Evaluation of Intestinal Barrier Dysfunction with Serum Iohexol Concentration in Dogs with Acute Hemorrhagic Diarrhea Syndrome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.2.1. Dogs with AHDS
2.2.2. Healthy Control Group
2.3. Iohexol Measurement
2.4. Evaluation of Disease Severity and Clinical Outcome
2.5. Statistical Analysis
3. Results
3.1. Signalment
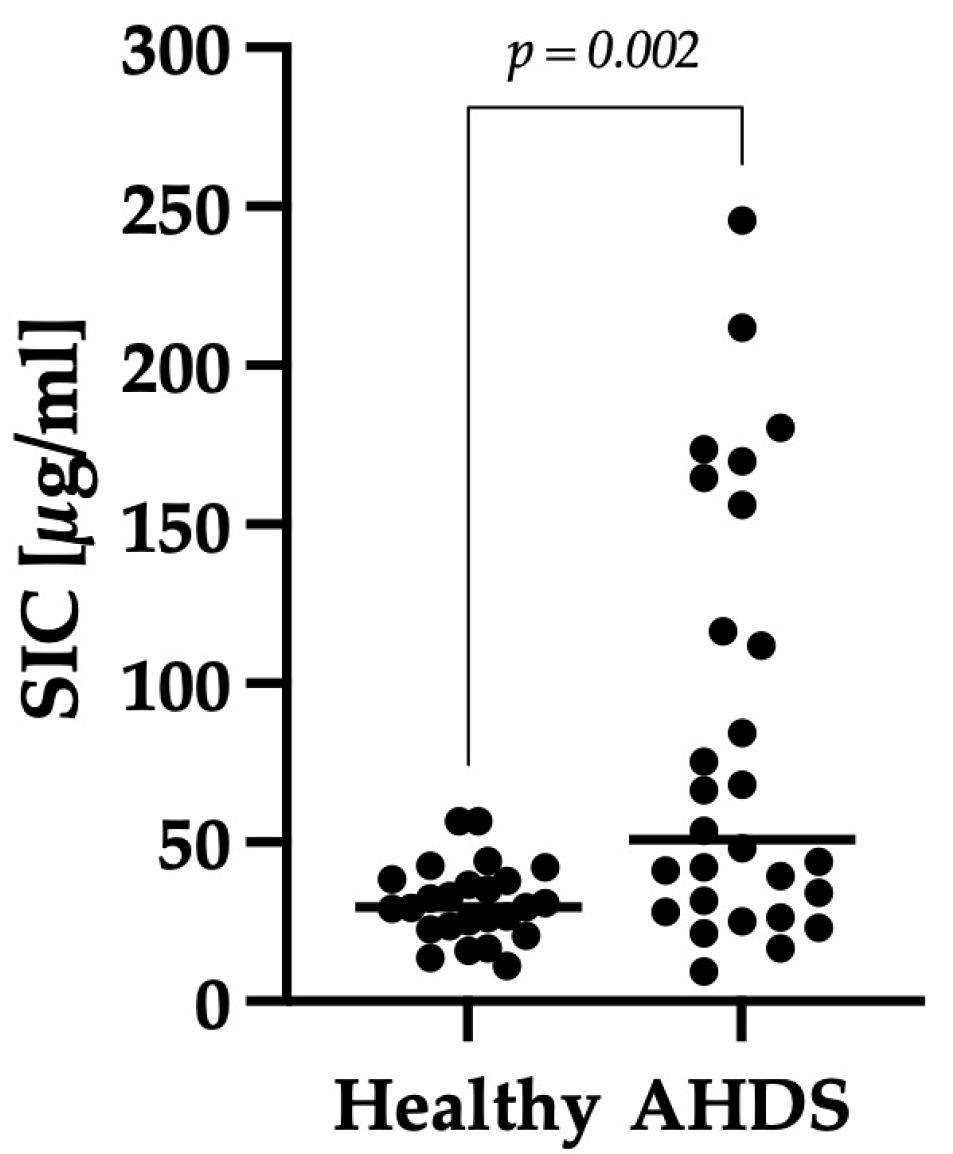
3.2. Comparison of SIC between the