021, 54, 4455–4469. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; Nava-Castro, K.E.; Palacios-Arreola, M.I.; Garay-Canales, C.; Morales-Montor, J. How Microplastic Components Influence the Immune System and Impact on Children Health: Focus on Cancer. Birth Defects Res. 2020, 112, 1341–1361. [Google Scholar] [CrossRef]
- Pei, Z.; Qin, Y.; Fu, X.; Yang, F.; Huo, F.; Liang, X.; Wang, S.; Cui, H.; Lin, P.; Zhou, G.; et al. Inhibition of Ferroptosis and Iron Accumulation Alleviates Pulmonary Fibrosis in a Bleomycin Model. Redox Biol. 2022, 57, 102509. [Google Scholar] [CrossRef]
- Wei, J.; Liu, J.; Liang, S.; Sun, M.; Duan, J. Low-Dose Exposure of Silica Nanoparticles Induces Neurotoxicity via Neuroactive Ligand–Receptor Interaction Signaling Pathway in Zebrafish Embryos. Int. J. Nanomed. 2020, 15, 4407–4415. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of Gut Microbiota Plays an Important Role in Micro/Nanoplastics-Induced Gut Barrier Dysfunction. Nanoscale 2021, 13, 8806–8816. [Google Scholar] [CrossRef]
- Wu, H.; Xu, T.; Chen, T.; Liu, J.; Xu, S. Oxidative Stress Mediated by the TLR4/NOX2 Signalling Axis Is Involved in Polystyrene Microplastic-Induced Uterine Fibrosis in Mice. Sci. Total Environ. 2022, 838, 155825. [Google Scholar] [CrossRef]
- Besseling, E.; Wang, B.; Lürling, M.; Koelmans, A.A. Correction to Nanoplastic Affects Growth of S. obliquus and Reproduction of D. magna. Environ. Sci. Technol. 2014, 48, 14065. [Google Scholar] [CrossRef]
- Toto, B.; Refosco, A.; O’Keeffe, M.; Barkhald, Ø.H.; Brønstad, A.; Lied, G.A.; Yadetie, F.; Goksøyr, A.; Kögel, T.; Dierkes, J. Intestinal Permeability and Gene Expression after Polyethylene and Polyamide Microplastic Ingestion in Wistar Rats. Toxicol. Lett. 2022, 370, 35–41. [Google Scholar] [CrossRef] [PubMed]
- De Souza-Silva, T.G.; Oliveira, I.A.; da Silva, G.G.; Giusti, F.C.V.; Novaes, R.D.; de Paula, H.A.A. Impact of Microplastics on the Intestinal Microbiota: A Systematic Review of Preclinical Evidence. Life Sci. 2022, 294, 120366. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue Accumulation of Microplastics in Mice and Biomarker Responses Suggest Widespread Health Risks of Exposure. Sci. Rep. 2017, 7, srep46687. [Google Scholar] [CrossRef]
- Xu, W.; Ye, S.; Liu, W.; Guo, H.; Zhang, L.; Wei, S.; Anwaier, A.; Chang, K.; Malafaia, G.; Zhang, H.; et al. Single-Cell RNA-Seq Analysis Decodes the Kidney Microenvironment Induced by Polystyrene Microplastics in Mice Receiving a High-Fat Diet. J. Nanobiotechnol. 2024, 22, 13. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Wang, C.; Su, X.-L.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.-H.; Cai, Z.; Zheng, C. Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells. Environ. Sci. Technol. 2022, 56, 12483–12493. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and Release of Chemicals from Plastics to the Environment and to Wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- López de las Hazas, M.-C.; Boughanem, H.; Dávalos, A. Untoward Effects of Micro- and Nanoplastics: An Expert Review of Their Biological Impact and Epigenetic Effects. Adv. Nutr. 2022, 13, 1310–1323. [Google Scholar] [CrossRef]
- Li, Y.-S.; Li, X.-T.; Yu, L.-G.; Wang, L.; Shi, Z.-Y.; Guo, X.-L. Roles of Galectin-3 in Metabolic Disorders and Tumor Cell Metabolism. Int. J. Biol. Macromol. 2020, 142, 463–473. [Google Scholar] [CrossRef]
- Qiang, L.; Cheng, J. Exposure to Polystyrene Microplastics Impairs Gonads of Zebrafish (Danio rerio). Chemosphere 2021, 263, 128161. [Google Scholar] [CrossRef]
- De Felice, B.; Sugni, M.; Casati, L.; Parolini, M. Molecular, Biochemical and Behavioral Responses of Daphnia magna under Long-Term Exposure to Polystyrene Nanoplastics. Environ. Int. 2022, 164, 107264. [Google Scholar] [CrossRef]
