Detection of Protein Biomarkers Relevant to Sperm Characteristics and Fertility in Semen in Three Wild Felidae: The Flat-Headed Cat (Prionailurus planiceps), Fishing Cat (Prionailurus viverrinus), and Asiatic Golden Cat (Catopuma temminckii)
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Anesthesia and Morphometric Measurements
2.3. Semen Collection and Evaluation
2.4. Western Immunoblotting and Evaluation
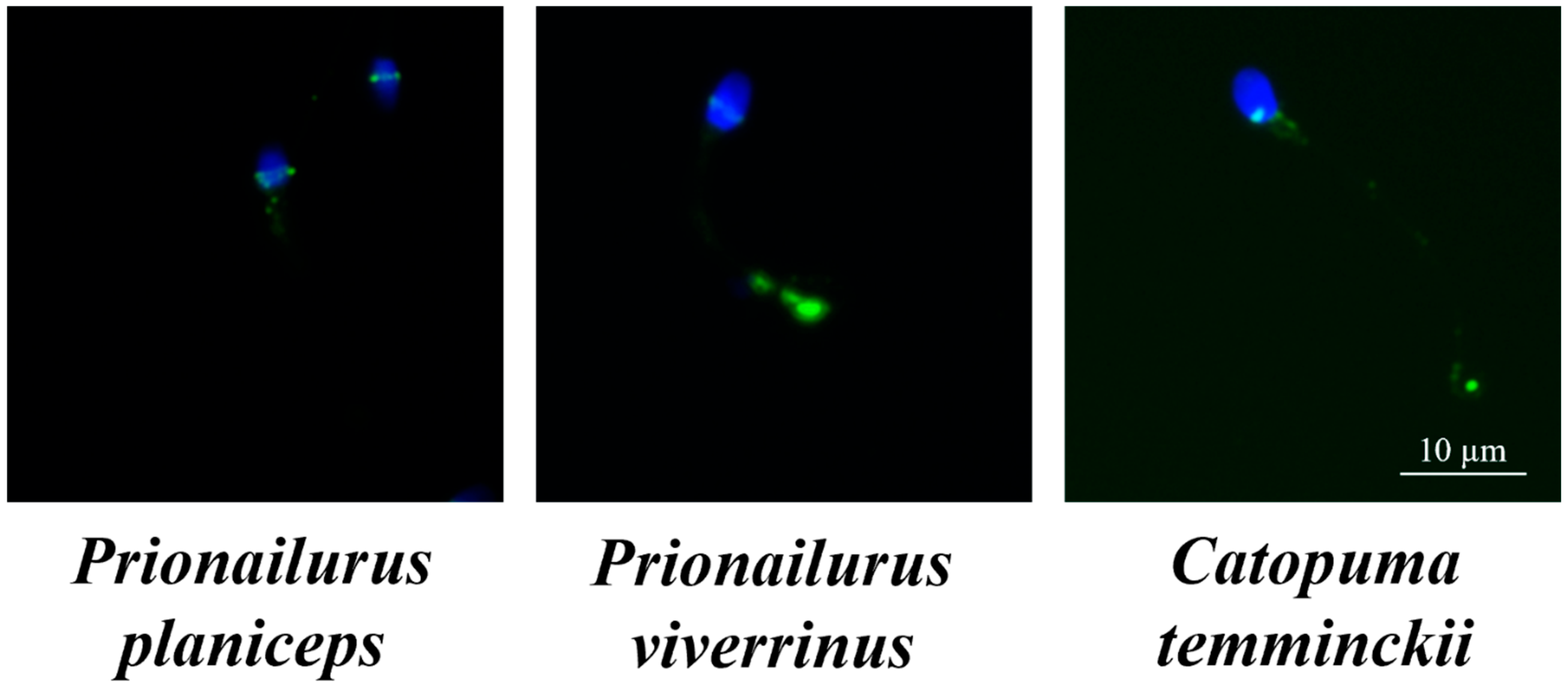
2.5. Sperm Immunofluorescence Microscopy
2.6. Statistical Analyses
3. Results
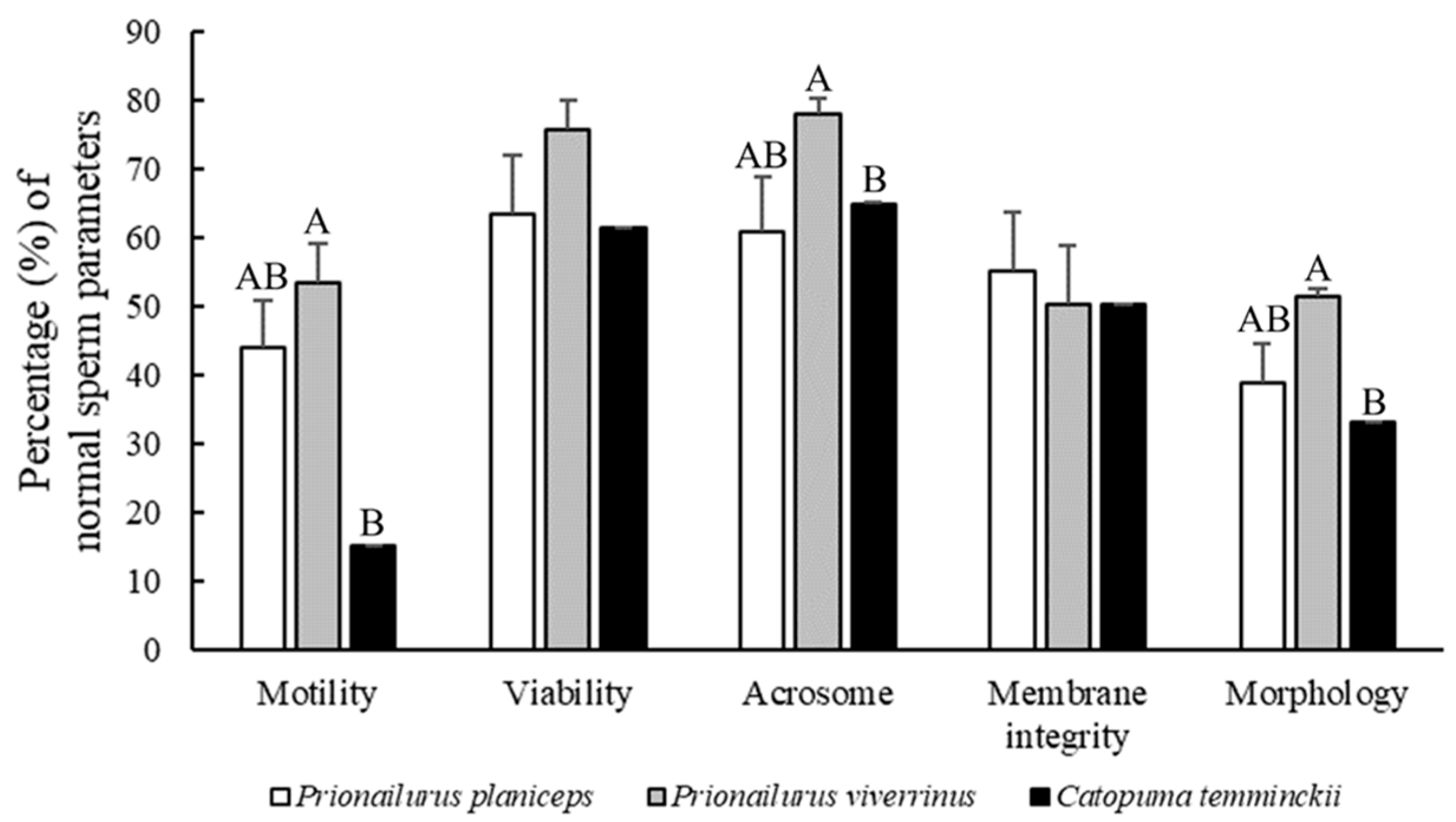
3.1. Differences in Ejaculated Semen Characteristics and Sperm Traits among Three Wild Cat Species
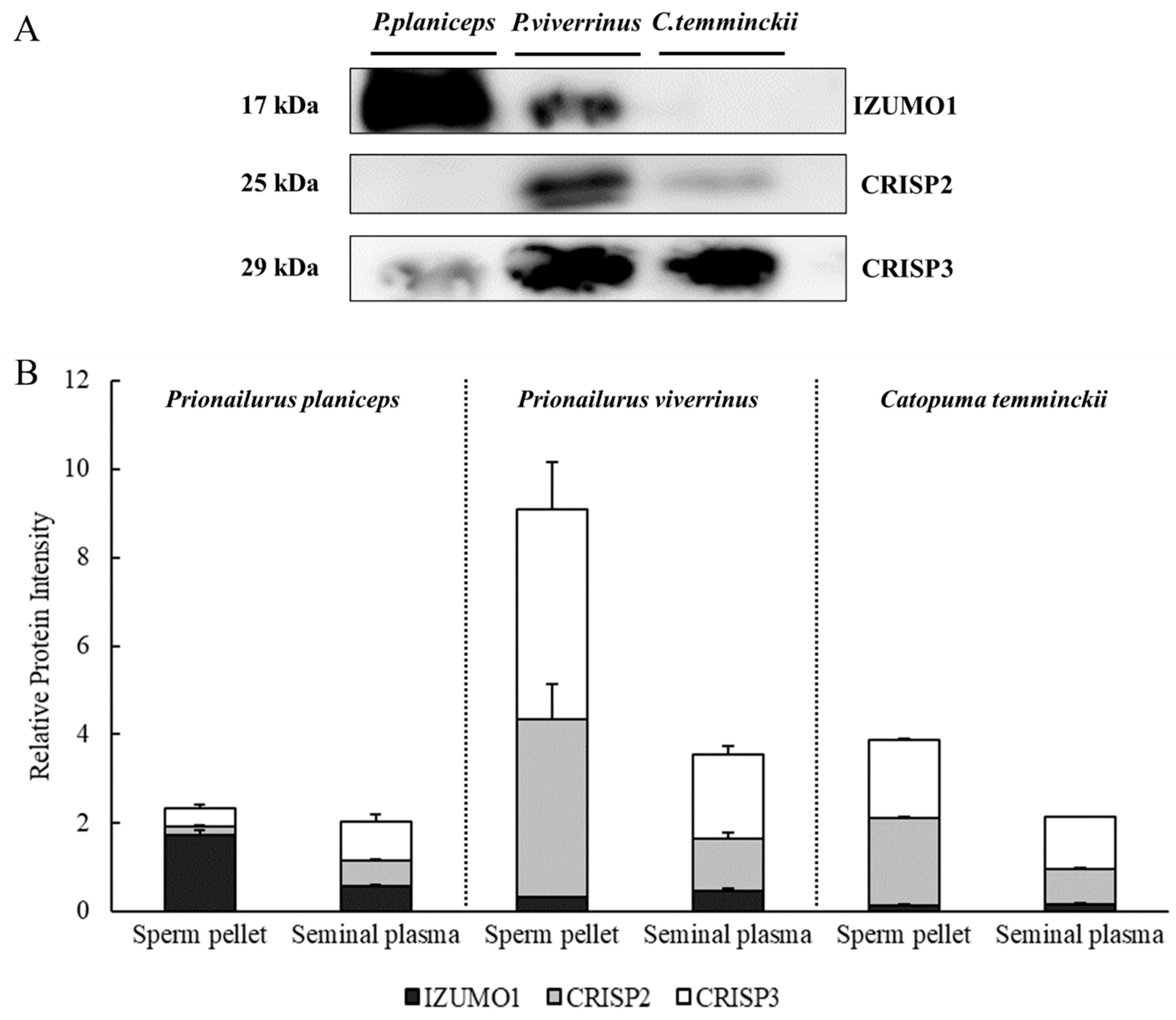
3.2. Expression of IZUMO1, CRISP2, and CRISP3 Proteins in Spermatozoa and Seminal Plasma among Three Wild Cat Species
3.3. Correlation between IZUMO1, CRISP2, and CRISP3 Protein Expression Levels and Ejaculate Characteristics among Three Wildcat Species
3.4. Percentage of Cleaved Caspase-3-Positive Sperm and Its Correlation with Semen Characteristics among Three Wildcat Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilting, A.; Cord, A.; Hearn, A.J.; Hesse, D.; Mohamed, A.; Traeholdt, C.; Cheyne, S.M.; Sunarto, S.; Jayasilan, M.-A.; Ross, J. Modelling the species distribution of flat-headed cats (Prionailurus planiceps), an endangered South-East Asian small felid. PloS ONE 2010, 5, e9612. [Google Scholar] [CrossRef] [PubMed]
- Phosri, K.; Tantipisanuh, N.; Chutipong, W.; Gore, M.L.; Giordano, A.J.; Ngoprasert, D. Fishing cats in an anthropogenic landscape: A multi-method assessment of local population status and threats. Glob. Ecol. Conserv. 2021, 27, e01615. [Google Scholar] [CrossRef]
- Petersen, W.J.; Savini, T.; Gray, T.N.; Baker-Whatton, M.; Bisi, F.; Chutipong, W.; Cremonesi, G.; Gale, G.A.; Mohamad, S.W.; Rayan, D.M. Identifying conservation priorities for an understudied species in decline: Golden cats (Catopuma temminckii) in mainland Tropical Asia. Glob. Ecol. Conserv. 2021, 30, e01762. [Google Scholar] [CrossRef]