Healthy Control Group and Dogs with AHDS
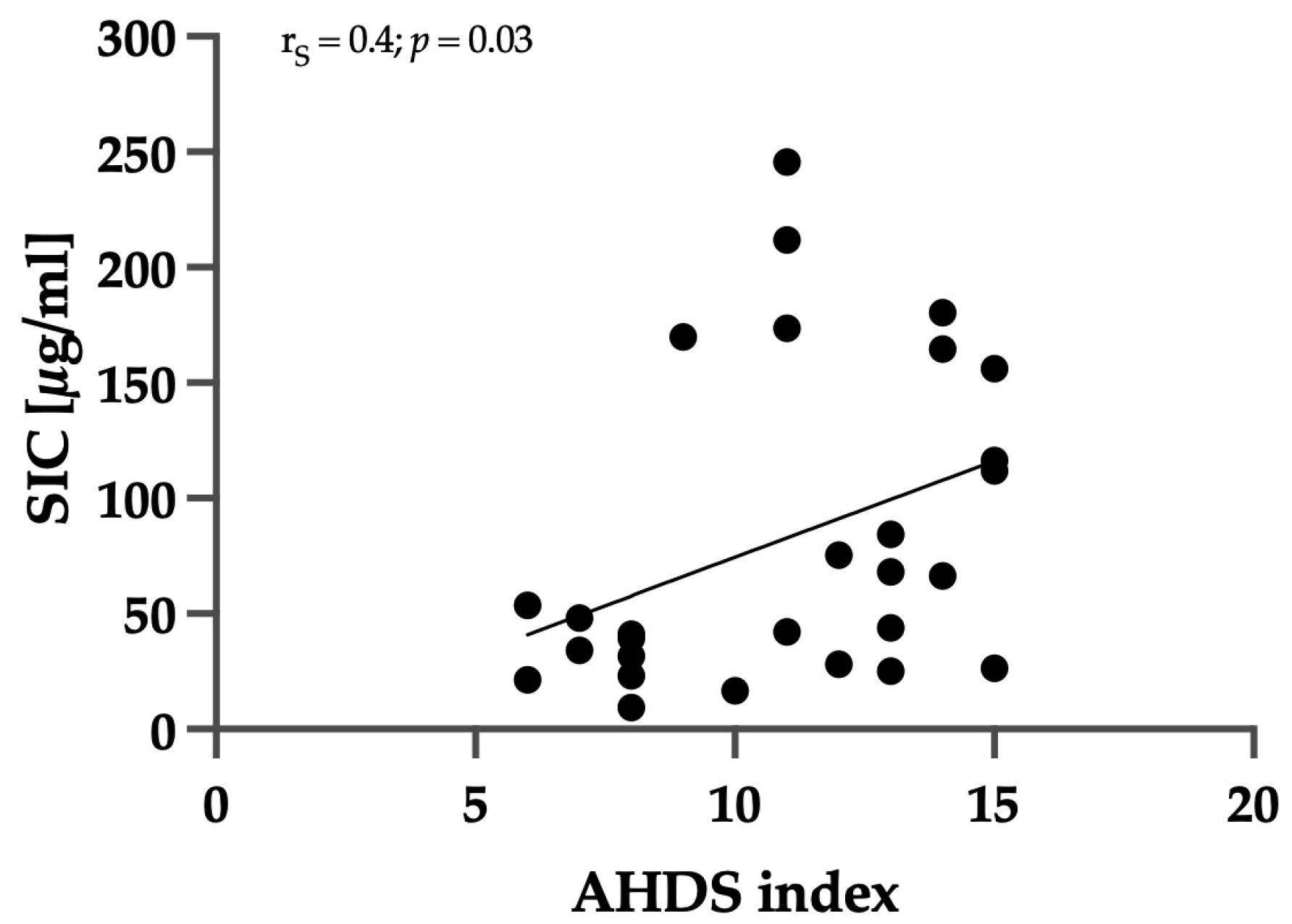
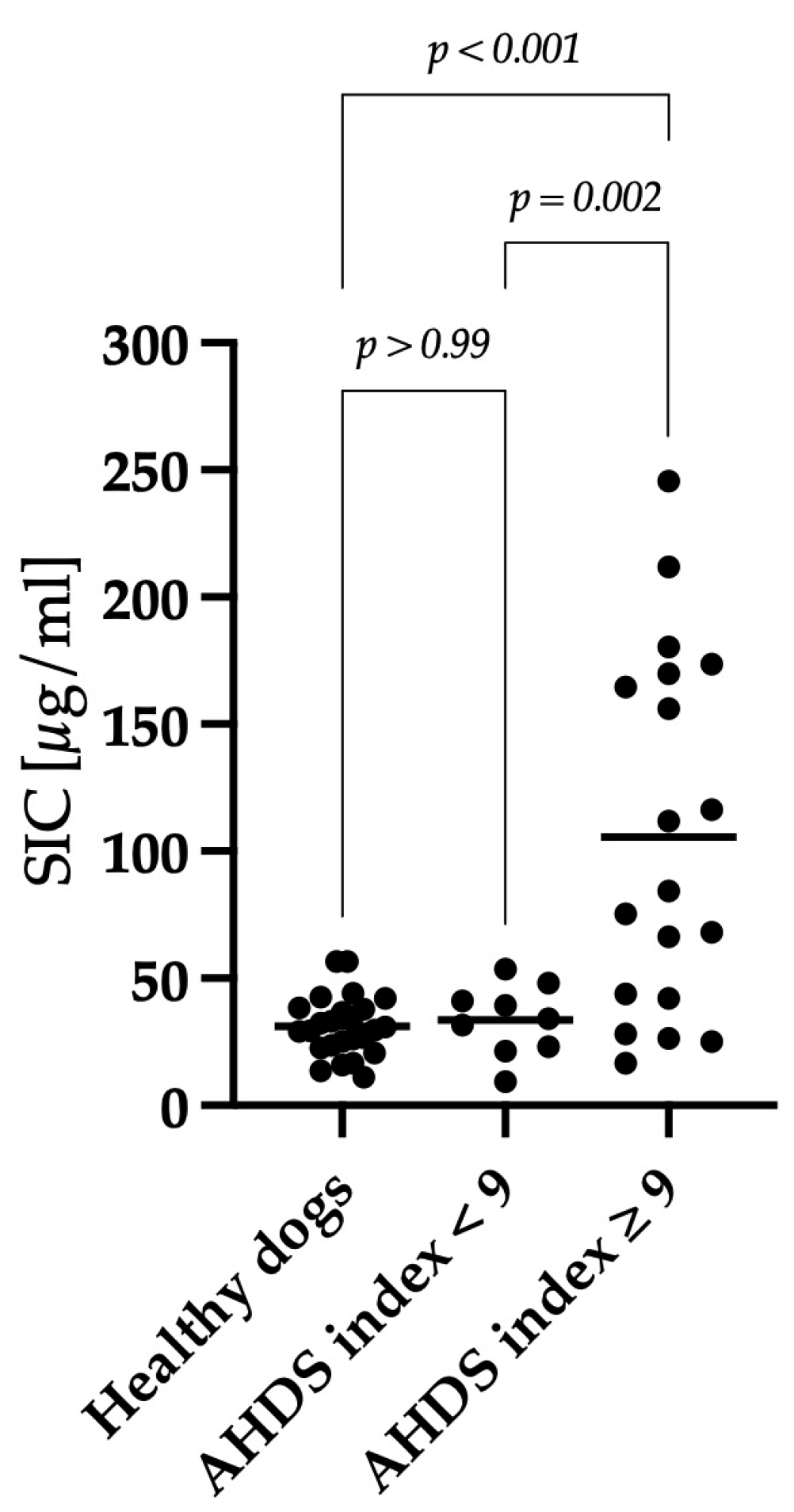
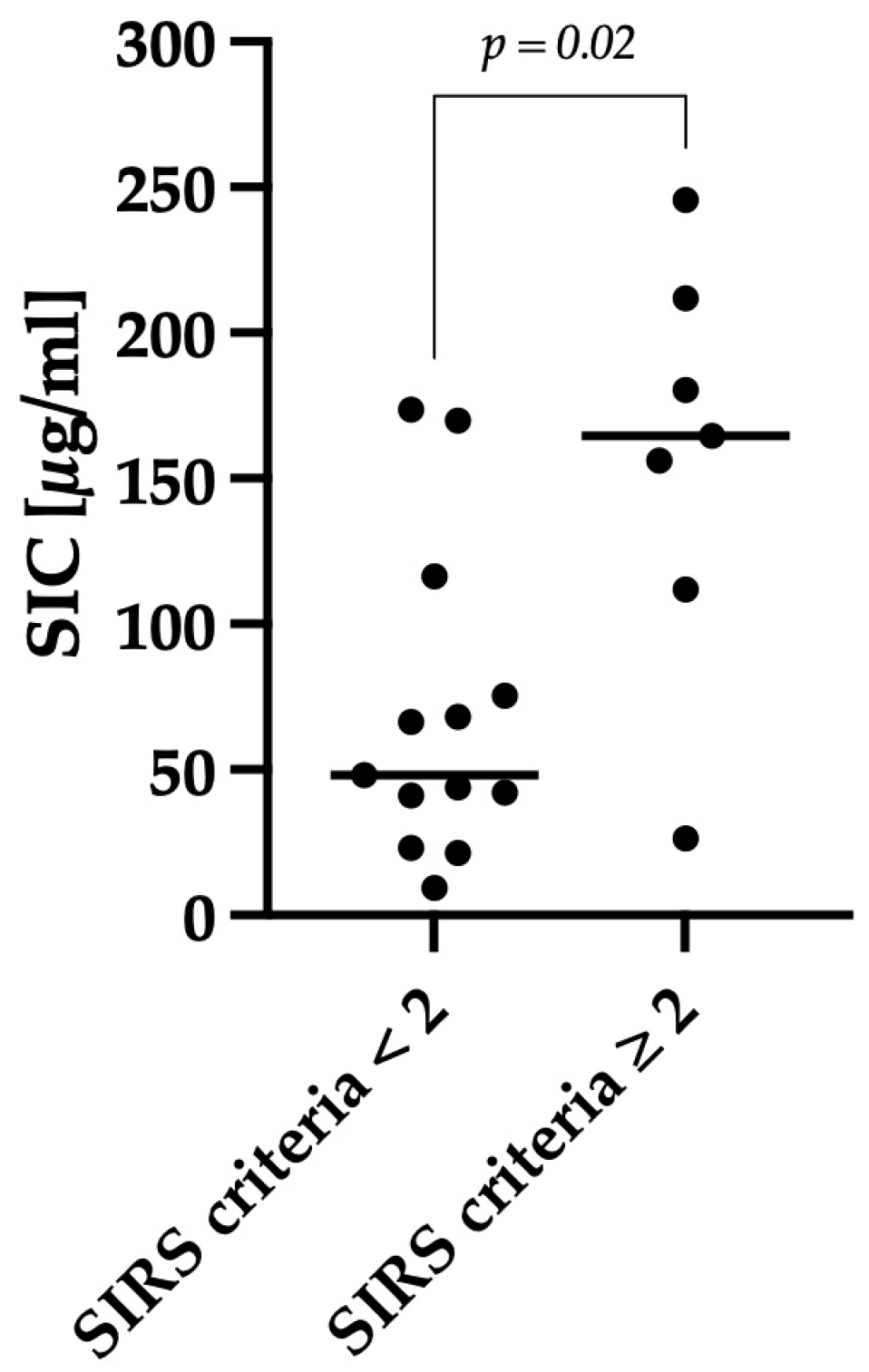
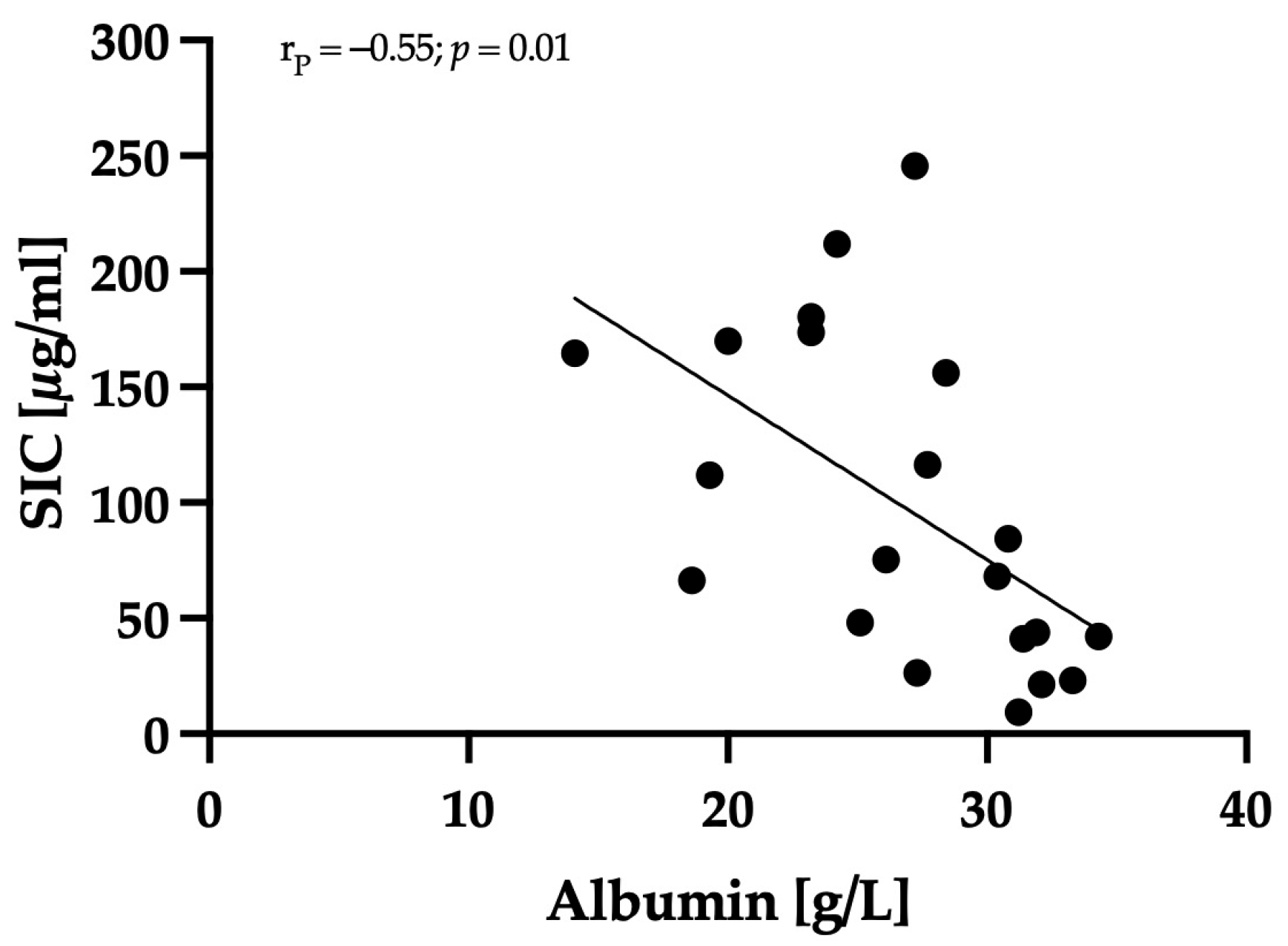
