Dietary Shifts in the Adaptation to Changing Marine Resources: Insights from a Decadal Study on Greater Lizardfish (Saurida tumbil) in the Beibu Gulf, South China Sea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection and Processing
2.2. Stable Isotopes Analysis (SIA)
2.3. Data Treatment
3. Results
3.1. Population Structure and Indices
3.2. Temporal Variation in Diet Composition and Types
3.3. Variations in Diet Composition with Individual Development
3.4. Individual Specialization and Trophic Niche Widths
3.5. Variations in Trophic Levels
4. Discussion
4.1. Temporal Shifts in Dietary Strategies
4.2. Shifts in Dietary Strategies throughout Individual Development
4.3. Variations in Trophic Levels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Hernández, J.; Nunn, A.D.; Adams, C.E.; Amundsen, P.A. Causes and consequences of ontogenetic dietary shifts: A global synthesis using fish models. Biol. Rev. 2018, 94, 539–554. [Google Scholar] [CrossRef]
- Nakazawa, T. Ontogenetic niche shifts matter in community ecology: A review and future perspectives. Popul. Ecol. 2014, 57, 347–354. [Google Scholar] [CrossRef]
- Nunn, A.D.; Tewson, L.H.; Cowx, I.G. The foraging ecology of larval and juvenile fishes. Rev. Fish Biol. Fish. 2011, 22, 377–408. [Google Scholar] [CrossRef]
- Juanes, F.; Conover, D.O. Rapid Growth, High Feeding Rates, and Early Piscivory in Young-of-the-Year Bluefish (Pomatomus saltatrix). Can. J. Fish. Aquat. Sci. 1994, 51, 1752–1761. [Google Scholar] [CrossRef]
- Post, D.M. Individual Variation in the Timing of Ontogenetic Niche Shifts in Largemouth Bass. Ecology 2003, 84, 1298–1310. [Google Scholar] [CrossRef]
- van Leeuwen, A.; Huss, M.; Gårdmark, A.; de Roos, A. Ontogenetic specialism in predators with multiple niche shifts prevents predator population recovery and establishment. Ecology 2014, 95, 2409–2422. [Google Scholar] [CrossRef][Green Version]
- Persson, A.; Brönmark, C. Foraging capacities and effects of competitive release on ontogenetic diet shift in bream, Abramis brama. Oikos 2002, 97, 271–281. [Google Scholar] [CrossRef]
- Huss, M.; Persson, L.; Borcherding, J.; Heermann, L. Timing of the diet shift from zooplankton to macroinvertebrates and size at maturity determine whether normally piscivorous fish can persist in otherwise fishless lakes. Freshw. Biol. 2013, 58, 1416–1424. [Google Scholar] [CrossRef]
- GÜL, G.; Demirel, N. Evaluation of the comprehensive feeding strategy and trophic role of overexploited mesopredator species in the Sea of Marmara (northeastern Mediterranean). Estuar. Coast. Shelf Sci. 2021, 259, 107448. [Google Scholar] [CrossRef]
- Matich, P.; Kiszka, J.J.; Heithaus, M.R.; Le Bourg, B.; Mourier, J. Inter-individual differences in ontogenetic trophic shifts among three marine predators. Oecologia 2019, 189, 621–636. [Google Scholar] [CrossRef]
- Hobday, A.J.; Arrizabalaga, H.; Evans, K.; Nicol, S.; Young, J.W.; Weng, K.C. Impacts of climate change on marine top predators: Advances and future challenges. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 113, 1–8. [Google Scholar] [CrossRef]
- Kratina, P.; LeCraw, R.M.; Ingram, T.; Anholt, B.R. Stability and persistence of food webs with omnivory: Is there a general pattern? Ecosphere 2012, 3, 1–18. [Google Scholar] [CrossRef]
- Shurin, J.B.; Borer, E.T.; Seabloom, E.W.; Anderson, K.; Blanchette, C.A.; Broitman, B.; Cooper, S.D.; Halpern, B.S. A cross-ecosystem comparison of the strength of trophic cascades. Ecol. Lett. 2002, 5, 785–791. [Google Scholar] [CrossRef]
