Early Life Nutrition and Its Effects on the Developing Heifer: Immune and Metabolic Responses to Immune Challenges
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
- High preweaning treatment—8 L of milk daily (HH & HL)
- Low preweaning treatment—4 L of milk daily (LH & LL)
2.2. Animal Management
2.3. Immune Challenge
2.4. Biomarker Analyses
2.5. Statistical Analysis
3. Results
3.1. 6-Week Immune Challenge
3.2. 8-Month Immune Challenge
3.3. 13-Month Immune Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BHB | Beta-hydroxybutyrate |
| NEFA | Non-esterified fatty acid |
| IGF-1 | Insulin-like growth factor 1 |
| HH | High–high |
| HL | High–low |
| LH | Low–high |
| LL | Low–low |
| BPI | Balanced Performance Index |
| WBC | White blood dell |
| RIA | Radioimmunoassay |
| NHPP | National Hormone and Peptide Program |
| QUICKI | Quantitative insulin sensitivity check index |
| SED | Standard error of the difference |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
References
- Dairy Australia. A Guide to Growing More Productive Heifers, 1st ed.; D. A. Limited: Melbourne, Australia, 2020; p. 13. [Google Scholar]
- Abuelo, A.; Havrlant, P.; Wood, N.; Hernandez-Jover, M. An investigation of dairy calf management practices, colostrum quality, failure of transfer of passive immunity, and occurrence of enteropathogens among Australian dairy farms. J. Dairy Sci. 2019, 102, 8352–8366. [Google Scholar] [CrossRef] [PubMed]
- Moran, J. Rearing Young Stock on Tropical Dairy Farms in ASIA, 1st ed.; CSIRO Publishing: Clayton, Australia, 2012. [Google Scholar]
- Kašná, E.; Zavadilová, L.; Vařeka, J.; Kyselová, J. General resilience in dairy cows: A review. Czech J. Anim. Sci. 2022, 67, 475–482. [Google Scholar] [CrossRef]
- Adriaens, I.; Friggens, N.C.; Ouweltjes, W.; Scott, H.; Aernouts, B.; Statham, J. Productive life span and resilience rank can be predicted from on-farm first-parity sensor time series but not using a common equation across farms. J. Dairy Sci. 2020, 103, 7155–7171. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A. Programming by Early Nutrition: An Experimental Approach. J. Nutr. 1998, 128, 401–406. [Google Scholar] [CrossRef]
- Heinrichs, A. A Short Review: The Immune System of the Dairy Calf and the Importance of Colostrum IgG. J. Dairy Vet. Anim. Res. 2017, 5, 104–107. [Google Scholar] [CrossRef]
- Chase, C.C.L. Enteric Immunity: Happy Gut, Healthy Animal. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 1–18. [Google Scholar] [CrossRef]
- Ockenden, E.M.; Russo, V.M.; Leury, B.J.; Giri, K.; Wales, W.J. Preweaning Nutrition and Its Effects on the Growth, Immune Competence and Metabolic Characteristics of the Dairy Calf. Animals 2023, 13, 829. [Google Scholar] [CrossRef]
- Ollivett, T.L.; Nydam, D.V.; Linden, T.C.; Bowman, D.D.; Van Amburgh, M.E. Effect of nutritional plane on health and performance in dairy calves after experimental infection with Cryptosporidium parvum. J. Am. Vet. Med. Assoc. 2012, 241, 1514–1520. [Google Scholar] [CrossRef]
- Nonnecke, B.J.; Foote, M.R.; Smith, J.M.; Pesch, B.A.; Van Amburgh, M.E. Composition and Functional Capacity of Blood Mononuclear Leukocyte Populations from Neonatal Calves on Standard and Intensified Milk Replacer Diets. J. Dairy Sci. 2003, 86, 3592–3604. [Google Scholar] [CrossRef]
- Foote, M.R.; Nonnecke, B.J.; Beitz, D.C.; Waters, W.R. High Growth Rate Fails to Enhance Adaptive Immune Responses of Neonatal Calves and Is Associated with Reduced Lymphocyte Viability. J. Dairy Sci. 2007, 90, 404–417. [Google Scholar] [CrossRef]
- Obeidat, B.S.; Cobb, C.J.; Sellers, M.D.; Pepper-Yowell, A.R.; Earleywine, T.J.; Ballou, M.A. Plane of nutrition during the preweaning period but not the grower phase influences the neutrophil activity of Holstein calves. J. Dairy Sci. 2013, 96, 7155–7166. [Google Scholar] [CrossRef] [PubMed]