- Murata, M.; Kang, J.-H. Bisphenol A (BPA) and Cell Signaling Pathways. Biotechnol. Adv. 2018, 36, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Eom, H.-J.; Choi, J. Effect of Early-Life Exposure of Polystyrene Microplastics on Behavior and DNA Methylation in Later Life Stage of Zebrafish. Arch. Environ. Contam. Toxicol. 2022, 82, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Rosellini, M.; Schulze, A.; Omer, E.A.; Ali, N.T.; Marini, F.; Küpper, J.-H.; Efferth, T. The Effect of Plastic-Related Compounds on Transcriptome-Wide Gene Expression on CYP2C19-Overexpressing HepG2 Cells. Molecules 2023, 28, 5952. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Ashour, E.A.; AlMalki, F.; Khafaga, A.F.; Moustafa, M.; Alshaharni, M.O.; Youssef, I.M.; Elolimy, A.A.; Świątkiewicz, S. Harmful Impacts of Microplastic Pollution on Poultry and Biodegradation Techniques Using Microorganisms for Consumer Health Protection: A Review. Poult. Sci. 2025, 104, 104456. [Google Scholar] [CrossRef]
- Da Costa Filho, P.A.; Andrey, D.; Eriksen, B.; Peixoto, R.P.; Carreres, B.M.; Ambühl, M.E.; Descarrega, J.B.; Dubascoux, S.; Zbinden, P.; Panchaud, A.; et al. Detection and Characterization of Small-Sized Microplastics (≥5 Μm) in Milk Products. Sci. Rep. 2021, 11, 24046. [Google Scholar] [CrossRef]
- Cary, C.M.; DeLoid, G.M.; Yang, Z.; Bitounis, D.; Polunas, M.; Goedken, M.J.; Buckley, B.; Cheatham, B.; Stapleton, P.A.; Demokritou, P. Ingested Polystyrene Nanospheres Translocate to Placenta and Fetal Tissues in Pregnant Rats: Potential Health Implications. Nanomaterials 2023, 13, 720. [Google Scholar] [CrossRef]
- Waring, R.H.; Harris, R.M.; Mitchell, S.C. Plastic Contamination of the Food Chain: A Threat to Human Health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef]
- Sharma, V.K.; Ma, X.; Lichtfouse, E.; Robert, D. Nanoplastics Are Potentially More Dangerous than Microplastics. Environ. Chem. Lett. 2023, 21, 1933–1936. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested Plastic Transfers Hazardous Chemicals to Fish and Induces Hepatic Stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Sun, N.; Shi, H.; Li, X.; Gao, C.; Liu, R. Combined Toxicity of Micro/Nanoplastics Loaded with Environmental Pollutants to Organisms and Cells: Role, Effects, and Mechanism. Environ. Int. 2023, 171, 107711. [Google Scholar] [CrossRef]



| Animal | Types of Microplastics | Amount Isolated | Organs Isolated From | Size of Microplastics | Reference |
|---|---|---|---|---|---|
| Cow | Polyvinyl Chloride, Polyethylene, Polymers of Styrene, and Polypropylene | PVC-P varies between 1.2 and 6.1 μg/g, Styr-P varies between 0.09 and 1.5 μg/g, and PE varies between 0.22 and 2.9 μg/g | Blood | ≥700 nanometers | [54] |
| Cow | Nylon (polyamide) | 0.14 items/g | Meat, Liver, and Tripe | <5 mm | [55] |
| Sheep | Fiber | 0.13 items/g | Meat, Liver, and Tripe | <5 mm | [55] |
| Pig | Polymers of Styrene | PVC-P ranges from 1.7 to 17 μg/g, Styr-P ranges from 0.3 to 10 μg/g, and PE ranges from 2.1 to 33 μg/g | Blood | ≥700 nanometers | [54] |
| Chicken | Not specified | Detected | Eggs | Not specified | [56] |
| Chicken | Polyvinyl Chloride, Low-Density Polyethylene, Polystyrene, and Polypropylene Homopolymer | Crop: 17.8 ± 12.1 particles/sample; Gizzard: 33.25 ± 17.8 particles/sample | Crop and Gizzard | 50–500 µm | [19] |
| Experimental Models | Plastic Particles | Bioaccumulation | Effects | Reference |
|---|---|---|---|---|
| Mice | Polystyrene MPs | Heart, liver, spleen, lung, kidney, brain, large intestine, small intestine, uterus, ovary, and blood | Increased inflammation and oxidative stress in ovaries, reduced oocyte quality, and impaired reproductive outcomes. | [57] |
| Mice | Polystyrene MPs | No Information | Reduced sperm count and motility, increased deformity, decreased enzyme activity and testosterone levels, and induced oxidative stress. | [58] |
| Murine osteoblastic cell culture | Polystyrene NPs | Bone | Impact cell viability, induce oxidative stress and apoptosis, and alter gene expression related to inflammation and bone formation. | [59] |
| Mice | Polystyrene MPs | Organs of the digestive system and lower limb bones | Hematotoxicity and altered gene expression related to immune and metabolic processes. | [60] |
| Mice | Polystyrene MPs | No information | Delayed skeletal muscle regeneration, inhibiting myogenic differentiation, and promoting adipogenic differentiation. | [61] |
