Effect of Moringa oleifera Leaf Extract on the Growth Performance, Hematology, Innate Immunity, and Disease Resistance of Nile Tilapia (Oreochromis niloticus) against Streptococcus agalactiae Biotype 2
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Moringa Leaf Aqeous Extract
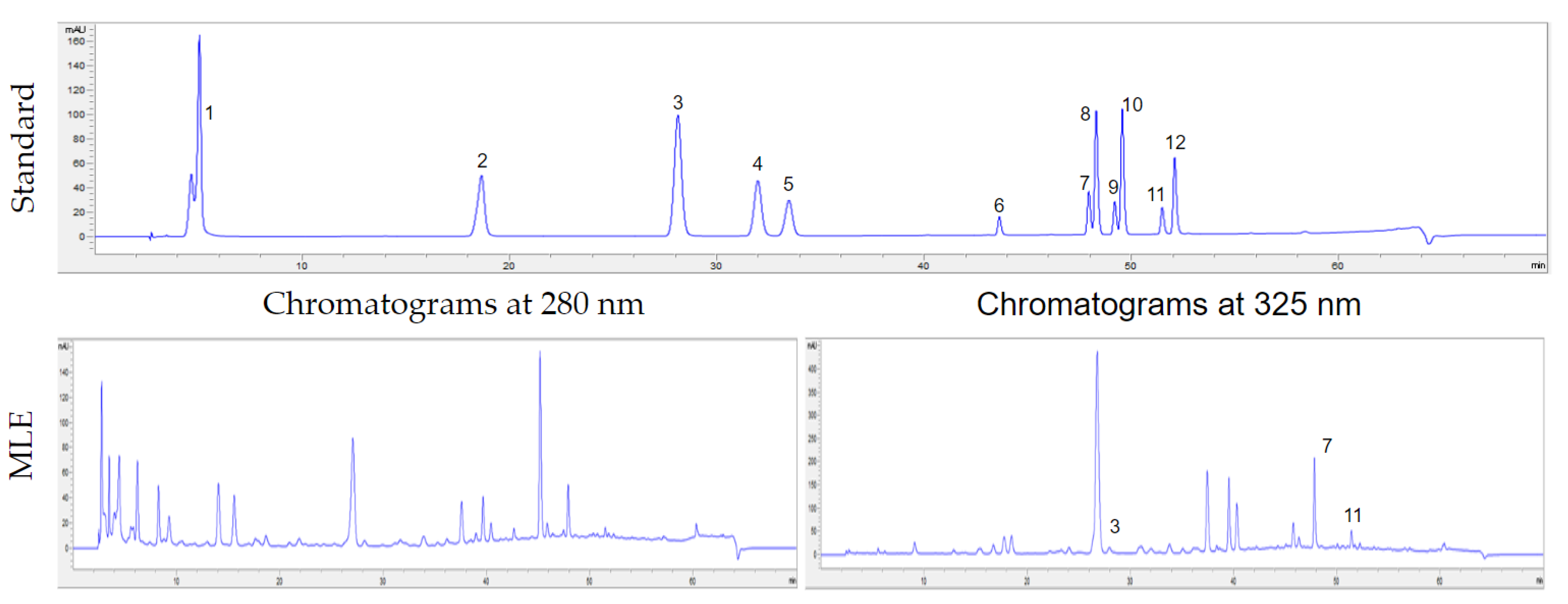
2.2. Identification of Phenolic Acids and Flavonoid Content in MLE Using High-Performance Liquid Chromatography with a Diode-Array Detection (HPLC-DAD) Analysis
2.3. Pathogen
2.4. Antimicrobial Activity of MLE
2.5. Minimum Inhibitory Concentraion (MIC) and Minimum Bactericidal Concentraion (MBC) of MLE
2.6. Fish
2.7. Preparation of the Experimental Diets Supplemented with MLE
2.8. Experimental Design
2.9. Growth Performance and Feed Utilization Efficicency of Nile Tilapia Fed Diets Supplemented with MLE
2.10. Blood and Serum Collection
2.11. Hematology Analysis of Nile Tilapia Fed Diets Supplemented with MLE
2.12. Innate Immune Paramter Analysis of Nile Tilapia Fed Diets Supplemented with MLE
2.13. Disease Resistance of Nile Tilapia Fed Diets Supplemented with MLE against S. agalctiae Biotype 2
2.14. Statistical Analysis
3. Results
3.1. Phenolic Acid and Flavonoid Content in MLE
3.2. Antimicrobial Activity
3.3. Improvement of Growth Performance and Feed Utilization Efficiency of Nile Tilapia Fed Diets Supplemented with MLE
3.4. Enhancement of the Hematological Parameter of Nile Tilapia Fed Diets Supplemented with MLE
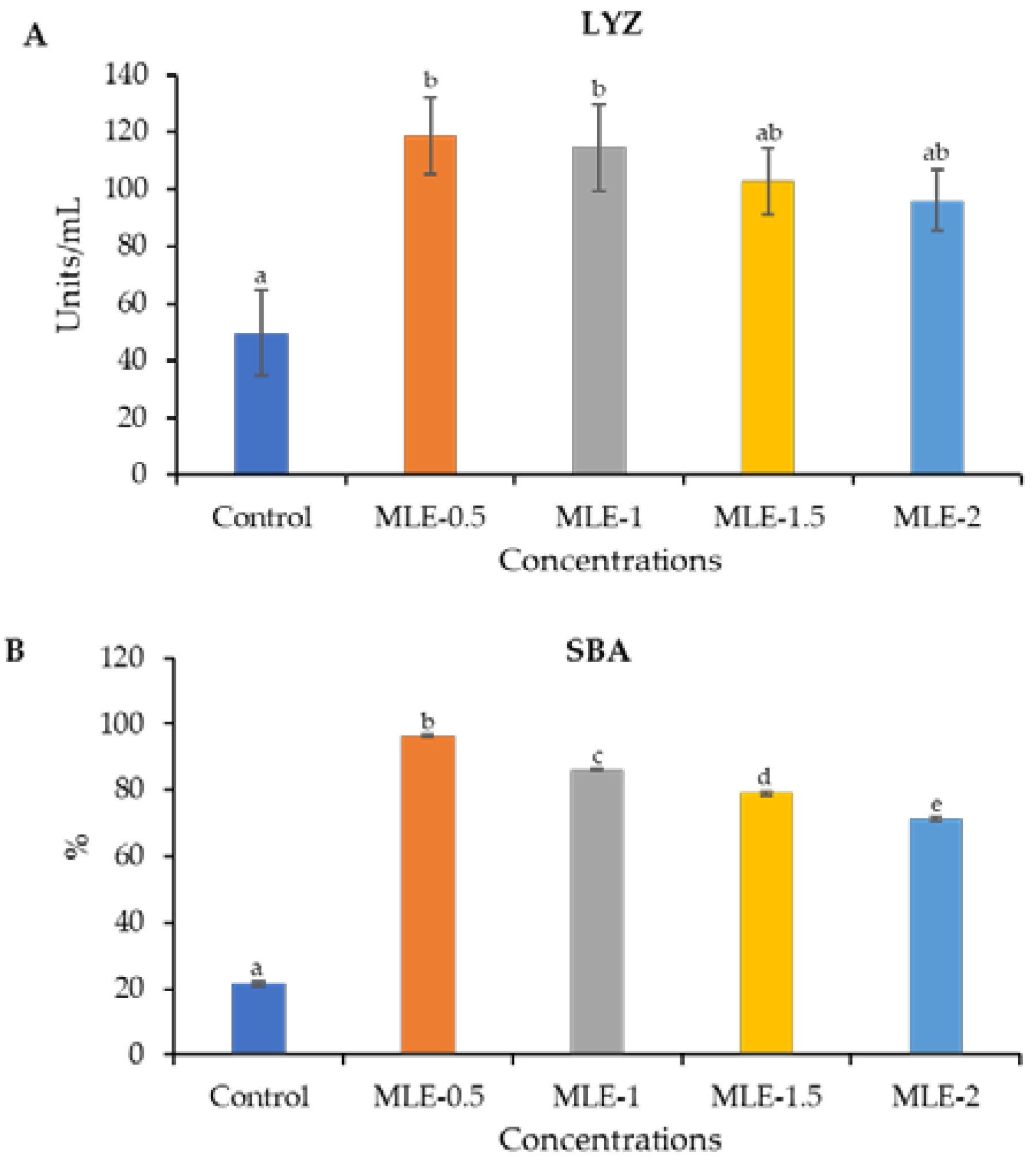
3.5. Augmentaion of the Innate Immune Response of Nile Tilapia Fed Diets Supplemented with MLE
3.6. Improvement of the Survival Rate of Nile Tilapia Fed Diets Supplemented with MLE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, G.; Lin, H.; Li, J. Tilapia is the fish for next-generation aquaculture. Int. J. Mar. Sci. Ocean. Technol. 2016, 3, 11–13. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2022—Towards Blue Transformation; FAO: Rome, Italy, 2022; p. 266. [Google Scholar]
- Nakharuthai, C.; Areechon, N.; Srisapoome, P. Molecular characterization, functional analysis, and defense mechanisms of two CC chemokines in Nile tilapia (Oreochromis niloticus) in response to severely pathogenic bacteria. Dev. Comp. Immunol. 2016, 59, 207–228. [Google Scholar] [CrossRef]
- Kamble, M.T.; Yakupitiyage, A.; Salin, K.R.; Chavan, B.R. Effect of Psidium guajava and Phyllanthus acidus leaf extract on immunostimulant response of Nile tilapia against Streptococcus agalactiae infection. Isr. J. Aquac. 2018, 70, 1–9. [Google Scholar] [CrossRef]
- Van Doan, H.; Soltani, M.; Leitão, A.; Shafiei, S.; Asadi, S.; Lymbery, A.J.; Ringø, E. Streptococcosis a Re-emerging disease in aquaculture: Significance and phytotherapy. Animals 2022, 12, 2443. [Google Scholar] [CrossRef]
- Delannoy, C.M.; Zadoks, R.N.; Crumlish, M.; Rodgers, D.; Lainson, F.A.; Ferguson, H.; Turnbull, J.; Fontaine, M.C. Genomic comparison of virulent and non-virulent Streptococcus agalactiae in fish. J. Fish Dis. 2016, 39, 13–29. [Google Scholar] [CrossRef]
