Performance of Culture Using a Semi-Automatic Needle as a Novel Tool for Collecting Lymph Node Samples for the Diagnosis of Canine Visceral Leishmaniasis
Simple Summary
Abstract
1. Introduction
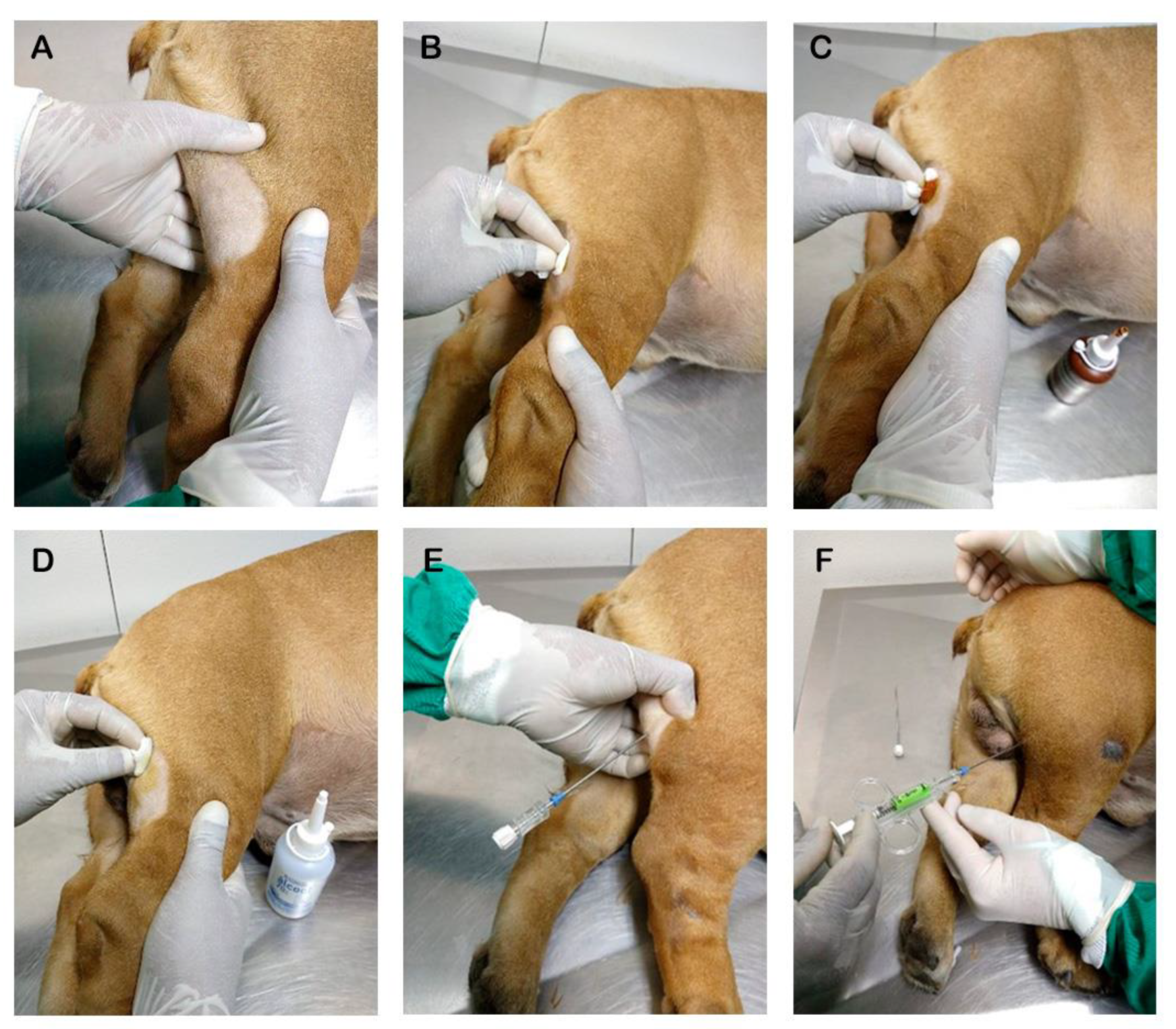
2. Materials and Methods
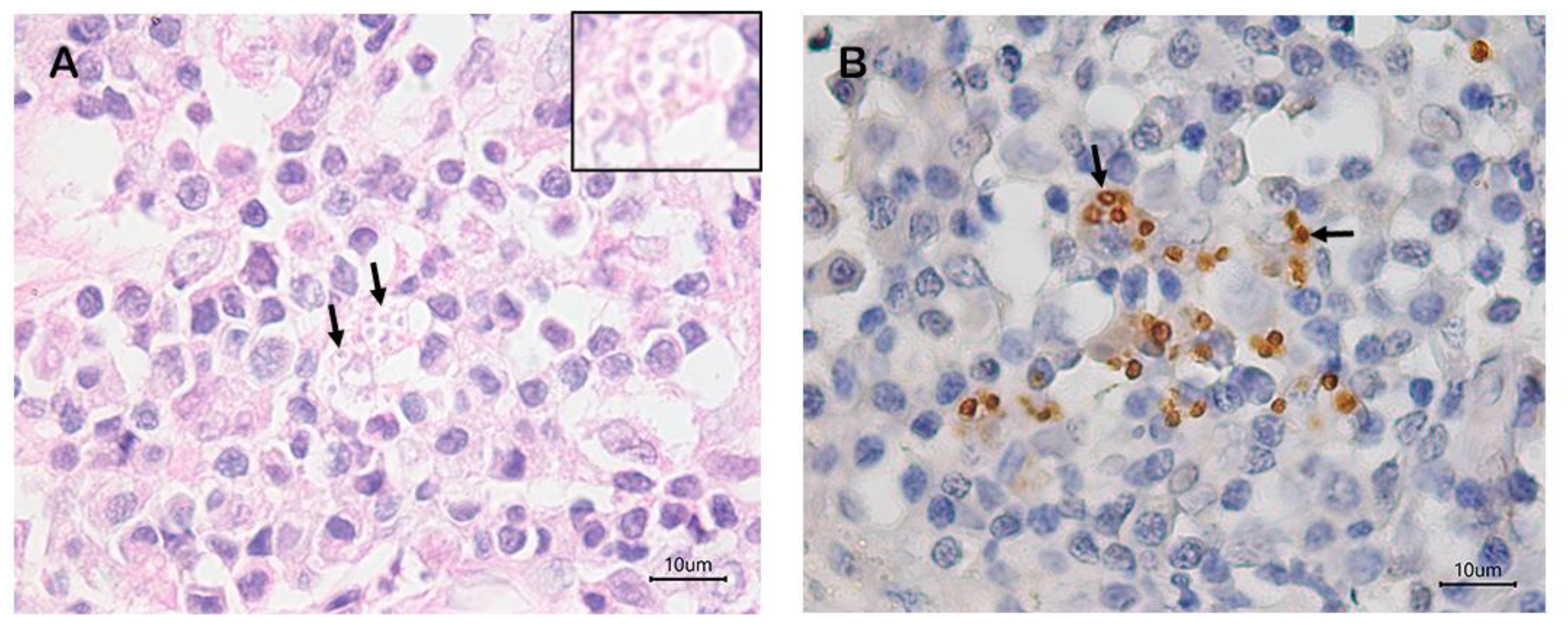
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Manual de Vigilância e Controle da Leishmaniose Visceral/Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica, 1st ed.; Ministério da Saúde: Brasília, Brazil, 2014; pp. 1–120.
- World Health Organization-Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 6 July 2024).
- Pessoa-E-Silva, R.; Vaitkevicius-Antão, V.; de Andrade, T.; de Oliveira Silva, A.; de Oliveira, G.; Trajano-Silva, L.; Nakasone, E.; de Paiva-Cavalcanti, M. The diagnosis of canine visceral leishmaniasis in Brazil: Confronting old problems. Exp. Parasitol. 2019, 199, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S.; Solano-Gallego, L.; Fondati, A.; Lubas, G.; Gradoni, L.; Castagnaro, M.; Crotti, A.; Maroli, M.; Oliva, G.; Roura, X.; et al. Canine Leishmaniasis Working Group, Italian Society of Veterinarians of Companion Animals. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J. Am. Vet Med. Assoc. 2010, 236, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.A.B.; Luvizotto, M.C.R.; Garcia, J.F.; Corbett, C.E.P.; Laurenti, M.D. Comparison of parasitological, immunological and molecular methods for the diagnosis of leishmaniasis in dogs with different clinical signs. Vet. Parasitol. 2007, 145, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.F.; Figueiredo, F.B.; Pinto, A.G.S.; Nascimento, L.D.; Furtado, M.; Mouta- Confort, E.; de Paula, C.C.; Bogio, A.; Gomes, M.C.A.; Bessa, A.M.S.; et al. Parasitological diagnosis of canine visceral leishmaniasis: Is intact skin a good target? Res. Vet. Sci. 2009, 87, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.B.P.F.; Sousa, V.R.F.; Boa Sorte, E.C.; Figueiredo, F.B.; de Paula, D.A.J.; Pimentel, M.F.A.; Dutra, V.; Madeira, M.F. Use of Parasitological culture to detect Leishmania (Leishmania) chagasi in naturally infected dogs. Vector Borne Zoonotic Dis. 2011, 11, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.V.A.; de Souza, T.L.; Figueiredo, F.B.; Mendes, A.A.V.; Ferreira, L.C.; Filgueira, P.C.B.; Cuervo, P.; Porrozzi, R.; Menezes, R.C.; Morgado, F.N. Detection of amastigotes and histopathological alterations in the thymus of Leishmania infantum-infected dogs. Immun. Inflamm. Dis. 2020, 8, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.C.; Figueiredo, F.B.; Wise, A.G.; Madeira, F.M.; Oliveira, R.V.C.; Schubach, T.M.P.; Kiupel, M.; Langohr, I.M. Sensitivity and specificity of in situ hybridization for diagnosis of cutaneous infection by Leishmania infantum in dogs. J. Clin. Microbiol. 