- Claw, K.G.; George, R.D.; Swanson, W.J. Detecting coevolution in mammalian sperm–egg fusion proteins. Mol. Reprod. Dev. 2014, 81, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Wittayarat, M.; Panyaboriban, S.; Kupthammasan, N.; Otoi, T.; Chatdarong, K. Effects of green tea polyphenols and α-tocopherol on the quality of chilled cat spermatozoa and sperm IZUMO1 protein expression during long-term preservation. Anim. Reprod. Sci. 2022, 237, 106926. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Hamada, D.; Kamikubo, H.; Hirata, K.; Kataoka, M.; Yamamoto, M.; Ikawa, M.; Okabe, M.; Hagihara, Y. Molecular dissection of IZUMO1, a sperm protein essential for sperm-egg fusion. Development 2013, 140, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Busso, D.; Da Ros, V.; Ellerman, D.A.; Maldera, J.A.; Goldweic, N.; Cuasnicu, P.S. Participation of cysteine-rich secretory proteins (CRISP) in mammalian sperm-egg interaction. Int. J. Dev. Biol. 2004, 52, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Busso, D.; Goldweic, N.M.; Hayashi, M.; Kasahara, M.; Cuasnicú, P.S. Evidence for the involvement of testicular protein CRISP2 in mouse sperm-egg fusion. Biol. Reprod. 2007, 76, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, V.G.; Muñoz, M.W.; Battistone, M.A.; Brukman, N.G.; Carvajal, G.; Curci, L.; Gómez-Elías, M.D.; Cohen, D.J.; Cuasnicu, P.S. From the epididymis to the egg: Participation of CRISP proteins in mammalian fertilization. Asian J. Androl. 2015, 17, 711. [Google Scholar] [PubMed]
- Doty, A.; Buhi, W.; Benson, S.; Scoggin, K.; Pozor, M.; Macpherson, M.; Mutz, M.; Troedsson, M. Equine CRISP3 modulates interaction between spermatozoa and polymorphonuclear neutrophils. Biol. Reprod. 2011, 85, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.N.; Sulzyk, V.; Weigel Muñoz, M.; Cuasnicu, P.S. Cysteine-rich secretory proteins (CRISP) are key players in mammalian fertilization and fertility. Front. Cell. Dev. Biol. 2021, 9, 800351. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Mason, M.C.; Govindaraju, A.; Belser, L.; Kaya, A.; Stokes, J.; Rowe, D.; Memili, E. Interrelationships between apoptosis and fertility in bull sperm. J. Reprod. Dev. 2013, 59, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Zorn, B.; Golob, B.; Ihan, A.; Kopitar, A.; Kolbezen, M. Apoptotic sperm biomarkers and their correlation with conventional sperm parameters and male fertility potential. J. Assist. Reprod. Genet. 