- Kannika, K.; Pisuttharachai, D.; Srisapoome, P.; Wongtavatchai, J.; Kondo, H.; Hirono, I.; Unajak, S.; Areechon, N. Molecular serotyping, virulence gene profiling and pathogenicity of Streptococcus agalactiae isolated from tilapia farms in Thailand by multiplex PCR. J. Appl. Microbiol. 2017, 122, 1497–1507. [Google Scholar] [CrossRef]
- Sheehan, B.; Labrie, L.; Lee, Y.; Wong, F.; Chan, J.; Komar, C.; Wendover, N.; Grisez, L. Streptococcosis in tilapia-vaccination effective against main strep species. Glob. Aquac. Advocate 2009, 5, 72–74. [Google Scholar]
- Amal, M.; Zamri-Saad, M. Streptococcosis in tilapia (Oreochromis niloticus): A review. Pertanika J. Trop. Agric. Sci. 2011, 34, 195–206. [Google Scholar]
- Phuoc, N.N.; Linh, N.T.H.; Crestani, C.; Zadoks, R.N. Effect of strain and enviromental conditions on the virulence of Streptococcus agalactiae (Group B Streptococcus; GBS) in red tilapia (Oreochromis sp.). Aquaculture 2021, 534, 736256. [Google Scholar] [CrossRef]
- Abidin, Z.; Huang, H.-T.; Liao, Z.-H.; Chen, B.-Y.; Wu, Y.-S.; Lin, Y.-J.; Nan, F.-H. Moringa oleifera leaves’ extract enhances nonspecific immune responses, resistance against Vibrio alginolyticus, and growth in whiteleg shrimp (Penaeus vannamei). Animals 2021, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Elumalai, P.; Kurian, A.; Lakshmi, S.; Faggio, C.; Esteban, M.A.; Ringø, E. Herbal immunomodulators in aquaculture. Rev. Fish. Sci. Aquac. 2020, 29, 33–57. [Google Scholar] [CrossRef]
- Mahfuz, S.; Piao, X.S. Application of Moringa (Moringa oleifera) as natural feed supplement in poultry diets. Animals 2019, 9, 431. [Google Scholar] [CrossRef]
- Coz-Bolaños, X.; Campos-Vega, R.; Reynoso-Camacho, R.; Ramos-Gómez, M.; Loarca-Piña, G.F.; Guzmán-Maldonado, S. Moringa infusion (Moringa oleifera) rich in phenolic compounds and high antioxidant capacity attenuate nitric oxide pro-inflammatory mediator in vitro. Ind. Crops Prod. 2018, 118, 95–101. [Google Scholar] [CrossRef]
- Ma, Z.; Ahmad, J.; Zhang, H.; Khan, I.; Muhammad, S. Evaluation of phytochemical and medicinal properties of Moringa (Moringa oleifera) as a potential functional food. South Afr. J. Bot. 2020, 129, 40–46. [Google Scholar] [CrossRef]
- Mwamatope, B.; Tembo, D.; Chikowe, I.; Kampira, E.; Nyirenda, C. Total phenolic contents and antioxidant activity of Senna singueana, Melia azedarach, Moringa oleifera and Lannea discolor herbal plants. Sci. Afr. 2020, 9, e00481. [Google Scholar] [CrossRef]
- Kamble, M.T.; Chavan, B.R.; Ataguba, G.; Azpeitia, T.; Medhe, S.; Jain, S.; Jadhav, R. Application of Moringa oleifera for development of sustainable and biosecure aquaculture. Aquac. Indones. 2015, 15, 64–73. [Google Scholar] [CrossRef]
- Abd El-Gawad, E.A.; El Asely, A.M.; Soror, E.I.; Abbass, A.A.; Austin, B. Effect of dietary Moringa oleifera leaf on the immune response and control of Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus) fry. Aquac. Int. 2020, 28, 389–402. [Google Scholar] [CrossRef]
- Elabd, H.; Soror, E.; El-Asely, A.; Abd El-Gawad, E.; Abbass, A. Dietary supplementation of Moringa leaf meal for Nile tilapia Oreochromis niloticus: Effect on growth and stress indices. Egypt. J. Aquat. Res. 2019, 45, 265–271. [Google Scholar] [CrossRef]