- Morris, T.; Letnic, M. Removal of an apex predator initiates a trophic cascade that extends from herbivores to vegetation and the soil nutrient pool. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170111. [Google Scholar] [CrossRef]
- Link, J.; Garrison, L. Trophic ecology of Atlantic cod Gadus morhua on the Northeast US Continental Shelf. Mar. Ecol. Prog. Ser. 2002, 227, 109–123. [Google Scholar] [CrossRef]
- Amundsen, P.-A.; Bøhn, T.; Popova, O.A.; Staldvik, F.J.; Reshetnikov, Y.S.; Kashulin, N.A.; Lukin, A.A. Ontogenetic niche shifts and resource partitioning in a subarctic piscivore fish guild. Hydrobiologia 2003, 497, 109–119. [Google Scholar] [CrossRef]
- Liu, D.; Tian, Y.; Ma, S.; Li, J.; Sun, P.; Ye, Z.; Fu, C.; Lan, K.; Zhou, S. Long-Term Variability of Piscivorous Fish in China Seas Under Climate Change with Implication for Fisheries Management. Front. Mar. Sci. 2021, 8, 581952. [Google Scholar] [CrossRef]
- Machado, R.; Oliveira, L.; Ott, P.; Denuncio, P.; Haimovici, M.; Cardoso, L.; Danilewicz, D.; Moreno, I.; Borges-Martins, M. Changes in the feeding ecology of South American sea lions on the southern Brazilian coast over the last two decades of excessive fishing exploration. Hydrobiologia 2018, 819, 17–37. [Google Scholar] [CrossRef]
- D’Iglio, C.; Porcino, N.; Savoca, S.; Profeta, A.; Perdichizzi, A.; Armeli Minicante, E.; Salvati, D.; Soraci, F.; Rinelli, P.; Giordano, D. Ontogenetic shift and feeding habits of the European hake (Merluccius merluccius L., 1758) in Central and Southern Tyrrhenian Sea (Western Mediterranean Sea): A comparison between past and present data. Ecol. Evol. 2022, 12, e8634. [Google Scholar] [CrossRef]
- Steneck, R.S. Human influences on coastal ecosystems: Does overfishing create trophic cascades? Trends Ecol. Evol. 1998, 13, 429–430. [Google Scholar] [CrossRef]
- Neubauer, P.; Jensen, O.P.; Hutchings, J.A.; Baum, J.K. Resilience and Recovery of Overexploited Marine Populations. Science 2013, 340, 347–349. [Google Scholar] [CrossRef]
- Stoffel, M.A.; Humble, E.; Paijmans, A.J.; Acevedo-Whitehouse, K.; Chilvers, B.L.; Dickerson, B.; Galimberti, F.; Gemmell, N.J.; Goldsworthy, S.D.; Nichols, H.J.; et al. Demographic histories and genetic diversity across pinnipeds are shaped by human exploitation, ecology and life-history. Nat. Commun. 2018, 9, 4836. [Google Scholar] [CrossRef]
- Lynam, C.P.; Llope, M.; Möllmann, C.; Helaouët, P.; Bayliss-Brown, G.A.; Stenseth, N.C. Interaction between top-down and bottom-up control in marine food webs. Proc. Natl. Acad. Sci. USA 2017, 114, 1952–1957. [Google Scholar] [CrossRef]
- van Leeuwen, A.; Huss, M.; Gårdmark, A.; Casini, M.; Vitale, F.; Hjelm, J.; Persson, L.; de Roos, A. Predators with Multiple Ontogenetic Niche Shifts Have Limited Potential for Population Growth and Top-Down Control of Their Prey. Am. Nat. 2013, 182, 53–66. [Google Scholar] [CrossRef]
- Reum, J.; Blanchard, J.; Holsman, K.K.; Aydin, K.; Punt, A. Species-specific ontogenetic diet shifts attenuate trophic cascades and lengthen food chains in exploited ecosystems. Oikos 2019, 128, 1051–1064. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, S.; Qiu, Y.; Lin, Z.; Jia, X. Modeling the effects of fishery management and marine protected areas on the Beibu Gulf using spatial ecosystem simulation. Fish. Res. 2009, 100, 222–229. [Google Scholar] [CrossRef]
