Simple Summary
Coccidiosis is one of the most prevalent diseases in poultry production, inflicting substantial economic losses exceeding USD 15 billion to the poultry industry. Coccidiosis is most common in young broilers but is not limited to them, and pullets and egg-laying hens are equally susceptible. Unfortunately, unlike broilers, low emphasis has been given to laying hens. This review aims to summarize the effect of coccidiosis in laying hens while exploring potential nutritional interventions to fight coccidiosis.
Abstract
Avian coccidiosis, despite advancements in management, nutrition, genetics, and immunology, still remains the most impactful disease, imposing substantial economic losses to the poultry industry. Coccidiosis may strike any avian species, and it may be mild to severe, depending on the pathogenicity of Eimeria spp. and the number of oocysts ingested by the bird. Unlike broilers, low emphasis has been given to laying hens. Coccidiosis in laying hens damages the gastrointestinal tract and causes physiological changes, including oxidative stress, immunosuppression, and inflammatory changes, leading to reduced feed intake and a drastic drop in egg production. Several countries around the world have large numbers of hens raised in cage-free/free-range facilities, and coccidiosis has already become one of the many problems that producers have to face in the future. However, limited research has been conducted on egg-laying hens, and our understanding of the physiological changes following coccidiosis in hens relies heavily on studies conducted on broilers. The aim of this review is to summarize the effect of coccidiosis in laying hens to an extent and correlate it with the physiological changes that occur in broilers following coccidiosis. Additionally, this review tries to explore the nutritional strategies successfully used in broilers to mitigate the negative effects of coccidiosis in improving the gut health and performance of broilers and if they can be used in laying hens.
1. Introduction
Avian coccidiosis, caused by various species of the protozoan Eimeria, poses a significant threat to the poultry industry. Despite advancements in management, nutrition, genetics, chemotherapy, and immunology, it remains the most prevalent disease in the poultry industry, imposing substantial economic losses of more than USD 15 billion [1]. The economic loss due to coccidiosis is not only due to clinical outbreaks resulting in low production and high mortality but also subclinical coccidiosis without visible clinical signs; even subclinical coccidiosis contributes to economic losses through reduced nutrient absorption and utilization, thereby compromising optimal performance and the feed conversion ratio (FCR). Reduced nutrient absorption during subclinical coccidiosis may result from intestinal enterocyte and morphometric damages, compromised gastrointestinal integrity, decreased ileal digestibility, and increased susceptibility to secondary diseases like necrotic enteritis [2,3,4,5,6,7].
Coccidiosis is common in young broilers or laying hen pullets ranging from 3 to 18 weeks of age but not limited to this age group. Newly hatched chicks have high maternal antibodies but have little protection against coccidiosis [8]. Chickens usually become infected when maternal antibodies deplete and the oocyst counts in litter peak at 3–6 weeks of age; however, immunity develops shortly after mild infections [2,5,8]. Older flocks without acquired immunity against Eimeria spp. might get the disease [2,8]. In addition, there is no cross-immunity among Eimeria species; thus, more than one outbreak is possible in the same flock with different species involved in each episode [2,8]. Generally, anticoccidial drugs are used in laying hens’ pullets and are withdrawn before they start laying eggs. Therefore, the acquired immunity against Eimeria during the pullet phase might not be enough to prevent infection throughout the production period [8,9,10]. Coccidiosis may strike any avian species, and it may be mild to severe, depending on the pathogenicity of the Eimeria spp. and the number of oocysts ingested by the bird [2,5].
Unlike broilers, limited attention has been given to laying hens regarding coccidiosis. One of the leading causes of laying hen mortality was reported as coccidiosis in cage-free systems [11,12,13]. A study conducted in Sweden observed 19% coccidiosis in laying hens raised on a litter-based cage-free system [14]. Laying hens recovered from coccidiosis but did not have optimal egg production and had a high FCR. Fossum et al. (2009) [12] observed higher incidences of coccidiosis in laying hens raised in cage-free systems (aviaries and free-range) compared with conventional cages in Sweden from 2001 to 2004, after the ban on conventional cages. Although there are several variations among cage-free systems (aviaries, free-range, or barn), the litter-based floor is typical in all housing systems that allow fecal–oral transmission of the oocyst, leading to disease outbreaks, unlike conventional cages. Therefore, preventing coccidiosis in these systems might become one of the many challenges for egg producers while transitioning from traditional cages to cage-free systems.
As the egg industry worldwide is steadily observing an increase in cage-free facilities, the broad topic of “gut health” associated with “coccidiosis” will have one of the most significant economic impacts on laying hen health and performance. Many countries in Europe have already transitioned to cage-free systems, whereas the United States is slowly transitioning to cage-free systems, and by 2023, more than 35% of laying hens in the U.S. were raised in cage-free environments, and this is still increasing (UEP, 2023). The evolution of these new production systems (aviaries; free-range) with large numbers of hens on the floor/range will influence the occurrence of coccidiosis. Laying hens raised in a litter-based aviary or free-range systems may provide a perfect environment for transmitting coccidiosis, and they have a higher chance of getting the disease than those raised in traditional cage systems. However, laying hens raised in conventional cages are also susceptible to coccidiosis, which contrasts with the traditional view that raising laying hens in conventional cages is unlikely to result in coccidiosis infection [10,15]. Hens raised in a cage system can still ingest enough oocysts from manure belts or edges of cages to induce clinical coccidiosis; however, the incidences are less [10,15]. Yet, there remains a dearth of knowledge regarding the effects of coccidiosis on laying hen performance, gastrointestinal health, oxidative stress, immune response, and skeletal health. However, broilers and laying hens have been intensively selected for many generations for different purposes (rapid growth and meat production vs. egg production), which might have changed their gastrointestinal physiology, immune response, and skeletal development. In this review, we aim to correlate findings from the broiler studies to best explain what might happen in laying hens infected with coccidiosis, particularly concerning gastrointestinal physiology, immune response, and potential mitigation strategies.
2. Avian Coccidiosis
Avian coccidiosis is caused by multiple species of the genus Eimeria belonging to the subkingdom protozoa and phylum Apicomplexa [5]. Eimeria is an intracellular parasite that infiltrates intestinal enterocytes at different locations, depending on the species. Only seven have been recognized to infect chickens among the hundreds of Eimeria species including the following: E. acervulina, E. brunetti, E. maxima, E. necatrix, E. mitis, E. praecox, and E. tenella [2,5]. Among these Eimeria species known to infect chickens, E. brunetti, E. maxima, E. necatrix, and E. tenella are considered highly pathogenic, whereas E. acervulina, E. mitis, and E. praecox exhibit low pathogenicity (Table 1) [2,5]. In the past, there were sporadic reports of outbreaks of E. hagani and E. mivati in chickens. However, their existence was doubted, and they are now recognized as nomina dubia [16].

Table 1.
List of Eimeria species important in laying hens with their predilection sites, characteristic lesions, pathogenicity, and immunogenicity.
Avian coccidiosis is a common and widespread disease of chickens around the world and is transmitted by the fecal–oral route. Outbreaks of coccidiosis are reported wherever chickens are raised [2,5]. Eimeria oocysts can remain viable for a long period of time, and the disease can become endemic to the areas where environmental and managemental conditions favor year-round survival and multiplication [2,5]. The incidences of coccidiosis are usually higher in litter-based intensive systems, which favor the survival and accumulation of oocysts [4].
Eimeria’s life cycle is short and has high reproductive potential with self-limiting infection [2,5]. The life cycle of the Eimeria consists of an exogenous phase involving the sporulation of the oocyst in the environment and an endogenous phase involving asexual reproduction (schizogony) followed by sexual reproduction (gametogony) within the avian intestine. Infection initiates with the ingestion of sporulated oocysts from the environment [2,5,17]. Each sporulated oocyst contains four sporocysts, each containing two sporozoites. Upon ingestion, mechanical and biochemical forces break down the oocyst, releasing the sporozoites in the digestive tract [2,5]. Subsequently, sporozoites migrate to specific sites depending on the species involved and invade the intestine’s epithelial enterocytes. Within enterocytes, sporozoites transform into trophozoites and begin multiple asexual multiplications, forming schizonts, known as schizogony. Mature schizonts release merozoites, which again invade epithelial enterocytes, repeat the asexual cycle, and complete the second schizogonous cycle. Depending on the species, they might complete another schizogonous cycle before forming male (microgametocytes) and female (macrogametocytes) gametocytes [2,5]. Mature gametocytes release microgametes that fertilize mature macrogametocytes, resulting in the formation of oocysts. Upon maturation, oocysts rupture epithelial cells, which are excreted in feces and, under favorable conditions, sporulate within 48–72 h, becoming infective [2,5]. With each successive cycle, the number of sporulated oocysts increases exponentially in the environment, which retains their infectivity for extended periods [18]. The development of Eimeria in a host results in the destruction of the host’s intestinal tissues, leading to clinical manifestation observed in a disease outbreak.
3. Host–Pathogen Interaction and Immune Responses against Coccidiosis
Eimeria species are highly host-specific and, once infected, can produce protective immunity against that particular species [19,20,21]. Notably, no cross-immunity is observed among Eimeria species. Generally, a large number of Eimeria oocysts are required to induce complete protective immunity, except for E. maxima, which is considered more immunogenic than other species [22]. The asexual life cycle of Eimeria is more immunogenic than the sexual life cycle. However, for the development of complete immunity and protection, both sexual and asexual phases of the life cycle are equally essential, especially for adaptive immunity [23,24].
Gut-associated lymphoid tissue (GALT) is an integral component of mucosal-associated lymphoid tissues and plays a vital role in the immune response as a first line of defense, preventing the progression of diseases and destroying infectious agents at an early stage [18,22]. GALT is a multilayered tissue with antigen-presenting cells, immunoregulatory cells, and effector cell types different from their systemic counterpart [19]. In poultry, during coccidiosis, GALT serves as antigen recognition and presentation, followed by the release of intestinal antibodies and activation of the cell-mediated immune response [18,19,25]. Various lymphoid tissues (Peyer’s patches, bursa of Fabricius, and cecal tonsils) have evolved in the GALT to produce verities of immune cells (epithelial, lymphoid, antigen-presenting, and natural killer cells) to protect against invading pathogens [18,19,22]. Peyer’s patches serve as antigen recognition and immune response activation, along with facilitating gastrointestinal IgA secretion [19,22,25]. Following this, activated B and T cells migrate to lamina propria, which serves as the effector site for immune responses, initiating antigen-specific/non-specific responses involving antibody production, leukocyte accumulation, and locally produced cytokines [19,25]. The activation of antigen-presenting cells, such as macrophages and dendritic cells, by coccidia upregulates the production of pro-inflammatory cytokines and chemokines from innate immune cells, which are essential for the development of the adaptive immune response. T lymphocytes play a crucial role in the response to coccidia infection, with various T cell subpopulations capable of recognizing multiple antigens and modulating humoral and cell-mediated immunity [18,19,25].
Cell-mediated immune responses are an important effector mechanism that includes antigen-specific and non-specific activation of various cell populations, including T lymphocytes, CD4+ helper T cells and CD8+ cytotoxic T cells, natural killer cells, and macrophages. T cell activation is associated with major histocompatibility complex (MHC), with cytotoxic T cells recognizing antigens presented in the context of MHC-I and helper T cells recognizing antigens associated with MHC-II molecules. Antigen-presenting and dendritic cells help activate naïve CD4+ T cells into different subsets such as Th1, Th2, Treg, and Th17 [19,26,27]. Activated helper T cells are involved in the humoral immune response, cytotoxic activity activation, macrophages, and natural killer cells [21]. Following coccidiosis, cytotoxic T cells, along with macrophages and natural killer cells, identify coccidia-infected host cells and modulate mononuclear cells to produce interferon-γ, activating proinflammatory pathways to inhibit the development of intracellular Eimeria within host cells [18,19,28]. Cytokines produced by CD4+ Th1 cells (IL-2, IL-12, IFN-γ, and TNF-α) aid in clearing the infection by promoting the activation of macrophages and other immune cells capable of eliminating intracellular coccidia [26,27]. The importance of cytotoxic T cells during coccidiosis has been demonstrated previously, as evidenced by the presence of intestinal intraepithelial lymphocytes expressing more than 75% CD8+ T cells, the presence of CD8+ cells in GALT within 24 h of infection, and the presence and activation of mono and polynuclear cells [19,22,29,30].
4. Coccidiosis and Its Effects on Pullets and Laying Hens
4.1. Gut Health and Oxidative Stress
The negative effect of coccidiosis on the parameters associated with gastrointestinal health, such as increased intestinal lesion scores, gastrointestinal permeability, a breach in tight junction integrity, damage to intestinal morphology, inflammation, and oxidative stress, are related to Eimeria multiplication and release in the gut lumen [2,5,6,7,31]. Continuous intestinal epithelial cell turnover occurs throughout the lifetime of the chickens. However, coccidiosis infection increases epithelial cell turnover by two times compared with noninfected birds [32]. It has been reported that the highest turnover of epithelial cells was observed between 5 and 7 days post-Eimeria inoculation due to the release of large numbers of first- and second-generation merozoites into the lumen. As a result, the mRNA and protein expression of a cell proliferative marker, the proliferating cell nuclear antigen, in the duodenum epithelium was upregulated, thus increasing crypt depth and resulting in a lower villus height to crypt depth ratio [33]. The difference between Eimeria-infected and non-infected birds in terms of villus height might range from 10 to 40% and is also dependent on the Eimeria species and level of infection [7,34,35,36,37,38,39,40]. This epithelial cell turnover during Eimeria infection might be a host defense mechanism to limit the multiplication of the Eimeria by the expulsion of infected cells [41]. Moreover, the tight junction proteins maintaining the tight junction integrity of the small intestine are composed of claudin, occludin, junctional adhesion molecules, and zonula occludens families [42]. These tight junction proteins were reported to be upregulated in Eimeria-infected birds along with increased permeability by more than 100%, supporting the observation of a rapid turnover rate in the intestinal epithelium during Eimeria infection [6,7,37,38,43]. Similar results were observed in pullets and laying hens of different age groups and at various stages of egg production [34,35,36].
During coccidiosis, the equilibrium between reactive oxygen species (ROS) and the host’s ability to neutralize ROS is disrupted, leading to oxidative stress. These ROS have a high affinity for the phospholipid bilayer of the cell, initiating lipid peroxidation and cytotoxic changes, which in turn damages the intestine, altering the gastrointestinal integrity and causing abnormal changes in the intestinal morphology [44,45]. Under normal circumstances, the production of ROS and its effects on cellular mechanisms are controlled by the host defense mechanism consisting of enzymes such as glutathione (GSH), glutathione peroxidases (GPx), and catalase [44]. During Eimeria infection, although oxidative defense mechanisms are active, the production of ROS exceeds the production of enzymes suppressing ROS, aggravating the situation. Previous studies have reported that Eimeria infection increased the markers associated with oxidative stress, such as reduced concentration of total antioxidant capacity (TAC) from 15 to 40% [31,46] and reduced GPx and GSH activity [31,47]. Furthermore, Eimeria infection also increased the markers of radical-induced damage, such as superoxide dismutase (SOD) and malondialdehyde (MDA) [34,35,36,44,48,49].
