Oregano Essential Oil as a Natural Plant Additive Affects Growth Performance and Serum Antibody Levels by Regulating the Rumen Microbiota of Calves
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Feeding Program
2.2. Nutritional Level of Feed
2.3. Sample Collection and Measurement
2.3.1. Growth Performance Measurement
2.3.2. Immunoglobulin Assay
2.3.3. Sequencing of Rumen Microorganisms
2.4. Statistics and Analysis of Data
3. Results
3.1. Effect of Oregano Essential Oil Supplementation on Calf Growth Performance
3.2. Effect of Oregano Essential Oil Supplementation on Calf Serum Immunoglobulin
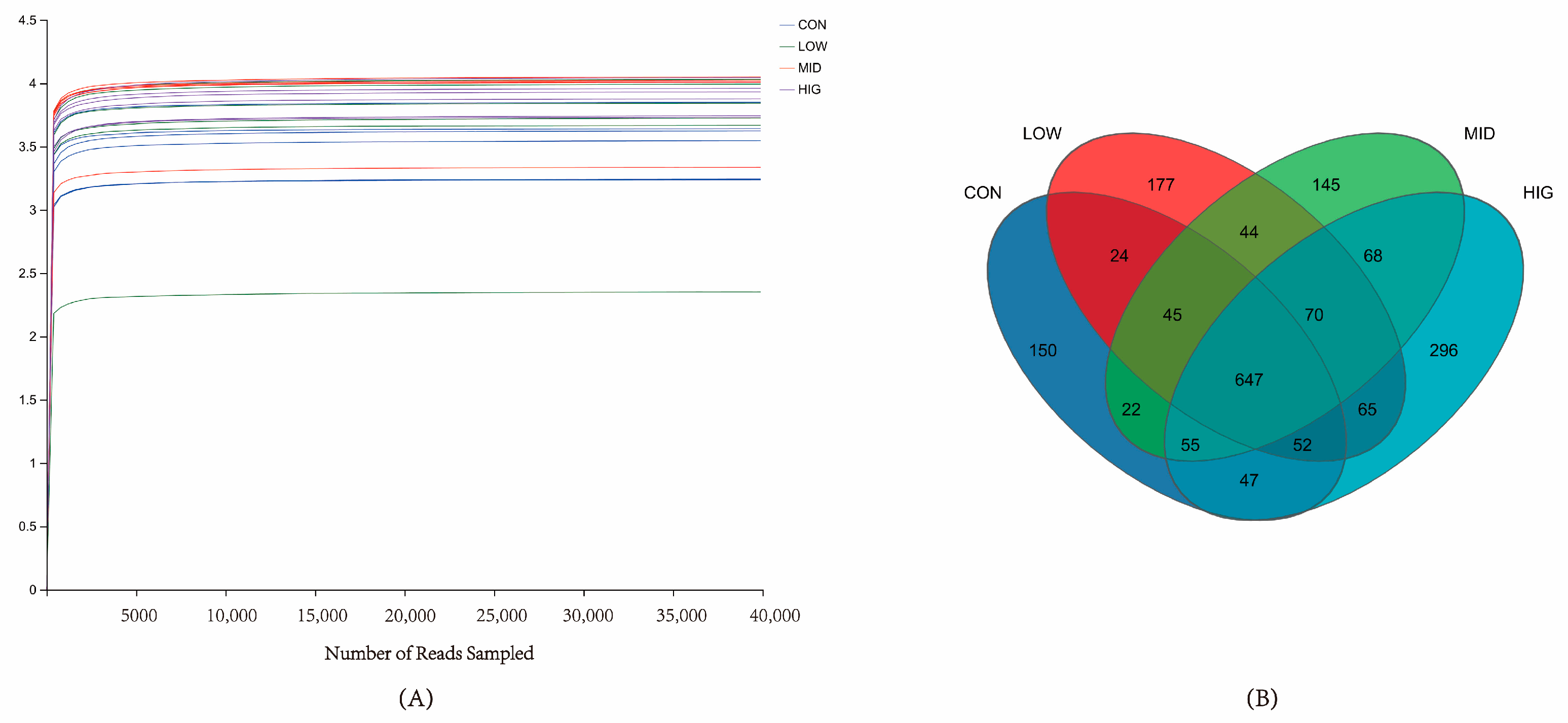
3.3. Calf Rumen Microbial Base Sequencing Results
3.4. Analysis of Rumen Microbial Diversity in Calves
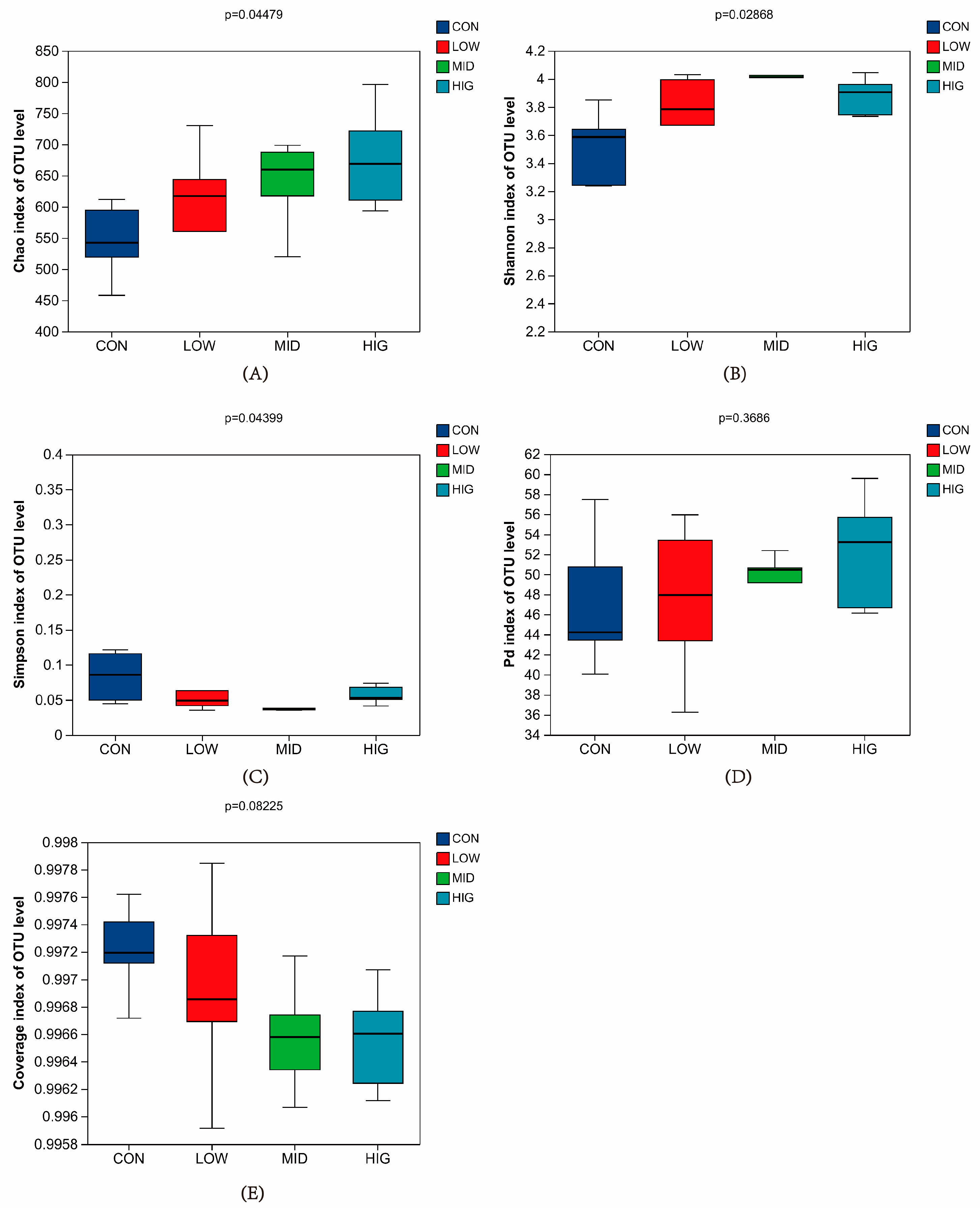
3.4.1. Alpha Diversity
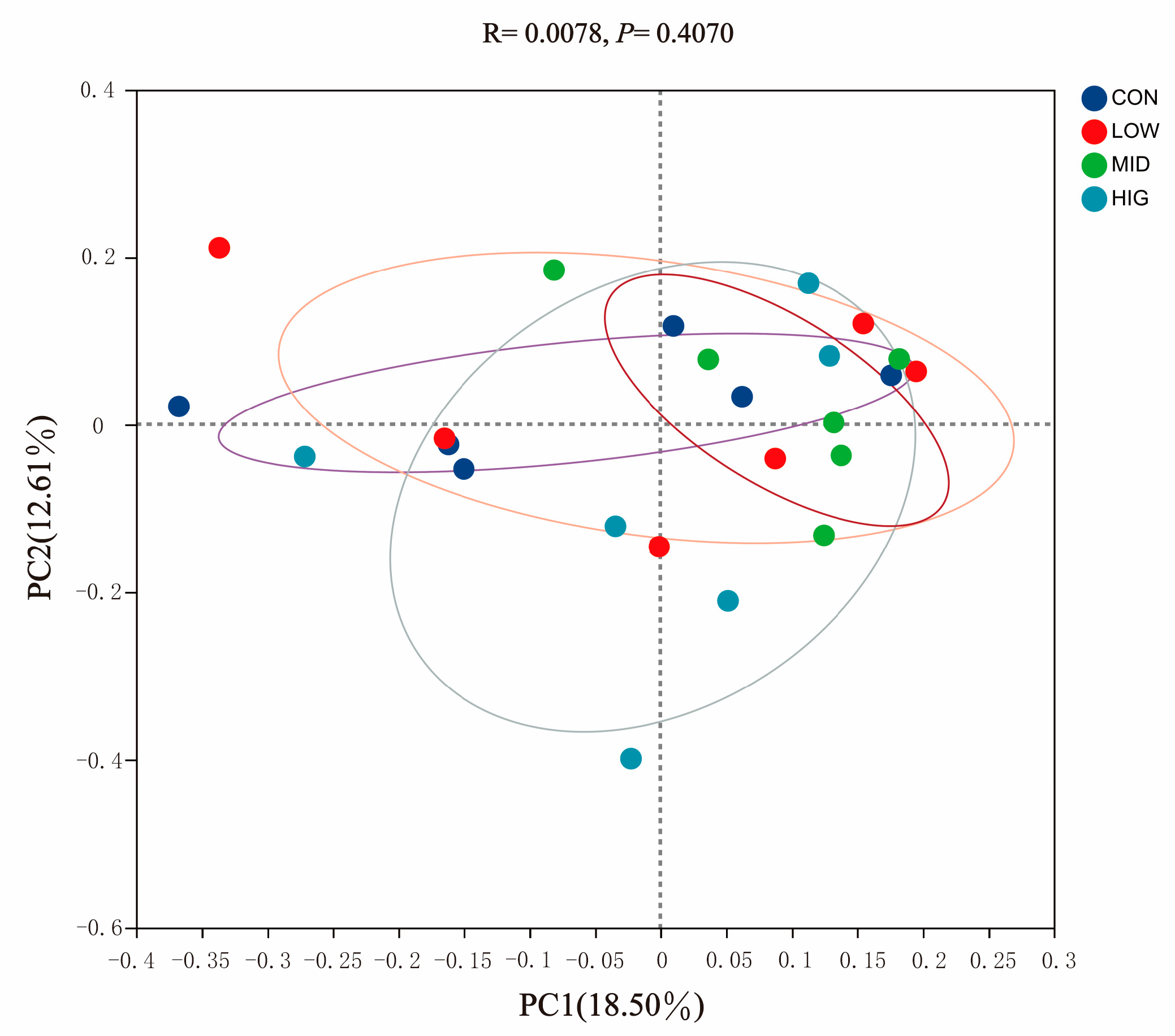
3.4.2. Beta Diversity
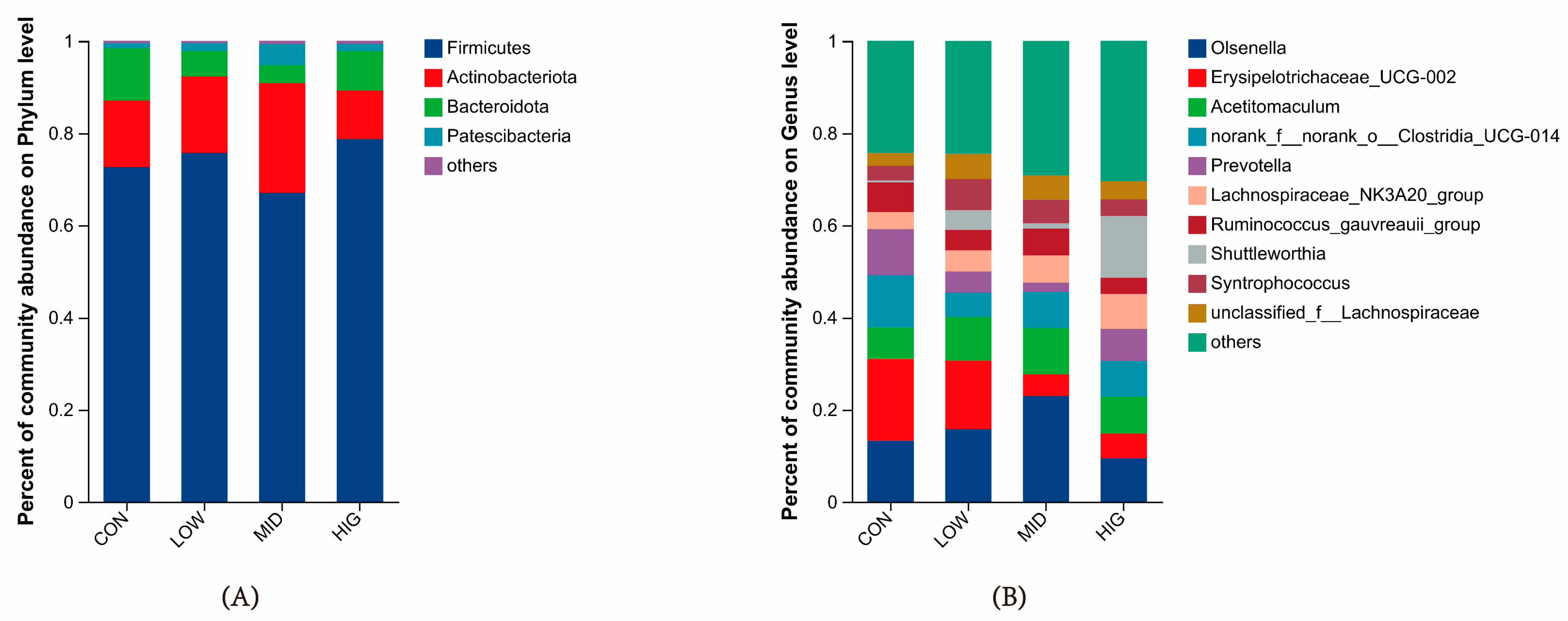
3.5. Analysis of Microbial Species Composition in the Rumen of Calves
3.6. Analysis of Differences in Microbial Species in the Rumen of Calves
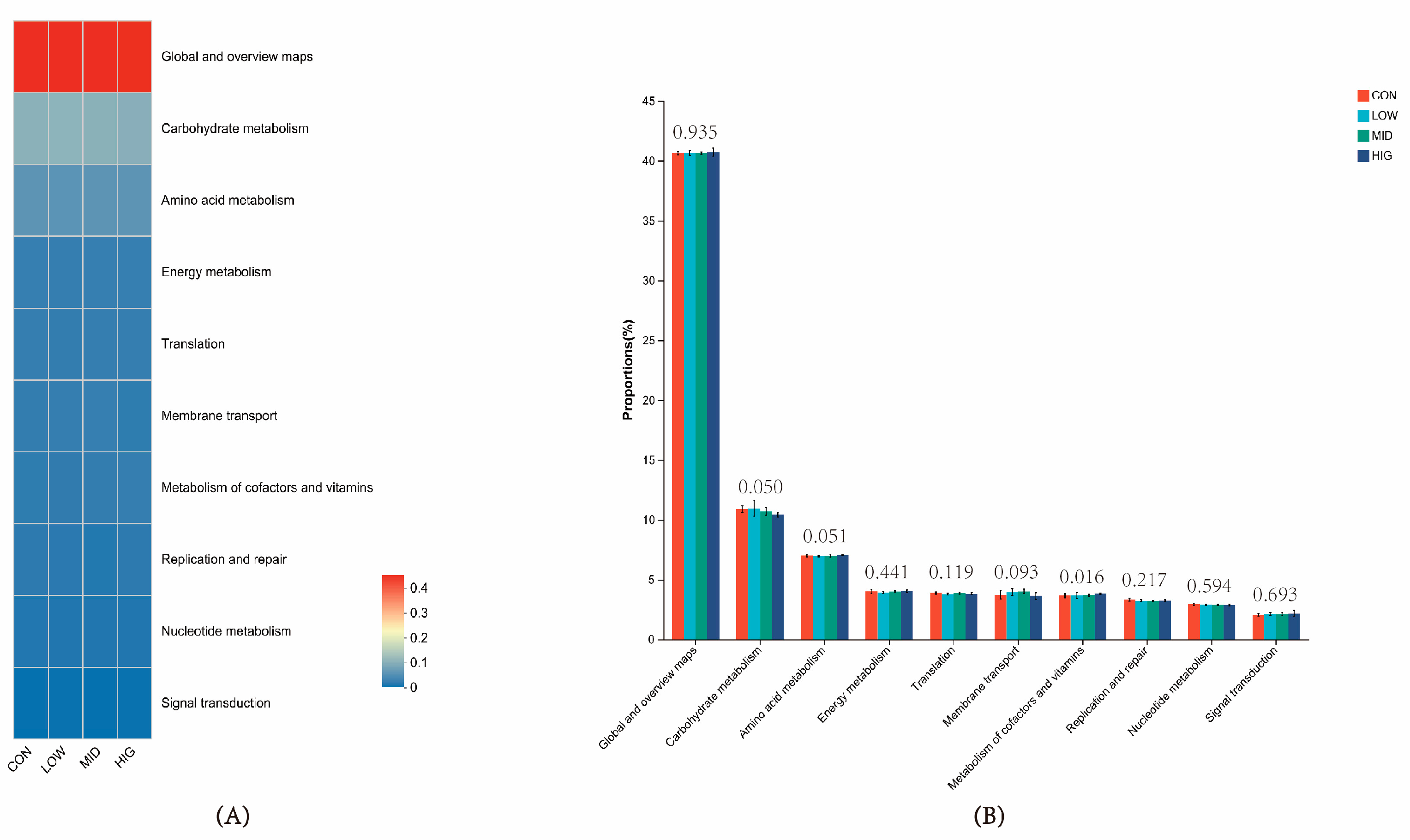
3.7. Prediction of Rumen Microbial Function in Calves
3.8. Correlation Analysis of Rumen Microorganisms with Growth Performance and Serum Immunoglobulin in Calves
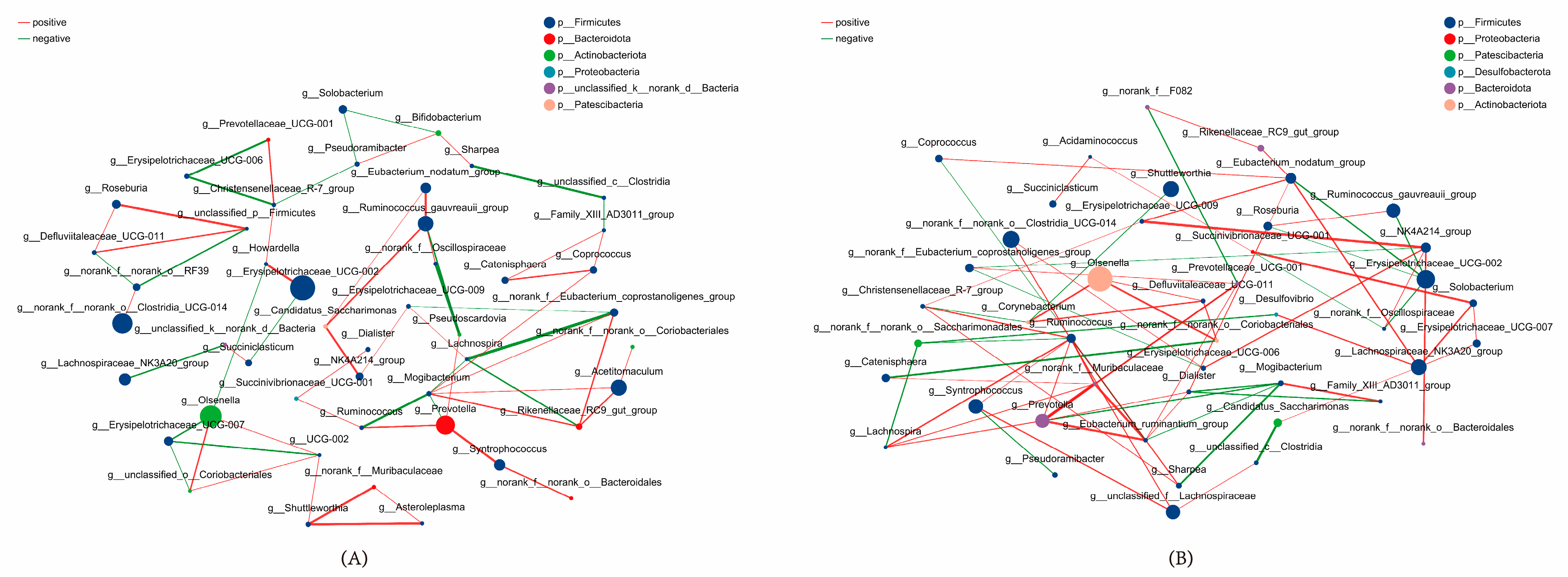
3.9. Core Species Screening for Rumen Microbiota in Calves
4. Discussion
4.1. Effect of Different Oregano Essential Oil Additions on Calf Growth Performance
4.2. Effect of Different Oregano Essential Oil Additions on Calf Serum Immunoglobulin
4.3. Effect of Oregano Essential Oil Supplementation on Rumen Microbial Community in Calves
4.4. Differences in Rumen Microbial Composition at Different Taxonomic Levels
4.5. Modulation of Rumen Microflora in Calves by Oregano Essential Oil Supplementation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, J.; Shah, A.M.; Shao, Y.; Wang, Z.; Zou, H.; Kang, K. Dietary supplementation of yeast cell wall improves the gastrointestinal development of weaned calves. Anim. Nutr. 2020, 6, 507–512. [Google Scholar] [CrossRef]
- Cao, Z.; Xiao, J.; Alugongo, G.M.; Ji, S.; Wu, Z.; Dong, S.; Li, S.; Yoon, I.; Chung, R. Effects of Saccharomyces Cerevisiae Fermentation Products on the Microbial Community throughout the Gastrointestinal Tract of Calves. Animals 2018, 9, 4. [Google Scholar] [CrossRef]
- Soltis, M.P.; Moorey, S.E.; Egert-McLean, A.M.; Voy, B.H.; Shepherd, E.A.; Myer, P.R. Rumen Biogeographical Regions and Microbiome Variation. Microorganisms 2023, 11, 747. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Yu, Z.; Xu, Q.; Zheng, N.; Zhao, S.; Huang, G.; Wang, J. Ruminal microbiota-host interaction and its effect on nutrient metabolism. Anim. Nutr. 2021, 7, 49–55. [Google Scholar] [CrossRef]
- Naeem, A.; Drackley, J.K.; Stamey, J.; Loor, J.J. Role of metabolic and cellular proliferation genes in ruminal development in response to enhanced plane of nutrition in neonatal Holstein calves. J. Dairy Sci. 2012, 95, 1807–1820. [Google Scholar] [CrossRef]