| Mice | Polystyrene MPs | Testis | Reproductive dysfunction, including decreased sperm quality, testosterone levels, testicular inflammation, and disruption of the blood–testis barrier. | [62] |
| Common fruit fly | Polystyrene MPs | Gut | Gut damage, shortened lifespan, disrupted sleep, reduced ovary size and egg-laying, and altered gene expression in various tissues. | [63] |
| Mice | Polystyrene MPs | Intestinal mucosa | Increased blood glucose and lipid levels, NAFLD activity, intestinal inflammation, and altered nutrient absorption. | [64] |
| Mice | Polyethylene MPs and NP | Gut | Induce gut macrophage activation and IL-1 signaling, leading to brain inflammation and cognitive decline in mice. | [65] |
| Mice | Polystyrene MPs | Gut | Gut microbiota imbalance, intestinal barrier dysfunction, and metabolic disorders in mice. | [66] |
| Mice | Polyethene MPs | No Information | Gut microbial changes, increased inflammation, and intestinal dysbiosis in mice. | [67] |
| Chicken | Polystyrene MPs | Liver | Significant liver damage through gut barrier disruption, microbial translocation, and tissue necrosis. | [68] |
| Mice | Polyethene NPs | Colon | Decreased colon mucin production, altered immune responses, and increased amino acid metabolism by changing colon microflora composition. | [69] |
| Lamb | Polystyrene MPs | No information | Decreased average daily gain, digestive disorders, gastrointestinal injury, and reduced meat quality. | [70] |
| Mice | Polystyrene NPs | Testes | Impaired sperm quality, disrupted testicular structures, and affected acrosome biogenesis in mice, with autophagy. | [71] |
| Mice | Polystyrene MPs and NPs | Organs of the digestive system, testis, lung, and bone marrow | Hematopoietic toxicity by disrupting bone marrow function, gut microbiota, metabolism, and inflammation. | [72] |
| Mice and their bone marrow cell culture | Polystyrene, polymethyl methacrylate, and polyethylene MPs | Heart, lung, kidney, spleen, organs of the digestive system, and bone marrow | Disrupt gut microbiota, impair hematopoietic stem cell function, and lead to their accumulation in multiple organs, including the gastrointestinal tract, liver, and bone marrow. | [73] |
| Mice | Polyethylene MPs | Stomach and spleen | Reduced body weight gain, altered immune responses, and negatively impacted reproductive and developmental outcomes in mice. | [74] |
| Chicken | No information | No information | Significantly impaired chicken growth performance and a notable imbalance in gut microbiota, altering microbial composition and structure. | [75] |
| Chicken | Polystyrene MPs | Muscle tissue | Affect metabolism, induce oxidative stress and neurotoxicity, alter metabolomic profiles, reduce meat quality, and impact muscle development by regulating neural function-related genes. | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahiduzzaman, F.; Rahman, M.Z.; Akhi, M.A.J.; Manik, M.; Khatun, M.M.; Islam, M.A.; Matin, M.N.; Haque, M.A. Potential Biological Impacts of Microplastics and Nanoplastics on Farm Animals: Global Perspectives with Insights from Bangladesh. Animals 2025, 15, 1394. https://doi.org/10.3390/ani15101394
Nahiduzzaman F, Rahman MZ, Akhi MAJ, Manik M, Khatun MM, Islam MA, Matin MN, Haque MA. Potential Biological Impacts of Microplastics and Nanoplastics on Farm Animals: Global Perspectives with Insights from Bangladesh. Animals. 2025; 15(10):1394. https://doi.org/10.3390/ani15101394
Chicago/Turabian StyleNahiduzzaman, FNU, Md Zaminur Rahman, Mst. Arjina Jannat Akhi, Mohammed Manik, Mst Minara Khatun, Md. Ariful Islam, Mohammad Nurul Matin, and Md Azizul Haque. 2025. "Potential Biological Impacts of Microplastics and Nanoplastics on Farm Animals: Global Perspectives with Insights from Bangladesh" Animals 15, no. 10: 1394. https://doi.org/10.3390/ani15101394
APA StyleNahiduzzaman, F., Rahman, M. Z., Akhi, M. A. J., Manik, M., Khatun, M. M., Islam, M. A., Matin, M. N., & Haque, M. A. (2025). Potential Biological Impacts of Microplastics and Nanoplastics on Farm Animals: Global Perspectives with Insights from Bangladesh. Animals, 15(10), 1394. https://doi.org/10.3390/ani15101394