4.2. Growth Performance
The effect of coccidiosis on the growth performance of birds depends on the Eimeria species and the severity of the infection. Previous studies have reported that broilers challenged with 105 oocysts of Eimeria acervulina reduced their body weight gain (BWG) by 10.3%, whereas 106 oocysts per bird decreased BWG by 26.7% [50]. Likewise, similar results were observed with E. maxima and E. tenella [50]. On average, coccidiosis reduced the body weight (BW) of the chickens by 10%, irrespective of the species involved in the infection [51]. E. maxima-induced coccidiosis was observed to have the most severe negative impact on the BW of broilers, ranging from 23% to 37% [6,51], followed by E. tenella, ranging from 15% to 27% [52,53], and E. acervulina, from 16% to 19% [54,55]. However, Choi et al. (2021) [56] did not observe any reduction in the BW of broilers when they were inoculated with sporulated oocysts of E. tenella ranging from 6250 to 50,000 [56]. In cases of mixed Eimeria (E. maxima, E. tenella, and E. acervulina) infections, the reduction in BW can range from 13% to 50% with an increase in Eimeria dosage from 6250 to 50,000 E. maxima, 6250 to 50,000 E. tenella, and 31,250 to 250,000 E. acervulina [7,37,46]. However, mixed Eimeria infection has been associated with higher mortality rates, reaching up to 47%, and reductions in BW ranging from 9% to 28% in laying hen pullets aged two weeks, 8% to 15% in pullets aged 15 weeks, and 9% to 11% in hens aged 25 weeks during the early phase of infection [34,35,36]. Most studies have reported reductions in BW during the early phase of infection (1–8 days post-Eimeria inoculation) but not during the recovery phase (8–14 days post-Eimeria inoculation). During the early phase of infection, the reduction in feed intake and damage to the intestinal linings are more pronounced compared with those in the recovery phase, where feed intake and intestinal damage recover [7,35]. Additionally, during the early phase, nutritional redistribution occurs towards the immune response to subside the infection [7,35,36]. This reduction in feed intake and nutritional redistribution are associated with reduced growth or body weight loss in pullets and laying hens.
4.3. Production Performance
Coccidiosis rarely occurs in laying hens because of previous infection and the resulting immunity [10]. However, pullets reared in cages/on floors with coccidiostat drugs might not have enough exposure to coccidia to stimulate immunity. Outbreaks can occur after moving to layer facilities, either conventional cages or alternative housing systems [5,8,10]. Coccidiosis in laying hens has been reported during the early egg production period, around 23–24 weeks of age, and is characterized by high morbidity and mortality with a dramatic reduction in egg production [4,10,57,58,59]. Temporary cessation of egg production has been observed in laying hens infected with E. maxima, E. acervulina, and E. tenella. Hegde and Reid (1969) [57] reported that total egg production dropped to less than 20% when susceptible laying hens were independently challenged with different Eimeria species (E. acervulina, E. maxima, E. brunetti, E. necatrix, or E. tenella), with a significant drop observed two weeks post-challenge. It took 4–6 weeks to recover from infection, which varied among species. The mortality was more significant with E. necatrix and E. maxima following infection; however, the number of culled birds was not different from the control unchallenged group. Single-comb white leghorns infected with E. mitis experienced significant reductions in egg production and eggshell quality, undergoing complete molting before resuming egg production [58]. Hens recovered from coccidiosis may not reach their full egg-laying potential [60]. An outbreak of E. necatrix in a breeder hen facility resulted in significant mortality among breeder hens and roosters, as well as a 4.3% reduction in egg production following the recovery period compared with hens of the same age [59]. Recent studies in our lab have observed that coccidiosis at the pre-lay stage of growth delayed the onset of maturity and egg production by two weeks, and infection at peak egg production drastically dropped egg production and temporarily ceased egg production in some hens [34,36]. Reduced egg production during a coccidiosis outbreak might be because of the malabsorption of nutrients, including amino acids, carbohydrates, and minerals, which are essential during the laying period [6,7,8,61]. The possible mechanism of how coccidiosis affects the performance of laying hens is shown in Figure 1. Reduced feed intake and damage to intestinal linings following coccidiosis are evident. This damage caused by Eimeria to the intestinal tract affects the digestion and absorption of the nutrients required for egg formation [36]. Furthermore, it has been observed that plasma amino acids are lowered, and amino acid imbalance occurs during coccidiosis [55]. The limited availability of nutrients and amino acids in laying hens might reduce the synthesis of albumin, an important component of an egg, thus reducing egg production. Increased oxidative stress during coccidiosis might damage the developing oocytes and granulosa cells, leading to follicular atresia, production, and deposition of yolk precursors in selected ovaries, or occurrence of apoptosis in the oviducts and ovarian follicles [36,62,63]. These disruptions to normal physiological and reproductive functions might contribute to a decline in egg production. Additionally, the redistribution of energy during coccidiosis for tissue repair, inflammation, and maintaining oxidative status might also be responsible for the decline in egg production [36]. These studies showed that laying hens are susceptible to coccidiosis during the laying phase, with a dramatic reduction in egg production posing substantial challenges to the egg industry. The economic loss to the industry is mostly from reduced egg production, reduced feed intake, and an increased FCR due to malabsorption of nutrients and mortality.
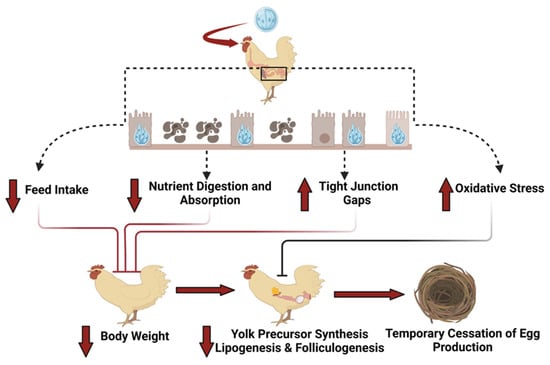
Figure 1.
Potential mechanism of action showing how coccidiosis affects egg production in laying hens.
5. Prevention and Control of Coccidiosis
5.1. Biosecurity and Management
Biosecurity is an important tool in poultry production to prevent disease outbreaks on farms. Once a coccidiosis outbreak occurs on a farm and the house becomes contaminated with oocysts, it is virtually impossible to completely decontaminate the environment [2,5]. Strict biosecurity measures should be implemented between farms and chicken houses to minimize the risk of cross-contamination and outbreaks. In litter-based intensive housing systems, Eimeria oocysts accumulate in large amounts and can remain viable for longer periods of time [2]. In those systems, management strategies should focus on maintaining the optimum level of moisture in the litter so that it can maintain the viable number of sporulated oocysts sufficient to generate protective immunity but not high enough to induce clinical disease. Furthermore, when employing vaccination as a preventive measure, it is also critical to maintain optimum litter moisture, as oocyst recycling is important in inducing a protective immune response against specific Eimeria species [64,65]. Additionally, immunosuppression from stressors such as heat, cold, or high stocking density in chicken houses might increase susceptibility to the disease; therefore, it is critical to maintain optimum environments in the houses [66].
5.2. Chemotherapy
Traditionally, chemotherapeutic (anticoccidial) drugs have been used in the poultry industry for years all around the world to control coccidiosis outbreaks in farms; however, the emergence of drug resistance among the zoonotic pathogens and concerns raised by the World Health Organization and consumers led to a ban of antibiotics use in food-producing animals including poultry as growth promoters and disease prevention [67,68]. In poultry, coccidiostat (inhibits the replication of Eimeria) or coccidiocidal (kills and destroys the Eimeria) drugs are used, which can be either synthetic or ionophores [64,68]. Synthetic anticoccidial drugs (chemicals) are produced by chemical synthesis from other chemical compounds that have diverse modes of action in controlling Eimeria, from inhibiting mitochondrial respiration and disrupting energy production (clopidol or decoquinate) to competitive inhibition of thiamine uptake (amprolium) or inhibiting folic acid pathways (sulphonamides). Some of the synthesized chemicals approved to be used in the poultry industry are diclazuril, halofuginone, clopidol, robenidine, decoquinate, nequinate, amprolium, and zoalene [68,69]. These chemicals act on the intracellular stage of Eimeria after they invade the host intestine and interfere with one or more stages of the life cycle [69]. However, while using these chemicals, it is important to consider that they are more susceptible to the development of resistance [68]. Additionally, these chemicals vary in their mode of action, efficacy, dose range, and susceptibility to resistance; it is important to rotate between them at certain intervals to minimize the development of resistance [68]. On the other hand, ionophores are fermented products from bacteria such as Streptomyces spp. and Actinomadura spp. [64,68]. Ionophores facilitate the transportation of ions across cell membranes, increasing the concentration of intracellular ions and disrupting the normal ion balance across cell membranes [70]. The common ionophores used in the poultry industry are monensin, narasin, salinomycin, maduramicin, semduramicin, and lasalocid. Even though these ionophores are not of human importance, the ban on antibiotics in animal production may include these, which have been the backbone of coccidiosis prevention programs for many years, forcing the poultry industry to look for alternatives [68]. Although the poultry industry has used shuttle or rotational programs to minimize resistance to these drugs in the past, most field strains of Eimeria have shown varying levels of resistance to more than one drug [64,71].
5.3. Vaccination
Vaccination against Eimeria species has become one of the most widely used methods in controlling coccidiosis outbreaks in the United States. The discovery of the self-limiting infection of Eimeria species and the development of resistance to reinfection by the same Eimeria species by Beach and Corl. (1925) [72] paved the way for the development and use of a live vaccine to control coccidiosis in the USA by 1952. In addition to self-limiting infection, Eimeria species are less susceptible to younger chicks, and the generation of acquired immunity against Eimeria species with limited to no pathogenesis made vaccination an efficient strategy to control coccidiosis in commercial poultry production [73]. The objective of immunization is to expose the birds to Eimeria species to elicit protective immunity against them, which is adequate to prevent future infections [9,15,65,66,74]. However, the major disadvantage of vaccination is the absence of cross-immunity among different Eimeria species; thus, it is more labor-intensive to prepare and has a high cost because of including multiple species in the vaccine [75]. When vaccinating meat birds against coccidiosis, the emphasis is placed on E. acervulina, E. maxima, and E. tenella. In contrast, for egg-laying hens, the focus shifts to E. tenella, E. acervulina, E. maxima, E. brunetti, E. necatrix, and E. praecox, primarily because of variations in the life spans of the respective hosts (Table 2) [15]. To ensure birds build strong immunity against Eimeria species, they must undergo oocyst recycling through three to four successive re-infections so that they have complete immunity against Eimeria by 3–4 weeks of age when most coccidiosis outbreaks occur [17,65,75,76]. In the United States, two types of live vaccines are available based on the pathogenicity of the parasites, including (i) nonattenuated and (ii) attenuated live oocyst vaccines [65,75]. Nonattenuated vaccines contain laboratory or field strains of Eimeria oocysts that have their virulence preserved to elicit protective immunity. With a nonattenuated live oocyst vaccine, immunity against Eimeria is produced by completing the life cycle and boosted by recycling oocysts from the litter for reinfections [9,17,65,73,75,76,77]. However, the significant disadvantages of nonattenuated vaccines are their short shelf life, high cost of production due to the inclusion of all Eimeria species, occasional outbreaks of coccidiosis on farms, halted growth, and a possible lack of compensation for growth after recovery [9,15,65,75,77]. On the other hand, live attenuated oocyst vaccines are selected via passage through embryonated hen eggs or by selection for precociousness and have demonstrated reduced pathogenicity while maintaining the ability to induce protective immunity [15,65,75,78]. This selection for precocious has decreased pathogenicity while still having the capacity to stimulate the immune response against selective Eimeria species [65,73,74,75,77]. Recombinant or subunit vaccines are composed of immunogenic antigens capable of producing protective immunity against Eimeria. However, identifying the antigens that elicit protective immunity and limited to no cross-immunity among Eimeria species are limiting factors for the development of recombinant vaccines [65,74,77]. Thus, for complete protection against the prevalent Eimeria species, it is essential to identify antigens against several isolates of the same species and for multiple species to ensure complete protection [65,77].

Table 2.
Commercial vaccines available in the U.S. for use in laying hens; adapted from Price (2014) [15] and Cervantes (2023) [68].
6. Nutritional Intervention for Coccidiosis
6.1. Role of Vitamins
6.1.1. Vitamin D
Vitamin D is a fat-soluble vitamin, and the active metabolites of vitamin D play an intrinsic role in calcium and phosphorus homeostasis and bone mineralization [79,80,81,82]. More recently, the immunomodulatory roles of vitamin D in improving the host defense against invading pathogens have been described [83,84,85,86]. The absorption of vitamin D is fat-dependent and undergoes hydroxylation into 25-hydroxyvitamin D3 [25(OH)D3] in the liver [86,87]. Subsequently, 25(OH)D3 is converted into its active form, 1,25-dihydroxy vitamin D [1,25(OH)2D3], in the kidney by the enzyme 25-hydroxyvitamin D-1α-hydroxylase. The hydroxylation of 25(OH)D3 to 1,25(OH)2D3 is not limited only to the kidney but also occurs in the bone, breast, and thigh muscles, small intestine, and immune cells [88].
The inclusion of 25(OH)D3 at varying levels in either layer pullets or turkey poults challenged with Eimeria reduced BW loss and fecal oocyst shedding, increased macrophage nitric oxide (NO) production, stimulated the activity of innate immune cells, and modulated adaptive immunity (CD4+, CD8+ and CD4+CD25+ T cells) and inflammatory cytokines (IL-1β and IL-10), as well as maintained tight junction [85,86,89]. Furthermore, supplementation of either vitamin D or 25(OH)D3 or in combination has been shown to improve the FCR and BWG in broilers vaccinated against Eimeria spp. at high doses; however, no beneficial effects were observed in reducing intestinal lesions and improving morphometry [90,91,92]. In broilers challenged with either E. maxima or mixed Eimeria spp. and fed reduced calcium/phosphorus diets, supplementation of 25(OH)D3 at the rate of either 3000 IU/kg or 4000 IU/kg has been shown to improve bone mineralization [83,84,93]. Furthermore, in laying hens challenged with lipopolysaccharides, 25(OH)D3 supplementation improved egg production by improving follicular development and oocyte maturation by increasing the expression of VDR in oocytes, maintaining the plasma estradiol/progesterone and luteinizing hormone levels [94,95,96,97]. Moreover, the lipopolysaccharide challenge increased the immune response (CD4+CD25+ T cells, proinflammatory cytokines, and plasma IgM levels) and oxidative stress (SOD, TAC, and GPx) in laying hens, which were normalized by the addition of 25(OH)D3 [97,98]. Previous studies have reported that inflammation and oxidative stress associated with coccidiosis are responsible for at least a 10% reduction in bone volume, bone mineral content and density, and osteoclastic activity [46,48,99]. These results suggest that vitamin D and its metabolites can potentially reduce the negative impacts of coccidiosis related to growth performance, egg production, oxidative stress, and bone mineralization.
6.1.2. Vitamin E
Similar to vitamin D, vitamin E is a fat-soluble vitamin with a potent antioxidant capacity to protect cells and tissues from lipoperoxidation damage induced by reactive oxygen species [100,101,102]. Vitamin E from diets gets absorbed and incorporated into cell membranes to protect unsaturated fatty acids inside and outside the cells from reactive oxygen species. Furthermore, vitamin E prevents the excessive generation of reactive oxygen species from respiratory processes [103,104]. In addition, vitamin E is also involved in reducing inflammation, production of cytokine (TNF-α and IL-8) protecting cells of immune responses (lymphocytes, macrophages, and plasma cells), and enhancing the proliferation and functions of immune cells [100,105].