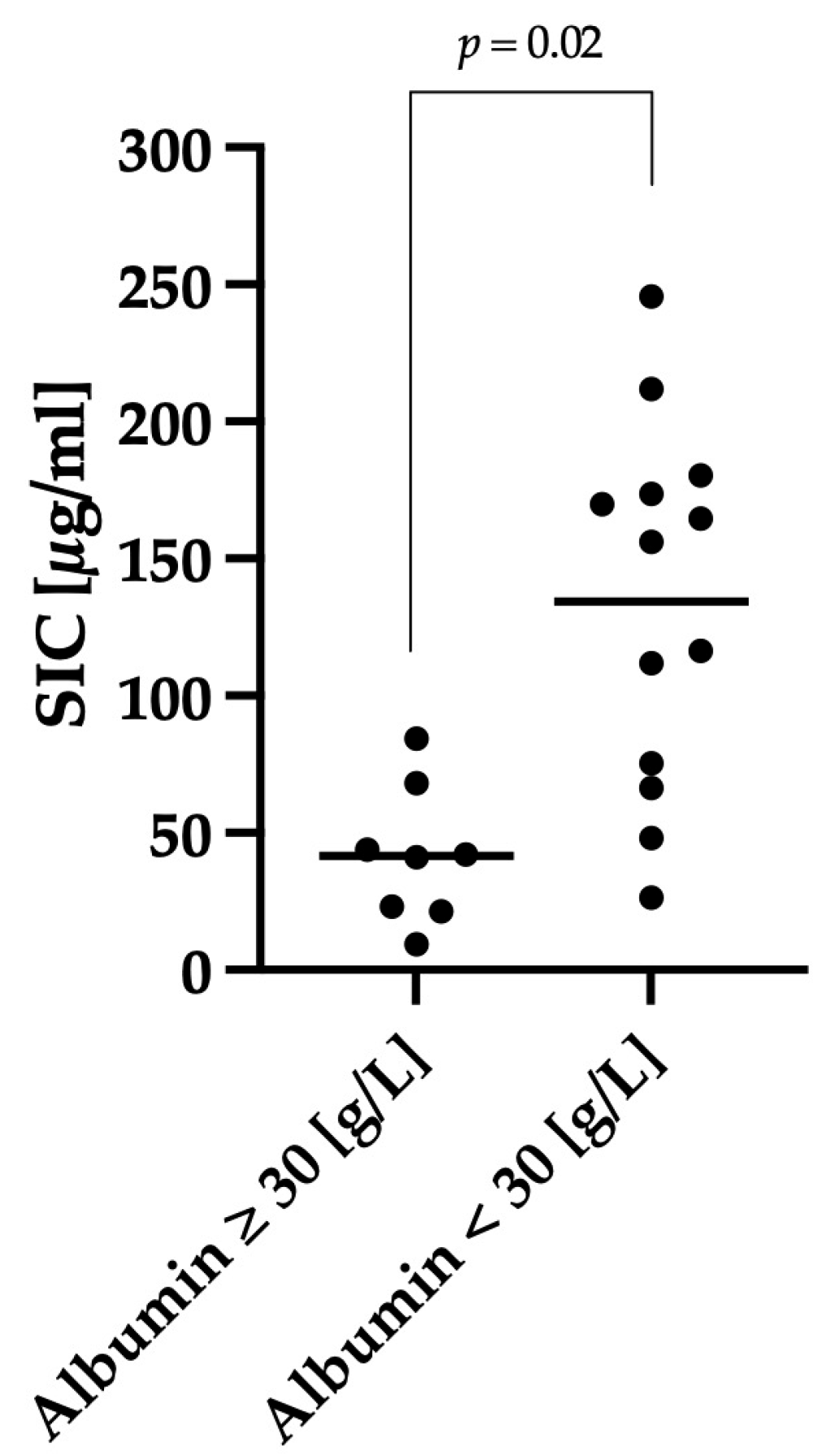
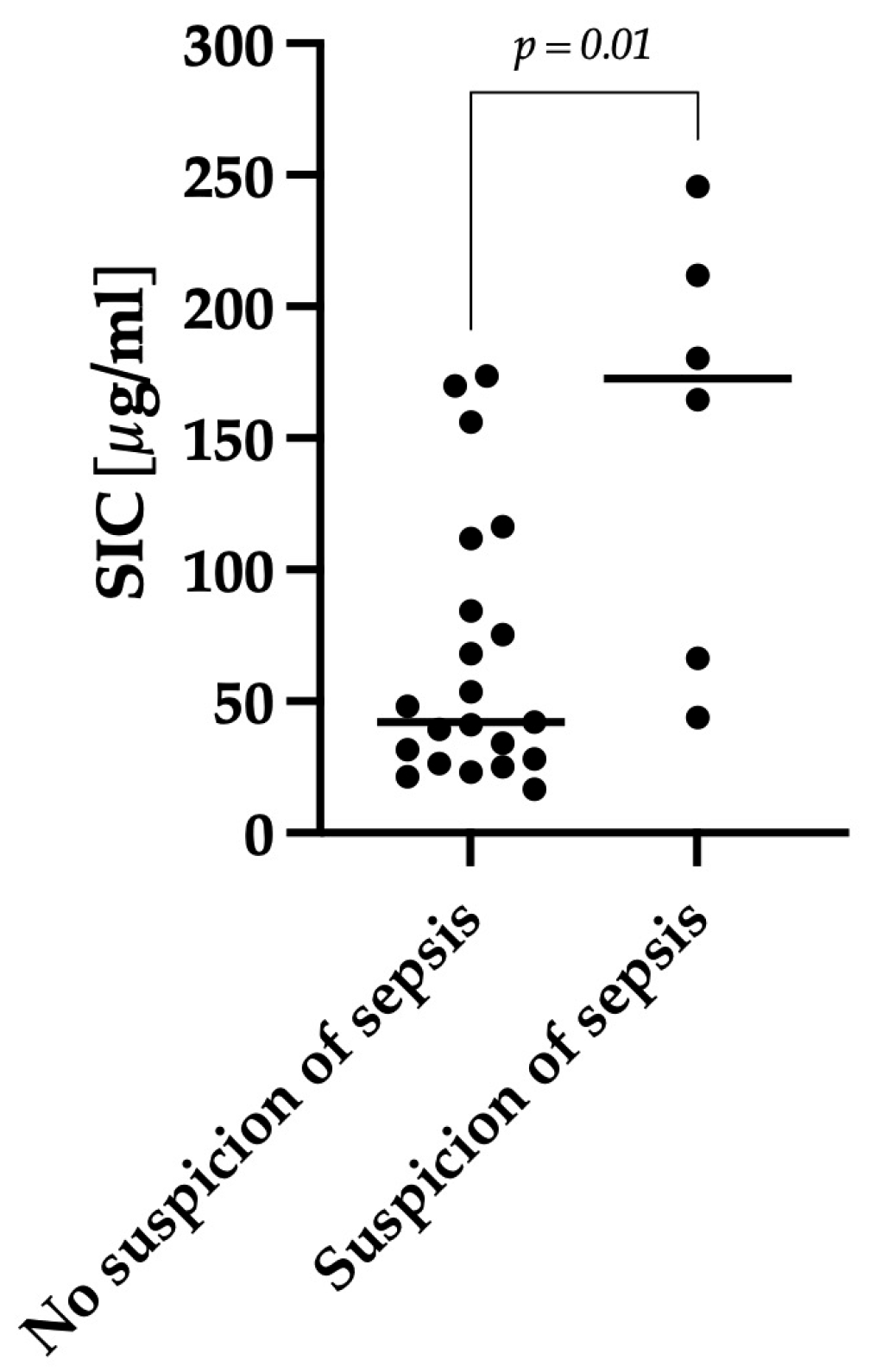
3.3. Correlation between Clinical Severity and Serum Iohexol Concentration
3.4. Antibiotic Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konig, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Deitch, E.A. Nutrition and the gut mucosal barrier. Curr. Opin. Gen. Surg. 1993, 85–91. Available online: https://europepmc.org/article/med/7584019 (accessed on 1 March 2024).
