Angiogenesis and Apoptosis: Data Comparison of Similar Microenvironments in the Corpus Luteum and Tumors
Abstract
Simple Summary
Abstract
1. Introduction
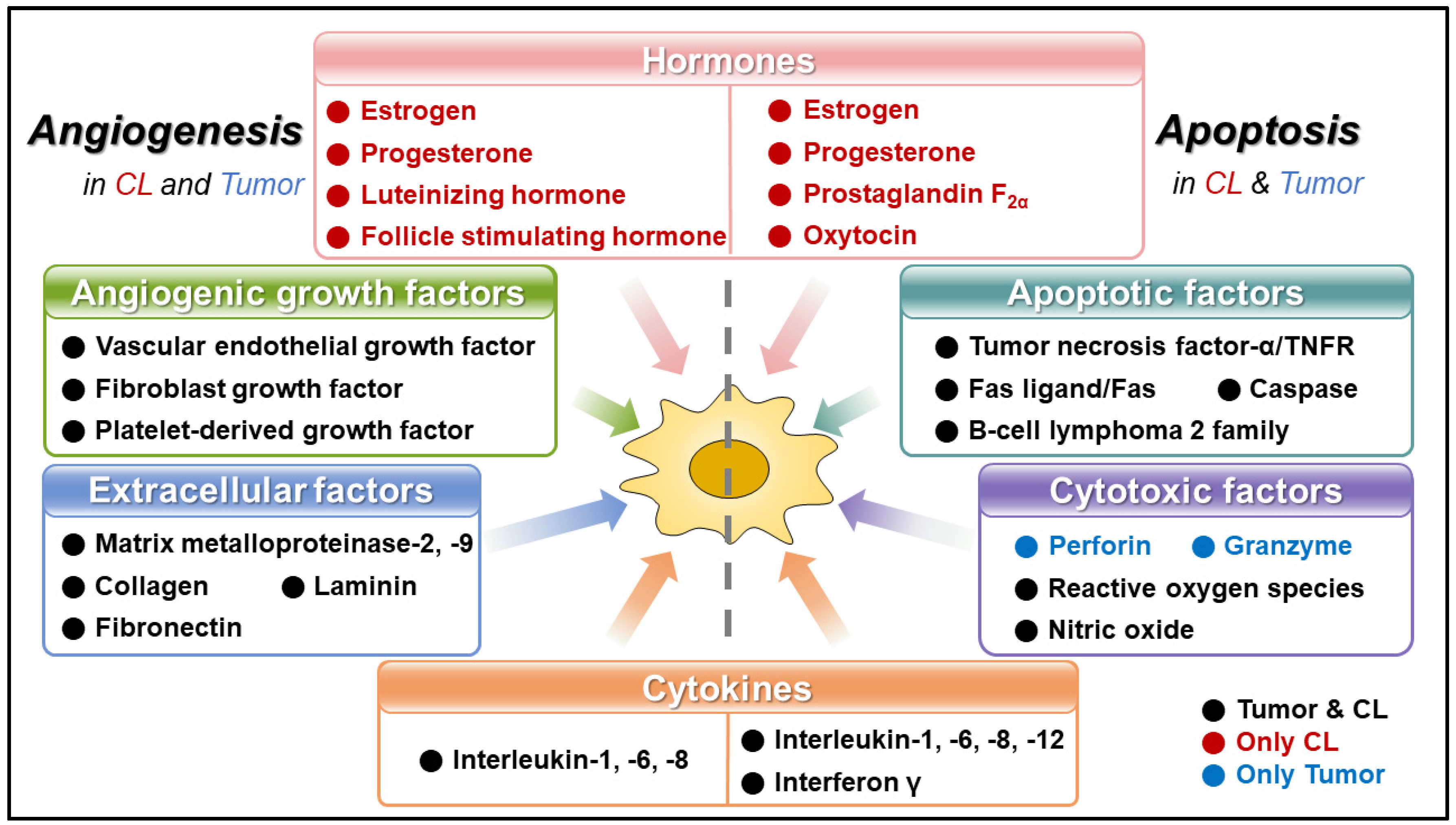
2. Angiogenesis in Corpus Luteum and Tumor
2.1. Angiogenesis in Corpus Luteum
2.2. Angiogenesis in Tumor
2.3. Cellular Interaction
2.4. Signaling Molecules
2.5. Extracellular Factors
2.6. Cytokines and Chemokines
2.7. Signaling Pathways
2.7.1. Vascular Endothelial Growth Factor (VEGF) Signaling Pathway
2.7.2. Fibroblast Growth Factor (FGF) Signaling Pathway
2.7.3. Platelet-Derived Growth Factor (PDGF) Signaling Pathway
3. Apoptosis in Corpus Luteum and Tumor
3.1. Apoptosis in Corpus Luteum
3.2. Apoptosis in Tumor
3.3. Cellular Interactions
3.4. Signaling Molecules
3.5. Extracellular Factors
3.6. Cytokines
3.7. Signaling Pathways
3.7.1. Tumor Suppressor Pathways
3.7.2. Mitochondrial Pathway
3.7.3. Death Receptor Pathway
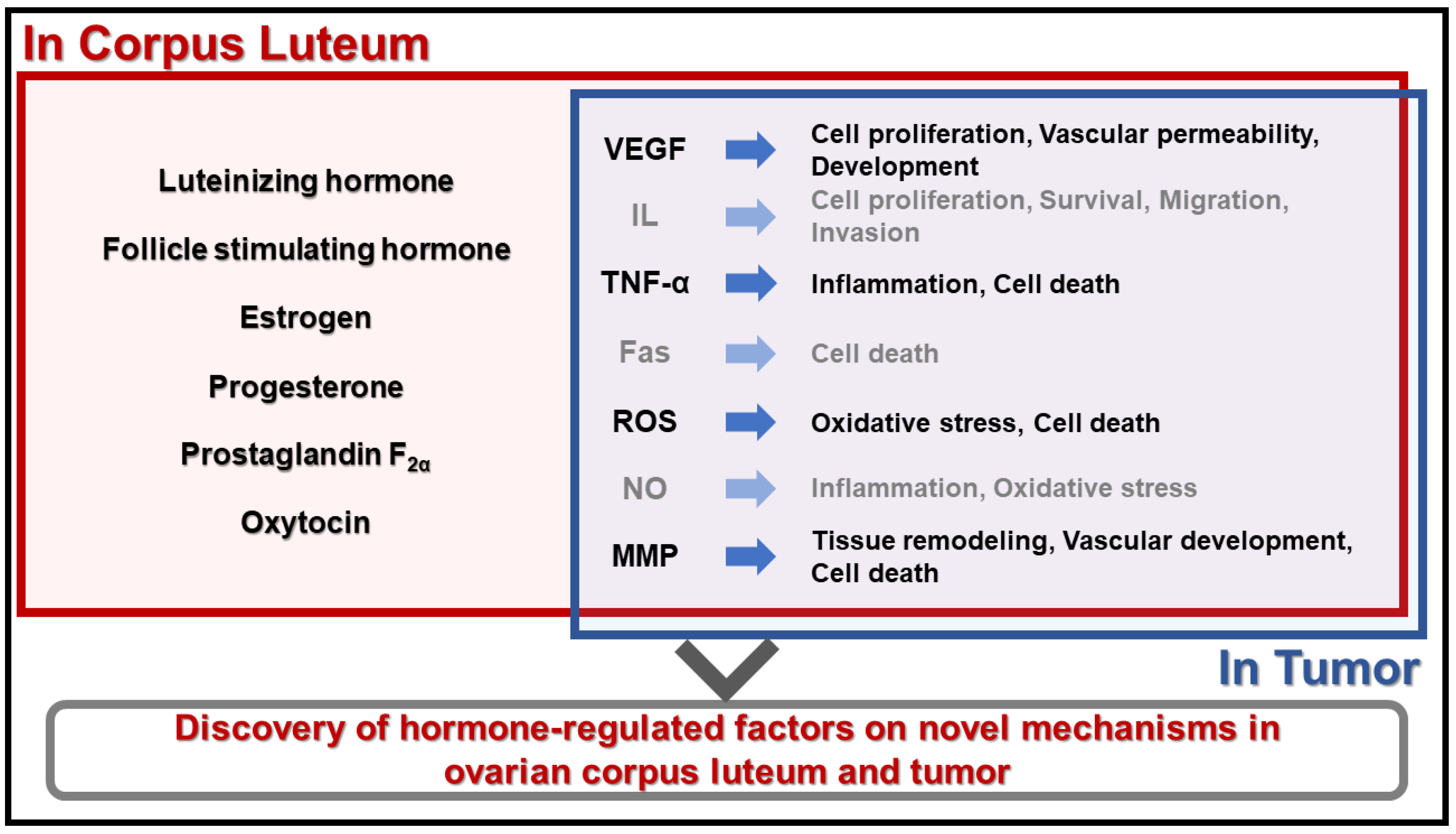
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Woad, K.J.; Robinson, R.S. Luteal angiogenesis and its control. Theriogenology 2016, 86, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xiao, L.J.; Zhang, X.S.; Liu, Y.X. Apotosis in ovary. Front. Biosci. 2011, 3, 680–697. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, C.; Zhou, S. Targeting tumor microenvironment in ovarian cancer: Premise and promise. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188361. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Okuda, K. Multiple roles of hypoxia in bovine corpus luteum. J. Reprod. Dev. 2020, 66, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hong, M.; Li, Y.; Chen, D.; Wu, Y.; Hu, Y. Programmed cell death tunes tumor immunity. Front. Immunol. 2022, 13, 847345. [Google Scholar] [CrossRef] [PubMed]
- Hojo, T.; Skarzynski, D.J.; Okuda, K. Apoptosis, autophagic cell death, and necroptosis: Different types of programmed cell death in bovine corpus luteum regression. J. Reprod. Dev. 2022, 68, 355–360. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; De Giorgis, M.; Ribatti, D. microRNAs biogenesis, functions and role in tumor angiogenesis. Front. Oncol. 2020, 10, 581007. [Google Scholar] [CrossRef]
- Rizov, M.; Andreeva, P.; Dimova, I. Molecular regulation and role of angiogenesis in reproduction. Taiwan. J. Obstet. Gynecol. 2017, 56, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Gavalas, N.G.; Liontos, M.; Trachana, S.P.; Bagratuni, T.; Arapinis, C.; Liacos, C.; Dimopoulos, M.A.; Bamias, A. Angiogenesis-related pathways in the pathogenesis of ovarian cancer. Int. J. Mol. Sci. 2013, 14, 15885–15909. [Google Scholar] [CrossRef]