- Chen, S.; Jia, Y.; Xu, W.; Peng, J.; He, Y.; Sun, J.; Pan, Q.; Peng, C.; Yang, J.; Chen, X. Effect of Moringa oleifera leaf meal supplementation on growth performance, morphological indexes, antioxidant status and resistance to Streptococcus agalactiae of Nile tilapia (Oreochromis niloticus). Turk. J. Fish. Aquat. Sci. 2020, 21, 29–39. [Google Scholar] [CrossRef]
- Emam, M.A.; Shourbela, R.M.; El-Hawarry, W.N.; Abo-Kora, S.Y.; Gad, F.A.-M.; Abd El-latif, A.M.; Dawood, M.A. Effects of Moringa oleifera aqueous extract on the growth performance, blood characteristics, and histological features of gills and livers in Nile tilapia. Aquac. Fish. 2024, 9, 85–92. [Google Scholar] [CrossRef]
- El-Kassas, S.; Abdo, S.E.; Abosheashaa, W.; Mohamed, R.; Moustafa, E.M.; Helal, M.A.; El-Naggar, K. Growth performance, serum lipid profile, intestinal morphometry, and growth and lipid indicator gene expression analysis of mono-sex Nile tilapia fed Moringa oleifera leaf powder. Aquac. Rep. 2020, 18, 100422. [Google Scholar] [CrossRef]
- Hamed, H.S.; El-Sayed, Y.S. Antioxidant activities of Moringa oleifera leaf extract against pendimethalin-induced oxidative stress and genotoxicity in Nile tilapia, Oreochromis niloticus (L.). Fish Physiol. Biochem. 2019, 45, 71–82. [Google Scholar] [CrossRef]
- El-Son, M.A.; Hendam, B.M.; Nofal, M.I.; Abdel-Latif, H.M. Effects of Moringa oleifera-based diets on growth, immunological responses, liver antioxidant biomarkers and expression of immune-related genes in Nile tilapia (Oreochromis niloticus) raised in hapa-in-pond system. Aquac. Res. 2022, 53, 4338–4352. [Google Scholar] [CrossRef]
- Mahmoud, H.K.; Farag, M.R.; Reda, F.M.; Alagawany, M.; Abdel-Latif, H.M. Dietary supplementation with Moringa oleifera leaves extract reduces the impacts of sub-lethal fipronil in Nile tilapia, Oreochromis niloticus. Sci. Rep. 2022, 12, 21748. [Google Scholar] [CrossRef]
- Ahmed, N.F.; Sadek, K.M.; Soliman, M.K.; Khalil, R.H.; Khafaga, A.F.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A. Moringa oleifera leaf extract repairs the oxidative misbalance following sub-chronic exposure to sodium fluoride in nile tilapia Oreochromis niloticus. Animals 2020, 10, 626. [Google Scholar] [CrossRef]
- Momin, M.; Memiş, D. Potential use of the miracle tree (Moringa oleifera) leaves in aquaculture: A recent update. Aquat. Sci. Eng. 2023, 38, 122–130. [Google Scholar] [CrossRef]
- Kamble, M.T.; Gallardo, W.; Yakupitiyage, A.; Chavan, B.R.; Rusydi, R.; Rahma, A. Antimicrobial activity of bioactive herbal extracts against Streptococcus agalactiae biotype 2. Int. J. Basic Appl. Biol. 2014, 2, 152–155. [Google Scholar]
- Merken, H.M.; Beecher, G.R. Liquid chromatographic method for the separation and quantification of prominent flavonoid aglycones. J. Chromatogr. A 2000, 897, 177–184. [Google Scholar] [CrossRef]
- Judprasong, K.; Charoenkiatkul, S.; Thiyajai, P.; Sukprasansap, M. Nutrients and bioactive compounds of Thai indigenous fruits. Food Chem. 2013, 140, 507–512. [Google Scholar] [CrossRef]
- Kamble, M.T.; Rudtanatip, T.; Soowannayan, C.; Nambunruang, B.; Medhe, S.V.; Wongprasert, K. Depolymerized fractions of sulfated galactans extracted from Gracilaria fisheri and their antibacterial activity against Vibrio parahaemolyticus and Vibrio harveyi. Mar. Drugs 2022, 20, 469. [Google Scholar] [CrossRef]