- Unterer, S.H.K. Akuter blutiger Durchfall beim Hund—Ursachen und diagnostische Aufarbeitung. Tierarztl. Prax. Ausg. K. Kleintiere Heimtiere 2009, 37, 261–268. [Google Scholar]
- Unterer, S.; Busch, K.; Leipig, M.; Hermanns, W.; Wolf, G.; Straubinger, R.K.; Mueller, R.S.; Hartmann, K. Endoscopically visualized lesions, histologic findings, and bacterial invasion in the gastrointestinal mucosa of dogs with acute hemorrhagic diarrhea syndrome. J. Vet. Intern. Med. 2014, 28, 52–58. [Google Scholar] [CrossRef]
- Will, K.; Nolte, I.; Zentek, J. Early enteral nutrition in young dogs suffering from haemorrhagic gastroenteritis. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2005, 52, 371–376. [Google Scholar] [CrossRef]
- Mehdizadeh Gohari, I.; Parreira, V.R.; Nowell, V.J.; Nicholson, V.M.; Oliphant, K.; Prescott, J.F. A novel pore-forming toxin in type A Clostridium perfringens is associated with both fatal canine hemorrhagic gastroenteritis and fatal foal necrotizing enterocolitis. PLoS ONE 2015, 10, e0122684. [Google Scholar] [CrossRef]
- Sindern, N.; Suchodolski, J.S.; Leutenegger, C.M.; Mehdizadeh Gohari, I.; Prescott, J.F.; Proksch, A.L.; Mueller, R.S.; Busch, K.; Unterer, S. Prevalence of Clostridium perfringens netE and netF toxin genes in the feces of dogs with acute hemorrhagic diarrhea syndrome. J. Vet. Intern. Med. 2019, 33, 100–105. [Google Scholar] [CrossRef]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome-A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef]
- Ziese, A.L.; Suchodolski, J.S.; Hartmann, K.; Busch, K.; Anderson, A.; Sarwar, F.; Sindern, N.; Unterer, S. Effect of probiotic treatment on the clinical course, intestinal microbiome, and toxigenic Clostridium perfringens in dogs with acute hemorrhagic diarrhea. PLoS ONE 2018, 13, e0204691. [Google Scholar] [CrossRef]
- Unterer, S.; Lechner, E.; Mueller, R.S.; Wolf, G.; Straubinger, R.K.; Schulz, B.S.; Hartmann, K. Prospective study of bacteraemia in acute haemorrhagic diarrhoea syndrome in dogs. Vet. Rec. 2015, 176, 309. [Google Scholar] [CrossRef]
- Gonzalez, L.M.; Moeser, A.J.; Blikslager, A.T. Animal models of ischemia-reperfusion-induced intestinal injury: Progress and promise for translational research. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G63–G75. [Google Scholar] [CrossRef]
- Mortier, F.; Strohmeyer, K.; Hartmann, K.; Unterer, S. Acute haemorrhagic diarrhoea syndrome in dogs: 108 cases. Vet. Rec. 2015, 176, 627. [Google Scholar] [CrossRef] [PubMed]
- Travis, S.; Menzies, I. Intestinal permeability: Functional assessment and significance. Clin. Sci. 1992, 82, 471–488. [Google Scholar] [CrossRef] [PubMed]
- Klenner, S.; Coenen, M.; Failing, K.; Hewicker-Trautwein, M.; Ternes, W.; Verspohl, J.; Spillmann, T. Estimation of intestinal permeability in healthy dogs using the contrast medium iohexol. Vet. Clin. Pathol. 2009, 38, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Frias, R.; Strube, K.; Ternes, W.; Collado, M.C.; Spillmann, T.; Sankari, S.; Westermarck, E. Comparison of 51chromium-labeled ethylenediamine tetra-acetic acid and iohexol as blood markers for intestinal permeability testing in Beagle dogs. Vet. J. 2012, 192, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.S.; Willard, M.D.; Williamson, K.K.; Steiner, J.M.; Williams, D.A. Sustained strenuous exercise increases intestinal permeability in racing Alaskan sled dogs. J. Vet. Intern. Med. 2005, 19, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.M.; Williams, D.A.; Moeller, E.M. Kinetics of urinary recovery of five sugars after orogastric administration in healthy dogs. Am. J. Vet. Res. 2002, 63, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Meddings, J.B.; Sutherland, L.R.; Byles, N.I.; Wallace, J.L. Sucrose: A novel permeability marker for gastroduodenal disease. Gastroenterology 1993, 104, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Ortín-Piqueras, V.S.T.; Pöytäkangas, M.; Vaccaro, D.E.; Sankari, S.; Frías, R. Determination of iohexol in canine plasma—Strong correlation between enzyme-linked immunosorbent assay, high- performance liquid chromatography, and neutron activation analysis. Scand. J. Lab. Anim. Sci. 2018, 44, 1–7. [Google Scholar]