- Nagaraja, T.G. Microbiology of the Rumen. In Rumenology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 39–61. [Google Scholar] [CrossRef]
- Hansen, C.H.; Nielsen, D.S.; Kverka, M.; Zakostelska, Z.; Klimesova, K.; Hudcovic, T.; Tlaskalova-Hogenova, H.; Hansen, A.K. Patterns of early gut colonization shape future immune responses of the host. PLoS ONE 2012, 7, e34043. [Google Scholar] [CrossRef] [PubMed]
- Low, C.X.; Tan, L.T.H.; Ab Mutalib, N.S.; Pusparajah, P.; Goh, B.H.; Chan, K.G.; Letchumanan, V.; Lee, L.H. Unveiling the Impact of Antibiotics and Alternative Methods for Animal Husbandry: A Review. Antibiotics 2021, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009, 62, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, M.S.; Guo, J.; Sun, H.; Aboagye, D.; Sobhi, M.; Muhmood, A.; Dong, R. A review targeting veterinary antibiotics removal from livestock manure management systems and future outlook. Bioresour. Technol. 2021, 333, 125069. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Li, X.; Ma, X.; Ru, S.; Qiu, T.; Lu, A. Veterinary antibiotics and estrogen hormones in manures from concentrated animal feedlots and their potential ecological risks. Environ. Res. 2021, 198, 110463. [Google Scholar] [CrossRef]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Castronovo, L.M.; Calonico, C.; Ascrizzi, R.; Del Duca, S.; Delfino, V.; Chioccioli, S.; Vassallo, A.; Strozza, I.; De Leo, M.; Biffi, S.; et al. The Cultivable Bacterial Microbiota Associated to the Medicinal Plant Origanum vulgare L.: From Antibiotic Resistance to Growth-Inhibitory Properties. Front. Microbiol. 2020, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Vitalini, S.; Iriti, M. Bioactivities of Origanum vulgare L.: An update. Phytochem 2017, 16, 1253–1268. [Google Scholar] [CrossRef]
- Abdelaali, B.; El Menyiy, N.; El Omari, N.; Benali, T.; Guaouguaou, F.E.; Salhi, N.; Naceiri Mrabti, H.; Bouyahya, A. Phytochemistry, Toxicology, and Pharmacological Properties of Origanum elongatum. Evid. Based Complement Altern. Med. 2021, 2021, 6658593. [Google Scholar] [CrossRef]
- Marrelli, M.; Statti, G.A.; Conforti, F. Origanum spp.: An update of their chemical and biological profiles. Phytochem. Rev. 2018, 17, 873–888. [Google Scholar] [CrossRef]
- Kampasakali, E.; Nakas, A.; Mertzanidis, D.; Kokkini, S.; Assimopoulou, A.N.; Christofilos, D. μ-Raman Determination of Essential Oils’ Constituents from Distillates and Leaf Glands of Origanum Plants. Molecules 2023, 28, 1221. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crop Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Jia, L.; Wu, J.; Lei, Y.; Kong, F.; Zhang, R.; Sun, J.; Wang, L.; Li, Z.; Shi, J.; Wang, Y.; et al. Oregano Essential Oils Mediated Intestinal Microbiota and Metabolites and Improved Growth Performance and Intestinal Barrier Function in Sheep. Front. Immunol. 2022, 13, 908015. [Google Scholar] [CrossRef]
- Olijhoek, D.W.; Hellwing, A.L.F.; Grevsen, K.; Haveman, L.S.; Chowdhury, M.R.; Løvendahl, P.; Weisbjerg, M.R.; Noel, S.J.; Højberg, O.; Wiking, L.; et al. Effect of driedoregano (Origanum vulgare L.) plant material in feed on methane production, rumen fermentation, nutrient digestibility, and milk fatty acid composition in dairy cows. J. Dairy Sci. 2019, 102, 9902–9918. [Google Scholar] [CrossRef]