A previous study by Colnago et al. (1984) [106] observed that supplementation of 100 IU/kg vitamin E (DL-α-tocopheryl acetate) in a broiler diet and subsequent challenge by E. tenella or E. maxima reduced mortality, improved weight gain and the FCR, and reduced lesion scores. Similar results were observed when broiler chicks were challenged twice at 10 and 38 days of age and supplemented with both vitamin A (8 g/kg) and E (300 mg/kg) [107]. However, Allen and Fetterer (2002) [101] conducted two consecutive studies with increasing levels of vitamin E (13–200 IU/kg) in broilers challenged with E. maxima and did not observe significant differences in performance except for a slight reduction in lesion score. In broilers fed vitamin E at either 40 or 80 IU/kg alone or in combination with arginine and challenged with mixed Eimeria spp., heterophil and monocyte oxidative bursts (ROS production) and NO production decreased at seven days post-inoculation. They also observed lower lesions in challenged birds fed vitamin E, and birds fed a high level of vitamin E (80 IU/kg) in combination with arginine had higher levels of humoral antibodies IgG, IgM, and IGA [108]. Increased humoral and cell-mediated immune response with lower inflammatory mediators were observed in laying hens challenged with Salmonella enteritidis and fed diets supplemented with 30 IU/kg vitamin E [109]. Furthermore, da Silva et al. (2011) [110] observed that the inclusion of vitamin E at the rate of 65 mg/kg of diet increased the cell-mediated immune response of chickens vaccinated against coccidiosis and New Castle disease as measured by the cutaneous basophil hypersensitivity test. The antioxidant status (TAC, MDA, GPx activity, or SOD) and plasma lipid peroxidation of the broilers were improved following the Eimeria challenge in broilers, but the effects were not enough to improve performance compared with non-challenged broilers [111,112]. Furthermore, vitamin E has been shown to enhance the phagocytic activity of macrophages in chickens against pathogens [100].
In the case of egg layers, vitamin E inclusion has increased egg production by enhancing follicular development by increasing the concentration of reproductive hormones such as follicle-stimulating hormone, luteinizing hormone, estrogens, and progesterone [102]. These results from previous studies showed that vitamin E can be used in laying hens to improve their performance and boost the immune response; however, further studies are needed to confirm the effective dose.
6.1.3. Other Vitamins
Vitamin A is a fat-soluble nutrient constituting a broad range of retinoid compounds and plays a crucial role in various physiological processes, including vision, growth, cell growth and differentiation, reproduction, immunity, skeletal development, and antioxidant properties [113,114,115]. The association between vitamin A deficiency and the increased incidence and severity of coccidiosis was noted as early as 1945 [116]. Dietary supplementation of vitamin A above the 1960 NRC recommendation (8000 IU/lb) has been shown to enhance broiler performance and the recovery of chicks infected with E. tenella, acervulina, or necartix and results in an improved performance after recovery [117,118]. Furthermore, Dalloul et al. (2002) [113] reported that vitamin A deficiency compromised the intestinal defense mechanism against E. acervulina infection, as evidenced by a reduced population of intraepithelial lymphocytes (CD4+ and CD8+ T cells) through alterations in concanavalin A-induced spleen lymphocyte proliferation. Moreover, vitamin A supplementation (12,000 IU/kg) enhanced intestinal morphometry and tight junction integrity in broiler chickens co-infected with Clostridium and Eimeria [119].
Unlike vitamins A, D, and E, vitamin C is a water-soluble vitamin with a strong antioxidant capacity and acts as a cofactor for collagen biosynthesis, thus maintaining epithelial barrier function and stimulating wound healing [120]. Moreover, it enhances innate immunity by increasing the phagocytic activity of mononuclear cells and adaptive immune responses by differentiation and proliferation of B- and T-cells [121]. Supplementing vitamin C (110–220 ppm) improved weight gain, increased feed intake, reduced mortality, and lowered the corticosterone level and heterophil: lymphocyte ratio in the blood [122,123]. Additionally, concurrent supplementation of vitamin C and protease improved mucin and NO production [124], and vitamin C and arginine or vitamin C and vitamin E in different experiments improved oxidative status but were not able to improve performance in Eimeria-infected broilers [112]. The effect of supplementing different vitamins on minimizing the effect of coccidiosis is summarized in Table 3. A hypothesis for using vitamins during coccidiosis could posit that both fat-soluble and water-soluble vitamins may be readily absorbed by the body, potentially exerting beneficial effects on modulating immune responses despite reduced feed intake and nutrient utilization. While there is a notable gap in the available literature concerning the utilization of vitamins to support laying hens during disease conditions, the positive outcomes observed in broilers suggest that similar benefits might extend to laying hens as well. However, further studies are necessary to confirm the optimal dosage for this beneficial effect in laying hens.

Table 3.
Effects of vitamin supplementation on performance and intestinal health of chickens infected with Eimeria species.
6.2. Role of Functional Amino Acids
6.2.1. Arginine
Arginine, an essential functional amino acid, is vital in protein synthesis and accretion in chickens. Additionally, it contributes to secondary functions such as immune modulation, wound healing, and antioxidant activity [126,127,128,129]. In response to inflammation, arginine is metabolized to nitric oxide (NO) by nitric oxide synthase, which serves as an immune modulator, antioxidant defense, and cytotoxic mediator for non-specific host defenses [126,127,128]. Additionally, metabolites derived from arginine, such as proline, hydroxyproline, and polyamines, have been demonstrated to promote cell proliferation (Figure 2). Furthermore, ornithine and proline are primary amino acids found within collagen that are essential for wound healing and skeletal growth [126,127,128,130,131].
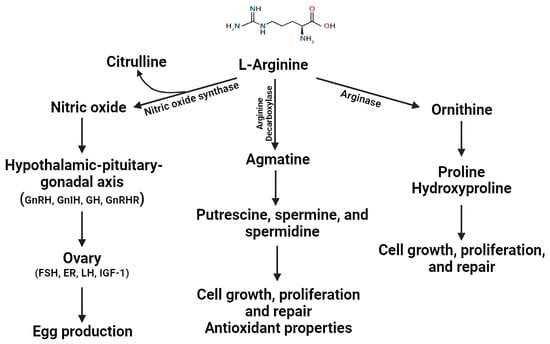
Figure 2.
Effect of L-arginine and its metabolites on gut health and reproductive functions in laying hens. FSH: follicular-stimulating hormone; ER: estrogen; LH: luteinizing hormone; IGF: insulin-like growth factor 1.
The secondary functions of arginine during coccidiosis have been extensively investigated in recent years and have shown beneficial effects in chickens. An early study by Allen and Fetterer (2000) [132] reported that E. acervulina infection reduced the plasma concentration of arginine, whereas its metabolite NO was significantly increased. The hypothesis for using arginine above the requirement could be that additional arginine might aid in balancing amino acids in plasma and provide a surplus for secondary functions. However, supplementation of arginine (500 mg/kg) did not reverse the negative effect associated with E. acervulina, maxima, or tenella infection except for a reduction in oocyst shedding of E. tenella [133]. Increasing dietary arginine concentration above the NRC requirement improved gut health by increasing villus height and goblet cell count and increasing mTOR pathways for healing [134,135]. The intestinal integrity of infected birds was improved when supplementing arginine, ranging from 1.24 to 1.48%, as observed by the upregulation of tight junction protein, permeability, and intestinal morphology [136,137]. Furthermore, arginine supplementation also reduced the expression of TLR-4 and IL-1β in broilers along with modulating the humoral immune response (secretory IgG and IgA) in broilers challenged with 20× Coccivac-B vaccine [134]. In addition, Yazdanabadi et al. (2020a; b) [138,139] reported that arginine supplemented 25–50% over the requirement increased the improved BWG, NO, and proinflammatory concentration along with gut morphometry. Furthermore, supplementation of arginine above the requirements has been found to improve the oxidative status of chickens following Eimeria challenge by GPx activity, reducing MDA and maintaining the levels of GSH and TAC [136,137,140].
In the case of laying hens, there is a lack of evidence for arginine supplementation and Eimeria challenge; however, in lipopolysaccharide-induced immune suppression, arginine supplementation above the requirements reduced the expression of inflammatory cytokines such as IL-1β, IL-10, TLR4, and NF-κB [141]. Furthermore, NO, a metabolite of arginine, plays a significant role in the hypothalamus–ovarian axis and plays a vital role in laying hen performance as well [142,143,144]. Based on previous studies in broilers and laying hens, supplementation of L-arginine above the requirements can be used in laying hens challenged with Eimeria to alleviate the symptoms associated with it.
6.2.2. Methionine
Methionine is considered the first limiting amino acid in corn- and soy-based diets for laying hens and broilers and the first amino acid to be incorporated into the polypeptide chain during translation [126,145]. Aside from protein synthesis, methionine has antioxidant functions, acting as a precursor of cysteine, which is essential for GSH synthesis [128,146]. Both GSH and cysteine protect the cells against ROS [146]. Because of its role in balancing oxidative status through GSH and cysteine, its efficacy has been tested in broilers exposed to coccidiosis. A study by Pourali et al. (2014) [147] reported that supplementing 150% of total sulfur amino acids improved BWG, oocyst shedding, and hepatic malondialdehyde concentration; however, no effect was observed in GPx in broilers challenged with mixed Eimeria species. A study by Lai et al. (2018, 2023) [148,149] reported that supplementing extra methionine above the requirement in vaccinated birds did not have any beneficial effect on the performance, immune response, or oxidative status of Partridge shank broilers. However, reducing the level of methionine in the diets during Eimeria infection significantly affects the performance, oxidative status, and immune responses in challenged broilers [31,47,150,151], and supplementing 0.75% methionine in low-crude protein diets increased the detrimental effect of Eimeria infection on broiler health and performance [6]. In laying hens under heat stress, similar results were observed as those for Eimeria-challenged broilers, where reducing the methionine level reduced the production performance and bone mineralization and increased the oxidative stress of laying hens [152]. The role of TSAA is evident in maintaining performance, immune response, and oxidative status during Eimeria infection; however, it is still unclear if supplementing methionine above the requirement has a beneficial effect on laying hens under coccidiosis.
6.2.3. Other Amino Acids
Glutamine, a conditionally essential amino acid, is the primary fuel for immune and intestinal epithelial cells and a precursor for GSH synthesis [153]. Supplementation of glutamine (0.5% or 1%) in coccidia-challenged broilers has been shown to reduce the expression of inflammatory cytokines IL-10 and IFN-γ, whereas it improves intestinal integrity and morphometry [154]. Furthermore, supplementing 0.75% glutamine in low-crude protein diets improved BWG during the recovery phase and maintained the expression of Claudin-1 compared to that of a normal protein diet in Eimeria-infected broilers [6].
Threonine is another essential amino acid and is essential for mucin and antibody (IgA, IgM) production in the gastrointestinal tract [155]. The intestinal mucus layer acts as the first line of defense against invading pathogens [155]. Therefore, factors increasing mucin production or intestinal secretions, as observed in coccidiosis, might increase threonine requirements during infection [156]. Supplementing 124% of threonine to its requirement in broilers challenged with mixed Eimeria species improved performance to the level of non-challenged birds, intestinal morphometry, oocyst shedding, and humoral immune response as measured by higher antibody production [157]. In contrast, threonine deficiency worsened the effects of coccidiosis in broilers challenged with coccidiosis by impairing intestinal morphometry and integrity, inflammatory responses, and lymphocyte population [158]. The effect of supplementing functional amino acids on the performance and intestinal health of chickens under coccidiosis infection is summarized in Table 4. Although these functional amino acids have shown beneficial effects in broilers under coccidiosis and in laying hens under normal conditions, their beneficial effect in laying hens under diseased conditions has not been fully explored. These amino acids have shown potential in mitigating the negative effect of coccidiosis in broilers and might have the same functions in laying hens, which need to be explored.

Table 4.
Effects of functional amino acids supplementation on the performance and intestinal health of chickens infected with Eimeria species.
7. Role of Phytogenic Feed Additives
Phytogenic feed additives (PFAs) are plant-derived natural bioactive compounds or products that, when fed to animals, have a beneficial effect on performance and health [161]. Phytogenic feed additives have a wide range of bioactive compounds with antimicrobial, antioxidant, or anti-inflammatory properties and are used in traditional human medicines [161,162]. Phytochemicals are generally recognized as safe in the United States, indicating their safety for consumption. This designation strengthens the potential utilization of phytochemicals in poultry production for coccidiosis control. In the poultry industry, PFAs are gaining considerable attention mainly because of improvements in the performance of birds by improving gastrointestinal health alongside antioxidative and immunomodulatory effects [161]. The efficacy of various phytogenic feed additives, including Artemisia annua, curcumin, oregano, thyme, and their essential oils, has been investigated in broiler chickens infected with coccidiosis, demonstrating some beneficial effects.
Dietary inclusion of curcumin powder (100–200 mg/kg) has been shown to reduce the lesion score and improve the oxidative status of broilers challenged with mixed Eimeria spp. [43]. Furthermore, essential oils of oregano have been shown to improve BWG and the FCR, reduce intestinal lesions and oxidative stress, and improve the gut morphology in broilers infected with coccidiosis [163,164]. The beneficial effects of these PFAs include supporting the host by immunomodulatory effects and providing protection against free radicals by scavenging reactive oxygen species and interfering directly with parasitic metabolism, reducing oocyst shedding and cecal short-chain fatty acid production [45,163,165]. Furthermore, Felici et al. (2023) [166] reported that bioactive compounds from a PFA can inhibit the intracellular replication of Eimeria and reduce schizont numbers.
Artemisinin, a bioactive flavonoid found in Artemisia annua leaves (AA), has been shown to inhibit the growth of several stages of Plasmodium spp. [167,168]. The use of AA and its extract artemisinin has been shown to demonstrate anticoccidial effects against E. tenella [169,170,171,172,173] when infected alone. The use of either dried AA leaves or artemisinin has been shown to improve lesion scores, reduce oocyst shedding and sporulation, and modulate the humoral and immune response in chickens [169,174,175,176,177,178]. The mechanism of action of artemisinin is promoting apoptosis of infected host cells, thus neutralizing parasites [172]. Furthermore, artemisinin from AA is able to alter the cell wall formation of the oocysts, leading to the death of developing oocysts and a reduced sporulation rate [170]. The use of phytogenic feed additives in laying hens infected with coccidiosis has not been studied. However, results from laying hen studies reported that PFA inclusion in diets improved the performance, immune response, and antioxidant status of laying hens [179,180,181,182,183]. Since coccidiosis mainly affects the performance of birds with immune suppression and increased oxidative stress, PFAs can be helpful in laying hens infected with coccidiosis, as in broilers.
8. Role of Prebiotics, Probiotics, and Symbiotics
8.1. Probiotics
Probiotics are selective nonpathogenic microorganisms that, when administered in adequate amounts, offer a beneficial advantage to the host and improve gut functions by altering the gut microflora and reducing pathogenic bacteria colonization in the gastrointestinal tract [184,185,186,187]. By selectively eliminating pathogenic microorganisms from the GI tract through competitive exclusion, probiotics promote the growth of beneficial bacteria. Additionally, their metabolites, including short-chain organic fatty acids and hydrogen peroxide, exhibit antimicrobial properties, effectively inhibiting the growth of pathogenic bacteria [188,189]. Probiotic microorganisms help develop immune components in the GI tract and innate or adaptive immune responses and modulate the phosphorylation of cytoskeletal and tight junction proteins, improving the intestinal barrier [188,190,191]. Some of the commonly used probiotics in the poultry industry include Bacillus, Lactobacillus, Enterococcus, Bifidobacterium, and Lactococcus, and yeasts such as Aspergillus, Candida, and Saccharomyces [188,192].