- Busch, K.; Suchodolski, J.S.; Kuhner, K.A.; Minamoto, Y.; Steiner, J.M.; Mueller, R.S.; Hartmann, K.; Unterer, S. Clostridium perfringens enterotoxin and Clostridium difficile toxin A/B do not play a role in acute haemorrhagic diarrhoea syndrome in dogs. Vet. Rec. 2015, 176, 253. [Google Scholar] [CrossRef]
- Chu, V.; Goggs, R.; Bichoupan, A.; Radhakrishnan, S.; Menard, J. Hypophosphatemia in Dogs With Presumptive Sepsis: A Retrospective Study (2008–2018). Front. Vet. Sci. 2021, 8, 636732. [Google Scholar] [CrossRef]
- Dupont, N.; Jessen, L.R.; Moberg, F.; Zyskind, N.; Lorentzen, C.; Bjørnvad, C.R. A retrospective study of 237 dogs hospitalized with suspected acute hemorrhagic diarrhea syndrome: Disease severity, treatment, and outcome. J. Vet. Intern. Med. 2021, 35, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Hauptman, J.G.; Walshaw, R.; Olivier, N.B. Evaluation of the sensitivity and specificity of diagnostic criteria for sepsis in dogs. Vet. Surg. 1997, 26, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, X.; Andersson, R. Role of intestinal permeability in monitoring mucosal barrier function. History, methodology, and significance of pathophysiology. Dig. Surg. 1998, 15, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Skotnitzki, E.; Suchodolski, J.S.; Busch, K.; Werner, M.; Zablotski, Y.; Ballhausen, B.D.; Neuerer, F.; Unterer, S. Frequency of signs of chronic gastrointestinal disease in dogs after an episode of acute hemorrhagic diarrhea. J. Vet. Intern. Med. 2022, 36, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Melton-Celsa, A.; Mohawk, K.; Teel, L.; O’Brien, A. Pathogenesis of Shiga-toxin producing escherichia coli. Curr. Top. Microbiol. Immunol. 2012, 357, 67–103. [Google Scholar] [CrossRef] [PubMed]
- Crisan, C.V.; Hammer, B.K. The Vibrio cholerae type VI secretion system: Toxins, regulators and consequences. Environ. Microbiol. 2020, 22, 4112–4122. [Google Scholar] [CrossRef]
- Mehdizadeh Gohari, I.; Unterer, S.; Whitehead, A.E.; Prescott, J.F. NetF-producing Clostridium perfringens and its associated diseases in dogs and foals. J. Vet. Diagn. Investig. 2020, 32, 230–238. [Google Scholar] [CrossRef]
- Rummell, L.M.; Steele, M.A.; Templeman, J.R.; Yohe, T.T.; Akhtar, N.; Lambie, J.G.; Singh, P.; Asquith, T.; Verbrugghe, A.; Pearson, W.; et al. A proof of principle study investigating the effects of supplemental concentrated brewer’s yeast on markers of gut permeability, inflammation, and fecal metabolites in healthy non-challenged adult sled dogs. J. Anim. Sci. 2022, 100, skac281. [Google Scholar] [CrossRef]
- Forsgard, R.A.; Korpela, R.; Holma, R.; Lindén, J.; Frias, R.; Spillmann, T.; Österlund, P. Intestinal permeability to iohexol as an in vivo marker of chemotherapy-induced gastrointestinal toxicity in Sprague-Dawley rats. Cancer Chemother. Pharmacol. 2016, 78, 863–874. [Google Scholar] [CrossRef]
- Mutzel, W.; Speck, U. Pharmacokinetics and biotransformation of iohexol in the rat and the dog. Acta Radiol. Suppl. 1980, 362, 87–92. [Google Scholar] [CrossRef]
- Lasser, E.C.; Farr, R.S.; Fujimagari, T.; Tripp, W.N. The significance of protein binding of contrast media in roentgen diagnosis. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1962, 87, 338–360. [Google Scholar] [PubMed]
- Bettmann, M.A.; Morris, T.W. Recent advances in contrast agents. Radiol. Clin. N. Am. 1986, 24, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Strube, K. Vergleichende Iohexol- und Iodbestimmung in Caninen und Equinen Serum- und Rattenurinproben Nach Oraler Verabreichung von Iohexol—Ein Beitrag zum Möglichen Einsatz von Iohexol als Marker Für Die Intestinale Permeabilität. Ph.D. Thesis, Stiftung Tierärztliche Hochschule Hannover, Hannover, Germany, 2007. [Google Scholar]