- Zalman, Y.; Klipper, E.; Farberov, S.; Mondal, M.; Wee, G.; Folger, J.K.; Smith, G.W.; Meidan, R. Regulation of angiogenesis-related prostaglandin f2alpha-induced genes in the bovine corpus luteum. Biol. Reprod. 2012, 86, 92. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Kang, G.; Wang, T.; Huang, H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol. Lett. 2018, 16, 687–702. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Dhanabal, M.; Griffioen, A.W.; Sukhatme, V.P.; Ramakrishnan, S. Synergy between angiostatin and endostatin: Inhibition of ovarian cancer growth. Cancer Res. 2000, 60, 2190–2196. [Google Scholar]
- Sugino, N.; Matsuoka, A.; Taniguchi, K.; Tamura, H. Angiogenesis in the human corpus luteum. Reprod. Med. Biol. 2008, 7, 91–103. [Google Scholar] [CrossRef]
- Devesa, J.; Caicedo, D. The Role of Growth Hormone on Ovarian Functioning and Ovarian Angiogenesis. Front. Endocrinol. 2019, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Schams, D.; Berisha, B. Angiogenic Factors (VEGF, FGF and IGF) in the Bovine Corpus Luteum. J. Reprod. Dev. 2002, 48, 233–242. [Google Scholar] [CrossRef][Green Version]
- Takenaga, K. Angiogenic signaling aberrantly induced by tumor hypoxia. Front. Biosci. 2011, 16, 31–48. [Google Scholar] [CrossRef]
- Samples, J.; Willis, M.; Klauber-Demore, N. Targeting angiogenesis and the tumor microenvironment. Surg. Oncol. Clin. N. Am. 2013, 22, 629–639. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, H.; Betsholtz, C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003, 314, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Murdoch, C. Macrophage responses to hypoxia: Implications for tumor progression and anti-cancer therapies. Am. J. Pathol. 2005, 167, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ji, Y.R.; Lee, Y.M. Crosstalk between angiogenesis and immune regulation in the tumor microenvironment. Arch. Pharm. Res. 2022, 45, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Shirasuna, K.; Shimizu, T.; Matsui, M. Impact of angiogenic and innate immune systems on the corpus luteum function during its formation and maintenance in ruminants. Reprod. Biol. 2013, 13, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Yee, K.O.; Lawler, J.; Khosravi-Far, R. Regulation of tumor angiogenesis by thrombospondin-1. Biochim. Biophys. Acta 2006, 1765, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Plendl, J. Angiogenesis and vascular regression in the ovary. Anat. Histol. Embryol. 2000, 29, 257–266. [Google Scholar] [CrossRef]
- Karaman, S.; Leppänen, V.M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145, dev151019. [Google Scholar] [CrossRef]
- Senger, D.R.; Davis, G.E. Angiogenesis. Cold Spring Harbor Perspect. Biol. 2011, 3, a005090. [Google Scholar] [CrossRef] [PubMed]
- Lepucki, A.; Orlińska, K.; Mielczarek-Palacz, A.; Kabut, J.; Olczyk, P.; Komosińska-Vassev, K. The Role of Extracellular Matrix Proteins in Breast Cancer. J. Clin. Med. 2022, 11, 1250. [Google Scholar] [CrossRef]