- Cahenzli, J.; Köller, Y.; Wyss, M.; Geuking, M.B.; McCoy, K.D. Intestinal Microbial Diversity during Early-Life Colonization Shapes Long-Term IgE Levels. Cell Host Microbe 2013, 14, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Olin, A.; Henckel, E.; Chen, Y.; Lakshmikanth, T.; Pou, C.; Mikes, J.; Gustafsson, A.; Bernhardsson, A.K.; Zhang, C.; Bohlin, K.; et al. Stereotypic Immune System Development in Newborn Children. Cell 2018, 174, 1277–1292.E14. [Google Scholar] [CrossRef]
- Ockenden, E.M.; Russo, V.M.; Leury, B.J.; Giri, K.; Wales, W.J. The Preservation of the Effects of Preweaning Nutrition on Growth, Immune Competence and Metabolic Characteristics of the Developing Heifer. Animals 2023, 13, 1309. [Google Scholar] [CrossRef]
- Chandler, T.L.; Pralle, R.S.; Dórea, J.R.R.; Poock, S.E.; Oetzel, G.R.; Fourdraine, R.H.; White, H.M. Predicting hyperketonemia by logistic and linear regression using test-day milk and performance variables in early-lactation Holstein and Jersey cows. J. Dairy Sci. 2018, 101, 2476–2491. [Google Scholar] [CrossRef]
- Tessari, R.; Berlanda, M.; Morgante, M.; Badon, T.; Gianesella, M.; Mazzotta, E.; Contiero, B.; Fiore, E. Changes of Plasma Fatty Acids in Four Lipid Classes to Understand Energy Metabolism at Different Levels of Non-Esterified Fatty Acid (NEFA) in Dairy Cows. Animals 2020, 10, 1410. [Google Scholar] [CrossRef]
- Khan, M.A.; Weary, D.M.; von Keyserlingk, M.A.G. Invited review: Effects of milk ration on solid feed intake, weaning, and performance in dairy heifers. J. Dairy Sci. 2011, 94, 1071–1081. [Google Scholar] [CrossRef]
- Schwarzkopf, S.; Kinoshita, A.; Kluess, J.; Kersten, S.; Meyer, U.; Huber, K.; Dänicke, S.; Frahm, J. Weaning Holstein Calves at 17 Weeks of Age Enables Smooth Transition from Liquid to Solid Feed. Animals 2019, 9, 1132. [Google Scholar] [CrossRef]
- MacPherson, J.A.R.; Berends, H.; Leal, L.N.; Cant, J.P.; Martín-Tereso, J.; Steele, M.A. Effect of plane of milk replacer intake and age on glucose and insulin kinetics and abomasal emptying in female Holstein Friesian dairy calves fed twice daily. J. Dairy Sci. 2016, 99, 8007–8017. [Google Scholar] [CrossRef]
- Byrne, C.J.; Fair, S.; English, A.M.; Johnston, D.; Lonergan, P.; Kenny, D.A. Effect of milk replacer and concentrate intake on growth rate, feeding behaviour and systemic metabolite concentrations of pre-weaned bull calves of two dairy breeds. Animal 2017, 11, 1531–1538. [Google Scholar] [CrossRef]
- Rosadiuk, J.P.; Bruinjé, T.C.; Moslemipur, F.; Fischer-Tlustos, A.J.; Renaud, D.L.; Ambrose, D.J.; Steele, M.A. Differing planes of pre- and postweaning phase nutrition in Holstein heifers: I. Effects on feed intake, growth efficiency, and metabolic and development indicators. J. Dairy Sci. 2021, 104, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Wilms, J.N.; Ghaffari, M.H.; Steele, M.A.; Sauerwein, H.; Martín-Tereso, J.; Leal, L.N. Macronutrient profile in milk replacer or a whole milk powder modulates growth performance, feeding behavior, and blood metabolites in ad libitum-fed calves. J. Dairy Sci. 2022, 105, 6670–6692. [Google Scholar] [CrossRef] [PubMed]
- Yunta, C.; Terré, M.; Bach, A. Short- and medium-term changes in performance and metabolism of dairy calves offered different amounts of milk replacers. Livest. Sci. 2015, 181, 249–255. [Google Scholar] [CrossRef]
- Bach, A.; Domingo, L.; Montoro, C.; Terré, M. Short communication: Insulin responsiveness is affected by the level of milk replacer offered to young calves. J. Dairy Sci. 2013, 96, 4634–4637. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity In Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Ockenden, E.M.; Russo, V.M.; Leury, B.J.; Giri, K.; Wales, W.J. Early Life Nutrition and its Effects on the Developing Heifer: Growth, nutritive intakes, and metabolism. J. Dairy Sci. 2025, 108, 3515–3528. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes; Australian Government: Canberra, Australian, 2013.