2013, 51, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.C.; Quintella, L.P.; Schubach, A.O.; Miranda, L.F.C.; Madeira, M.F.; Pimentel, M.I.F.; Vasconcellos, E.C.F.; Lyra, M.R.; Oliveira, R.V.C.; Menezes, R.C. Comparison between colorimetric in situ hybridization, histopathology, and immunohistochemistry for the diagnosis of New World cutaneous leishmaniasis in human skin samples. Trop. Med. Infect. Dis. 2022, 7, 344. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.C.; Menezes, R.C.; Kiupel, M.; Madeira, M.F.; Oliveira, R.V.C.; Langohr, I.M.; Figueiredo, F.B. Comparative study of in situ hybridization, immunohistochemistry and parasitological culture for the diagnosis of canine leishmaniasis. Parasit. Vectors 2015, 8, 620. [Google Scholar] [CrossRef]
- Fleming, K.L.; Howells, E.J.; Villiers, E.J.; Maddox, T.W. A Randomized controlled comparison of aspiration and non-aspiration fine-needle techniques for obtaining ultrasound-guided cytological samples from canine livers. Vet. J. 2019, 252, 105372. [Google Scholar] [CrossRef] [PubMed]
- Karakitsou, V.; Christopher, M.M.; Meletis, E.; Kostoulas, P.; Pardali, D.; Koutinas, C.K.; Mylonakis, M.E. A Comparison of cytologic quality in fine-needle specimens obtained with and without aspiration from superficial lymph nodes in the dog. J. Small Anim. Pract. 2022, 63, 16–21. [Google Scholar] [CrossRef]
- Özel, D.; Aydın, T.A. Clinical compilation of lymph node pathologies comparing the diagnostic performance of biopsy methods. J. Ultrasound 2019, 22, 59–64. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde e Ambiente. Departamento de Ações Estratégicas de Epidemiologia e Vigilância em Saúde e Ambiente. Guia de Vigilância em Saúde: Volume 2 [Recurso Eletrônico]/Ministério da Saúde, Secretaria de Vigilância em Saúde e Ambiente, Departamento de Ações Estratégicas de Epidemiologia e Vigilância em Saúde e Ambiente, 6th ed.; Ministério da Saúde: Brasília, Brazil, 2024; pp. 901–919.
- Miranda, L.F.C.; Pacheco, R.S.; Pimentel, M.I.F.; Salgueiro, M.M.; Silva, A.F.; Mello, C.X.; Barros, J.H.S.; Valete-Rosalino, C.M.; Madeira, M.F.; Xavier, S.C.D.C.; et al. Geospatial analysis of tegumentary leishmaniasis in Rio de Janeiro state, Brazil from 2000 to 2015: Species typing and flow of travelers and migrants with leishmaniasis. PLoS Negl. Trop. Dis. 2019, 13, e0007748. [Google Scholar] [CrossRef] [PubMed]