- Conway, E.M.; Collen, D.; Carmeliet, P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 2001, 49, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Takeyama, H.; Guha, S. Cytokine network: New targeted therapy for pancreatic cancer. Curr. Pharm. Des. 2012, 18, 2416–2419. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Varney, M.L.; Valasek, J.; Godfrey, M.; Dave, B.J.; Singh, R.K. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis 2005, 8, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Jiemtaweeboon, S.; Shirasuna, K.; Nitta, A.; Kobayashi, A.; Schuberth, H.J.; Shimizu, T.; Miyamoto, A. Evidence that polymorphonuclear neutrophils infiltrate into the developing corpus luteum and promote angiogenesis with interleukin-8 in the cow. Reprod. Biol. Endocrinol. 2011, 9, 79. [Google Scholar] [CrossRef]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef] [PubMed]
- Galvão, A.M.; Ferreira-Dias, G.; Skarzynski, D.J. Cytokines and angiogenesis in the corpus luteum. Mediat. Inflamm. 2013, 2013, 420186. [Google Scholar] [CrossRef]
- Ono, M. Molecular links between tumor angiogenesis and inflammation: Inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008, 99, 1501–1506. [Google Scholar] [CrossRef]
- Holmes, D.I.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Geva, E.; Jaffe, R.B. Role of vascular endothelial growth factor in ovarian physiology and pathology. Fertil. Steril. 2000, 74, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.M.; Wulff, C. Angiogenesis in the corpus luteum. Reprod. Biol. Endocrinol. 2003, 1, 88. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Welsh, M. VEGFA and tumor angiogenesis. J. Intern. Med. 2013, 273, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Tugues, S.; Li, X.; Gualandi, L.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 2011, 437, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signaling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev. 2010, 21, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kisliouk, T.; Podlovni, H.; Meidan, R. Unique expression and regulatory mechanisms of EG-VEGF/prokineticin-1 and its receptors in the corpus luteum. Ann. Anat. 2005, 187, 529–537. [Google Scholar] [CrossRef]
- Corlan, A.S.; Cimpean, A.M.; Jitariu, A.A.; Melnic, E.; Raica, M. Endocrine gland-derived vascular endothelial growth factor/prokineticin-1 in cancer development and tumor angiogenesis. Int. J. Endocrinol. 2017, 2017, 3232905. [Google Scholar] [CrossRef]
- Baryla, M.; Goryszewska-Szczurek, E.; Kaczynski, P.; Balboni, G.; Waclawik, A. Prokineticin 1 is a novel factor regulating porcine corpus luteum function. Sci. Rep. 2023, 13, 5085. [Google Scholar] [CrossRef] [PubMed]