- Ruan, D.; Fan, Q.; Fouad, A.M.; Sun, Y.; Huang, S.; Wu, A.; Lin, C.; Kuang, Z.; Zhang, C.; Jiang, S. Effects of dietary oregano essential oil supplementation on growth performance, intestinal antioxidative capacity, immunity, and intestinal microbiota in yellow-feathered chickens. J. Anim. Sci. 2021, 99, skab033. [Google Scholar] [CrossRef]
- Ritt, L.A.; Modesto, E.C.; Guimarães, J.A.; Heisler, G.; Oliveira, A.T.D.; Fischer, V. Oregano extract fed to pre-weaned dairy calves. Part 2: Effect on ruminal and intestinal morphology of pre-weaned calves. Livest. Sci. 2023, 270, 105194. [Google Scholar] [CrossRef]
- Kubiliene, A.; Munius, E.; Songailaite, G.; Kokyte, I.; Baranauskaite, J.; Liekis, A.; Sadauskiene, I. A Comparative Evaluation of Antioxidant Activity of Extract and Essential Oil of Origanum onites L. In Vivo. Molecules 2023, 28, 5302. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.; Ege, G.; Aysul, N.; Akşit, H.; Tüzün, A.E.; Küçükyılmaz, K.; Borum, A.E.; Uygun, M.; Akşit, D.; Aypak, S.; et al. Effect of anticoccidial monensin with oregano essential oil on broilers experimentally challenged with mixed Eimeria spp. Poult. Sci. 2016, 95, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, J.; Lei, Y.; Bai, Y.; Jia, L.; Li, Z.; Liu, T.; Xu, Y.; Sun, J.; Wang, Y.; et al. Oregano Essential Oils Promote Rumen Digestive Ability by Modulating Epithelial Development and Microbiota Composition in Beef Cattle. Front. Nutr. 2021, 8, 722557. [Google Scholar] [CrossRef]
- Zhu, C.; Yao, J.; Zhu, M.; Zhu, C.; Yuan, L.; Li, Z.; Cai, D.; Chen, S.; Hu, P.; Liu, H.Y. A meta-analysis of Lactobacillus-based probiotics for growth performance and intestinal morphology in piglets. Front. Vet. Sci. 2022, 9, 1045965. [Google Scholar] [CrossRef] [PubMed]
- Ritt, L.A.; Orso, C.; Silveira, A.K.; Frazzon, J.; de Vargas, D.P.; Wagner, R.; de Oliveira, F.C.; Nörnberg, J.L.; Fischer, V. Oregano extract fed to pre-weaned dairy calves. Part 1: Effects on intake, digestibility, body weight, and rumen and intestinal bacteria microbiota. Livest. Sci. 2023, 269, 105165. [Google Scholar] [CrossRef]
- El-Azrak, K.E.M.; Morsy, A.S.; Soltan, Y.A.; Hashem, N.M.; Sallam, S.M.A. Impact of specific essential oils blend on milk production, serum biochemical parameters and kid performance of goats. Anim. Biotechnol. 2022, 33, 1344–1352. [Google Scholar] [CrossRef]
- Kazemi-Bonchenari, M.; Falahati, R.; Poorhamdollah, M.; Heidari, S.R.; Pezeshki, A. Essential oils improved weight gain, growth and feed efficiency of young dairy calves fed 18 or 20% crude protein starter diets. J. Anim. Physiol. Anim. Nutr. 2018, 102, 652–661. [Google Scholar] [CrossRef]
- Tan, C.; Wei, H.; Sun, H.; Ao, J.; Long, G.; Jiang, S.; Peng, J. Effects of Dietary Supplementation of Oregano Essential Oil to Sows on Oxidative Stress Status, Lactation Feed Intake of Sows, and Piglet Performance. Biomed. Res. Int. 2015, 2015, 525218. [Google Scholar] [CrossRef]