2012, 29, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell. Death. Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Gown, A.M.; Willingham, M.C. Improved detection of apoptotic cells in archival paraffin sections: Immunohistochemistry using antibodies to cleaved caspase 3. J. Histochem. Cytochem. 2002, 50, 449–454. [Google Scholar] [CrossRef]
- Weng, S.-L.; Taylor, S.L.; Morshedi, M.; Schuffner, A.; Duran, E.H.; Beebe, S.; Oehninger, S. Caspase activity and apoptotic markers in ejaculated human sperm. Mol. Hum. Reprod. 2002, 8, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.F.; Cardoso, M.; Sousa, M.; Viana, P.; Gonçalves, A.; Silva, J.; Barros, A. Quantitative study of caspase-3 activity in semen and after swim-up preparation in relation to sperm quality. Hum. Reprod. 2005, 20, 1307–1313. [Google Scholar] [CrossRef]
- Panyaboriban, S.; Singh, R.P.; Songsasen, N.; Padilla, L.; Brown, J.; Reed, D.; Techakumphu, M.; Pukazhenthi, B. Reproductive seasonality and sperm cryopreservation in the male tufted deer (Elaphodus cephalophus). Theriogenology 2016, 86, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Pukazhenthi, B.S.; Togna, G.D.; Padilla, L.; Smith, D.; Sanchez, C.; Pelican, K.; Sanjur, O.I. Ejaculate traits and sperm cryopreservation in the endangered Baird’s tapir (Tapirus bairdii). J. Androl. 2011, 32, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Pukazhenthi, B.; Quse, V.; Hoyer, M.; van Engeldorp Gastelaars, H.; Sanjur, O.; Brown, J.L. A review of the reproductive biology and breeding management of tapirs. Integr. Zool. 2013, 8, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Kupthammasan, N.; Wittayarat, M.; Panichayupakaranant, P.; Didas, N.; Wattanachant, C.; Panyaboriban, S. Effects of water-soluble curcuminoid-rich extract in a solid dispersion form (CRE-SD) on the sperm characteristics, longevity and casein kinase II catalytic subunit alpha protein stability in chilled goat semen. Cryobiology 2022, 109, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wittayarat, M.; Pukazhenthi, B.S.; Tipkantha, W.; Techakumphu, M.; Srisuwatanasagul, S.; Panyaboriban, S. CRISP protein expression in semen of the endangered Malayan tapir (Tapirus indicus). Theriogenology 2021, 172, 106–115. [Google Scholar] [CrossRef]