- Presta, M.; Dell’Era, P.; Mitola, S.; Moroni, E.; Ronca, R.; Rusnati, M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Tomala, K.K.; Jonczyk, A.W.; Kordowitzki, P.; Jalali, B.M.; Skarzynski, D.J. The effect of basic fibroblast growth factor 2 on the bovine corpus luteum depends on the stage of the estrous cycle and modulates prostaglandin F(2alpha) action. Animal 2021, 15, 100048. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liaw, L.; Prudovsky, I.; Brooks, P.C.; Vary, C.; Oxburgh, L.; Friesel, R. Fibroblast growth factor signaling in the vasculature. Curr. Atheroscleros. Rep. 2015, 17, 509. [Google Scholar] [CrossRef] [PubMed]
- Manzat Saplacan, R.M.; Balacescu, L.; Gherman, C.; Chira, R.I.; Craiu, A.; Mircea, P.A.; Lisencu, C.; Balacescu, O. The Role of PDGFs and PDGFRs in Colorectal Cancer. Mediat. Inflamm. 2017, 2017, 4708076. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, J.B.; Essaghir, A. PDGF receptor signaling networks in normal and cancer cells. Cytokine Growth Factor Rev. 2014, 25, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Multifarious functions of PDGFs and PDGFRs in tumor growth and metastasis. Trends Mol. Med. 2013, 19, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Ishi, Y.; Hamashima, T.; Yamamoto, S.; Sasahara, M. Pathogenetic significance and possibility as a therapeutic target of platelet derived growth factor. Pathol. Int. 2017, 67, 235–246. [Google Scholar] [CrossRef]
- Zou, X.; Tang, X.Y.; Qu, Z.Y.; Sun, Z.W.; Ji, C.F.; Li, Y.J.; Guo, S.D. Targeting the PDGF/PDGFR signaling pathway for cancer therapy: A review. Int. J. Biol. Macromol. 2022, 202, 539–557. [Google Scholar] [CrossRef]
- Kazlauskas, A. PDGFs and their receptors. Gene 2017, 614, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Niba, E.T.; Nagaya, H.; Kanno, T.; Tsuchiya, A.; Gotoh, A.; Tabata, C.; Kuribayashi, K.; Nakano, T.; Nishizaki, T. Crosstalk between PI3 kinase/PDK1/Akt/Rac1 and Ras/Raf/MEK/ERK pathways downstream PDGF receptor. Cell. Physiol. Biochem. 2013, 31, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Razmara, M.; Heldin, C.H.; Lennartsson, J. Platelet-derived growth factor-induced Akt phosphorylation requires mTOR/Rictor and phospholipase C-γ1, whereas S6 phosphorylation depends on mTOR/Raptor and phospholipase D. Cell Commun. Signal. 2013, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, J.E.; Frandsen, S.; Chang, J.; Celestino, B.; Tucker, E.; Poole, D.H. Progesterone inhibits cytokine/TNF-alpha production by porcine CL macrophages via the genomic progesterone receptor. Domest. Anim. Endocrinol. 2020, 72, 106426. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Banu, S.K.; McCracken, J.A.; Arosh, J.A. Early pregnancy modulates survival and apoptosis pathways in the corpus luteum in sheep. Reproduction 2016, 151, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging-US 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Balogh, O.; Müller, L.; Boos, A.; Kowalewski, M.P.; Reichler, I.M. Expression of insulin-like growth factor 1 and its receptor in preovulatory follicles and in the corpus luteum in the bitch. Gen. Comp. Endocrinol. 