- Su, L.; Chen, Z.; Zhang, K.; Xu, Y.; Xu, S.; Wang, K. Decadal-Scale Variation in Mean Trophic Level in Beibu Gulf Based on Bottom-Trawl Survey Data. Mar. Coast. Fish. 2021, 13, 174–182. [Google Scholar] [CrossRef]
- Russell, B.C.; Houston, W.A. Offshore fishes of the Arafura Sea. Beagle Rec. Mus. Art Gall. North. Territ. 1989, 6, 69–84. [Google Scholar] [CrossRef]
- Jawad, L.; Abed, J. Morphological asymmetry in the greater lizardfish Saurida tumbil (Bloch, 1795) collected from the marine waters of Iraq. Mar. Pollut. Bull. 2020, 159, 111523. [Google Scholar] [CrossRef]
- Du, J.; Lu, Z.; Yang, S.; Chen, M. Studies on ecological characteristics variation and population dynamics of four lizardfishes in the southern Taiwan Straits. Acta Oceanol. Sin. 2011, 30, 72–81. [Google Scholar] [CrossRef]
- Deng, Y.J.; Yi, M.; Li, B.; Liu, S.B.; Qiu, K.W.; Shen, C.Y.; He, X.B.; Yan, Y.R. Biological characteristics and inter-annual changes of Saurida tumbil in spring in the Beibu Gulf, South China Sea. Prog. Fish. Sci. 2021, 42, 36–44. [Google Scholar] [CrossRef]
- Carassou, L.; Al-kindi, A.; Dobretsov, S. Preliminary assessment of the trophic structure of demersal fish community in the Sea of Oman. Reg. Stud. Mar. Sci. 2018, 16, 145–151. [Google Scholar] [CrossRef][Green Version]
- Manojkumar, P.P.; Pavithran, P.P. Diet and feeding habits of Saurida tumbil (Bloch, 1795) from northern Kerala, South-west coast of India. Indian J. Fish. 2016, 63, 41–47. [Google Scholar] [CrossRef][Green Version]
- Vahabnezhad, A.; Motlagh, S.; Shojaei, M. Seasonal variations in diet and feeding habits of Saurida tumbil (Bloch, 1795), Netuma thalassina (Rüppell, 1837) and Pomadasys kaakan (Cuvier, 1830) in the northern coasts of the Oman Sea. Iran. J. Fish. Sci. 2021, 20, 694–709. [Google Scholar]
- Yan, Y.; Chen, J.; Lu, H.; Hou, G.; Lai, J. Feeding habits and ontogenetic diet shifts of hairtail, Trichiurus margarites, in the Beibu Gulf of the South China Sea. Acta Ecol. Sin. 2012, 32, 18–25. [Google Scholar] [CrossRef]
- Carbonara, P.; Porcu, C.; Donnaloia, M.; Pesci, P.; Sion, L.; Spedicato, M.T.; Zupa, W.; Vitale, F.; Follesa, M. The spawning strategy of European hake (Merluccius merluccius, L. 1758) across the Western and Central Mediterranean Sea. Fish. Res. 2019, 219, 105333. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach contents analysis—A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Cortés, E. A critical review of methods of studying fish feeding based on analysis of stomach contents: Application to elasmobranch fishes. Can. J. Fish. Aquat. Sci. 1997, 54, 726–738. [Google Scholar] [CrossRef]
- Roughgarden, J. Evolution of Niche Width. Am. Nat. 1972, 106, 683–718. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Svanbäck, R.; Fordyce, J.A.; Yang, L.H.; Davis, J.; Hulsey, C.D.; Forister, M.L. The Ecology of Individuals: Incidence and Implications of Individual Specialization. Am. Nat. 2003, 161, 1. [Google Scholar] [CrossRef]
- Schoener, T.W. The Anolis Lizards of Bimini: Resource Partitioning in a Complex Fauna. Ecology 1968, 49, 704–726. [Google Scholar] [CrossRef]
- Zaccarelli, N.; Bolnick, D.; Mancinelli, G. RInSp: An R package for the analysis of individual specialization in resource use. Methods Ecol. Evol. 2013, 4, 1018–1023. [Google Scholar] [CrossRef]
- Pinnegar, J.K.; Polunin, N.V.C. Differential fractionation of δ13C and δ15N among fish tissues: Implications for the study of trophic interactions. Funct. Ecol. 2002, 13, 225–231. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org. (accessed on 5 October 2022).