The inclusion of Bacillus spp. in diets has been shown to improve the performance, tight junction integrity of the intestine, and immune response as well as positively influenced the cecal microbiome of broilers challenged with coccidiosis [193,194,195,196]. Similar results were observed in birds fed lactobacillus-based probiotics, including reduced mortality and oocyst shedding, upregulation of intestinal integrity, increased intestinal intraepithelial lymphocytes expressing CD4+ and CD8+ cells, and antibody titers in broilers challenged with coccidiosis [197,198,199]. In the case of laying hens, the dietary inclusion of probiotics (Saccharomyces, Pediococcus, Lactobacillus, or Bacillus), either alone or in combination, has improved the performance, oxidative status, immune responses, intestinal morphometry, and microbial composition of ceca positively in a non-challenge model [197,200,201]. The effectiveness of probiotics (Bacillus amyloliquefaciens, Bacillus licheniformis, and Bacillus pumilus) against Salmonella Enteritidis in laying hens and observed a significant reduction in salmonella colonization in ceca of mature laying hens [202,203].
8.2. Prebiotics
Prebiotics are non-digestible or selectively fermentable feed ingredients that, when incorporated into diets, are utilized by the host intestinal microbiota, selectively promoting the growth or activity of specific bacterial populations, positively influencing the microbiome, and improving the gut health of the host [18,204,205]. While selecting prebiotics, it is essential to consider (i) digestibility (non-digestible by host enzymes), (ii) absorption (should not be absorbed directly by host cells), (iii) selective fermentation by intestinal microbiota, (iv) selective promotion of the growth of beneficial bacterial population, and (v) stimulation of the immune response of the host [188,204,205,206]. Some of the prebiotics commonly used in the poultry industry are oligosaccharides (mannan-, galacto-, and xylo-oligosaccharides), β-glucan, and fructans [188,206,207,208].
The efficacy of prebiotics in improving the gut health of poultry has been investigated using both challenged and unchallenged models [209,210,211,212,213]. In experimentally infected broilers with coccidiosis, the administration of chitosan oligosaccharide (1 g/kg) improved various parameters, including BWG, FCR, oocyst shedding, intestinal tight junctions, and morphometry and reduced intestinal inflammation [214]. Similarly, broilers fed mannooligosaccharides (0.8 g/kg), xylooligosaccharides (0.5 g/kg), galactoglucomannan oligosaccharide (4%), or yeast cell wall polysaccharides (0.5 g/kg) exhibited improved performance, intestinal tight junction integrity and nutrient digestibility and mitigation of hostile cecal microbial populations and fermentation induced by Eimeria infection [209,210,211,215].
Feeding prebiotics such as dry whey powder (6 g/kg), mannan oligosaccharides (0.25–2 kg/kg), or fructooligosaccharides (0.25–1 g/kg) to laying hens has shown positive effects on their intestinal microbial populations. Specifically, it promotes beneficial bacteria like Lactobacillus spp. and Olensella spp. while reducing the abundance of harmful bacteria such as Clostridium perfringens, Escherichia coli, and Salmonella enteritidis [216,217,218]. Additionally, oligosaccharide supplementation has been linked to improved performance, digestibility, and upregulation of toll-like receptor-4, interferon-γ, and antibody production in laying hens [217,218].
8.3. Synbiotics
Synbiotics refer to the combined application of both prebiotics and probiotics, which exhibit synergistic effects on the host by enhancing gut health, the immune response, and microbial balance [219]. The rationale behind synbiotic uses lies in the premise that prebiotics facilitate the survival and colonization of probiotics in the host’s gastrointestinal tract, thereby positively influencing the health of the host [188,219]. In broiler chickens, both in ovo and in vivo studies have demonstrated the beneficial effects of synbiotics on performance, gut health, and the immune response, irrespective of whether the birds were challenged with coccidiosis or necrotic enteritis [220,221,222,223,224,225]. Similarly, in laying hens, supplementation with synbiotics containing both prebiotics and probiotics has been shown to enhance production performance, decrease levels of inflammatory cytokines, and increase populations of beneficial bacteria in the intestinal tract [226,227]. The efficacy of probiotics, prebiotics, and synbiotics in chickens infected with coccidiosis is summarized in Table 5. The effect of probiotics, prebiotics, and synbiotics in laying hens has been investigated in both normal and diseased (Salmonellosis) conditions and has shown positive impacts on the performance and wellness of hens. Although they have shown beneficial effects in broilers under coccidiosis, their effect in laying hens infected with Eimeria spp. hens has not been tested yet.

Table 5.
Effects of prebiotics, probiotics, and synbiotics supplementation on performance and intestinal health of chickens infected with Eimeria species.
8.4. Postbiotics
In recent years, it has been established that the beneficial effects of probiotics are not only limited to when the microorganisms are alive but also after their death, leading to the development of new antibiotic alternatives, named postbiotics [234,235]. Postbiotics are non-viable bacterial cells or cell walls or metabolites derived from probiotics that have biological activity and offer physiological benefits to the host [235]. Different bioactive metabolites from probiotics with biological activity may include a range of compounds such as short-chain fatty acids, peptides, enzymes, polysaccharides, vitamins, cell surface proteins, and organic acids [235]. Recent studies conducted in chickens have shown that postbiotics confer beneficial effects on the host by positively modulating the intestinal microbiome, immune responses, and oxidative status [233,236,237,238]. In laying hen pullets challenged with Salmonella spp., dietary inclusion of postbiotics from Saccharomyces cerevisiae (1–1.5 kg/MT) or oral inoculation of Lactobacillus spp. postbiotics reduced the intestinal colonization of Salmonella [239,240]. Furthermore, Lactobacillus-derived postbiotics have been shown to enhance the performance, oxidative status, and intestinal morphology of broilers under heat stress [236,241]. Additionally, in broilers with experimentally induced necrotic enteritis, postbiotics from Enterococcus spp. Pediococcus spp., Lactobacillus spp., Enterococcus spp., and Lactiplantibacillus spp. were able to improve performance, lesion scores, and oxidative status and reduce proinflammatory responses [237,238,242]. Moreover, the inclusion of maltol (a metabolite of Bacillus subtilis) in a broiler diet infected with E. maxima was able to reduce intestinal damage and inflammatory response [233]. Although the use of postbiotics in broilers and laying hens infected with Eimeria spp. has not been extensively explored, previous studies have demonstrated their efficacy in reducing the symptoms associated with coccidiosis in other stress conditions.
9. Conclusions
To conclude, this review summarizes the effect of coccidiosis on laying hen health and performance and its potential to cause a significant economic loss to the egg industry. Since the ban on antibiotics as growth promoters in animal production, there has been an increase in the incidences of economically important diseases in poultry, such as coccidiosis and necrotic enteritis. Several antibiotic alternatives (vitamins, functional amino acids, phytogenic feed additives, prebiotics, probiotics, synbiotics, and postbiotics) have been tested in broilers with positive effects on minimizing the effect of coccidiosis, but their beneficial effects have not been explored in laying hens. These nutritional strategies have been shown to have the potential to mitigate the negative effects of coccidiosis in laying hens as well. However, before applying these nutritional strategies either to boost immunity after vaccination or to mitigate the negative effects of coccidiosis, they need to be tested in laying hens. Additionally, while implementing these nutritional strategies to mitigate the adverse effects of coccidiosis, it is important to consider that these strategies are not capable of curing coccidiosis completely, and poultry producers must evaluate the severity of the conditions. Future studies should focus on evaluating nutritional strategies either alone or in combination, focusing on performance, gut health, and immune responses against coccidiosis in laying hens.
Author Contributions
Conceptualization M.K.S. and W.K.K.; writing—review and editing, M.K.S. and W.K.K.; supervision, W.K.K.; funding acquisition, W.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-Calculating the Cost of Coccidiosis in Chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.P.; Mckenzie, M.E.; Conway, D.P.; Mckenzie, M.E. Poultry Coccidiosis; Wiley-Blackwell: Oxford, UK, 2007; ISBN 9780813822020. [Google Scholar]
- Su, S.; Miska, K.B.; Fetterer, R.H.; Jenkins, M.C.; Wong, E.A. Expression of Digestive Enzymes and Nutrient Transporters in Eimeria acervulina-Challenged Layers and Broilers. Poult. Sci. 2014, 93, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Abdisa, T.; Hasen, R.; Tagesu, T.; Regea, G.; Tadese, G. Poultry Coccidiosis and Its Prevention, Control. J. Vet. Anim. Res. 2019, 2, 1–6. [Google Scholar]
- Swayne, D.E.; Glisson, J.R.; McDougald, L.R.; Nolan, L.K.; Suarez, D.L.; Nair, V. Diseases of Poultry; Blackwell Pub: Oxford, UK, 2019; ISBN 9781119371199. [Google Scholar]
- Teng, P.Y.; Choi, J.; Tompkins, Y.; Lillehoj, H.; Kim, W. Impacts of Increasing Challenge with Eimeria Maxima on the Growth Performance and Gene Expression of Biomarkers Associated with Intestinal Integrity and Nutrient Transporters. Vet. Res. 2021, 52, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.Y.; Yadav, S.; Castro, F.L.d.S.; Tompkins, Y.H.; Fuller, A.L.; Kim, W.K. Graded Eimeria Challenge Linearly Regulated Growth Performance, Dynamic Change of Gastrointestinal Permeability, Apparent Ileal Digestibility, Intestinal Morphology, and Tight Junctions of Broiler Chickens. Poult. Sci. 2020, 99, 4203–4216. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.D. Coccidiosis in Egg Laying Poultry; Elsevier Inc.: Philadelphia, PA, USA, 2017; ISBN 9780128011515. [Google Scholar]
- Chapman, H.D. Applied Strategies for the Control of Coccidiosis in Poultry. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Soares, R.; Cosstick, T.; Lee, E.H. Control of Coccidiosis in Caged Egg Layers: A Paper Plate Vaccination Method. J. Appl. Poult. Res. 2004, 13, 360–363. [Google Scholar] [CrossRef]
- Rodenburg, T.B.; Tuyttens, F.A.M.; Sonck, B.; De Reu, K.; Herman, L.; Zoons, J. Welfare, Health, and Hygiene of Laying Hens Housed in Furnished Cages and in Alternative Housing Systems. J. Appl. Anim. Welf. Sci. 2005, 8, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Fossum, O.; Jansson, D.S.; Etterlin, P.E.; Vgsholm, I. Causes of Mortality in Laying Hens in Different Housing Systems in 2001 to 2004. Acta Vet. Scand. 2009, 51, 3. [Google Scholar] [CrossRef]
- Kaufmann-Bat, M.; Hoop, R.K. Diseases in Chicks and Laying Hens during the First 12 Years after Battery Cages Were Banned in Switzerland. Vet. Rec. 2009, 164, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Lunden, A.; Thebo, P.; Gunnarsson, S.; Hooshmand-Rad, P.; Tauson, R.; Uggla, A. Eimeria Infections in Litter-Based, High Stocking Density Systems for Loose-Housed Laying Hens in Sweden. Br. Poult. Sci. 2000, 41, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Price, K.R. Use of Live Vaccines for Coccidiosis Control in Replacement Layer Pullets. J. Appl. Poult. Res. 2012, 21, 679–692. [Google Scholar] [CrossRef]
- Blake, D.P.; Vrba, V.; Xia, D.; Jatau, I.D.; Spiro, S.; Nolan, M.J.; Underwood, G.; Tomley, F.M. Genetic and Biological Characterisation of Three Cryptic Eimeria Operational Taxonomic Units That Infect Chickens (Gallus gallus domesticus). Int. J. Parasitol. 2021, 51, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M.W.; Smith, A.L.; Tomley, F.M. The Biology of Avian Eimeria with an Emphasis on Their Control by Vaccination. Adv. Parasitol. 2005, 60, 285–330. [Google Scholar] [PubMed]
- Lee, Y.; Lu, M.; Lillehoj, H.S. Coccidiosis: Recent Progress in Host Immunity and Alternatives to Antibiotic Strategies. Vaccines 2022, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Shivaramaiah, C.; Barta, J.R.; Hernandez-Velasco, X.; Téllez, G.; Hargis, B.M. Coccidiosis: Recent Advancements in the Immunobiology of Eimeria Species, Preventive Measures, and the Importance of Vaccination as a Control Tool against These Apicomplexan Parasites. Vet. Med. Res. Rep. 2014, 5, 23–34. [Google Scholar]
