Positive Correlation of Social Rank and Hair Cortisol Concentration in Group-Housed Pregnant Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
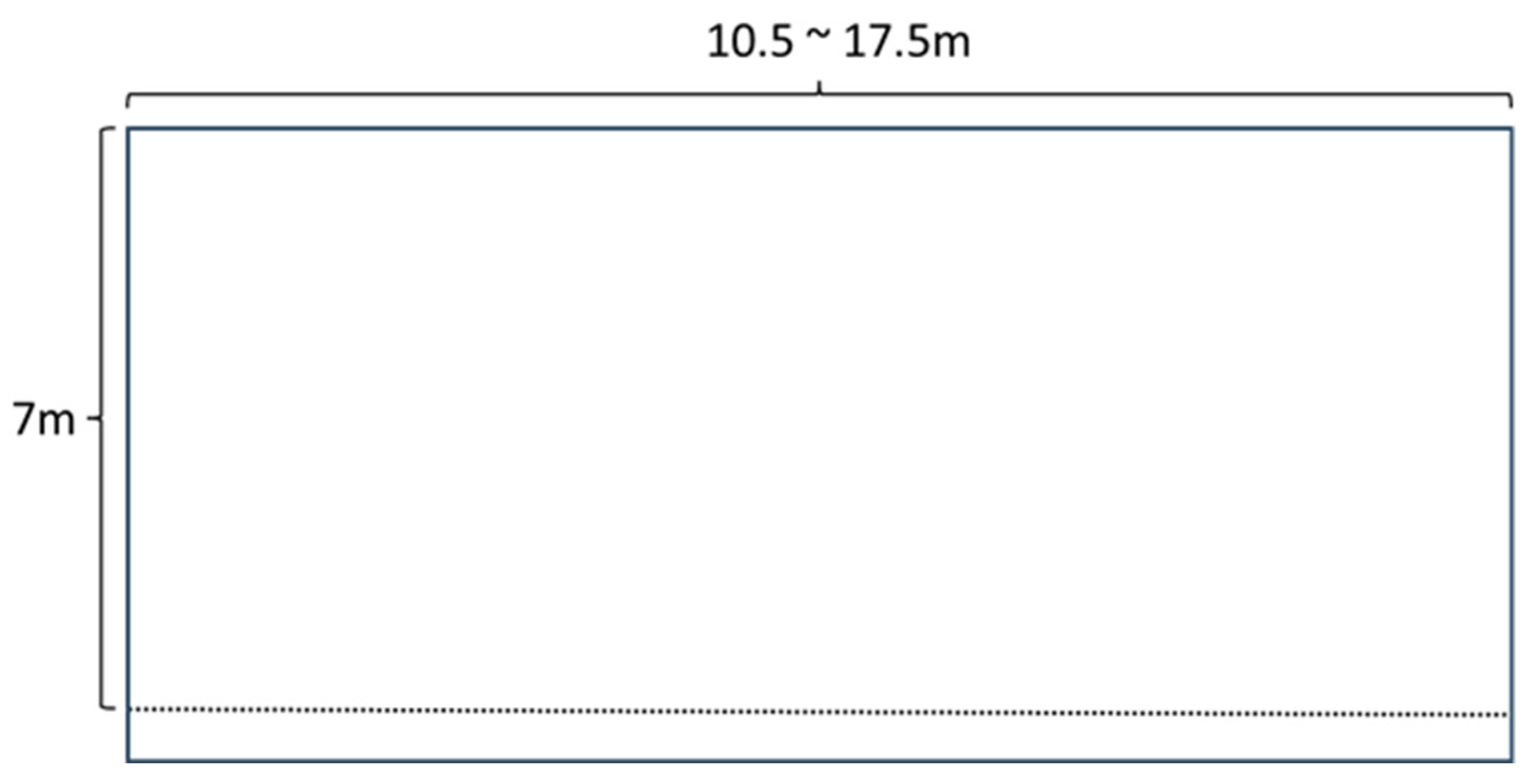
2.2. Husbandry Conditions
2.3. Examinations
2.3.1. Social Behaviours