- Horton, R.E.; Vidarsson, G. Antibodies and their receptors: Different potential roles in mucosal defense. Front. Immunol. 2013, 4, 200. [Google Scholar] [CrossRef]
- Pabst, O. New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 2012, 12, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, Z.; Lu, J.; Ables, E.; Scemama, J.L.; Yang, L.V.; Lu, J.Q.; Hu, X.H. Quantitative analysis and comparison of 3D morphology between viable and apoptotic MCF-7 breast cancer cells and characterization of nuclear fragmentation. PLoS ONE 2017, 12, e0184726. [Google Scholar] [CrossRef] [PubMed]
- Urie, N.J.; Lombard, J.E.; Shivley, C.B.; Kopral, C.A.; Adams, A.E.; Earleywine, T.J.; Olson, J.D.; Garry, F.B. Preweaned heifer management on US dairy operations: Part, V. Factors associated with morbidity and mortality in preweaned dairy heifer calves. J. Dairy Sci. 2018, 101, 9229–9244. [Google Scholar] [CrossRef] [PubMed]
- Dewell, R.D.; Hungerford, L.L.; Keen, J.E.; Laegreid, W.W.; Griffin, D.D.; Rupp, G.P.; Grotelueschen, D.M. Association of neonatal serum immunoglobulin G1 concentration with health and performance in beef calves. J. Am. Vet. Med. Assoc. 2006, 228, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Xiong, E.; Hong, R.; Lu, Q.; Ohno, H.; Wang, J.Y. Role of the IgM Fc Receptor in Immunity and Tolerance. Front. Immunol. 2019, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Keyt, B.A.; Baliga, R.; Sinclair, A.M.; Carroll, S.F.; Peterson, M.S. Structure, Function, and Therapeutic Use of IgM Antibodies. Antibodies 2020, 9, 53. [Google Scholar] [CrossRef]
- Gasmi, A.; Piscopo, S.; Menzel, A.; Noor, S. A Review on Metabolic Paradoxes and their Impact on Metabolism. Arch. Razi Inst. 2022, 77, 929–941. [Google Scholar] [CrossRef]
- Tourkochristou, E.; Triantos, C.; Mouzaki, A. The Influence of Nutritional Factors on Immunological Outcomes. Front. Immunol. 2021, 12, 665968. [Google Scholar] [CrossRef]
- Kulkarni, D.H.; Newberry, R.D. Intestinal Macromolecular Transport Supporting Adaptive Immunity. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, D.; Sun, H. Herba Origani alleviated DSS-induced ulcerative colitis in mice through remolding gut microbiota to regulate bile acid and short-chain fatty acid metabolisms. Biomed. Pharmacother. 2023, 161, 114409. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.B.; Gonzalez, E.L.; Choy, K.; Faull, K.F.; Jewell, T.; Arellano, A.; Liang, J.; Yu, K.B.; Paramo, J.; Hsiao, E.Y. Gut microbiota Turicibacter strains differentially modify bile acids and host lipids. Nat. Commun. 2023, 14, 3669. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Li, P.; Hao, S.; Kim, S.W.; Du, T.; Hua, J.; Huang, R. Characteristics of Gut Microbiota in Sows and Their Relationship with Apparent Nutrient Digestibility. Int. J. Mol. Sci. 2019, 20, 870. [Google Scholar] [CrossRef] [PubMed]