- Berche, P.; Gaillard, J.; Simonet, M. Nosocomial Infections Caused by Bacteria and Their Prevention in Bacteriology. Ed Flammarion Med. Sci. 1988, 64–71. [Google Scholar]
- National Research Council. Nutrient Requirements of Fish and Shrimp; The National Academies Press: Washington, DC, USA, 2011; p. 392. [Google Scholar]
- Mane, A.M.; Dube, K.; Varghese, T.; Chavan, B.R.; Kamble, M.T. Effects of stocking density on growth performance, survival and production of Catla catla and Labeo rohita during nursery rearing in cages. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 275–281. [Google Scholar] [CrossRef]
- Sukkarun, P.; Kitiyodom, S.; Kamble, M.T.; Bunnoy, A.; Boonanuntanasarn, S.; Yata, T.; Boonrungsiman, S.; Thompson, K.D.; Rodkhum, C.; Pirarat, N. Systemic and mucosal immune responses in red tilapia (Oreochromis sp.) following immersion vaccination with a chitosan polymer-based nanovaccine against Aeromonas veronii. Fish Shellfish. Immunol. 2024, 146, 109383. [Google Scholar] [CrossRef]
- Suwanbumrung, D.; Wongkhieo, S.; Keaswejjareansuk, W.; Dechbumroong, P.; Kamble, M.T.; Yata, T.; Kitiyodom, S.; Rodkhum, C.; Thompson, K.D.; Namdee, K. Oral delivery of a Streptococcus agalactiae vaccine to Nile tilapia (Oreochromis niloticus) using a novel cationic-based nanoemulsion containing bile salts. Fish Shellfish. Immunol. 2023, 139, 108913. [Google Scholar] [CrossRef]
- Amend, D.F. Potency testing of fish vaccines. Fish Biol. Serodiagn. Vaccines 1981, 49, 447–454. [Google Scholar]
- Hussain, K.; Draz, S.N.; Khalil, S.M.; Ebrahiem, A. Estimation of total phenolic, flavonoid contents and in vitro antioxidant activity of Moringa oleifera variety grown in Egypt. Menoufia J. Agric. Biotechnol. 2017, 2, 15–24. [Google Scholar] [CrossRef]
- Dhibi, M.; Amri, Z.; Bhouri, A.M.; Hammami, S.; Hammami, M. Comparative study of the phenolic profile and antioxidant activities of Moringa (Moringa oleifera Lam.) and Jujube (Ziziphus lotus Linn.) leaf extracts and their protective effects in frying stability of corn oil. Meas. Food 2022, 7, 100045. [Google Scholar] [CrossRef]
- Abo El-Fadl, S.; Osman, A.; Al-Zohairy, A.; Dahab, A.A.; Abo El Kheir, Z.A. Assessment of total phenolic, flavonoid content, antioxidant potential and hplc profile of three moringa species leaf extracts. Sci. J. Flowers Ornam. Plants 2020, 7, 53–70. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Prasad, V.G.N.V.; Swamy, P.L.; Rao, T.S.; Rao, G.S. Antibacterial synergy between quercetin and polyphenolic acids against bacterial pathogens of fish. Asian Pac. J. Trop. Dis. 2014, 4, S326–S329. [Google Scholar] [CrossRef]
- Cruz, B.G.; Dos Santos, H.S.; Bandeira, P.N.; Rodrigues, T.H.S.; Matos, M.G.C.; Nascimento, M.F.; de Carvalho, G.G.; Braz-Filho, R.; Teixeira, A.M.; Tintino, S.R. Evaluation of antibacterial and enhancement of antibiotic action by the flavonoid kaempferol 7-O-β-D-(6 ″-O-cumaroyl)-glucopyranoside isolated from Croton piauhiensis müll. Microb. Pathog. 2020, 143, 104144. [Google Scholar] [CrossRef]