- Lillehoj, H.S.; Lillehoj, E.P. Avian Coccidiosis. A Review of Acquired Intestinal Immunity and Vaccination Strategies. Avian Dis. 2000, 44, 408–425. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.S.; Trout, J.M. Coccidia: A Review of Recent Advances on Immunity and Vaccine Development. Avian Pathol. 1993, 22, 3–31. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.H.; Lillehoj, H.S.; Lillehoj, E.P. Intestinal Immune Responses to Coccidiosis. Dev. Comp. Immunol. 2000, 24, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Wallach, M.; Smith, N.C.; Petracca, M.; Miller, C.M.D.; Eckert, J.; Braun, R. Eimeria Maxima Gametocyte Antigens: Potential Use in a Subunit Maternal Vaccine against Coccidiosis in Chickens. Vaccine 1995, 13, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wallach, M.; Pillemer, G.; Yarus, S.; Halabi, A.; Pugatsch, T.; Mencher, D. Passive Immunization of Chickens against Eimeria Maxima Infection with a Monoclonal Antibody Developed against a Gametocyte Antigen. Infect. Immun. 1990, 58, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.S.; Trout, J.M. Avian Gut-Associated Lymphoid Tissues and Intestinal Immune Responses to Eimeria Parasites. Clin. Microbiol. Rev. 1996, 9, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Regmi, P.; Applegate, T.; Chai, L.; Kim, W.K.; Sharma, M.K.; Regmi, P.; Applegate, T.; Chai, L.; Kim, W.K. Osteoimmunology: A Link between Gastrointestinal Diseases and Skeletal Health in Chickens. Animals 2023, 13, 1816. [Google Scholar] [CrossRef] [PubMed]
- Fathima, S.; Al Hakeem, W.G.; Shanmugasundaram, R.; Selvaraj, R.K. Necrotic Enteritis in Broiler Chickens: A Review on the Pathogen, Pathogenesis, and Prevention. Microorganisms 2022, 10, 1958. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.S.; Choi, K.D. Recombinant Chicken Interferon-Gamma-Mediated Inhibition of Eimeria tenella Development in Vitro and Reduction of Oocyst Production and Body Weight Loss Following Eimeria acervulina Challenge Infection. Avian Dis. 1998, 42, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.E.; Hesketh, P.; Rennie, M. Coccidiosis: Rapid Depletion of Circulating Lymphocytes after Challenge of Immune Chickens with Parasite Antigens. Infect. Immun. 1984, 45, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.S.; Min, W.; Dalloul, R.A. Recent Progress on the Cytokine Regulation of Intestinal Immune Responses to Eimeria. Poult. Sci. 2004, 83, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sharma, M.K.; Tompkins, Y.H.; Teng, P.Y.; Kim, W.K. Impacts of Varying Methionine to Cysteine Supplementation Ratios on Growth Performance, Oxidative Status, Intestinal Health, and Gene Expression of Immune Response and Methionine Metabolism in Broilers under Eimeria Spp. Challenge. Poult. Sci. 2024, 103, 103300. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.A.; McCraw, B.M. Mucosal Morphology and Cellular Renewal in the Intestine of Chickens Following a Single Infection of Eimeria acervulina. J. Parasitol. 1973, 59, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Major, P.; Tóth, Š.; Goldová, M.; Révajová, V.; Kožárová, I.; Levkut, M.; Mojžišová, J.; Hisira, V.; Mihok, T. Dynamic of Apoptosis of Cells in Duodenal Villi Infected with Eimeria acervulina in Broiler Chickens. Biologia 2011, 66, 696–700. [Google Scholar] [CrossRef]
- Sharma, M.K.; Liu, G.; White, D.L.; Kim, W.K. Graded Levels of Eimeria Infection Linearly Reduced the Growth Performance, Altered the Intestinal Health, and Delayed the Onset of Egg Production of Hy-Line W-36 Laying Hens When Infected at the Prelay Stage. Poult. Sci. 2024, 103, 103174. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Liu, G.; White, D.L.; Tompkins, Y.H.; Kim, W.K. Effects of Mixed Eimeria Challenge on Performance, Body Composition, Intestinal Health, and Expression of Nutrient Transporter Genes of Hy-Line W-36 Pullets (0–6 Wks of Age). Poult. Sci. 2022, 101, 102083. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Singh, A.K.; Goo, D.; Choppa, V.S.R.; Ko, H.; Shi, H.; Kim, W.K. Graded Levels of Eimeria Infection Modulated Gut Physiology and Temporarily Ceased the Egg Production of Laying Hens at Peak Production. Poult. Sci. 2024, 103, 103229. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Goo, D.; Sharma, M.K.; Ko, H.; Liu, G.; Paneru, D.; Choppa, V.S.R.; Lee, J.; Kim, W.K. Effects of Different Eimeria Inoculation Doses on Growth Performance, Daily Feed Intake, Gut Health, Gut Microbiota, Foot Pad Dermatitis, and Eimeria Gene Expression in Broilers Raised in Floor Pens for 35 Days. Animals 2023, 13, 2237. [Google Scholar] [CrossRef] [PubMed]
- Hansen, V.L.; Kahl, S.; Proszkowiec-Weglarz, M.; Jiménez, S.C.; Vaessen, S.F.C.; Schreier, L.L.; Jenkins, M.C.; Russell, B.; Miska, K.B. The Effects of Tributyrin Supplementation on Weight Gain and Intestinal Gene Expression in Broiler Chickens during Eimeria Maxima-Induced Coccidiosis. Poult. Sci. 2021, 100, 100984. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.C.; Danforth, H.D.; Caldwell, D.J.; Pierson, F.W.; McElroy, A.P. Intestinal Mucosal Mast Cell Immune Response and Pathogenesis of Two Eimeria acervulina Isolates in Broiler Chickens. Poult. Sci. 2004, 83, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Belote, B.L.; Soares, I.; Tujimoto-Silva, A.; Sanches, A.W.D.; Kraieski, A.L.; Santin, E. Applying I See inside Histological Methodology to Evaluate Gut Health in Broilers Challenged with Eimeria. Vet. Parasitol. 2019, 276, 100004. [Google Scholar] [CrossRef] [PubMed]
- Cliffe, L.J.; Humphreys, N.E.; Lane, T.E.; Potten, C.S.; Booth, C.; Grencis, R.K. Immunology—Accelerated Intestinal Epithelial Cell Turnover: A New Mechanism of Parasite Expulsion. Science 2005, 308, 1463–1465. [Google Scholar] [CrossRef] [PubMed]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef]
- Yadav, S.; Teng, P.Y.; Souza dos Santos, T.; Gould, R.L.; Craig, S.W.; Lorraine Fuller, A.; Pazdro, R.; Kim, W.K. The Effects of Different Doses of Curcumin Compound on Growth Performance, Antioxidant Status, and Gut Health of Broiler Chickens Challenged with Eimeria Species. Poult. Sci. 2020, 99, 5936–5945. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, N.V.; Gabrashanska, M.; Georgieva, N.V.; Gabrashanska, M.; Koinarski, V.; Ermidou-Pollet, S. Antioxidant Status in Eimeria acervulina Infected Chickens after Dietary Selenium Treatment. Trace Elem. Electrolytes 2011, 28, 42. [Google Scholar] [CrossRef]
- Idris, M.; Abbas, R.Z.; Masood, S.; Rehman, T.; Farooq, U.; Babar, W.; Hussain, R.; Raza, A.; Riaz, U. World’s Poultry Science Journal The Potential of Antioxidant Rich Essential Oils against Avian Coccidiosis. Poult. Sci. J. 2017, 73, 89–104. [Google Scholar]
- Tompkins, Y.H.; Choi, J.; Teng, P.Y.; Yamada, M.; Sugiyama, T.; Kim, W.K. Reduced Bone Formation and Increased Bone Resorption Drive Bone Loss in Eimeria Infected Broilers. Sci. Rep. 2023, 13, 616. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.Y.; Liu, G.; Choi, J.; Yadav, S.; Wei, F.; Kim, W.K. Effects of Levels of Methionine Supplementations in Forms of L- or DL-Methionine on the Performance, Intestinal Development, Immune Response, and Antioxidant System in Broilers Challenged with Eimeria spp. Poult. Sci. 2023, 102, 102586. [Google Scholar] [CrossRef]
- Tompkins, Y.H.; Teng, P.; Pazdro, R.; Kim, W.K. Long Bone Mineral Loss, Bone Microstructural Changes and Oxidative Stress after Eimeria Challenge in Broilers. Front. Physiol. 2022, 13, 945740. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.M.; Baldissera, M.D.; Griss, L.G.; Souza, C.F.; Fortuoso, B.F.; Boiago, M.M.; Gris, A.; Mendes, R.E.; Stefani, L.M.; da Silva, A.S. Intestinal Injury Caused by Eimeria spp. Impairs the Phosphotransfer Network and Gain Weight in Experimentally Infected Chicken Chicks. Parasitol. Res. 2019, 118, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.P.; Sasai, K.; Gaafar, S.M.; Smothers, C.D. Effects of Different Levels of Oocyst Inocula of Eimeria acervulina, E. Tenella, and E. Maxima on Plasma Constituents, Packed Cell Volume, Lesion Scores, and Performance in Chickens. Avian Dis. 1993, 37, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Kipper, M.; Andretta, I.; Lehnen, C.R.; Lovatto, P.A.; Monteiro, S.G. Meta-Analysis of the Performance Variation in Broilers Experimentally Challenged by Eimeria spp. Vet. Parasitol. 2013, 196, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Bun, S.D.; Guo, Y.M.; Guo, F.C.; Ji, F.J.; Cao, H. Influence of Organic Zinc Supplementation on the Antioxidant Status and Immune Responses of Broilers Challenged with Eimeria tenella. Poult. Sci. 2011, 90, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Botsoglou, N. Effect of Supplementing Feed with Oregano and/or α-Tocopheryl Acetate on Growth of Broiler Chickens and Oxidative Stability of Meat. Artic. J. Anim. Feed. Sci. 2005, 14, 521–535. [Google Scholar]
- Iuspa, M.A.M.; Soares, I.; Belote, B.L.; Kawazoe, U.; Santin, E. Comparing Performance and Resistance of Two Broilers Breeds Challenged by Eimeria acervulina. Vet. Parasitol. 2020, 287, 109235. [Google Scholar] [CrossRef] [PubMed]
- Rochell, S.J.; Parsons, C.M.; Dilger, R.N. Effects of Eimeria acervulina Infection Severity on Growth Performance, Apparent Ileal Amino Acid Digestibility, and Plasma Concentrations of Amino Acids, Carotenoids, and A1-Acid Glycoprotein in Broilers. Poult. Sci. 2016, 95, 1573–1581. [Google Scholar] [CrossRef]
- Choi, J.; Ko, H.; Tompkins, Y.H.; Teng, P.Y.; Lourenco, J.M.; Callaway, T.R.; Kim, W.K. Effects of Eimeria tenella Infection on Key Parameters for Feed Efficiency in Broiler Chickens. Animals 2021, 11, 3428. [Google Scholar] [CrossRef] [PubMed]
- Hegde, K.S.; Reid, W.M. Effects of Six Single Species of Coccidia on Egg Production and Culling Rate of Susceptible Layers. Poult. Sci. 1969, 48, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Fitz-coy, A.S.H.; Edgar, S.A.; Edgar, S.A. Effects of Eimeria mitis on Egg Production of Single-Comb White Leghorn Hens. Am. Assoc. Avian Pathol. Stable JSTOR 2020, 36, 718–721. [Google Scholar] [CrossRef]
- McDougald, L.R.; Fuller, A.L.; McMurray, B.L. An Outbreak of Eimeria necatrix Coccidiosis in Breeder Pullets: Analysis of Immediate and Possible Long-Term Effects on Performance. Avian Dis. 1990, 34, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, R.L. Studies on Coccidiosis. Poult. Sci. 1934, 13, 148–154. [Google Scholar] [CrossRef]
- Adams, C.; Vahl, H.A.; Veldman, A. Interaction between Nutrition and Eimeria acervulina Infection in Broiler Chickens: Diet Compositions That Improve Fat Digestion during Eimeria acervulina Infection. Br. J. Nutr. 1996, 75, 875–880. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Lin, X.; Mi, Y.; Li, J.; Zhang, C. Grape Seed Proanthocyanidin Extract Prevents Ovarian Aging by Inhibiting Oxidative Stress in the Hens. Oxid. Med. Cell Longev. 2018, 2018, 9390810. [Google Scholar] [CrossRef]
- Zhu, M.; Miao, S.; Zhou, W.; Elnesr, S.S.; Dong, X.; Zou, X. MAPK, AKT/FoxO3a and MTOR Pathways Are Involved in Cadmium Regulating the Cell Cycle, Proliferation and Apoptosis of Chicken Follicular Granulosa Cells. Ecotoxicol. Environ. Saf. 2021, 214, 112091. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.D. Milestones in Avian Coccidiosis Research: A Review. Poult. Sci. 2014, 93, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Hauck, R.; Macklin, K.S. Vaccination Against Poultry Parasites. Avian Dis. 2023, 67, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Kiess, A.; Adhikari, R.; Jha, R. An Approach to Alternative Strategies to Control Avian Coccidiosis and Necrotic Enteritis. J. Appl. Poult. Res. 2020, 29, 515–534. [Google Scholar] [CrossRef]
- Good, E.; Practice, C. Guidance for Industry Guidance for Industry. Fed. Regist. 2004, 505, 79. [Google Scholar]
- Cervantes, H.M.; McDougald, L.R. Raising Broiler Chickens without Ionophore Anticoccidials. J. Appl. Poult. Res. 2023, 32, 100347. [Google Scholar] [CrossRef]
- Martins, R.R.; Silva, L.J.G.; Pereira, A.M.P.T.; Esteves, A.; Duarte, S.C.; Pena, A. Coccidiostats and Poultry: A Comprehensive Review and Current Legislation. Foods 2022, 11, 2738. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.K.; Galloway, R.B.; White, S.L. Effect of Ionophores on Survival, Penetration, and Development of Eimeria tenella Sporozoites In Vitro. J. Parasitol. 1981, 67, 511–516. [Google Scholar] [CrossRef]
- Chapman, H.D. Biochemical, Genetic and Applied Aspects of Drug Resistance in Eimeria Parasites of the Fowl. Avian Pathol. 1997, 26, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Beach, J.R.; Corl, J.C. Studies in the Control of Avian Coccidiosis. Poult. Sci. 1925, 4, 83–93. [Google Scholar] [CrossRef]
- McDonald, V.; Shirley, M.W. Past and Future: Vaccination against Eimeria. Parasitology 2009, 136, 1477–1489. [Google Scholar] [CrossRef]
- Chapman, H.D.; Blake, D.P. Genetic Selection of Eimeria Parasites in the Chicken for Improvement of Poultry Health: Implications for Drug Resistance and Live Vaccine Development. Avian Pathol. 2022, 51, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Dalloul, R.A.; Lillehoj, H.S. Poultry Coccidiosis: Recent Advancements in Control Measures and Vaccine Development. Expert. Rev. Vaccines 2006, 5, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.D.; Cherry, T.E.; Danforth, H.D.; Richards, G.; Shirley, M.W.; Williams, R.B. Sustainable Coccidiosis Control in Poultry Production: The Role of Live Vaccines. Int. J. Parasitol. 2002, 32, 617–629. [Google Scholar] [CrossRef]
- Sharman, P.A.; Smith, N.C.; Wallach, M.G.; Katrib, M. Chasing the Golden Egg: Vaccination against Poultry Coccidiosis. Parasite Immunol. 2010, 32, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Dalloul, R.A.; Lillehoj, H.S. Recent Advances in Immunomodulation and Vaccination Strategies against Coccidiosis. Avian Dis. 2005, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Bloomfield, S.A.; Ricke, S.C. Effects of Age, Vitamin D3, and Fructooligosaccharides on Bone Growth and Skeletal Integrity of Broiler Chicks. Poult. Sci. 2011, 90, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- White, D.; Chen, C.; Kim, W.K. Effect of the Combination of 25-Hydroxyvitamin D3 and Higher Level of Calcium and Phosphorus in the Diets on Bone 3D Structural Development in Pullets. Front. Physiol. 2023, 14, 1056481. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Turner, B.; Applegate, T.J.; Litta, G.; Kim, W.K. Role of Long-Term Supplementation of 25-Hydroxyvitamin D3 on Laying Hen Bone 3-Dimensional Structural Development. Poult. Sci. 2020, 99, 5771–5782. [Google Scholar] [CrossRef] [PubMed]