2018, 269, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Galvao, A.M.; Ramilo, D.W.; Skarzynski, D.J.; Lukasik, K.; Tramontano, A.; Mollo, A.; Mateus, L.M.; Ferreira-Dias, G.M. Is FAS/Fas ligand system involved in equine corpus luteum functional regression? Biol. Reprod. 2010, 83, 901–908. [Google Scholar] [CrossRef]
- Sakumoto, R.; Hayashi, K.G.; Takahashi, T. Different expression of PGE synthase, PGF receptor, TNF, Fas and oxytocin in the bovine corpus luteum of the estrous cycle and pregnancy. Reprod. Biol. 2014, 14, 115–121. [Google Scholar] [CrossRef]
- Antonsson, B. Bax and other pro-apoptotic Bcl-2 family “killer-proteins” and their victim the mitochondrion. Cell Tissue Res. 2001, 306, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Sakumoto, R. Multiple roles of TNF superfamily members in corpus luteum function. Reprod. Biol. Endocrinol. 2003, 1, 95. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Pate, J.L.; Landis Keyes, P. Immune cells in the corpus luteum: Friends or foes? Reproduction 2001, 122, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Zocchi, M.R. Mechanisms of tumor escape: Role of tumor microenvironment in inducing apoptosis of cytolytic effector cells. Arch. Immunol. Ther. Exp. 2006, 54, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yang, J.; Jones, D.P. Mitochondrial control of apoptosis: The role of cytochrome c. Biochim. Biophys. Acta. 1998, 1366, 139–149. [Google Scholar] [CrossRef]
- Thorburn, A. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) pathway signaling. J. Thorac. Oncol. 2007, 2, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Waring, P.; Müllbacher, A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol. Cell Biol. 1999, 77, 312–317. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.H.; Yoon, J.T. Expression of matrix metalloproteinases to induce the expression of genes associated with apoptosis during corpus luteum development in bovine. PeerJ 2019, 7, e6344. [Google Scholar] [CrossRef]
- Boyd, D.F.; Thomas, P.G. Towards integrating extracellular matrix and immunological pathways. Cytokine 2017, 98, 79–86. [Google Scholar] [CrossRef]
- Folgueras, A.R.; Pendás, A.M.; Sánchez, L.M.; López-Otín, C. Matrix metalloproteinases in cancer: From new functions to improved inhibition strategies. Int. J. Dev. Biol. 2004, 48, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Petroff, M.G.; Petroff, B.K.; Pate, J.L. Mechanisms of cytokine-induced death of cultured bovine luteal cells. Reproduction 2001, 121, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xiao, Z.; Zhao, Q.; Li, M.; Wu, X.; Zhang, L.; Hu, W.; Cho, C.H. Anti-cancer therapy with TNFα and IFNγ: A comprehensive review. Cell Prolif. 2018, 51, e12441. [Google Scholar] [CrossRef] [PubMed]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L. The transcriptional targets of p53 in apoptosis control. Biochem. Biophys. Res. Commun. 2005, 331, 851–858. [Google Scholar] [CrossRef]