- Abdelhiee, E.Y.; Elbialy, Z.I.; Saad, A.H.; Dawood, M.A.; Aboubakr, M.; El-Nagar, S.H.; El-Diasty, E.M.; Salah, A.S.; Saad, H.M.; Fadl, S.E. The impact of Moringa oleifera on the health status of Nile tilapia exposed to aflatoxicosis. Aquaculture 2021, 533, 736110. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Kalhor, N.; Dawood, M.A.; Ahmadifar, M.; Shahriari Moghadam, M.; Yousefi, M. Effects of dietary p-coumaric acid on the growth performance, digestive enzyme activity, humoral immunity and immune-related gene expression in common carp, Cyprinus carpio. Aquac. Nutr. 2021, 27, 747–756. [Google Scholar] [CrossRef]
- Kong, Y.; Tian, J.; Niu, X.; Li, M.; Kong, Y.; Li, R.; Chen, X.; Wang, G. Effects of dietary quercetin on growth, antioxidant capacity, immune response and immune-related gene expression in snakehead fish, Channa argus. Aquac. Rep. 2022, 26, 101314. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, H.; Li, X.; Xu, X.; Tan, H.; Leng, X. Dietary supplementation of kaempferol improved the growth, lipid metabolism and flesh quality of juvenile grass carp (Ctenopharyngodon idellus) based on metabolomics. Anim. Feed. Sci. Technol. 2023, 295, 115520. [Google Scholar] [CrossRef]
- Makkar, H.; Becker, K. Nutritional value and antinutritional components in different parts of Moringa oleifera tree. J. Agric. Sci. 1997, 128, 311–322. [Google Scholar] [CrossRef]
- Giner-Chavez, B.I. Condensed Tannins in Tropical Forages; Cornell University: Ithaca, NY, USA, 1996. [Google Scholar]
- Richardson, N.L.; Higgs, D.A.; Beames, R.M.; McBride, J.R. Influence of dietary calcium, phosphorus, zinc and sodium phytate level on cataract incidence, growth and histopathology in juvenile chinook salmon (Oncorhynchus tshawytscha). J. Nutr. 1985, 115, 553–567. [Google Scholar] [CrossRef]
- Spinelli, J.; Houle, C.R.; Wekell, J.C. The effect of phytates on the growth of rainbow trout (Salmo gairdneri) fed purified diets containing varying quantities of calcium and magnesium. Aquaculture 1983, 30, 71–83. [Google Scholar] [CrossRef]
- Hossain, M.; Jauncey, K. The effects of varying dietary phytic acid, calcium and magnesium levels on the nutrition of common carp, Cyprinus carpio. In Proceedings of the Fish Nutrition in Practice. Proceedings of International Conference, Biarritz, France, 24–27 June 1991; UNDP/FAO: Rome, Italy, 1993; pp. 705–715. [Google Scholar]
- Dongmeza, E.; Siddhuraju, P.; Francis, G.; Becker, K. Effects of dehydrated methanol extracts of moringa (Moringa oleifera Lam.) leaves and three of its fractions on growth performance and feed nutrient assimilation in Nile tilapia (Oreochromis niloticus (L.)). Aquaculture 2006, 261, 407–422. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Lionetti, L.; Faggio, C. Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci. Total Environ. 2019, 670, 1170–1183. [Google Scholar] [CrossRef]
- Sherif, A.H.; Adel, M.; Adel, E. Incorporation of Moringa oleifera leaf in Nile tilapia Oreochromis niloticus diet and its effect on growth performance and immune status. J. Vet. Sci. 2014, 1, 806–814. [Google Scholar]
- Mbokane, E.M.; Moyo, N.A.G. Effects of dietary levels of essential oil extracts from Moringa oleifera and Artemisia afra on kidney histology, haemato-immunological parameters and disease resistance in Clarias gariepinus. Aquac. Res. 2020, 51, 410–425. [Google Scholar] [CrossRef]