- Cupolillo, E.; Grimaldi, G.; Momen, H. A general classification of New World Leishmania using numerical zymotaxonomy. Am. J. Trop. Med. Hyg. 1994, 50, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Carson, F.L.; Cappellano, C.H. Histotechnology A Self-Instructional Text, 4th ed.; ASCP: Chicago, IL, USA, 2015; pp. 1–352. [Google Scholar]
- Oliveira, V.C.; Junior, A.A.V.M.; Ferreira, L.C.; Calvet, T.M.Q.; Santos, S.A.; Figueiredo, F.B.; Campos, M.P.; Rodrigues, F.C.C.; Oliveira, R.V.C.; Lemos, E.R.S.; et al. Frequency of co- seropositivities for certain pathogens and their relationship with clinical and histopathological changes and parasite load in dogs infected with Leishmania infantum. PLoS ONE 2021, 16, e0247560. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Koutinas, A.; Miró, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniasis. Vet. Parasitol 2009, 165, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.I.F.; Alves, E.L.M.; Da Silva, M.H.F.F.; Moza, P.G.; De Almeida, P.M.P.; Cunha, C.S.; De Mello, C.X.; De Oliveira Schubach, A. High visceral leishmaniasis mortality rate in Barra Mansa, a new area of visceral leishmaniasis transmission in the state of Rio de Janeiro, Brazil. Rev. Soc. Bras. Med. Trop. 2014, 47, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.X.; Figueiredo, F.B.; Mendes Júnior, A.A.V.; Furtado, M.C.; Miranda, L.F.C.; Madeira, M.F. Outbreak of canine visceral leishmaniasis in Barra Mansa, state of Rio de Janeiro. Rev. Soc. Bras. Med. Trop. 2014, 47, 788–790. [Google Scholar] [CrossRef]
- Maia, C.; Ramada, J.; Cristóvão, J.M.; Gonçalves, L.; Campino, L. Diagnosis of canine leishmaniasis: Conventional and molecular techniques using different tissues. Vet. J. 2009, 179, 142–144. [Google Scholar] [CrossRef]
- Sundar, S.; Rai, M. Laboratory diagnosis of visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 2002, 9, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.I.P.; Silva, D.M.; Vital, T.; Nitz, N.; de Carvalho, B.C.; Hecht, M.; Oliveira, D.; Oliveira, E.; Rabello, A.; Romero, G.A.S. Improving the reference standard for the diagnosis of canine visceral leishmaniasis: A challenge for current and future tests. Mem. Inst. Oswaldo Cruz 2019, 114, e180452. [Google Scholar] [CrossRef]
| Sampling Technique + Parasitological Examination | Frequency of Leishmania Positivity in the Popliteal Lymph Node (N = 30) | |
|---|---|---|
| N | % | |
| Necropsy + culture | 23 | 76.7 |