- Fritts, C.A.; Waldroup, P.W. Effect of Source and Level of Vitamin D on Live Performance and Bone Development in Growing Broilers. J. Appl. Poult. Res. 2003, 12, 45–52. [Google Scholar] [CrossRef]
- Oikeh, I.; Sakkas, P.; Blake, D.P.; Kyriazakis, I. Interactions between Dietary Calcium and Phosphorus Level, and Vitamin D Source on Bone Mineralization, Performance, and Intestinal Morphology of Coccidia-Infected Broilers. Poult. Sci. 2019, 98, 5679–5690. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, P.; Oikeh, I.; Blake, D.P.; Smith, S.; Kyriazakis, I. Dietary Vitamin D Improves Performance and Bone Mineralisation, but Increases Parasite Replication and Compromises Gut Health in Eimeria-Infected Broilers. Br. J. Nutr. 2019, 122, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.; Shanmugasundaram, R.; McDonald, J.; Selvaraj, R.K. Effect of In Vitro and In Vivo 25-Hydroxyvitamin D Treatment on Macrophages, T Cells, and Layer Chickens during a Coccidia Challenge. J. Anim. Sci. 2015, 93, 2894–2903. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, H.M.A.; Kvietys, P.R.; AlKattan, W.; Al Anouti, F.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and Intestinal Homeostasis: Barrier, Microbiota, and Immune Modulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. N. Am. 2017, 46, 815. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.; Selvaraj, R.K. In Vitro 25-Hydroxycholecalciferol Treatment of Lipopolysaccharide-Stimulated Chicken Macrophages Increases Nitric Oxide Production and MRNA of Interleukin-1beta and 10. Vet. Immunol. Immunopathol. 2014, 161, 265–270. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Morris, A.; Selvaraj, R.K. Effect of 25-Hydroxycholecalciferol Supplementation on Turkey Performance and Immune Cell Parameters in a Coccidial Infection Model. Poult. Sci. 2019, 98, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.D.; Cho, S.H.; Chung, T.K.; Kim, I.H. Anti-Coccidial Effect of Essential Oil Blends and Vitamin D on Broiler Chickens Vaccinated with Purified Mixture of Coccidian Oocyst from Eimeria tenella and Eimeria maxima. Poult. Sci. 2019, 98, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Jimenez, H.; Gardner, K.; Al-Jumaa, Y.; Padgett, J.C.; Bailey, C.A. Partial Replacement of Dietary Cholecalciferol with 25-Hydroxycholecalciferol on Broiler Chickens Subjected to a Coccidiosis Vaccine Challenge. J. Appl. Poult. Res. 2019, 28, 743–754. [Google Scholar] [CrossRef]
- Suarez, J.C.; Knape, K.; Ali, N.A.; Aljuboori, J.; Al-Alwani, T.; Gutierrez, O.; Carey, J.B. Dietary Supplementation of 25-Hydroxycholecalciferol and Its Impact on Performance, Intestinal Morphology, and Vitamin D Status in Broilers Subjected to Coccidiosis Vaccine. J. Appl. Poult. Res. 2023, 32, 100360. [Google Scholar] [CrossRef]
- Lopes, T.S.B.; Shi, H.; White, D.; Araújo, I.C.S.; Kim, W.K. Effects of 25-Hydroxycholecalciferol on Performance, Gut Health, and Bone Quality of Broilers Fed with Reduced Calcium and Phosphorus Diet during Eimeria Challenge. Poult. Sci. 2024, 103, 103267. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, M. Vitamin D3 Action within the Ovary—An Updated Review. Physiol. Res. 2020, 69, 371. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wolf, S.; Green, O.; Xu, J. Vitamin D in Follicular Development and Oocyte Maturation. Reproduction 2021, 161, R129–R137. [Google Scholar] [CrossRef] [PubMed]
- Ruschkowski, S.R.; Hart, L.E. Ionic and Endocrine Characteristics of Reproductive Failure in Calcium-Deficient and Vitamin D-Deficient Laying Hens. Poult. Sci. 1992, 71, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, X.; Zeng, Q.; Bai, S.; Zhang, K.; Mao, X.; Xu, S.; Zhuo, Y.; Xuan, Y.; Peng, H.; et al. Dietary 25-Hydroxyvitamin D Improves Productive Performance and Intestinal Health of Laying Hens under Escherichia Coli Lipopolysaccharide Challenge. Poult. Sci. 2023, 102, 102371. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Ma, Q.; Wang, Z.; Guo, Y. Dietary Vitamin D3 Supplementation Protects Laying Hens against Lipopolysaccharide-Induced Immunological Stress. Nutr. Metab. 2018, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Liu, G.; White, D.L.; Tompkins, Y.H.; Kim, W.K. Graded Levels of Eimeria Challenge Altered the Microstructural Architecture and Reduced the Cortical Bone Growth of Femur of Hy-Line W-36 Pullets at Early Stage of Growth (0–6 Wk of Age). Poult. Sci. 2023, 102, 102888. [Google Scholar] [CrossRef] [PubMed]
- Konjufca, V.K.; Bottje, W.G.; Bersi, T.K.; Erf, G.F. Influence of Dietary Vitamin E on Phagocytic Functions of Macrophages in Broilers. Poult. Sci. 2004, 83, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.C.; Fetterer, R.H. Interaction of Dietary Vitamin E with Eimeria maxima Infections in Chickens. Poult. Sci. 2002, 81, 41–48. [Google Scholar] [CrossRef]
- Fu, Z.; Zhong, T.; Wan, X.; Xu, L.; Yang, H.; Han, H.; Wang, Z.; Fu, Z.; Zhong, T.; Wan, X.; et al. Effects of Dietary Vitamin E Supplementation on Reproductive Performance, Egg Characteristics, Antioxidant Capacity, and Immune Status in Breeding Geese during the Late Laying Period. Antioxidants 2022, 11, 2070. [Google Scholar] [CrossRef] [PubMed]
- Tappel, A.L. Vitamin E and free radical peroxidation of lipids. Ann. N. Y. Acad. Sci. 1972, 203, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lin, H.; Wang, X.J.; Song, Z.G.; Jiao, H.C. Vitamin E Supplementation Alleviates the Oxidative Stress Induced by Dexamethasone Treatment and Improves Meat Quality in Broiler Chickens. Poult. Sci. 2010, 89, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Atkinson, J. Vitamin E, Antioxidant and Nothing More. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Colnago, G.L.; Jensen, L.S.; Long, P.L. Effect of Selenium and Vitamin E on the Development of Immunity to Coccidiosis in Chickens. Poult. Sci. 1984, 63, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamadani, A.A.; Al-Dhanki, Z.T.M. Effect of Locally Produced Dual Virulent Coccidian Vaccine and Supplementation of Vitamin A and E on Performance, Some Physiological Traits, Protein and Fat Digestibility of Broiler. Al-Anbar J. Vet. Sci. 2013, 6, 80–95. [Google Scholar]
- Perez-Carbajal, C.; Caldwell, D.; Farnell, M.; Stringfellow, K.; Pohl, S.; Casco, G.; Pro-Martinez, A.; Ruiz-Feria, C.A. Immune Response of Broiler Chickens Fed Different Levels of Arginine and Vitamin E to a Coccidiosis Vaccine and Eimeria Challenge. Poult. Sci. 2010, 89, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhao, L.H.; Mosenthin, R.; Zhang, J.Y.; Ji, C.; Ma, Q.G. Protective Effect of Vitamin E on Laying Performance, Antioxidant Capacity, and Immunity in Laying Hens Challenged with Salmonella Enteritidis. Poult. Sci. 2019, 98, 5847–5854. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.C.M.; Ribeiro, A.M.L.; Canal, C.W.; Vieira, M.M.; Pinheiro, C.C.; Gonçalves, T.; de Moraes, M.L.; Ledur, V.S. Effect of Vitamin E Levels on the Cell-Mediated Immunity of Broilers Vaccinated against Coccidiosis. Braz. J. Poult. Sci. 2011, 13, 53–56. [Google Scholar] [CrossRef][Green Version]
- Jafari, R.A. Effect of Dietary Vitamin E on Eimeria tenella-Induced Oxidative Stress in Broiler Chickens. Afr. J. Biotechnol. 2012, 11. [Google Scholar]
- Dominguez, P.A.; Pro-Martinez, A.; Narciso-Gaytán, C.; Hernández-Cázares, A.; Sosa-Montes, E.; Perez-Hernandez, P.; Caldwell, D.; Ruiz-Feria, C.A. Supplémentation Simultanée En Arginine et En Vitamines E et c Antioxydantes Réduit Le Stress Oxydatif Chez Les Poulets à Griller Après Exposition à Eimeria spp. Can. J. Anim. Sci. 2015, 95, 143–153. [Google Scholar] [CrossRef]
- Dalloul, R.A.; Lillehoj, H.S.; Shellem, T.A.; Doerr, J.A. Effect of Vitamin A Deficiency on Host Intestinal Immune Response to Eimeria acervulina in Broiler Chickens. Poult. Sci. 2002, 81, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Stephensen, C.B. Vitamin A, infection, and immune function*. Annu. Rev. Nutr. 2003, 21, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Green, A.S.; Fascetti, A.J. Meeting the Vitamin A Requirement: The Efficacy and Importance of β-Carotene in Animal Species. Sci. World J. 2016, 2016, 7393620. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Russell, W.C. The Provitamin A Requirement of Growing Chickens. Poult. Sci. 1947, 26, 234–242. [Google Scholar] [CrossRef]
- Erasmus, J.; Scott, M.L.; Levine, P.P. A Relationship Between Coccidiosis and Vitamin A Nutrition in Chickens. Poult. Sci. 1960, 39, 565–572. [Google Scholar] [CrossRef]
- Panda, B.; Combs, G.F.; Devolt, H.M. Studies on Coccidiosis and Vitamin A Nutrition of Broilers 1. Poult. Sci. 1964, 43, 154–164. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Niu, J.; Zhang, Y.; Li, C.; Zhang, Z.; Guo, S.; Ding, B. Effects of Dietary Supplementation with Vitamin A on Antioxidant and Intestinal Barrier Function of Broilers Co-Infected with Coccidia and Clostridium Perfringens. Animals 2022, 12, 3431. [Google Scholar] [CrossRef] [PubMed]
- Boyera, N.; Galey, I.; Bernard, B.A. Effect of Vitamin C and Its Derivatives on Collagen Synthesis and Cross-Linking by Normal Human Fibroblasts. Int. J. Cosmet. Sci. 1998, 20, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- McKee, J.S.; Harrison, P.C. Effects of Supplemental Ascorbic Acid on the Performance of Broiler Chickens Exposed to Multiple Concurrent Stressors. Poult. Sci. 1995, 74, 1772–1785. [Google Scholar] [CrossRef]
- Little, P.L.; Edgar, S.A. The Effect of Vitamin C on Performance of Coccidia Infected Chickens Fed Omplete and Vitamin Deficient Semi-Purified Diets. Poult. Sci. 1971, 50, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Hutsko, S.; Wick, M.; Bielke, L.; Selvaraj, R. The Effects of an Anti-Coccidial Vaccination in Conjunction with Supplemental Protease, Vitamin C and Differing Levels of Dietary Protein on the Production and Gut. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2017. [Google Scholar]
- Stephens, J.F.; Tugwell, R.L. Sources and Levels of Vitamin K in Relation to Cecal Coccidiosis. Poult. Sci. 1960, 39, 1183–1187. [Google Scholar] [CrossRef]
- Liu, G.; Kim, W.K. The Functional Roles of Methionine and Arginine in Intestinal and Bone Health of Poultry: Review. Animals 2023, 13, 2949. [Google Scholar] [CrossRef] [PubMed]
- de Souza Castro, F.L.; Kim, W.K. Secondary Functions of Arginine and Sulfur Amino Acids in Poultry Health: Review. Animals 2020, 10, 2106. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Rochell, S.J.; Kriseldi, R.; Kim, W.K.; Mitchell, R.D. Functional Properties of Amino Acids: Improve Health Status and Sustainability. Poult. Sci. 2023, 102, 102288. [Google Scholar] [CrossRef] [PubMed]
- Rochell, S.J.; Helmbrecht, A.; Parsons, C.M.; Dilger, R.N. Interactive Effects of Dietary Arginine and Eimeria acervulina Infection on Broiler Growth Performance and Metabolism. Poult. Sci. 2017, 96, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Albina, J.E.; Abate, J.A.; Mastrofrancesco, B. Role of Ornithine as a Proline Precursor in Healing Wounds. J. Surg. Res. 1993, 55, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Stechmiller, J.K.; Childress, B.; Cowan, L. Arginine Supplementation and Wound Healing. Nutr. Clin. Pract. 2005, 20, 52–61. [Google Scholar] [CrossRef]
- Allen, P.C.; Fetterer, R.H. Effect of Eimeria acervulina Infections on Plasma L-Arginine. Poult. Sci. 2000, 79, 1414–1417. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.C. Effects of Daily Oral Doses of L-Arginine on Coccidiosis Infections in Chickens. Poult. Sci. 1999, 78, 1506–1509. [Google Scholar] [CrossRef]
- Tan, J.; Applegate, T.J.; Liu, S.; Guo, Y.; Eicher, S.D. Supplemental Dietary L-Arginine Attenuates Intestinal Mucosal Disruption during a Coccidial Vaccine Challenge in Broiler Chickens. Br. J. Nutr. 2014, 112, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Laika, M.; Jahanian, R. Increase in Dietary Arginine Level Could Ameliorate Detrimental Impacts of Coccidial Infection in Broiler Chickens. Livest. Sci. 2016, 195, 38–44. [Google Scholar] [CrossRef]
- Castro, F.L.S.; Teng, P.Y.; Yadav, S.; Gould, R.L.; Craig, S.; Pazdro, R.; Kim, W.K. The Effects of L-Arginine Supplementation on Growth Performance and Intestinal Health of Broiler Chickens Challenged with Eimeria spp. Poult. Sci. 2020, 99, 5844–5857. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ajao, A.M.; Shanmugasundaram, R.; Taylor, J.; Ball, E.; Applegate, T.J.; Selvaraj, R.; Kyriazakis, I.; Olukosi, O.A.; Kim, W.K. The Effects of Arginine and Branched-Chain Amino Acid Supplementation to Reduced-Protein Diet on Intestinal Health, Cecal Short-Chain Fatty Acid Profiles, and Immune Response in Broiler Chickens Challenged with Eimeria spp. Poult. Sci. 2023, 102, 102773. [Google Scholar] [CrossRef]
- Yazdanabadi, F.I.; Moghaddam, G.H.; Nematollahi, A.; Daghighkia, H.; Sarir, H. Effect of Arginine Supplementation on Growth Performance, Lipid Profile, and Inflammatory Responses of Broiler Chicks Challenged with Coccidiosis. Prev. Vet. Med. 2020, 180, 105031. [Google Scholar] [CrossRef] [PubMed]
- Yazdanabadi, F.I.; Mohebalian, H.; Moghaddam, G.; Abbasabadi, M.; Sarir, H.; Vashan, S.J.H.; Haghparast, A. Influence of Eimeria Spp. Infection and Dietary Inclusion of Arginine on Intestine Histological Parameters, Serum Amino Acid Profile and Ileal Amino Acids Digestibility in Broiler Chicks. Vet. Parasitol. 2020, 286, 109241. [Google Scholar] [CrossRef]