- Leahy, T.; Rickard, J.P.; Pini, T.; Gadella, B.M.; de Graaf, S.P. Quantitative proteomic analysis of seminal plasma, sperm membrane proteins, and seminal extracellular vesicles suggests vesicular mechanisms aid in the removal and addition of proteins to the ram sperm membrane. Proteomics 2020, 20, 1900289. [Google Scholar] [CrossRef] [PubMed]
- Guasti, P.; Souza, F.; Scott, C.; Papa, P.; Camargo, L.; Schmith, R.; Monteiro, G.; Hartwig, F.; Papa, F. Equine seminal plasma and sperm membrane: Functional proteomic assessment. Theriogenology 2020, 156, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Navaneetham, D.; Sivashanmugam, P.; Rajalakshmi, M. Changes in binding of lectins to epididymal, ejaculated, and capacitated spermatozoa of the rhesus monkey. Anat. Rec. 1996, 245, 500–508. [Google Scholar] [CrossRef]
- Brukman, N.G.; Miyata, H.; Torres, P.; Lombardo, D.; Caramelo, J.J.; Ikawa, M.; Da Ros, V.G.; Cuasnicu, P.S. Fertilization defects in sperm from Cysteine-rich secretory protein 2 (Crisp2) knockout mice: Implications for fertility disorders. Mol. Hum. Reprod. 2016, 22, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Fedorka, C.; Scoggin, K.; Squires, E.; Ball, B.; Troedsson, M. Expression and localization of cysteine-rich secretory protein-3 (CRISP-3) in the prepubertal and postpubertal male horse. Theriogenology 2017, 87, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Udby, L.; Bjartell, A.; Malm, J.; Egesten, A.; Lundwall, Å.; Cowland, J.B.; Borregaard, N.; Kjeldsen, L. Characterization and localization of cysteine-rich secretory protein 3 (CRISP-3) in the human male reproductive tract. J. Androl. 2005, 26, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Ellerman, D.A.; Busso, D.; Morgenfeld, M.M.; Piazza, A.D.; Hayashi, M.; Young, E.T.; Kasahara, M.; Cuasnicu, P.S. Evidence that human epididymal protein ARP plays a role in gamete fusion through complementary sites on the surface of the human egg. Biol. Reprod. 