| FNAP + culture | 22 | 73.3 |
| Necropsy + immunohistochemistry | 21 | 70.0 |
| SANP + culture | 19 | 63.3 |
| Necropsy + histopathology | 10 | 33.3 |
| Sampling Technique | Frequency of Leishmania sp. Positivity in Popliteal Lymph Nodes | |
|---|---|---|
| Lymphadenomegaly (N = 12) | No Lymphadenomegaly (N = 18) | |
| SANP | 9 (75%) | 10 (56%) |
| FNAP | 9 (75%) | 13 (72%) |
| Necropsy | 11 (92%) | 12 (67%) |
| Sampling Technique | Frequency of Leishmania sp. Positivity (N = 64) by Culture | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Week | 2nd Week | 3rd Week | 4th Week | Total | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| SANP | 18 | 95 | 1 | 5 | 0 | 0 | 0 | 0 | 19 | 100 |
| Necropsy | 20 | 87 | 3 | 13 | 0 | 0 | 0 | 0 | 23 | 100 |
| FNAP | 18 | 82 | 2 | 9 | 1 | 4.5 | 1 | 4.5 | 22 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes Júnior, A.A.V.; Figueiredo, F.B.; Ferreira, L.C.; Keidel, L.; Ornellas, R.O.; Almeida, A.B.; Santos, F.N.; Miranda, L.d.F.C.; Marcelino, A.P.; Pereira, S.A.; et al. Performance of Culture Using a Semi-Automatic Needle as a Novel Tool for Collecting Lymph Node Samples for the Diagnosis of Canine Visceral Leishmaniasis. Animals 2025, 15, 107. https://doi.org/10.3390/ani15010107
Mendes Júnior AAV, Figueiredo FB, Ferreira LC, Keidel L, Ornellas RO, Almeida AB, Santos FN, Miranda LdFC, Marcelino AP, Pereira SA, et al. Performance of Culture Using a Semi-Automatic Needle as a Novel Tool for Collecting Lymph Node Samples for the Diagnosis of Canine Visceral Leishmaniasis. Animals. 2025; 15(1):107. https://doi.org/10.3390/ani15010107
Chicago/Turabian StyleMendes Júnior, Artur Augusto Velho, Fabiano Borges Figueiredo, Luiz Cláudio Ferreira, Lucas Keidel, Renato Orsini Ornellas, Adilson Benedito Almeida, Fernanda Nunes Santos, Luciana de Freitas Campos Miranda, Andreza Pain Marcelino, Sandro Antonio Pereira, and et al. 2025. "Performance of Culture Using a Semi-Automatic Needle as a Novel Tool for Collecting Lymph Node Samples for the Diagnosis of Canine Visceral Leishmaniasis" Animals 15, no. 1: 107. https://doi.org/10.3390/ani15010107
APA StyleMendes Júnior, A. A. V., Figueiredo, F. B., Ferreira, L. C., Keidel, L., Ornellas, R. O., Almeida, A. B., Santos, F. N., Miranda, L. d. F. C., Marcelino, A. P., Pereira, S. A., & Menezes, R. C. (2025). Performance of Culture Using a Semi-Automatic Needle as a Novel Tool for Collecting Lymph Node Samples for the Diagnosis of Canine Visceral Leishmaniasis. Animals, 15(1), 107. https://doi.org/10.3390/ani15010107