- Izadi, F.; Moghaddam, G.; Nematollahi, A.; Khodadmehr, M.; Abbasabadi, M. The Effects of Different Levels of Arginine on Cecum Microbial Population and Serum Antioxidant Properties of Healthy and Coccidia-Challenged Broiler Chicks. Vet. Clin. Pathol. 2020, 14, 127–145. [Google Scholar]
- Tan, J.; Liu, S.; Guo, Y.; Applegate, T.J.; Eicher, S.D. Dietary L-Arginine Supplementation Attenuates Lipopolysaccharide-Induced Inflammatory Response in Broiler Chickens. Br. J. Nutr. 2014, 111, 1394–1404. [Google Scholar] [CrossRef]
- Manwar, S.J.; Moudgal, R.P.; Sastry, K.V.H.; Tyagi, I.S.; Saxena, Y.K. Nitric Oxide Regulating Immune Functions in Egg Type Japanese Quail. Indian J. Poult. Sci. 2003, 38, 133–136. [Google Scholar]
- Basiouni, G.F. The Effect of Feeding an Extra Amounts of Arginine to Local Saudi Hens on Luteinizing Hormone Secretion. J. Biol. Sci. 2009, 9, 617–620. [Google Scholar] [CrossRef][Green Version]
- Uyanga, V.A.; Xin, Q.; Sun, M.; Zhao, J.; Wang, X.; Jiao, H.; Onagbesan, O.M.; Lin, H. Research Note: Effects of Dietary L-Arginine on the Production Performance and Gene Expression of Reproductive Hormones in Laying Hens Fed Low Crude Protein Diets. Poult. Sci. 2022, 101, 101816. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.; Nakashima, N. Methionine-Independent Initiation of Translation in the Capsid Protein of an Insect RNA Virus. Proc. Natl. Acad. Sci. USA 2000, 97, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Swennen, Q.; Geraert, P.A.; Mercier, Y.; Everaert, N.; Stinckens, A.; Willemsen, H.; Li, Y.; Decuypere, E.; Buyse, J. Effects of Dietary Protein Content and 2-Hydroxy-4-Methylthiobutanoic Acid or Dl-Methionine Supplementation on Performance and Oxidative Status of Broiler Chickens. Br. J. Nutr. 2011, 106, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Pourali, M.; Kermanshahi, H.; Golian, A.; Razmi, G.R.; Soukhtanloo, M. Antioxidant and Anticoccidial Effects of Garlic Powder and Sulfur Amino Acids on Eimeria-Infected and Uninfected Broiler Chickens. J. Biol. Sci. 2014, 15, 227–232. [Google Scholar]
- Lai, A.; Dong, G.; Song, D.; Yang, T.; Zhang, X. Responses to Dietary Levels of Methionine in Broilers Medicated or Vaccinated against Coccidia under Eimeria tenella-Challenged Condition. BMC Vet. Res. 2018, 14, 140. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Yuan, Z.; Wang, Z.; Chen, B.; Zhi, L.; Huang, Z.; Zhang, Y.; Lai, A.; Yuan, Z.; Wang, Z.; et al. Dietary Methionine Increased the Growth Performances and Immune Function of Partridge Shank Broilers after Challenged with Coccidia. Animals 2023, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.L.S.; Tompkins, Y.H.; Pazdro, R.; Kim, W.K. The Effects of Total Sulfur Amino Acids on the Intestinal Health Status of Broilers Challenged with Eimeria spp. Poult. Sci. 2020, 99, 5027–5036. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Bütz, D.E.; Whelan, R.; Naranjo, V.; Arendt, M.K.; Ramuta, M.D.; Yang, X.; Crenshaw, T.D.; Cook, M.E. Effects of Dietary Methionine plus Cysteine Levels on Growth Performance and Intestinal Antibody Production in Broilers during Eimeria Challenge. Poult. Sci. 2020, 99, 374–384. [Google Scholar] [CrossRef]
- Castro, F.L.S.; Kim, Y.; Xu, H.; Kim, W.K. The Effect of Total Sulfur Amino Acid Levels on Growth Performance and Bone Metabolism in Pullets under Heat Stress. Poult. Sci. 2020, 99, 5783–5791. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Jiang, Z.M.; Li, D.M. Glutamine: A Precursor of Glutathione and Its Effect on Liver. World J. Gastroenterol. 1999, 5, 143. [Google Scholar] [CrossRef] [PubMed]
- Oxford, J.H.; Selvaraj, R.K. Effects of Glutamine Supplementation on Broiler Performance and Intestinal Immune Parameters During an Experimental Coccidiosis Infection. J. Appl. Poult. Res. 2019, 28, 1279–1287. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Rochell, S.J.; Applegate, T.J. Threonine, Arginine, and Glutamine: Influences on Intestinal Physiology, Immunology, and Microbiology in Broilers. Poult. Sci. 2018, 97, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, S.A.; Ajuwon, K.M.; Romero, L.F.; Adeola, O. Ileal Endogenous Amino Acid Losses: Response of Broiler Chickens to Fiber and Mild Coccidial Vaccine Challenge. Poult. Sci. 2012, 91, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, Z.; Golian, A.; Nassiri-Moghadam, H.; Javadmanesh, A. Effect of Threonine Supplementation on Growth Performance, Metabolizable Energy, Morphological Changes and Immune Response in Broiler Chickens Challenged with Coccidia. Poult. Sci. J. 2020, 8, 95–107. [Google Scholar]
- Zhang, Q.; Chen, X.; Eicher, S.D.; Ajuwon, K.M.; Applegate, T.J. Effect of Threonine Deficiency on Intestinal Integrity and Immune Response to Feed Withdrawal Combined with Coccidial Vaccine Challenge in Broiler Chicks. Br. J. Nutr. 2016, 116, 2030–2043. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.Y.; Choi, J.; Yadav, S.; Tompkins, Y.H.; Kim, W.K. Effects of Low-Crude Protein Diets Supplemented with Arginine, Glutamine, Threonine, and Methionine on Regulating Nutrient Absorption, Intestinal Health, and Growth Performance of Eimeria-Infected Chickens. Poult. Sci. 2021, 100, 101427. [Google Scholar] [CrossRef] [PubMed]
- Wils-Plotz, E.L.; Jenkins, M.C.; Dilger, R.N. Modulation of the Intestinal Environment, Innate Immune Response, and Barrier Function by Dietary Threonine and Purified Fiber during a Coccidiosis Challenge in Broiler Chicks. Poult. Sci. 2013, 92, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Applegate, T.J.; Klose, V.; Steiner, T.; Ganner, A.; Schatzmayr, G. Probiotics and Phytogenics for Poultry: Myth or Reality? J. Appl. Poult. Res. 2010, 19, 194–210. [Google Scholar] [CrossRef]
- Mohammadi Gheisar, M.; Kim, I.H. Phytobiotics in Poultry and Swine Nutrition–a Review. Ital. J. Anim. Sci. 2018, 17, 92–99. [Google Scholar] [CrossRef]
- Bozkurt, M.; Ege, G.; Aysul, N.; Akşit, H.; Tüzün, A.E.; Küçükyllmaz, K.; Borum, A.E.; Uygun, M.; Akşit, D.; Aypak, S.; et al. Effect of Anticoccidial Monensin with Oregano Essential Oil on Broilers Experimentally Challenged with Mixed Eimeria spp. Poult. Sci. 2016, 95, 1858–1868. [Google Scholar] [CrossRef]
- Mohiti-Asli, M.; Ghanaatparast-Rashti, M. Dietary Oregano Essential Oil Alleviates Experimentally Induced Coccidiosis in Broilers. Prev. Vet. Med. 2015, 120, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Gordillo Jaramillo, F.X.; Kim, D.H.; Lee, S.H.; Kwon, S.K.; Jha, R.; Lee, K.W. Role of Oregano and Citrus Species-Based Essential Oil Preparation for the Control of Coccidiosis in Broiler Chickens. J. Anim. Sci. Biotechnol. 2021, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Felici, M.; Tugnoli, B.; De Hoest-Thompson, C.; Piva, A.; Grilli, E.; Marugan-Hernandez, V. Thyme, Oregano, and Garlic Essential Oils and Their Main Active Compounds Influence Eimeria tenella Intracellular Development. Animals 2023, 14, 77. [Google Scholar] [CrossRef]
- Golenser, J.; Waknine, J.H.; Krugliak, M.; Hunt, N.H.; Grau, G.E. Current Perspectives on the Mechanism of Action of Artemisinins. Int. J. Parasitol. 2006, 36, 1427–1441. [Google Scholar] [CrossRef]
- Meshnick, S.R. Artemisinin: Mechanisms of Action, Resistance and Toxicity. Int. J. Parasitol. 2002, 32, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.C.; Lydon, J.; Danforth, H.D. Effects of Components of Artemisia annua on Coccidia Infections in Chickens. Poult. Sci. 1997, 76, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Del Cacho, E.; Gallego, M.; Francesch, M.; Quílez, J.; Sánchez-Acedo, C. Effect of Artemisinin on Oocyst Wall Formation and Sporulation during Eimeria tenella Infection. Parasitol. Int. 2010, 59, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Drǎgan, L.; Györke, A.; Ferreira, J.F.S.; Pop, I.A.; Dunca, I.; Drǎgan, M.; Mircean, V.; Dan, I.; Cozma, V. Effects of Artemisia annua and Foeniculum vulgare on Chickens Highly Infected with Eimeria tenella (Phylum Apicomplexa). Acta Vet. Scand. 2014, 56, 22. [Google Scholar] [CrossRef]
- Jiao, J.Y.; Yang, Y.Q.; Liu, M.J.; Li, J.G.; Cui, Y.; Yin, S.J.; Tao, J.P. Artemisinin and Artemisia Annua Leaves Alleviate Eimeria tenella Infection by Facilitating Apoptosis of Host Cells and Suppressing Inflammatory Response. Vet. Parasitol. 2018, 254, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Hady, M.M.; Zaki, M.M. Efficacy of Some Herbal Feed Additives on Performance and Control of Cecal Coccidiosis in Broilers. APCBEE Procedia 2012, 4, 163–168. [Google Scholar] [CrossRef]
- Kaboutari, J.; Arab, H.A.; Ebrahimi, K.; Rahbari, S. Prophylactic and Therapeutic Effects of a Novel Granulated Formulation of Artemisia Extract on Broiler Coccidiosis. Trop. Anim. Health Prod. 2014, 46, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.A.; Katadj, J.K.; Rahbari, S.; Nabian, S.; Mohammadi, A.R.S.; Pirali-Kheirabadi, K. Comparison between Anticoccidial Effect of Granulated Artemisia Siberi Extract and Pure Artemisinin in Affected Broilers. J. Vet. Res. 2012, 67, 119–125. [Google Scholar]
- Song, Z.; Cheng, K.; Zhang, L.; Wang, T. Dietary Supplementation of Enzymatically Treated Artemisia Annua Could Alleviate the Intestinal Inflammatory Response in Heat-Stressed Broilers. J. Therm. Biol. 2017, 69, 184–190. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Jiang, Y.; Xu, Y.Q.; Jin, X.; Yan, S.M.; Shi, B.L. Effects of Artemisia argyi Flavonoids on Growth Performance and Immune Function in Broilers Challenged with Lipopolysaccharide. Anim. Biosci. 2021, 34, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.L.; Niu, Y.; Zheng, X.C.; Huang, Q.; Su, W.P.; Zhang, J.F.; Zhang, L.L.; Wang, T. Antioxidant Capacities of Artemisia annua L. Leaves and Enzymatically Treated Artemisia annua L. in Vitro and in Broilers. Anim. Feed Sci. Technol. 2016, 221, 27–34. [Google Scholar] [CrossRef]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic Feed Additives in Poultry: Achievements, Prospective and Challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef] [PubMed]
- Aktaran Bala, D.; Matur, E.; Ergul Ekiz, E.; Akyazi, I.; Eraslan, E.; Ozcan, M.; Ergen, E.; Erek, M.; Gursel, F.E.; Eseceli, H.; et al. Effects of dietary thyme on immune cells, the antioxidant defence system, cytokine cascade, productive performance and egg quality in laying hens. J. Anim. Plant Sci. 2020, 31, 394–402. [Google Scholar]
- Mao, X.; Dou, Y.; Fan, X.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Luo, J.; Luo, Y.; Yan, H.; et al. The Effect of Dietary Yucca schidigera Extract Supplementation on Productive Performance, Egg Quality, and Gut Health in Laying Hens with Clostridium Perfringens and Coccidia Challenge. Poult. Sci. 2023, 102, 102822. [Google Scholar] [CrossRef] [PubMed]
- Laptev, G.Y.; Yildirim, E.A.; Ilina, L.A.; Filippova, V.A.; Kochish, I.I.; Gorfunkel, E.P.; Dubrovin, A.V.; Brazhnik, E.A.; Narushin, V.G.; Novikova, N.I.; et al. Effects of Essential Oils-Based Supplement and Salmonella Infection on Gene Expression, Blood Parameters, Cecal Microbiome, and Egg Production in Laying Hens. Animals 2021, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Dinh, T.; Adhikari, P.A. Production Performance, Egg Quality, and Small Intestine Histomorphology of the Laying Hens Supplemented with Phytogenic Feed Additive. J. Appl. Poult. Res. 2020, 29, 362–371. [Google Scholar] [CrossRef]
- Sharma, M.K.; White, D.L.; Singh, A.K.; Liu, H.; Tan, Z.; Peng, X.; Kim, W.K. Effect of Dietary Supplementation of Probiotic Aspergillus Niger on Performance and Cecal Microbiota in Hy-Line W-36 Laying Hens. Animals 2022, 12, 2406. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, C.D.; Dittoe, D.K.; Wamsley, K.G.S.; McDaniel, C.D.; Blanch, A.; Sandvang, D.; Kiess, A.S. In Ovo Inoculation of an Enterococcus Faecium—Based Product to Enhance Broiler Hatchability, Live Performance, and Intestinal Morphology. Poult. Sci. 2020, 99, 6163–6172. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Lee, C.H.; Cosby, D.E.; Cox, N.A.; Kim, W.K. Effect of Probiotics on Fecal Excretion, Colonization in Internal Organs and Immune Gene Expression in the Ileum of Laying Hens Challenged with Salmonella enteritidis. Poult. Sci. 2019, 98, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.A.; Cosby, D.E.; Cox, N.A.; Lee, J.H.; Kim, W.K. Effect of Dietary Bacteriophage Supplementation on Internal Organs, Fecal Excretion, and Ileal Immune Response in Laying Hens Challenged by Salmonella enteritidis. Poult. Sci. 2017, 96, 3264–3271. [Google Scholar] [CrossRef]
- Markowiak, P.; Ślizewska, K. The Role of Probiotics, Prebiotics and Synbiotics in Animal Nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Mountzouris, K.C.; Tsitrsikos, P.; Palamidi, I.; Arvaniti, A.; Mohnl, M.; Schatzmayr, G.; Fegeros, K. Effects of Probiotic Inclusion Levels in Broiler Nutrition on Growth Performance, Nutrient Digestibility, Plasma Immunoglobulins, and Cecal Microflora Composition. Poult. Sci. 2010, 89, 58–67. [Google Scholar] [CrossRef]
- Kogut, M.H.; Lee, A.; Santin, E. Microbiome and Pathogen Interaction with the Immune System. Poult. Sci. 2020, 99, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J.; Kogut, M.H. The Role of the Gut Microbiome in Shaping the Immune System of Chickens. Vet. Immunol. Immunopathol. 2018, 204, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Das, R.; Oak, S.; Mishra, P. Probiotics (Direct-Fed Microbials) in Poultry Nutrition and Their Effects on Nutrient Utilization, Growth and Laying Performance, and Gut Health: A Systematic Review. Animals 2020, 10, 1863. [Google Scholar] [CrossRef] [PubMed]