- Ortin-Piqueras, V.; Freitag, T.L.; Andersson, L.C.; Lehtonen, S.H.; Meri, S.K.; Spillmann, T.; Frias, R. Urinary Excretion of Iohexol as a Permeability Marker in a Mouse Model of Intestinal Inflammation: Time Course, Performance and Welfare Considerations. Animals 2021, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Gerova, V.A.; Stoynov, S.G.; Katsarov, D.S.; Svinarov, D.A. Increased intestinal permeability in inflammatory bowel diseases assessed by iohexol test. World J. Gastroenterol. 2011, 17, 2211–2215. [Google Scholar] [CrossRef]
- Finco, D.R.; Braselton, W.E.; Cooper, T.A. Relationship between plasma iohexol clearance and urinary exogenous creatinine clearance in dogs. J. Vet. Intern. Med. 2001, 15, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Laroute, V.; Lefebvre, H.P.; Costes, G.; Toutain, P.L. Measurement of glomerular filtration rate and effective renal plasma flow in the conscious beagle dog by single intravenous bolus of iohexol and p-aminohippuric acid. J. Pharmacol. Toxicol. Methods 1999, 41, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Baklouti, S.; Concordet, D.; Borromeo, V.; Pocar, P.; Scarpa, P.; Cagnardi, P. Population Pharmacokinetic Model of Iohexol in Dogs to Estimate Glomerular Filtration Rate and Optimize Sampling Time. Front. Pharmacol. 2021, 12, 634404. [Google Scholar] [CrossRef] [PubMed]
- Leipig-Rudolph, M.; Busch, K.; Prescott, J.F.; Mehdizadeh Gohari, I.; Leutenegger, C.M.; Hermanns, W.; Wolf, G.; Hartmann, K.; Verspohl, J.; Unterer, S. Intestinal lesions in dogs with acute hemorrhagic diarrhea syndrome associated with netF-positive Clostridium perfringens type A. J. Vet. Diagn. Investig. 2018, 30, 495–503. [Google Scholar] [CrossRef]
- Mueller, R.S.; Unterer, S. Adverse food reactions: Pathogenesis, clinical signs, diagnosis and alternatives to elimination diets. Vet. J. 2018, 236, 89–95. [Google Scholar] [CrossRef]
| 0 | 1 | 2 | 3 | |
|---|---|---|---|---|
| Activity | Normal | Mildly decreased | Moderately decreased | Severely decreased |
| Appetite | Normal | Mildly decreased | Moderately decreased | Severely decreased |
| Fecal consistency | PFS 1–2 | PFS 3–4 | PFS 5–6 | PFS 7 |
| Fecal frequency (times/day) | 1 | 2–3 | 4–5 | >5 |
| Vomiting (times/day) | 0 | 1 | 2–3 | >3 |
| Dehydration (%) | 0 | <5 | 5–10 | >10 |
| Clinical SIRS Criteria | |
|---|---|
| Hypo- or hyperthermia | <37.5 °C or >39.3 °C <99.5 °F or >102.74 °F |
| Tachycardia | >140 beats/min |
| Tachypnea | >40 breaths/min |
| Laboratory SIRS criteria | |
| Leukopenia or leukocytosis | <6 G/L or >25 G/L |
| Band neutrophils | >3% |
| Hypoglycemia | <70.2 mg/dL or <3.9 mmol/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reisinger, A.; Stübing, H.; Ishii, P.E.; Suchodolski, J.S.; Lidbury, J.A.; Busch, K.; Unterer, S. Evaluation of Intestinal Barrier Dysfunction with Serum Iohexol Concentration in Dogs with Acute Hemorrhagic Diarrhea Syndrome. Animals 2024, 14, 963. https://doi.org/10.3390/ani14060963
Reisinger A, Stübing H, Ishii PE, Suchodolski JS, Lidbury JA, Busch K, Unterer S. Evaluation of Intestinal Barrier Dysfunction with Serum Iohexol Concentration in Dogs with Acute Hemorrhagic Diarrhea Syndrome. Animals. 2024; 14(6):963. https://doi.org/10.3390/ani14060963
Chicago/Turabian StyleReisinger, Andrea, Helene Stübing, Patricia E. Ishii, Jan S. Suchodolski, Jonathan A. Lidbury, Kathrin Busch, and Stefan Unterer. 2024. "Evaluation of Intestinal Barrier Dysfunction with Serum Iohexol Concentration in Dogs with Acute Hemorrhagic Diarrhea Syndrome" Animals 14, no. 6: 963. https://doi.org/10.3390/ani14060963
APA StyleReisinger, A., Stübing, H., Ishii, P. E., Suchodolski, J. S., Lidbury, J. A., Busch, K., & Unterer, S. (2024). Evaluation of Intestinal Barrier Dysfunction with Serum Iohexol Concentration in Dogs with Acute Hemorrhagic Diarrhea Syndrome. Animals, 14(6), 963. https://doi.org/10.3390/ani14060963