- Xian, M.; Shen, L.; Zhan, S.; Chen, S.; Lin, H.; Cai, J.; Hu, T.; Wang, S. Integrated 16S rRNA gene sequencing and LC/MS-based metabolomics ascertained synergistic influences of the combination of acupuncture and NaoMaiTong on ischemic stroke. J. Ethnopharmacol. 2022, 293, 115281. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.J.; Ke, X.L.; Jiang, L.J.; Zhang, Z.Y.; Yi, M.M.; Liu, Z.J.; Cao, K.M.; Lu, M.X.; Wang, M. Genomic and biochemical analysis reveals fermented product of a putative novel Romboutsia species involves the glycometabolism of tilapia. Aquaculture 2024, 581, 740483. [Google Scholar] [CrossRef]
- Cao, S.; Huang, K.; Wen, X.; Gao, J.; Cui, B.; Yao, K.; Zhan, X.; Hu, S.; Wu, Q.; Xiao, H.; et al. Dietary supplementation with potassium-magnesium sulfate modulates the antioxidant capacity, immunity, and gut microbiota in weaned piglets. Front. Microbiol. 2022, 13, 961989. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Pu, Q.; Zhao, Q.; Zhou, Y.; Jiang, X.; Han, T. Effects of Fucoidan Isolated from Laminaria japonica on Immune Response and Gut Microbiota in Cyclophosphamide-Treated Mice. Front. Immunol. 2022, 13, 916618. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Huang, K.; Yang, B.; Zhang, Y.; Yu, Z.; Wang, J. Fecal microbiota dynamics and its relationship to diarrhea and health in dairy calves. J. Anim. Sci. Biotechnol. 2022, 13, 132. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, H.; Jafari, H.; Liu, B.; Wang, Z.; Su, J.; Wang, F.; Yang, G.; Sun, M.; Cheng, J.; et al. Metabolic changes before and after weaning in Dezhou donkey foals in relation to gut microbiota. Front. Microbiol. 2023, 14, 1306039. [Google Scholar] [CrossRef]
- Xu, B.; Yan, Y.; Yin, B.; Zhang, L.; Qin, W.; Niu, Y.; Tang, Y.; Zhou, S.; Yan, X.; Ma, L. Dietary glycyl-glutamine supplementation ameliorates intestinal integrity, inflammatory response, and oxidative status in association with the gut microbiota in LPS-challenged piglets. Food Funct. 2021, 12, 3539–3551. [Google Scholar] [CrossRef]
- Chang, T.T.; Chen, J.W. Direct CCL4 inhibition modulates gut microbiota, reduces circulating trimethylamine N-oxide, and improves glucose and lipid metabolism in high-fat-diet-induced diabetes mellitus. J. Inflamm. Res. 2021, 14, 6237. [Google Scholar] [CrossRef]
- Hao, Y.; Ouyang, T.; Wang, W.; Wang, Y.; Cao, Z.; Yang, H.; Guan, L.L.; Li, S. Competitive Analysis of Rumen and Hindgut Microbiota Composition and Fermentation Function in Diarrheic and Non-Diarrheic Postpartum Dairy Cows. Microorganisms 2024, 12, 23. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, Q.; Xie, Q.; Jiang, C.; Zhou, L.; Liu, J.; Li, B.; Jiang, S. 2′-Fucosyllactose Promotes the Production of Short-Chain Fatty Acids and Improves Immune Function in Human-Microbiota-Associated Mice by Regulating Gut Microbiota. J. Agric. Food Chem. 2022, 70, 13615–13625. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, C.; Gong, Y.; Sun, X.; Wang, W.; Wang, Y.; Yang, H.; Cao, Z.; Li, S. Rumen Fermentation, Digestive Enzyme Activity, and Bacteria Composition between Pre-Weaning and Post-Weaning Dairy Calves. Animals 2021, 11, 2527. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Lu, D.; Wu, D.; Zhang, P.; Zhou, Y. Quorum quenching enhanced methane production in anaerobic systems—Performance and mechanisms. Water Res. 2023, 235, 119841. [Google Scholar] [CrossRef]