2.3.2. Avoidance Distance Measurement
2.3.3. Hair Sampling and Analysis
2.4. Data Calculation and Statistical Analyses
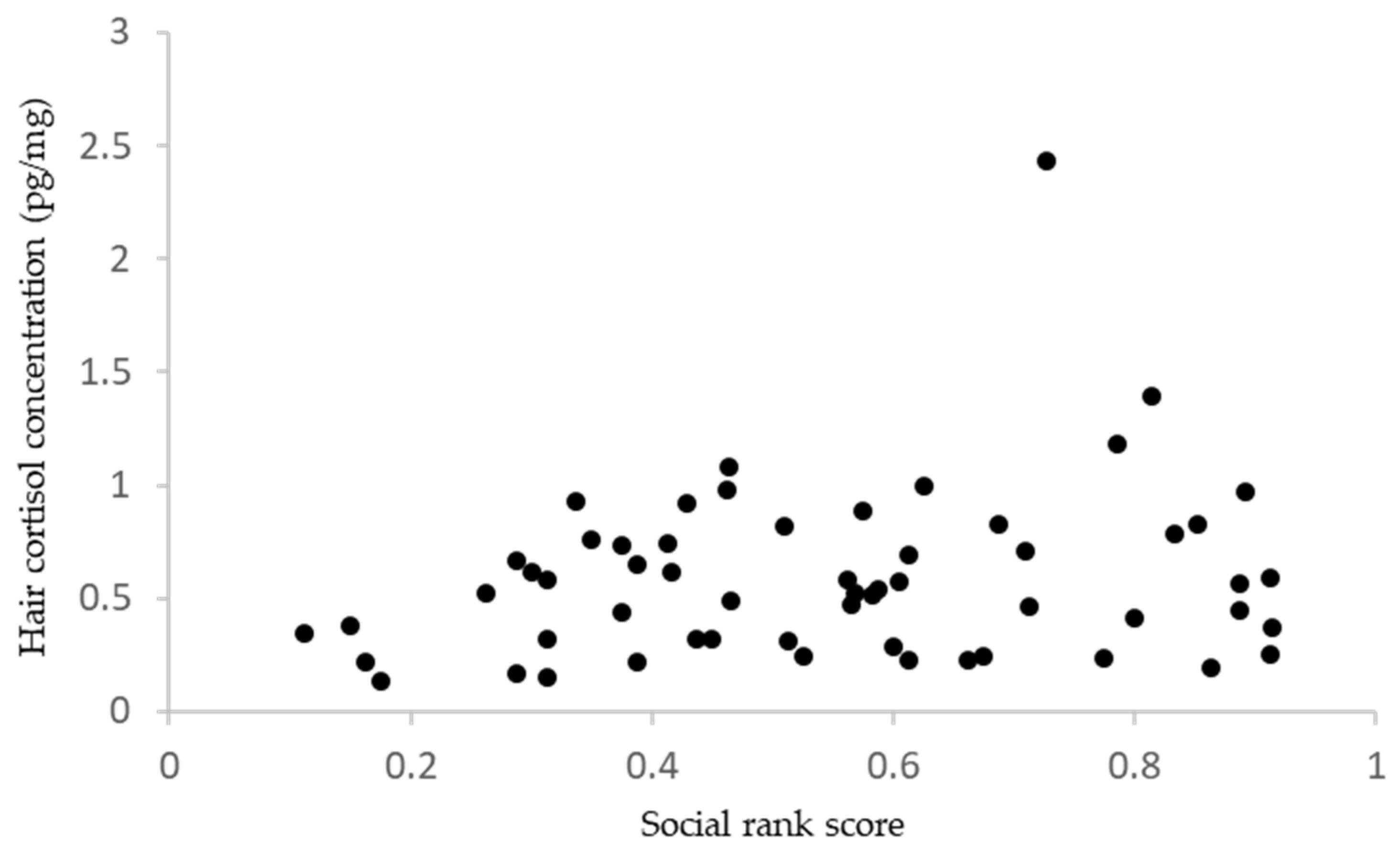
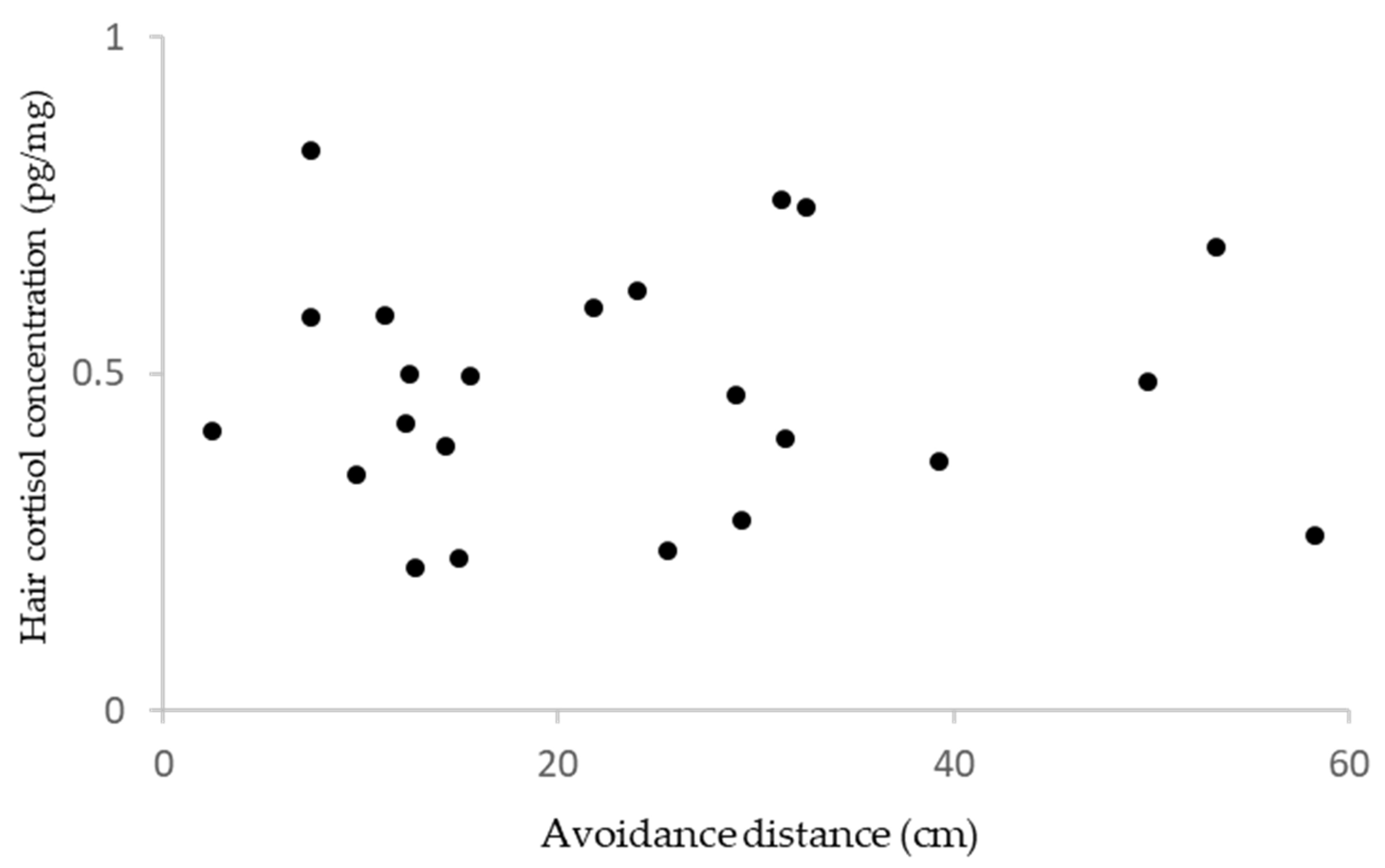
3. Results
4. Discussion
4.1. Social Rank
4.2. Avoidance Distance
4.3. Implication for Animal Welfare
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WOAH. Animal Welfare and Beef Cattle Production Systems. In Terrestrial Animal Health Code. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/ (accessed on 30 October 2024).