2001, 65, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, M.; Gutknecht, J.; Brew, K.; Spurr, N.; Goodfellow, P.N. Cloning and mapping of a testis-specific gene with sequence similarity to a sperm-coating glycoprotein gene. Genomics 1989, 5, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Obert, G.; Deffosez, A.; Formstecher, P.; Marchetti, P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum. Reprod. 2002, 17, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, M.; Moffatt, O.; Manicardi, G.C.; Bizzaro, D.; Afnan, M.; Sakkas, D. Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: Implications for assisted conception. Hum. Reprod. 2001, 16, 2160–2165. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Mesbah-Namin, S.A.; Movahedin, M. Attenuation of aquaporin-3 may be contributing to low sperm motility and is associated with activated caspase-3 in asthenozoospermic individuals. Andrologia 2021, 53, e14119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sharma, R.K.; Sikka, S.C.; Thomas Jr, A.J.; Falcone, T.; Agarwal, A. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil. Steril. 2003, 80, 531–535. [Google Scholar] [CrossRef] [PubMed]




| Parameters | IZUMO1 | CRISP2 | CRISP3 | |||
|---|---|---|---|---|---|---|
| Sperm Pellet | Seminal Plasma | Sperm Pellet | Seminal Plasma | Sperm Pellet | Seminal Plasma | |
| Semen volume (µL) | 0.229 (p = 0.361) | −0.004 (p = 0.987) | 0.253 (p = 0.309) | 0.385 (p = 0.085) | 0.536 (p < 0.05) | 0.197 (p = 0.393) |
| Sperm concentration (spermatozoa/mL) | 0.492 (p < 0.05) | 0.495 (p < 0.05) | 0.198 (p = 0.432) | 0.275 (p = 0.227) | 0.479 (p < 0.05) | 0.137 (p = 0.563) |
| Total sperm number (spermatozoa) | 0.492 (p < 0.05) | 0.495 (p < 0.05) | 0.198 (p = 0.432) | 0.275 (p = 0.227) | 0.479 (p < 0.05) | 0.137 (p = 0.563) |
| Motility (%) | 0.582 (p < 0.01) | 0.596 (p < 0.01) | 0.500 (p < 0.05) | 0.367 (p = 0.102) | 0.280 (p = 0.185) | 0.175 (p = 0.447) |
| Viability (%) | 0.441 (p < 0.05) | 0.484 (p < 0.05) | 0.615 (p < 0.01) | 0.397 (p = 0.075) | 0.402 (p = 0.052) | 0.291 (p = 0.201) |
| Intact acrosome (%) | 0.221 (p = 0.3) | 0.739 (p < 0.01) | 0.355 (p = 0.089) | 0.503 (p < 0.05) | 0.047 (p = 0.826) | 0.137 (p = 0.563) |