- de Souza, R.S.; Dos Santos, L.B.C.; Melo, I.O.; Cerqueira, D.M.; Dumas, J.V.; Leme, F.O.P.; Moreira, T.F.; Meneses, R.M.; de Carvalho, A.U.; Facury-Filho, E.J. Current Diagnostic Methods for Assessing Transfer of Passive Immunity in Calves and Possible Improvements: A Literature Review. Animals 2021, 11, 2936. [Google Scholar] [CrossRef]
- Lombard, J.; Urie, N.; Garry, F.; Godden, S.; Quigley, J.; Earleywine, T.; McGuirk, S.; Moore, D.; Branan, M.; Chamorro, M.; et al. Consensus recommendations on calf- and herd-level passive immunity in dairy calves in the United States. J. Dairy Sci. 2020, 103, 7611–7624. [Google Scholar] [CrossRef]
- Aleri, J.W.; Hine, B.C.; Pyman, M.F.; Mansell, P.D.; Wales, W.J.; Mallard, B.; Fisher, A.D. Assessing adaptive immune response phenotypes in Australian Holstein-Friesian heifers in a pasture-based production system. J. Anim. Sci. 2015, 93, 3713–3721. [Google Scholar] [CrossRef]
- Aleri, J.W.; Hine, B.C.; Pyman, M.F.; Mansell, P.D.; Wales, W.J.; Mallard, B.; Stevenson, M.A.; Fisher, A.D. Associations between immune competence, stress responsiveness, and production in Holstein-Friesian and Holstein-Friesian × Jersey heifers reared in a pasture-based production system in Australia. J. Dairy Sci. 2019, 102, 3282–3294. [Google Scholar] [CrossRef]
- Singh, B.; Saxena, A. Surrogate markers of insulin resistance: A review. World J. Diabetes 2010, 1, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J. Logarithmic transformation bias in allometry. Am. J. Phys. Anthropol. 1993, 90, 215–228. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Henderson, A.J.; Dow, S.W. Suppression of vaccine immunity by inflammatory monocytes. J. Immunol. 2012, 189, 5612–5621. [Google Scholar] [CrossRef]
- Nakano, H.; Lin, K.L.; Yanagita, M.; Charbonneau, C.; Cook, D.N.; Kakiuchi, T.; Gunn, M.D. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat. Immunol. 2009, 10, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.F.; Spencer, L.A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017, 17, 746–760. [Google Scholar] [CrossRef]
- Soberon, F.; Van Amburgh, M.E. LACTATION BIOLOGY SYMPOSIUM: The effect of nutrient intake from milk or milk replacer of preweaned dairy calves on lactation milk yield as adults: A meta-analysis of current data. J. Anim. Sci. 2013, 91, 706–712. [Google Scholar] [CrossRef]