- Nasirin, C.; Najm, M.A.; Chen, T.-C.; Dhamija, A.; Lionardo, A.; Bokov, D.O.; Shahbazi Naserabad, S. The protective effects of quercetin on the physiological responses in malathion-exposed common carp, Cyprinus carpio. Trop. Anim. Health Prod. 2023, 55, 22. [Google Scholar] [CrossRef]
- Eyiwunmi, F.; Augustine, O.; Ovie, W. The hematological parameters of catfish (Clarias gariepinus) fed fish feeds with replaced premix using moringa leaf meal (MLM). J. Aquac. Res. Dev. 2018, 2, 35–39. [Google Scholar]
- Stadtlander, T.; Sander, C.; Kumar, V.; Makkar, H.; Becker, K. Effects of Moringa oleifera Lam. dietary seed protein extracts on growth, nutrient utilization and blood parameters in common carp (Cyprinus carpio, L.) and Nile tilapia (Oreochromis niloticus, L.). Planta Medica 2013, 79, PL21. [Google Scholar] [CrossRef]
- Hrubec, T.C.; Cardinale, J.L.; Smith, S.A. Hematology and plasma chemistry reference intervals for cultured tilapia (Oreochromis hybrid). Vet. Clin. Pathol. 2000, 29, 7–12. [Google Scholar] [CrossRef]
- Misra, S.; Sahu, N.; Pal, A.; Xavier, B.; Kumar, S.; Mukherjee, S. Pre-and post-challenge immuno-haematological changes in Labeo rohita juveniles fed gelatinised or non-gelatinised carbohydrate with n-3 PUFA. Fish Shellfish. Immunol. 2006, 21, 346–356. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Ayoub, H.F.; El Tantawy, M.M.; Abdel-Latif, H.M. Influence of moringa (Moringa oleifera) and rosemary (Rosmarinus officinalis), and turmeric (Curcuma longa) on immune parameters and challenge of Nile tilapia to Aeromonas hydrophila. Life Sci. J. 2019, 16, 8–15. [Google Scholar]
- Kamble, M.T.; Chaiyapechara, S.; Salin, K.R.; Bunphimpapha, P.; Chavan, B.R.; Bhujel, R.C.; Medhe, S.V.; Kettawan, A.; Thiyajai, P.; Thompson, K.D.; et al. Guava and Star gooseberry leaf extracts improve growth performance, innate immunity, intestinal microbial community, and disease resistance in Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Aquac. Rep. 2024, 35, 101947. [Google Scholar] [CrossRef]
- Biller, J.D.; Polycarpo, G.d.V.; Moromizato, B.S.; Sidekerskis, A.P.D.; Silva, T.D.d.; Reis, I.C.d.; Fierro-Castro, C. Lysozyme activity as an indicator of innate immunity of tilapia (Oreochromis niloticus) when challenged with LPS and Streptococcus agalactiae. Rev. Bras. Zootec. 2021, 50, e20210053. [Google Scholar] [CrossRef]
- Ellis, A. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 2001, 25, 827–839. [Google Scholar] [CrossRef]
- Awad, E.; Austin, D.; Lyndon, A.R. Effect of black cumin seed oil (Nigella sativa) and nettle extract (Quercetin) on enhancement of immunity in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture 2013, 388, 193–197. [Google Scholar] [CrossRef]
- Talpur, A.D.; Ikhwanuddin, M.; Bolong, A.-M.A. Nutritional effects of ginger (Zingiber officinale Roscoe) on immune response of Asian sea bass, Lates calcarifer (Bloch) and disease resistance against Vibrio harveyi. Aquaculture 2013, 400, 46–52. [Google Scholar] [CrossRef]