| Normal sperm membrane (%) | 0.566 (p < 0.01) | 0.620 (p < 0.01) | −0.057 (p = 0.681) | 0.004 (p = 0.975) | 0.372 (p < 0.01) | 0.037 (p = 0.775) |
| Normal sperm morphology (%) | 0.524 (p < 0.05) | 0.389 (p = 0.081) | −0.028 (p = 0.912) | 0.279 (p = 0.220) | 0.367 (p = 0.134) | 0.090 (p = 0.697) |
| Normal DNA (%) | 0.254 (p = 0.266) | 0.548 (p < 0.05) | 0.572 (p < 0.01) | 0.548 (p < 0.05) | 0.286 (p = 0.209) | 0.153 (p = 0.508) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wittayarat, M.; Kiatsomboon, S.; Kupthammasan, N.; Tipkantha, W.; Yimprasert, S.; Thongphakdee, A.; Panyaboriban, S. Detection of Protein Biomarkers Relevant to Sperm Characteristics and Fertility in Semen in Three Wild Felidae: The Flat-Headed Cat (Prionailurus planiceps), Fishing Cat (Prionailurus viverrinus), and Asiatic Golden Cat (Catopuma temminckii). Animals 2024, 14, 1027. https://doi.org/10.3390/ani14071027
Wittayarat M, Kiatsomboon S, Kupthammasan N, Tipkantha W, Yimprasert S, Thongphakdee A, Panyaboriban S. Detection of Protein Biomarkers Relevant to Sperm Characteristics and Fertility in Semen in Three Wild Felidae: The Flat-Headed Cat (Prionailurus planiceps), Fishing Cat (Prionailurus viverrinus), and Asiatic Golden Cat (Catopuma temminckii). Animals. 2024; 14(7):1027. https://doi.org/10.3390/ani14071027
Chicago/Turabian StyleWittayarat, Manita, Supalak Kiatsomboon, Navapol Kupthammasan, Wanlaya Tipkantha, Surasak Yimprasert, Ampika Thongphakdee, and Saritvich Panyaboriban. 2024. "Detection of Protein Biomarkers Relevant to Sperm Characteristics and Fertility in Semen in Three Wild Felidae: The Flat-Headed Cat (Prionailurus planiceps), Fishing Cat (Prionailurus viverrinus), and Asiatic Golden Cat (Catopuma temminckii)" Animals 14, no. 7: 1027. https://doi.org/10.3390/ani14071027
APA StyleWittayarat, M., Kiatsomboon, S., Kupthammasan, N., Tipkantha, W., Yimprasert, S., Thongphakdee, A., & Panyaboriban, S. (2024). Detection of Protein Biomarkers Relevant to Sperm Characteristics and Fertility in Semen in Three Wild Felidae: The Flat-Headed Cat (Prionailurus planiceps), Fishing Cat (Prionailurus viverrinus), and Asiatic Golden Cat (Catopuma temminckii). Animals, 14(7), 1027. https://doi.org/10.3390/ani14071027