- Tsai, S.; Clemente-Casares, X.; Zhou, A.C.; Lei, H.; Ahn, J.J.; Chan, Y.T.; Choi, O.; Luck, H.; Woo, M.; Dunn, S.E.; et al. Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection. Cell Metab. 2018, 28, 922–934.E4. [Google Scholar] [CrossRef]
- van Niekerk, G.; Christowitz, C.; Conradie, D.; Engelbrecht, A.-M. Insulin as an immunomodulatory hormone. Cytokine Growth Factor Rev. 2020, 52, 34–44. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, J.A.; Sanz, A.; Tamanini, C.; Casasús, I. Metabolic, endocrine, and reproductive responses of beef heifers submitted to different growth strategies during the lactation and rearing periods. J. Anim. Sci. 2015, 93, 3871–3885. [Google Scholar] [CrossRef]
- De Paula, C.; Rennó, L.N.; Ferreira, M.F.D.L.; Da Silva, Á.E.M.; Moreira, S.S.; De Freitas Assis, G.J.; Detmann, E.; De Campos Valadares Filho, S.; Fonseca Paulino, M.; Dos Santos, G.M. Effect of pre- and post-weaning supplementation on performance, nutritional, and metabolic characteristics in Nellore heifers under grazing. Anim. Prod. Sci. 2022, 62, 1706–1719. [Google Scholar] [CrossRef]
- McGuire, M.A.; Vicini, J.L.; Bauman, D.E.; Veenhuizen, J.J. Insulin-like growth factors and binding proteins in ruminants and their nutritional regulation. J. Anim. Sci. 1992, 70, 2901–2910. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.C.L.; Hurley, D.J.; Reber, A.J. Neonatal Immune Development in the Calf and Its Impact on Vaccine Response. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Francis, T. On the Doctrine of Original Antigenic Sin. Proc. Am. Philos. Soc. 1960, 104, 572–578. [Google Scholar]
- Vatti, A.; Monsalve, D.M.; Pacheco, Y.; Chang, C.; Anaya, J.M.; Gershwin, M.E. Original antigenic sin: A comprehensive review. J. Autoimmun. 2017, 83, 12–21. [Google Scholar] [CrossRef]
- Chandra, R.K. Nutrition and the immune system: An introduction. Am. J. Clin. Nutr. 1997, 66, 460S–463S. [Google Scholar] [CrossRef]
- Schultze, A.B. Condition in Dairy Calves and Level of Circulating Eosinophils. J. Dairy Sci. 1957, 40, 672–676. [Google Scholar] [CrossRef]
- Knowles, T.G.; Edwards, J.E.; Bazeley, K.J.; Brown, S.N.; Butterworth, A.; Warriss, P.D. Changes in the blood biochemical and haematological profile of neonatal calves with age. Vet. Rec. 2000, 147, 593–598. [Google Scholar] [CrossRef]