- Meulmeester, E.; Jochemsen, A.G. p53: A guide to apoptosis. Curr. Cancer Drug Targets 2008, 8, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Nevins, J.R. The Rb/E2F pathway and cancer. Hum. Mol. Genet. 2001, 10, 699–703. [Google Scholar] [CrossRef]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Bratton, S.B.; Salvesen, G.S. Regulation of the Apaf-1-caspase-9 apoptosome. J. Cell Sci. 2010, 123, 3209–3214. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Z.T.; Kornbluth, S. The apoptosome: Physiological, developmental, and pathological modes of regulation. Dev. Cell 2006, 10, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Apoptosome: The cellular engine for the activation of caspase-9. Structure 2002, 10, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harbor Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef] [PubMed]
- Ukrainskaya, V.M.; Stepanov, A.V.; Glagoleva, I.S.; Knorre, V.D.; Belogurov, A.A.J.; Gabibov, A.G. Death Receptors: New Opportunities in Cancer Therapy. Acta Naturae 2017, 9, 55–63. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawasaki-Koyanagi, A.; Sueyoshi, N.; Kanai, A.; Yagita, H.; Okumura, K. Localization of Fas ligand in cytoplasmic granules of CD8+ cytotoxic T lymphocytes and natural killer cells: Participation of Fas ligand in granule exocytosis model of cytotoxicity. Biochem. Biophys. Res. Commun. 2002, 296, 328–336. [Google Scholar] [CrossRef]
- Lavrik, I.N.; Krammer, P.H. Regulation of CD95/Fas signaling at the DISC. Cell Death Differ. 2012, 19, 36–41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, T.; Lee, S.-H.; Lee, S. Angiogenesis and Apoptosis: Data Comparison of Similar Microenvironments in the Corpus Luteum and Tumors. Animals 2024, 14, 1118. https://doi.org/10.3390/ani14071118
Min T, Lee S-H, Lee S. Angiogenesis and Apoptosis: Data Comparison of Similar Microenvironments in the Corpus Luteum and Tumors. Animals. 2024; 14(7):1118. https://doi.org/10.3390/ani14071118
Chicago/Turabian StyleMin, Taehee, Sang-Hee Lee, and Seunghyung Lee. 2024. "Angiogenesis and Apoptosis: Data Comparison of Similar Microenvironments in the Corpus Luteum and Tumors" Animals 14, no. 7: 1118. https://doi.org/10.3390/ani14071118
APA StyleMin, T., Lee, S.-H., & Lee, S. (2024). Angiogenesis and Apoptosis: Data Comparison of Similar Microenvironments in the Corpus Luteum and Tumors. Animals, 14(7), 1118. https://doi.org/10.3390/ani14071118