- Memon, F.U.; Yang, Y.; Zhang, G.; Leghari, I.H.; Lv, F.; Wang, Y.; Laghari, F.; Khushk, F.A.; Si, H. Chicken Gut Microbiota Responses to Dietary Bacillus subtilis Probiotic in the Presence and Absence of Eimeria Infection. Microorganisms 2022, 10, 1548. [Google Scholar] [CrossRef]
- Lee, K.W.; Lillehoj, H.S.; Jang, S.I.; Li, G.; Lee, S.H.; Lillehoj, E.P.; Siragusa, G.R. Effect of Bacillus-Based Direct-Fed Microbials on Eimeria maxima Infection in Broiler Chickens. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, e105–e110. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Lee, Y.; Goo, D.; Zimmerman, N.P.; Smith, A.H.; Rehberger, T.; Lillehoj, H.S. The Effects of Dietary Bacillus subtilis Supplementation, as an Alternative to Antibiotics, on Growth Performance, Intestinal Immunity, and Epithelial Barrier Integrity in Broiler Chickens Infected with Eimeria maxima. Poult. Sci. 2020, 99, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.A.; Lee, Y.; Lillehoj, H.S. Beneficial Effects of Dietary Supplementation of Bacillus Strains on Growth Performance and Gut Health in Chickens with Mixed Coccidiosis Infection. Vet. Parasitol. 2020, 277, 109009. [Google Scholar] [CrossRef] [PubMed]
- Awais, M.M.; Jamal, M.A.; Akhtar, M.; Hameed, M.R.; Anwar, M.I.; Ullah, M.I. Immunomodulatory and Ameliorative Effects of Lactobacillus and Saccharomyces Based Probiotics on Pathological Effects of Eimeriasis in Broilers. Microb. Pathog. 2019, 126, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zheng, L.; Qi, Y.; Liu, Z.; Du, E.; Wei, J.; Zhang, Z.; Guo, S.; Ding, B. Dietary Lactobacillus fermentum and Lactobacillus paracasei Improve the Intestinal Health of Broilers Challenged with Coccidia and Clostridium perfringens. Front. Vet. Sci. 2022, 9, 1025677. [Google Scholar] [CrossRef] [PubMed]
- Dalloul, R.A.; Lillehoj, H.S.; Shellem, T.A.; Doerr, J.A. Enhanced Mucosal Immunity against Eimeria acervulina in Broilers Fed a Lactobacillus-Based Probiotic. Poult. Sci. 2003, 82, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Xie, Q.M.; Ji, J.; Yang, W.H.; Wu, Y.B.; Li, C.; Ma, J.Y.; Bi, Y.Z. Different Combinations of Probiotics Improve the Production Performance, Egg Quality, and Immune Response of Layer Hens. Poult. Sci. 2012, 91, 2755–2760. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Wang, C.; Zhang, H.; Lai, W.; Wei, H.; Peng, J. Effects of Different Probiotics on Laying Performance, Egg Quality, Oxidative Status, and Gut Health in Laying Hens. Animals 2019, 9, 1110. [Google Scholar] [CrossRef]
- Connor Padgett, J.; Allen Byrd, J.; Bailey, C.; Thomas Price, P.; Anthony Bailey, C. Salmonella Enteritidis Control in Mature Laying Hens Through Dry Fed Parietal Yeast Fraction or Bacillus Blend Probiotic. Int. J. Anim. Sci. Technol. 2021, 5, 1–6. [Google Scholar] [CrossRef]
- Price, P.T.; Gaydos, T.A.; Berghaus, R.D.; Baxter, V.; Hofacre, C.L.; Sims, M.D. Salmonella Enteritidis Reduction in Layer Ceca with a Bacillus Probiotic. Vet. World 2020, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.A.; Burkholder, K.M. Application of Prebiotics and Probiotics in Poultry Production. Poult. Sci. 2003, 82, 627–631. [Google Scholar] [CrossRef]
- Adhikari, P.A.; Kim, W.K. Overview Of prebiotics and probiotics: Focus On performance, Gut Health and Immunity—A Review. Anim. Sci 2017, 17, 949–966. [Google Scholar] [CrossRef]
- Teng, P.Y.; Kim, W.K. Review: Roles of Prebiotics in Intestinal Ecosystem of Broilers. Front. Vet. Sci. 2018, 5, 398676. [Google Scholar] [CrossRef]
- Teng, P.Y.; Adhikari, R.; Llamas-Moya, S.; Kim, W.K. Effects of Combination of Mannan-Oligosaccharides and β-Glucan on Growth Performance, Intestinal Morphology, and Immune Gene Expression in Broiler Chickens. Poult. Sci. 2021, 100, 101483. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Singh, A.K.; Selvaraj, R.K.; Applegate, T.J.; Bhattacharya, P.; Shinall, S.B.; Fenn, L.S.; Shanmugasundaram, R.; Kim, W.K. Research Note: Effect of Dietary Xylo-Oligosaccharide on Growth Performance, Intestinal Histomorphology, and Specific Cecal Bacteria in Broiler Chickens. Poult. Sci. 2024, 103, 103189. [Google Scholar] [CrossRef]
- Faber, T.A.; Dilger, R.N.; Hopkins, A.C.; Price, N.P.; Fahey, J.C. The Effects of a Galactoglucomannan Oligosaccharide-Arabinoxylan (GGMO-AX) Complex in Broiler Chicks Challenged with Eimeria acervulina. Poult. Sci. 2012, 91, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.; Yitbarek, A.; Snyder, R.; Patterson, R.; Barta, J.R.; Karrow, N.; Kiarie, E. Responses of Broiler Chickens to Eimeria Challenge When Fed a Nucleotide-Rich Yeast Extract. Poult. Sci. 2019, 98, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Teng, P.Y.; Olukosi, O.A. The Effects of Xylo-Oligosaccharides on Regulating Growth Performance, Nutrient Utilization, Gene Expression of Tight Junctions, Nutrient Transporters, and Cecal Short Chain Fatty Acids Profile in Eimeria-Challenged Broiler Chickens. Poult. Sci. 2022, 101, 102125. [Google Scholar] [CrossRef] [PubMed]
- Baurhoo, B.; Ferket, P.R.; Zhao, X. Effects of Diets Containing Different Concentrations of Mannanoligosaccharide or Antibiotics on Growth Performance, Intestinal Development, Cecal and Litter Microbial Populations, and Carcass Parameters of Broilers. Poult. Sci. 2009, 88, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, F.; Hinton, M.; Van Gils, B. Dietary Mannan-Oligosaccharides and Their Effect on Chicken Caecal Microflora in Relation to Salmonella Enteritidis Colonization. Avian Pathol. 2002, 31, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Osho, S.O.; Adeola, O. Impact of Dietary Chitosan Oligosaccharide and Its Effects on Coccidia Challenge in Broiler Chickens. Br. Poult. Sci. 2019, 60, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Chand, N.; Faheem, H.; Khan, R.U.; Qureshi, M.S.; Alhidary, I.A.; Abudabos, A.M. Anticoccidial Effect of Mananoligosacharide against Experimentally Induced Coccidiosis in Broiler. Environ. Sci. Pollut. Res. 2016, 23, 14414–14421. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Quiroga, C.; Borda-Molina, D.; Chaves-Moreno, D.; Ruiz, R.; Atxaerandio, R.; Camarinha-Silva, A.; García-Rodríguez, A. Microbial and Functional Profile of the Ceca from Laying Hens Affected by Feeding Prebiotics, Probiotics, and Synbiotics. Microorganisms 2019, 7, 123. [Google Scholar] [CrossRef]
- Ghasemian, M.; Jahanian, R. Dietary Mannan-Oligosaccharides Supplementation Could Affect Performance, Immunocompetence, Serum Lipid Metabolites, Intestinal Bacterial Populations, and Ileal Nutrient Digestibility in Aged Laying Hens. Anim. Feed Sci. Technol. 2016, 213, 81–89. [Google Scholar] [CrossRef]
- Adhikari, P.; Cosby, D.E.; Cox, N.A.; Franca, M.S.; Williams, S.M.; Gogal, R.M.; Ritz, C.W.; Kim, W.K. Effect of Dietary Fructooligosaccharide Supplementation on Internal Organs Salmonella Colonization, Immune Response, Ileal Morphology, and Ileal Immunohistochemistry in Laying Hens Challenged with Salmonella Enteritidis. Poult. Sci. 2018, 97, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Chalorsuntisakul, S.; Sirithunyalug, J.; Chaiyasuta, C.; Aengwanich, W.; Pewnim, T. Effect of Synbiotics on Caecal Morphology and Lesion Score in Broilers Infected with Eimeria tenella. Avian Biol. Res. 2010, 3, 187–190. [Google Scholar] [CrossRef]
- Duff, A.F.; Briggs, W.N.; Chasser, K.M.; Lilburn, M.S.; Syed, B.; Ramirez, S.; Murugesan, R.; Pender, C.; Bielke, L.R. Effect of Dietary Synbiotic Supplementation on Performance Parameters in Turkey Poults Administered a Mixed Eimeria Species Inoculation I. Poult. Sci. 2020, 99, 4235–4241. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, R.; Markazi, A.; Mortada, M.; Ng, T.T.; Applegate, T.J.; Bielke, L.R.; Syed, B.; Pender, C.M.; Curry, S.; Murugesan, G.R.; et al. Research Note: Effect of Synbiotic Supplementation on Caecal Clostridium perfringens Load in Broiler Chickens with Different Necrotic Enteritis Challenge Models. Poult. Sci. 2020, 99, 2452–2458. [Google Scholar] [CrossRef]
- White, M.B. In Ovo and Feed Application of Probiotics or Synbiotics and Response of Broiler Chicks to Post-Hatch Necrotic Enteritis Academic Abstract. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2021. [Google Scholar]
- Ghasemi, H.A.; Shivazad, M.; Esmaeilnia, K.; Kohram, H.; Karimi, M.A. The Effects of a Synbiotic Containing Enterococcus Faecium and Inulin on Growth Performance and Resistance to Coccidiosis in Broiler Chickens. J. Poult. Sci. 2010, 47, 149–155. [Google Scholar] [CrossRef]
- Shah, B.R.; Hakeem, W.A.; Shanmugasundaram, R.; Selvaraj, R.K. Effect of Synbiotic Supplementation on Production Performance and Severity of Necrotic Enteritis in Broilers during an Experimental Necrotic Enteritis Challenge. Poult. Sci. 2023, 102, 102959. [Google Scholar] [CrossRef]
- Song, D.; Wang, W.; Chen, B.; Li, A.; Song, G.; Cheng, J.; Qiao, L.; Zhu, R.; Min, Y. Dietary Supplemental Synbiotic—Yucca Extract Compound Preparation Modulates Production Performance, Immune Status and Faecal Microflora Diversity in Laying Hens. Food Agric. Immunol. 2022, 33, 360–376. [Google Scholar] [CrossRef]
- Murate, L.S.; Paião, F.G.; de Almeida, A.M.; Berchieri, A.; Shimokomaki, M. Efficacy of Prebiotics, Probiotics, and Synbiotics on Laying Hens and Broilers Challenged with Salmonella Enteritidis. J. Poult. Sci. 2015, 52, 52–56. [Google Scholar] [CrossRef]
- Craig ID, A.D.; Khattak, F.; Hastie, P.; Bedford, M.R.; Olukosi, O.A. The Similarity of the Effect of Carbohydrase or Prebiotic Supplementation in Broilers Aged 21 Days, Fed Mixed Cereal Diets and Challenged with Coccidiosis Infection. PLoS ONE 2020, 15, e0229281. [Google Scholar] [CrossRef]
- Lin, Y.; Lourenco, J.M.; Olukosi, O.A. The Effects of Protease, Xylanase, and Xylo-Oligosaccharides on Growth Performance, Nutrient Utilization, Short-Chain Fatty Acids, and Microbiota in Eimeria-Challenged Broiler Chickens Fed Low-Protein Diet. Poult. Sci. 2023, 102, 102789. [Google Scholar] [CrossRef]
- M’Sadeq, S.A. Ameliorative Effect of Yeast Cell Walls on Broiler Chickens’ Performance and Gut Health under Coccidiosis Challenge. Czech J. Anim. Sci. 2023, 68, 346–355. [Google Scholar]
- Gelinas, A.; Sudan, S.; Patterson, R.; Li, J.; Huyben, D.; Barta, J.R.; Kiarie, E.G. Growth Performance, Organs Weight, Intestinal Histomorphology, and Oocyst Shedding in Broiler Chickens Offered Novel Single Strain Bacillus Subtilis Isolated from Camel Dung and Challenged with Eimeria. Poult. Sci. 2024, 103, 103519. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Ma, Y.; Chai, J.; Li, Z.; You, X.; Wang, X.; Huang, Y.; Shi, H. In Ovo Injection Dosage of Lactobacillus Rhamnosus on Intestinal Health and Microbial Composition of Yellow Broilers with or without Eimeria Challenge. J. Appl. Poult. Res. 2024, 33, 100411. [Google Scholar] [CrossRef]
- Park, I.; Goo, D.; Nam, H.; Wickramasuriya, S.S.; Lee, K.; Zimmerman, N.P.; Smith, A.H.; Rehberger, T.G.; Lillehoj, H.S. Effects of Dietary Maltol on Innate Immunity, Gut Health, and Growth Performance of Broiler Chickens Challenged with Eimeria maxima. Front. Vet. Sci. 2021, 8, 667425. [Google Scholar] [CrossRef] [PubMed]
- Reuben, R.C.; Sarkar, S.L.; Roy, P.C.; Anwar, A.; Hossain, M.A.; Jahid, I.K. Prebiotics, Probiotics and Postbiotics for Sustainable Poultry Production. Worlds Poult. Sci. J. 2021, 77, 825–882. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Izuddin, W.I.; Awad, E.A.; Idrus, Z.; Samsudin, A.A.; Mustapha, N.M. Dietary Supplementation of Postbiotics Mitigates Adverse Impacts of Heat Stress on Antioxidant Enzyme Activity, Total Antioxidant, Lipid Peroxidation, Physiological Stress Indicators, Lipid Profile and Meat Quality in Broilers. Animals 2020, 10, 982. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Loh, T.C.; Foo, H.L.; Lim, E.T.C. Lactiplantibacillus Plantarum Postbiotics: Alternative of Antibiotic Growth Promoter to Ameliorate Gut Health in Broiler Chickens. Front. Vet. Sci. 2022, 9, 883324. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Ghany, W.A.; Abdel-Latif, M.A.; Hosny, F.; Alatfeehy, N.M.; Noreldin, A.E.; Quesnell, R.R.; Chapman, R.; Sakai, L.; Elbestawy, A.R. Comparative Efficacy of Postbiotic, Probiotic, and Antibiotic against Necrotic Enteritis in Broiler Chickens. Poult. Sci. 2022, 101, 101988. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, E.; Frana, T.; Logue, C.M.; Smith, D.P.; Pavlidis, H.O.; Chaney, W.E. Effect of Feeding a Postbiotic Derived from Saccharomyces Cerevisiae Fermentation as a Preharvest Food Safety Hurdle for Reducing Salmonella Enteritidis in the Ceca of Layer Pullets. J. Food Prot. 2021, 84, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.C.; Mogollón-García, H.D.; de Moraes, A.C.I.; Dias, G.S.; de Brito Viana, G.; Milbradt, E.L.; Andreatti-Filho, R.L.; Okamoto, A.S. Research Note: The Effects of a Lactobacillus Helveticus ATCC 15009-Derived Postbiotic Mitigating Salmonella Gallinarum Colonization in Commercial Layer Chicks. Poult. Sci. 2023, 102, 103095. [Google Scholar] [CrossRef] [PubMed]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Mustapha, N.M.; Zulkifli, I.; Izuddin, W.I. Effects of Feeding Different Postbiotics Produced by Lactobacillus Plantarum on Growth Performance, Carcass Yield, Intestinal Morphology, Gut Microbiota Composition, Immune Status, and Growth Gene Expression in Broilers under Heat Stress. Animals 2019, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Kogut, M.H.; Genovese, K.; He, H.; Kazemi, S.; Arsenault, R.J. Administration of a Postbiotic Causes Immunomodulatory Responses in Broiler Gut and Reduces Disease Pathogenesis Following Challenge. Microorganisms 2019, 7, 268. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).