- Brun-Hansen, H.C.; Kampen, A.H.; Lund, A. Hematologic values in calves during the first 6 months of life. Vet. Clin. Pathol. 2006, 35, 182–187. [Google Scholar] [CrossRef]
- Mohri, M.; Sharifi, K.; Eidi, S. Hematology and serum biochemistry of Holstein dairy calves: Age related changes and comparison with blood composition in adults. Res. Vet. Sci. 2007, 83, 30–39. [Google Scholar] [CrossRef]


| Time | Bodyweight (kg) | Concentrate Intake | ||||||
|---|---|---|---|---|---|---|---|---|
| HH | HL | LH | LL | HH | HL | LH | LL | |
| Preweaning phase | (kg DM/heifer per week) | |||||||
| Birth | 38.7 | 39.8 | 38.7 | 39.1 | 0.00 | 0.00 | 0.07 | 0.07 |
| 6-week immune challenge | 72.2 | 73.5 | 61.3 | 60.5 | 1.33 | 1.19 | 4.90 | 4.62 |
| 12 weeks (weaning) | 117.8 | 119.0 | 106.2 | 103.1 | 9.80 | 9.52 | 12.18 | 12.18 |
| Postweaning Phase | (kg DM/heifer per month) | |||||||
| 8-month immune challenge | 244.9 | 239.6 | 232.8 | 222.7 | 1.1 | 0.49 | 10.9 | 0.0 |
| 13-month immune challenge | 292.3 | 269.4 | 294.6 | 264.2 | 23.6 | 0.0 | 25.1 | 0.0 |
| 20 months (completion) | 493.8 | 486.0 | 495.9 | 450.8 | 46.4 | 0.4 | 66.3 | 11.9 |
| 6-Weeks | p-Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 Main Effect | Time Main Effect | T1 × Time | |||||||
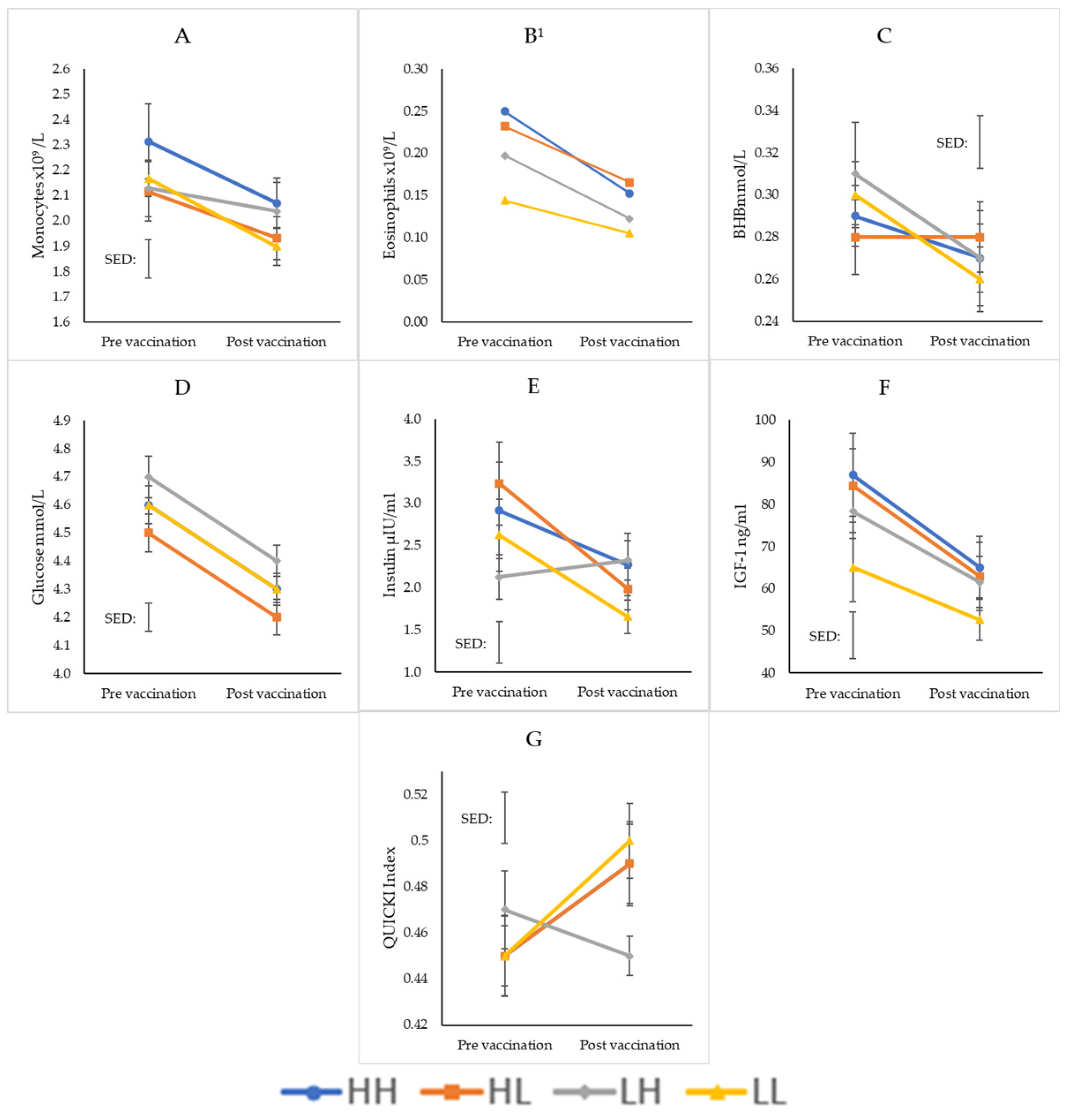
| WBC | 0.421 | <0.001 | 0.504 | ||||||
| Neutrophils | 0.647 | 0.043 | 0.594 | ||||||
| Monocytes | 0.017 | <0.001 | 0.138 | ||||||
| Lymphocytes | 0.273 | <0.001 | 0.371 | ||||||
| Basophils | 0.102 | 0.032 | 0.099 | ||||||
| Eosinophils | 0.008 | 0.002 | 0.321 | ||||||
| BHB | <0.001 | <0.001 | 0.201 | ||||||
| NEFA | 0.042 | <0.001 | 0.502 | ||||||
| Glucose | <0.001 | 0.022 | 0.079 | ||||||
| Insulin | <0.001 | 0.261 | 0.009 | ||||||
| IGF-1 | <0.001 | <0.001 | 0.603 | ||||||
| QUICKI | <0.001 | 0.339 | 0.008 | ||||||
| 8-months | T1 main effect | T2 main effect | Time main effect | T1 × T2 | T1 × Time | T2 × Time | T1 × T2 × Time | ||
| Monocytes | 0.609 | 0.250 | <0.001 | 0.543 | 0.744 | 0.567 | 0.256 | ||
| Eosinophils | <0.001 | 0.252 | <0.001 | 0.229 | 0.891 | 0.295 | 0.979 | ||
| BHB | 0.649 | 0.736 | 0.026 | 0.892 | 0.254 | 0.880 | 0.714 | ||
| Glucose | 0.091 | 0.258 | <0.001 | 0.932 | 0.870 | 0.793 | 0.645 | ||