- Gu, F.; Liang, S.; Zhu, S.; Liu, J.; Sun, H.Z. Multi-omics revealed the effects of rumen-protected methionine on the nutrient profile of milk in dairy cows. Food Res. Int. 2021, 149, 110682. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Tang, G.; Yu, J.J.; Chen, J.; Li, Z.J.; Cao, Y.C.; Lei, X.J.; Deng, L.; Wu, S.R.; et al. Multi-omics revealed the long-term effect of ruminal keystone bacteria and the microbial metabolome on lactation performance in adult dairy goats. Microbiome 2023, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Tian, Y.; Hou, M.; Zhao, Y.; Li, J.; Aftab, U.; Rousseau, X.; Jiang, R.; Kang, X.; Tian, Y.; et al. Evaluation of stimbiotic on growth performance and intestinal development of broilers fed corn- or wheat-based diets. Poult. Sci. 2023, 102, 103094. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Zhao, C.; Kuan, K.C.S.; Cao, X.Y.; Wang, B.W.; Ren, Y.C. Effects of dietary oregano essential oil-mediated intestinal microbiota and transcription on amino acid metabolism and Aeromonas salmonicida resistance in rainbow trout (Oncorhynchus mykiss). Aquacult. Int. 2023, P1, 21. [Google Scholar] [CrossRef]
- Shen, H.; Xu, Z.; Shen, Z.; Lu, Z. The Regulation of Ruminal Short-Chain Fatty Acids on the Functions of Rumen Barriers. Front. Physiol. 2019, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Eslick, S.; Thompson, C.; Berthon, B.; Wood, L. Short-chain fatty acids as anti-inflammatory agents in overweight and obesity: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 838–856. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lu, M.; Wang, J.; Zhang, H.; Qiu, K.; Qi, G.; Wu, S. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Anim. Sci. Biotechnol. 2021, 12, 72. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Zhang, L.; Liu, L.; Casper, D.P.; Jiao, T.; Liu, T.; Wang, J.; Lang, X.; Song, S.; et al. Effects of oregano essential oil on the ruminal pH and microbial population of sheep. PLoS ONE 2019, 14, e0217054. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, J.; Jia, L.; He, P.; Wang, Y.; Zhang, X.; Huang, Y.; Cheng, Q.; Zhang, Z.; Dai, Y.; et al. Oregano essential oil modulates colonic homeostasis and intestinal barrier function in fattening bulls. Front. Microbiol. 2023, 14, 1293160. [Google Scholar] [CrossRef]
- Chen, A.J.; Li, J.; Jannasch, A.; Mutlu, A.S.; Wang, M.C.; Cheng, J.X. Fingerprint Stimulated Raman Scattering Imaging Reveals Retinoid Coupling Lipid Metabolism and Survival. Chemphyschem 2018, 19, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Tiwari, R.; Yatoo, M.I.; Karthik, K.; Michalak, I.; Dhama, K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health—A comprehensive review. Vet. Q. 2020, 41, 1–29. [Google Scholar] [CrossRef]