- Biller-Takahashi, J.; Takahashi, L.; Pilarski, F.; Sebastião, F.; Urbinati, E. Serum bactericidal activity as indicator of innate immunity in pacu Piaractus mesopotamicus (Holmberg, 1887). Arq. Bras. Med. Veterinária Zootec. 2013, 65, 1745–1751. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Kalhor, N.; Dawood, M.A.; Ahmadifar, M.; Moghadam, M.S.; Yousefi, M. The blood and mRNA levels of antioxidant-related factors in common carp (Cyprinus carpio) fed p-Coumaric acid. Fish Physiol. Biochem. 2021, 47, 59–68. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Zhang, J.; Xie, J.; Yang, L.; Xing, Y.; Li, Z. The effects of quercetin on immunity, antioxidant indices, and disease resistance in zebrafish (Danio rerio). Fish Physiol. Biochem. 2020, 46, 759–770. [Google Scholar] [CrossRef]
- Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N.; Maita, M. Modulation of intestinal morphology and immunity in nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res. Vet. Sci. 2011, 91, e92–e97. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fan, D.; Pan, L.; Su, C.; Ding, Y.; Lu, M. Study of effects of dietary quercetin (Que) on growth performance and disease resistance mechanism of Litopenaeus vannamei. Aquaculture 2023, 563, 738887. [Google Scholar] [CrossRef]


| Treatments | Crude Protein (%) | Crude Lipid (%) | Moisture (%) | Ash (%) | NFE (%) | GE (Kcal/g) | P/E Ratio |
|---|---|---|---|---|---|---|---|
| Control | 30.43 ± 0.12 | 7.02 ± 0.10 | 8.12 ± 0.13 | 10.20 ± 0.08 | 44.22 ± 0.13 | 419.63 ± 0.15 | 72.52 ± 0.31 |
| MLE-0.5 | 32.73 ± 1.26 | 7.09 ± 0.1 | 8.15 ± 0.12 | 10.15 ± 0.02 | 41.88 ± 1.31 | 423.60 ± 1.90 | 77.25 ± 2.63 |
| MLE-1 | 33.36 ± 1.93 | 7.04 ± 0.10 | 8.23 ± 0.06 | 10.15 ± 0.03 | 41.22 ± 1.94 | 424.03 ± 3.15 | 78.66 ± 3.97 |
| MLE-1.5 | 35.20 ± 0.73 | 7.09 ± 0.11 | 8.08 ± 0.07 | 10.19 ± 0.09 | 39.44 ± 0.79 | 427.60 ± 1.00 | 82.32 ± 1.51 |
| MLE-2 | 36.00 ± 0.43 | 7.04 ± 0.09 | 8.23 ± 0.06 | 10.16 ± 0.01 | 38.58 ± 0.28 | 438.10 ± 0.90 | 84.10 ± 0.83 |
| MLE | Content | µg/100 g |
|---|---|---|
| Phenolic acid contents | Gallic acid | ND |
| Caffeic acid | ND | |
| p-Coumaric acid | 96.50 ± 3.41 | |
| Ferulic acid | ND | |
| Sinapic acid | ND | |
| Flavonoid contents | Myricetin | ND |
| Quercetin | 1397.82 ± 138.84 | |
| Kaempferol | 221.69 ± 25.57 | |
| Luteolin | ND | |
| Agpigenin | ND | |
| Naringenin | ND | |
| Hesperitin | ND |
| Parameters | Aqueous Extract | DMSO |
|---|---|---|
| Zone of Inhibition (mm) | 17.5 ± 2.3 | 0.0 ± 0.0 |
| MIC (µg/mL) | 78.13 | ND |
| MBC (µg/mL) | 78.13 | ND |
| Reaction | + | ND |
| Growth Performance | Feed Utilization Efficiency | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatments | IW (g/fish) | FW (g/fish) | WG (g/fish) | SGR (%/day) | SR (%) | FI (Kg) | MDI (g/fish/day) | FCR | PER |
| Control | 32.2 ± 0.1 | 53.6 ± 1.2 a | 21.4 ± 1.2 a | 1.70 ± 0.08 a | 93.3 ± 5.8 | 1.24 ± 0.02 | 1.37 ± 0.02 | 1.93 ± 0.09 a | 71.3 ± 4.1 a |
| MLE-0.5 | 32.4 ± 0.1 | 61.2 ± 1.2 c | 28.8 ± 1.2 b | 2.12 ± 0.07 b | 92.2 ± 6.9 | 1.25 ± 0.06 | 1.39 ± 0.07 | 1.45 ± 0.03 b | 95.9 ± 3.9 b |
| MLE-1 | 32.5 ± 0.1 | 55.3 ± 1.9 ab | 22.8 ± 1.9 a | 1.77 ± 0.11 a | 94.4 ± 5.1 | 1.24 ± 0.10 | 1.37 ± 0.11 | 1.82 ± 0.15 a | 75.9 ± 6.2 a |