| Insulin | 0.169 | 0.904 | <0.001 | 0.863 | 0.129 | 0.017 | 0.447 | ||
| IGF-1 | 0.129 | 0.326 | <0.001 | 0.519 | 0.380 | 0.766 | 0.807 | ||
| QUICKI | 0.879 | 0.597 | 0.002 | 0.526 | 0.367 | 0.023 | 0.056 | ||
| 13-months | T1 main effect | T2 main effect | Time main effect | T1 × T2 | T1 × Time | T2 × Time | T1 × T2 × Time | ||
| WBC | 0.483 | 0.828 | 0.805 | 0.625 | 0.894 | 0.184 | 0.002 | ||
| Neutrophils | 0.446 | 0.928 | 0.055 | 0.929 | 0.886 | 0.300 | 0.004 | ||
| Monocytes | 0.468 | 0.202 | <0.001 | 0.180 | 0.923 | 0.735 | 0.495 | ||
| Lymphocytes | 0.702 | 0.674 | <0.001 | 0.709 | 0.709 | 0.229 | 0.514 | ||
| Basophils | 0.171 | 0.533 | 0.002 | 0.986 | 0.844 | 0.081 | 0.633 | ||
| Eosinophils | 0.028 | 0.162 | 0.456 | 0.013 | 0.273 | 0.274 | 0.010 | ||
| BHB | 0.172 | 0.071 | <0.001 | 0.588 | 0.679 | 0.258 | 0.364 | ||
| NEFA | 0.967 | 0.013 | 0.020 | 0.216 | 0.426 | 0.239 | 0.667 | ||
| Glucose | 0.106 | 0.960 | <0.001 | 0.125 | 0.374 | 0.006 | 0.108 | ||
| Insulin | 0.535 | <0.001 | <0.001 | 0.308 | 0.657 | 0.315 | 0.182 | ||
| IGF-1 | 0.760 | 0.030 | 0.508 | 0.396 | 0.436 | 0.016 | 0.894 | ||
| QUICKI | 0.660 | <0.001 | <0.001 | 0.199 | 0.557 | 0.002 | 0.472 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ockenden, E.M.; Russo, V.M.; Leury, B.J.; Giri, K.; Wales, W.J. Early Life Nutrition and Its Effects on the Developing Heifer: Immune and Metabolic Responses to Immune Challenges. Animals 2025, 15, 1379. https://doi.org/10.3390/ani15101379
Ockenden EM, Russo VM, Leury BJ, Giri K, Wales WJ. Early Life Nutrition and Its Effects on the Developing Heifer: Immune and Metabolic Responses to Immune Challenges. Animals. 2025; 15(10):1379. https://doi.org/10.3390/ani15101379
Chicago/Turabian StyleOckenden, Emma M., Victoria M. Russo, Brian J. Leury, Khageswor Giri, and William J. Wales. 2025. "Early Life Nutrition and Its Effects on the Developing Heifer: Immune and Metabolic Responses to Immune Challenges" Animals 15, no. 10: 1379. https://doi.org/10.3390/ani15101379
APA StyleOckenden, E. M., Russo, V. M., Leury, B. J., Giri, K., & Wales, W. J. (2025). Early Life Nutrition and Its Effects on the Developing Heifer: Immune and Metabolic Responses to Immune Challenges. Animals, 15(10), 1379. https://doi.org/10.3390/ani15101379