- Hubbard, A.J.; Foster, M.J.; Daigle, C.L. Social dominance in beef cattle—A scoping review. Appl. Anim. Behav. Sci. 2021, 241, 105390. [Google Scholar] [CrossRef]
- Boissy, A.; Bouissou, M.F. Effects of early handling on heifers’ subsequent reactivity to humans and to unfamiliar situations. Appl. Anim. Behav. Sci. 1988, 20, 259–273. [Google Scholar] [CrossRef]
- Probst, J.K.; Spengler, N.A.; Leiber, F.; Kreuzer, M.; Hillmann, E. Gentle touching in early life reduces avoidance distance and slaughter stress in beef cattle. Appl. Anim. Behav. Sci. 2012, 139, 42–49. [Google Scholar] [CrossRef]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the hypothalamic–pituitary–adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Miranda-de la Lama, G.C.; Pascual-Alonso, M.; Guerrero, A.; Alberti, P.; Alierta, S.; Sans, P.; Gajan, J.P.; Villarroel, M.; Dalmau, A.; Velarde, A.; et al. Influence of social dominance on production, welfare and the quality of meat from beef bulls. Meat Sci. 2013, 94, 432–437. [Google Scholar] [CrossRef]
- Solano, J.; Galindo, F.; Orihuela, A.; Galina, C.S. The effect of social rank on the physiological response during repeated stressful handling in Zebu cattle (Bos indicus). Physiol. Behav. 2004, 82, 679–683. [Google Scholar] [CrossRef]
- Ebinghaus, A.; Knierim, U.; Simantke, C.; Palme, R.; Ivemeyer, S. Fecal Cortisol Metabolites in Dairy Cows: A Cross-Sectional Exploration of Associations with Animal, Stockperson, and Farm Characteristics. Animals 2020, 10, 1787. [Google Scholar] [CrossRef] [PubMed]
- Heimbürge, S.; Kanitz, E.; Tuchscherer, A.; Otten, W. Is it getting in the hair?—Cortisol concentrations in native, regrown and segmented hairs of cattle and pigs after repeated ACTH administrations. Gen. Comp. Endocrinol. 2020, 295, 113534. [Google Scholar] [CrossRef] [PubMed]
- Galindo, F.; Broom, D.M. The relationships between social behaviour of dairy cows and the occurrence of lameness in three herds. Res. Vet. Sci. 2000, 69, 75–79. [Google Scholar] [CrossRef]
- Windschnurer, I.; Schmied, C.; Boivin, X.; Waiblinger, S. Reliability and inter-test relationship of tests for on-farm assessment of dairy cows’ relationship to humans. Appl. Anim. Behav. Sci. 2008, 114, 37–53. [Google Scholar] [CrossRef]
- Mendl, M.; Deag, J.M. How useful are the concepts of alternative strategy and coping strategy in applied studies of social behaviour? Appl. Anim. Behav. Sci. 1995, 44, 119–137. [Google Scholar] [CrossRef]
- Hasegawa, N.; Nishiwaki, A.; Sugawara, K.; Iwao, I. The effects of social exchange between two groups of lactating primiparous heifers on milk production, dominance order, behavior and adrenocortical response. Appl. Anim. Behav. Sci. 1997, 51, 15–27. [Google Scholar] [CrossRef]
- Arave, C.W.; Mickelsen, C.H.; Lamb, R.C.; Svejda, A.J.; Canfield, R.V. Effects of Dominance Rank Changes, Age, and Body Weight on Plasma Corticoids of Mature Dairy Cattle. J. Dairy Sci. 1977, 60, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, O.; Heath, E. Social behaviour and adrenal cortical activity in heifers. Appl. Anim. Ethol. 1982, 8, 99–108. [Google Scholar] [CrossRef]
- Mench, J.A.; Swanson, J.C.; Stricklin, W.R. Social stress and dominance among group members after mixing beef cows. Can. J. Anim. Sci. 1990, 70, 345–354. [Google Scholar] [CrossRef]
- Mülleder, C.; Palme, R.; Menke, C.; Waiblinger, S. Individual differences in behaviour and in adrenocortical activity in beef-suckler cows. Appl. Anim. Behav. Sci. 2003, 84, 167–183. [Google Scholar] [CrossRef]
- Partida, J.A.; Olleta, J.L.; Campo, M.M.; Sañudo, C.; María, G.A. Effect of social dominance on the meat quality of young Friesian bulls. Meat Sci. 2007, 76, 266–273. [Google Scholar] [CrossRef]