| MLE-1.5 | 32.7 ± 0.2 | 55.7 ± 2.4 ab | 23.0 ± 2.5 a | 1.77 ± 0.16 a | 94.5 ± 6.9 | 1.26 ± 0.02 | 1.40 ± 0.02 | 1.84 ± 0.19 a | 76.6 ± 8.4 a |
| MLE-2 | 33.0 ± 0.1 | 57.6 ± 1.3 b | 24.6 ± 1.3 a | 1.85 ± 0.08 a | 96.7 ± 3.4 | 1.32 ± 0.04 | 1.47 ± 0.05 | 1.80 ± 0.11 a | 82.0 ± 4.4 a |
| Treatments | Control | MLE-0.5 | MLE-1 | MLE-1.5 | MLE-2 |
|---|---|---|---|---|---|
| RBC (×106 cells/µL) | 1.82 ± 0.23 a | 2.22 ± 0.27 b | 2.01 ± 0.15 ab | 1.77 ± 0.54 a | 1.86 ± 0.14 a |
| WBC (×102 cells/µL) | 8.04 ± 0.16 ab | 9.83 ± 0.61 b | 8.43 ± 0.32 ab | 9.05 ± 0.31 b | 8.36 ± 0.36 a |
| Hb (g/dL) | 6.51 ± 0.52 a | 9.05 ± 0.61 b | 7.10 ± 0.72 ab | 6.00 ± 2.27 a | 6.66 ± 0.46 a |
| Hct (%) | 24.78 ± 4.05 a | 30.01 ± 3.66 b | 27.96 ± 2.52 a | 26.38 ± 9.87 a | 28.61 ± 2.09 ab |
| MCV (fl) | 138.6 ± 2.73 a | 154.2 ± 5.08 b | 146.1 ± 6.66 ab | 149 ± 8.58 ab | 146.6 ±12.10 ab |
| MCH (pg) | 32.85 ± 3.61 a | 38.15 ± 1.75 b | 35.63 ± 0.75 ab | 35.16 ± 0.96 ab | 34.8 ± 0.85 ab |
| MCHC (%) | 24.06 ± 1.87 abc | 26.18 ± 2.14 c | 25.4 ± 0.30 bc | 22.4 ± 1.13 a | 22.61 ± 1.31 ab |
| Treatments | Mortality Rate (%) | Survival Rate (%) | RPS (%) |
|---|---|---|---|
| Control | 66.7 ± 8.8 a | 33.3 ± 8.8 a | - |
| MLE-0.5 | 10.0 ± 0.0 b | 90.0 ± 0.0 b | 84.4 ± 2.3 a |
| MLE-1 | 16.7 ± 6.7 bc | 83.3 ± 6.7 bc | 74.9 ± 9.1 ab |
| MLE-1.5 | 23.3 ± 3.3 bc | 76.7 ± 3.3 bc | 64.0 ± 5.5 bc |
| MLE-2 | 33.3 ± 6.7 c | 66.7 ± 6.7 c | 51.0 ± 5.0 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamble, M.T.; Gallardo, W.; Salin, K.R.; Pumpuang, S.; Chavan, B.R.; Bhujel, R.C.; Medhe, S.V.; Kettawan, A.; Thompson, K.D.; Pirarat, N. Effect of Moringa oleifera Leaf Extract on the Growth Performance, Hematology, Innate Immunity, and Disease Resistance of Nile Tilapia (Oreochromis niloticus) against Streptococcus agalactiae Biotype 2. Animals 2024, 14, 953. https://doi.org/10.3390/ani14060953
Kamble MT, Gallardo W, Salin KR, Pumpuang S, Chavan BR, Bhujel RC, Medhe SV, Kettawan A, Thompson KD, Pirarat N. Effect of Moringa oleifera Leaf Extract on the Growth Performance, Hematology, Innate Immunity, and Disease Resistance of Nile Tilapia (Oreochromis niloticus) against Streptococcus agalactiae Biotype 2. Animals. 2024; 14(6):953. https://doi.org/10.3390/ani14060953
Chicago/Turabian StyleKamble, Manoj Tukaram, Wenresti Gallardo, Krishna Rugmini Salin, Suntree Pumpuang, Balasaheb Ramdas Chavan, Ram Chandra Bhujel, Seema Vijay Medhe, Aikkarach Kettawan, Kim D. Thompson, and Nopadon Pirarat. 2024. "Effect of Moringa oleifera Leaf Extract on the Growth Performance, Hematology, Innate Immunity, and Disease Resistance of Nile Tilapia (Oreochromis niloticus) against Streptococcus agalactiae Biotype 2" Animals 14, no. 6: 953. https://doi.org/10.3390/ani14060953
APA StyleKamble, M. T., Gallardo, W., Salin, K. R., Pumpuang, S., Chavan, B. R., Bhujel, R. C., Medhe, S. V., Kettawan, A., Thompson, K. D., & Pirarat, N. (2024). Effect of Moringa oleifera Leaf Extract on the Growth Performance, Hematology, Innate Immunity, and Disease Resistance of Nile Tilapia (Oreochromis niloticus) against Streptococcus agalactiae Biotype 2. Animals, 14(6), 953. https://doi.org/10.3390/ani14060953