- O’Connor, C.; Varshosaz, P.; Moise, A.R. Mechanisms of Feedback Regulation of Vitamin A Metabolism. Nutrients 2022, 14, 1312. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.D.S.; Olivier, D.S.; Eberle, R.J.; de Moraes, F.R.; Amaral, M.S.; Arni, R.K.; Coronado, M.A. Binding studies of a putative C. pseudotuberculosis target protein from Vitamin B12 Metabolism. Sci. Rep. 2019, 9, 6350. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, R.; Chang, J.; Chen, L.; Nabi, M.; Zhang, H.; Zhang, G.; Zhang, P. Rumen microbes, enzymes, metabolisms, and application in lignocellulosic waste conversion—A comprehensive review. Biotechnol. Adv. 2024, 71, 108308. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Zhou, S.; O’Hair, J.; Al Nasr, K.; Ropelewski, A.; Li, H. Exploring the microbial diversity and characterization of cellulase and hemicellulase genes in goat rumen: A metagenomic approach. BMC Biotechnol. 2023, 23, 51. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Ghyselinck, J.; Marzorati, M.; Koch, A.M.; Lambert, W.; Michiels, J.; Chalvon-Demersay, T. The Effect of Amino Acids on Production of SCFA and bCFA by Members of the Porcine Colonic Microbiota. Microorganisms 2022, 10, 762. [Google Scholar] [CrossRef]
- Yi, S.; Wu, H.; Liu, Y.; Dai, D.; Meng, Q.; Chai, S.; Liu, S.; Zhou, Z. Concentrate supplementation improves cold-season environmental fitness of grazing yaks: Responsive changes in the rumen microbiota and metabolome. Front. Microbiol. 2023, 14, 1247251. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Zhang, Y.; Li, X.; Li, L.; Zhang, R.; Zhang, S. Differential Responses of Digesta- and Mucosa-Associated Jejunal Microbiota of Hu Sheep to Pelleted and Non-Pelleted High-Grain Diets. Animals 2022, 12, 1695. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, J.; Liu, X.; Liu, R.; Wang, Y.; Huang, X.; Li, Y.; Liu, R.; Yang, X. Dietary folic acid addition reduces abdominal fat deposition mediated by alterations in gut microbiota and SCFA production in broilers. Anim. Nutr. 2022, 12, 54–62. [Google Scholar] [CrossRef] [PubMed]
- La Reau, A.J.; Suen, G. The Ruminococci: Key symbionts of the gut ecosystem. J. Microbiol. 2018, 56, 199–208. [Google Scholar] [CrossRef]


| Ingredient Composition | Content | Nutrient Level | Content |
|---|---|---|---|
| Corn | 37.57 | DM 1 | 90.87 |
| Soybean meal | 27.21 | CP 2 | 21.58 |
| Bran | 12.40 | CF 3 | 4.18 |
| Cottonseed meal | 6.80 | Ash | 5.60 |
| Puffed soybeans | 2.50 | NDF 4 | 22.58 |
| Whey powder | 4.00 | ADF 5 | 8.86 |
| Molasses | 4.00 | Ca 6 | 1.08 |
| Calcium carbonate | 2.20 | P 7 | 0.48 |
| Soybean oil | 0.80 | ||
| Nacl | 0.80 | ||
| Calcium biphosphate | 0.90 | ||
| Magnesium oxide | 0.10 | ||
| Yeast selenium | 0.02 | ||
| Premix | 0.70 | ||
| Total | 100 |
| Index | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | LOW | MID | HIG | |||
| Initial weight | 92.42 | 91.17 | 91.50 | 92.58 | 0.47 | 0.689 |
| Final weight | 153.92 c | 170.92 b | 168.08 bc | 177.42 a | 3.15 | 0.046 |
| Average daily gain | 1.10 c | 1.42 b | 1.37 bc | 1.51 a | 0.05 | 0.037 |
| Index | Days | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| CON | LOW | MID | HIG | ||||
| IgA | 0 | 1.28 | 1.25 | 1.32 | 1.29 | 0.03 | 0.937 |