- Val-Laillet, D.; Passillé, A.M.D.; Rushen, J.; von Keyserlingk, M.A.G. The concept of social dominance and the social distribution of feeding-related displacements between cows. Appl. Anim. Behav. Sci. 2008, 111, 158–172. [Google Scholar] [CrossRef]
- Hubbard, A.J.; Foster, M.J.; Daigle, C.L. Impact of social mixing on beef and dairy cattle—A scoping review. Appl. Anim. Behav. Sci. 2021, 241, 105389. [Google Scholar] [CrossRef]
- Kondo, S.; Hurnik, J.F. Stabilization of social hierarchy in dairy cows. Appl. Anim. Behav. Sci. 1990, 27, 287–297. [Google Scholar] [CrossRef]
- Von Keyserlingk, M.A.G.; Olenick, D.; Weary, D.M. Acute behavioral effects of regrouping dairy cows. J. Dairy Sci. 2008, 91, 1011–1016. [Google Scholar] [CrossRef]
- Fisher, D.D.; Wilson, L.L.; Leach, R.M.; Scholz, R.W. Switch hair as an indicator of magnesium and copper status of beef cows. Am. J. Vet. Res. 1985, 46, 2235–2240. [Google Scholar] [PubMed]
- Ninomiya, S. Satisfaction of farm animal behavioral needs in behaviorally restricted systems: Reducing stressors and environmental enrichment. Anim. Sci. J. 2014, 85, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, S.; Sato, S. Effects of ‘Five freedoms’ environmental enrichment on the welfare of calves reared indoors. Anim. Sci. J. 2009, 80, 347–351. [Google Scholar] [CrossRef]
- Rizzo, A.; Maresca, L.; Ceci, E.; Guaricci, A.; Sciorsci, R.L. Kisspeptin-10 and gonadotropin inhibiting hormone during pregnancy in dairy cows. Vet. Ital. 2022, 58, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, P.H.; Coleman, G.J. Human-Livestock Interactions: The Stockperson and the Productivity and Welfare of Intensively-Farmed Animals, 2nd ed.; CABI: Wallingford, UK, 2011; p. ix. 187p. [Google Scholar]
- Dawkins, M.S. The Science of Animal Welfare: Understanding What Animals Want; Oxford University Press: Oxford, UK, 2021; pp. 78–80. [Google Scholar] [CrossRef]
- Ralph, C.R.; Tilbrook, A.J. Invited Review: The usefulness of measuring glucocorticoids for assessing animal welfare. J. Anim. Sci. 2016, 94, 457–470. [Google Scholar] [CrossRef]
- Farm Animal Welfare Council. FAWC updates the five freedoms. Vet. Rec. 1992, 131, 357. [Google Scholar]
| Behaviour | Definition |
|---|---|
| butt | cow using the front of her head to make contact with another cow |
| threat | cow turning toward or approaching another cow with head down and then lunging without making contact |
| chases | cow actively moving toward another cow, chasing the latter to walk or run away |
| avoidance | cow actively moving away from another cow whether or not a previous interaction has occurred between the two cows |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ninomiya, S.; Nishi, A.; Nakamura, R.; Shibata, M. Positive Correlation of Social Rank and Hair Cortisol Concentration in Group-Housed Pregnant Cows. Animals 2025, 15, 13. https://doi.org/10.3390/ani15010013
Ninomiya S, Nishi A, Nakamura R, Shibata M. Positive Correlation of Social Rank and Hair Cortisol Concentration in Group-Housed Pregnant Cows. Animals. 2025; 15(1):13. https://doi.org/10.3390/ani15010013
Chicago/Turabian StyleNinomiya, Shigeru, Ayumi Nishi, Ririka Nakamura, and Mitsuhiro Shibata. 2025. "Positive Correlation of Social Rank and Hair Cortisol Concentration in Group-Housed Pregnant Cows" Animals 15, no. 1: 13. https://doi.org/10.3390/ani15010013
APA StyleNinomiya, S., Nishi, A., Nakamura, R., & Shibata, M. (2025). Positive Correlation of Social Rank and Hair Cortisol Concentration in Group-Housed Pregnant Cows. Animals, 15(1), 13. https://doi.org/10.3390/ani15010013