| 56 | 1.99 b | 2.12 ab | 2.09 ab | 2.28 a | 0.04 | 0.028 | |
| IgG | 0 | 8.16 | 7.63 | 7.23 | 7.59 | 0.20 | 0.475 |
| 56 | 13.44 | 13.27 | 14.38 | 14.15 | 0.30 | 0.509 | |
| IgM | 0 | 1.10 | 0.96 | 1.02 | 0.96 | 0.04 | 0.550 |
| 56 | 1.65 b | 1.83 ab | 1.92 a | 1.99 a | 0.47 | 0.045 | |
| Index | Relative Abundance (%) | ||||
|---|---|---|---|---|---|
| CON | LOW | MID | HIG | p-Value | |
| Phylum level | |||||
| Firmicutes | 72.68 ± 15.85 | 75.76 ± 6.55 | 67.05 ± 10.99 | 78.66 ± 10.63 | 0.311 |
| Actinobacteriota | 14.33 ± 10.80 | 16.56 ± 7.71 | 23.83 ± 6.29 | 10.6 ± 5.95 | 0.042 |
| Bacteroidota | 11.47 ± 12.48 | 5.47 ± 6.35 | 3.87 ± 1.67 | 8.61 ± 9.58 | 0.397 |
| Patescibacteria | 0.99 ± 0.61 | 1.70 ± 0.44 | 4.58 ± 7.64 | 1.54 ± 0.64 | 0.236 |
| Proteobacteria | 0.23 ± 0.24 | 0.17 ± 0.17 | 0.18 ± 0.07 | 0.20 ± 0.15 | 0.928 |
| Genus level | |||||
| Olsenella | 13.28 ± 10.23 | 15.73 ± 7.49 | 22.9 ± 6.08 | 9.46 ± 5.97 | 0.039 |
| Erysipelotrichaceae_UCG-002 | 17.74 ± 13.49 | 14.96 ± 22.71 | 4.80 ± 3.64 | 5.34 ± 5.20 | 0.354 |
| Acetitomaculum | 6.87 ± 3.72 | 9.46 ± 5.37 | 10.04 ± 2.81 | 8.00 ± 4.48 | 0.646 |
| norank_f_norank_o_Clostridia_UCG-014 | 11.39 ± 9.35 | 5.28 ± 2.87 | 7.77 ± 5.24 | 7.77 ± 3.52 | 0.435 |
| Prevotella | 9.88 ± 12.52 | 4.56 ± 6.03 | 2.0 ± 1.23 | 6.97 ± 9.65 | 0.717 |
| Lachnospiraceae_NK3A20_group | 3.67 ± 1.42 | 4.58 ± 2.54 | 5.95 ± 1.76 | 7.54 ± 7.09 | 0.286 |
| Ruminococcus_gauvreauii_group | 6.51 ± 3.71 | 4.44 ± 2.59 | 5.84 ± 1.84 | 3.57 ± 0.74 | 0.227 |
| Shuttleworthia | 0.39 ± 0.31 | 4.30 ± 7.10 | 1.15 ± 0.90 | 13.38 ± 10.55 | 0.003 |
| Syntrophococcus | 3.17 ± 1.96 | 6.76 ± 3.86 | 5.12 ± 2.09 | 3.58 ± 2.15 | 0.257 |
| unclassified_f_Lachnospiraceae | 2.85 ± 1.68 | 5.42 ± 5.99 | 5.31 ± 3.06 | 4.03 ± 2.82 | 0.528 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Liu, T.; Cairang, D.; Cheng, S.; Hu, J.; Shi, B.; Zhu, H.; Chen, H.; Zhang, T.; Yi, X. Oregano Essential Oil as a Natural Plant Additive Affects Growth Performance and Serum Antibody Levels by Regulating the Rumen Microbiota of Calves. Animals 2024, 14, 820. https://doi.org/10.3390/ani14060820
Luo Z, Liu T, Cairang D, Cheng S, Hu J, Shi B, Zhu H, Chen H, Zhang T, Yi X. Oregano Essential Oil as a Natural Plant Additive Affects Growth Performance and Serum Antibody Levels by Regulating the Rumen Microbiota of Calves. Animals. 2024; 14(6):820. https://doi.org/10.3390/ani14060820
Chicago/Turabian StyleLuo, Zhihao, Ting Liu, Dongzhu Cairang, Shuru Cheng, Jiang Hu, Bingang Shi, Hui Zhu, Huan Chen, Tao Zhang, and Xuejiao Yi. 2024. "Oregano Essential Oil as a Natural Plant Additive Affects Growth Performance and Serum Antibody Levels by Regulating the Rumen Microbiota of Calves" Animals 14, no. 6: 820. https://doi.org/10.3390/ani14060820
APA StyleLuo, Z., Liu, T., Cairang, D., Cheng, S., Hu, J., Shi, B., Zhu, H., Chen, H., Zhang, T., & Yi, X. (2024). Oregano Essential Oil as a Natural Plant Additive Affects Growth Performance and Serum Antibody Levels by Regulating the Rumen Microbiota of Calves. Animals, 14(6), 820. https://doi.org/10.3390/ani14060820