- Colles, A.; Liow, L.H.; Prinzing, A. Are specialists at risk under environmental change? Neoecological, paleoecological and phylogenetic approaches. Ecol. Lett. 2009, 12, 849–863. [Google Scholar] [CrossRef]
- Clutton-Brock, T.; Sheldon, B. Individuals and populations: The role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol. Evol. 2010, 25, 562–573. [Google Scholar] [CrossRef]
- Salisbury, C.; Seddon, N.; Cooney, C.; Tobias, J. The latitudinal gradient in dispersal constraints: Ecological specialisation drives diversification in tropical birds. Ecol. Lett. 2012, 15, 847–855. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Amarasekare, P.; Araújo, M.S.; Bürger, R.; Levine, J.M.; Novak, M.; Rudolf, V.H.W.; Schreiber, S.J.; Urban, M.C.; Vasseur, D.A. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 2011, 26, 183–192. [Google Scholar] [CrossRef]
- Doustdar, M.; Hashemi, S.A.; Rahmati, R. The feeding and reproductive habits of Saurida tumbil and Rastrelliger kanagurata in the northern Oman Sea. Iran. J. Fish. Sci. 2022, 21, 816–828. [Google Scholar] [CrossRef]
- Budnichenko, V.A. The feeding of Saurida undosquamis and Saurida tumbil (Synodontidae) along the Oman Coast. J. Ichthyol. 1974, 14, 267–272. [Google Scholar]
- Schoener, T.W. Theory of Feeding Strategies. Annu. Rev. Ecol. Evol. Syst. 1971, 2, 369–404. [Google Scholar] [CrossRef]
- Cohen, D. The Equilibrium Distribution of Optimal Search and Sampling Effort of Foraging Animals in Patchy Environments. In Adaptation in Stochastic Environments; Lecture Notes in Biomathematics; Springer: Berlin/Heidelberg, Germany, 1993; pp. 173–191. ISBN 978-3-642-51483-8. [Google Scholar] [CrossRef]
- Reader, S. Causes of Individual Differences in Animal Exploration and Search. Top. Cogn. Sci. 2015, 7, 451–468. [Google Scholar] [CrossRef]
- Matich, P.; Heithaus, M.; Layman, C. Contrasting patterns of individual specialization and trophic coupling in two marine apex predators. J. Anim. Ecol. 2011, 80, 294–305. [Google Scholar] [CrossRef]
- Baylis, A.M.M.; Orben, R.A.; Arnould, J.P.Y.; Peters, K.; Knox, T.; Costa, D.P.; Staniland, I.J. Diving deeper into individual foraging specializations of a large marine predator, the southern sea lion. Oecologia 2015, 179, 1053–1065. [Google Scholar] [CrossRef]
- Phillips, R.A.; Lewis, S.; González-Solís, J.; Daunt, F. Causes and consequences of individual variability and specialization in foraging and migration strategies of seabirds. Mar. Ecol. Prog. Ser. 2017, 578, 117–150. [Google Scholar] [CrossRef]
- He, X.B.; Yan, Y.Y.; Feng, B. Resources and Environment of Beibu Gulf; Oceanpress: Beijing, China, 2023; pp. 35–63. ISBN 978-7-5210-1029-9. [Google Scholar]
- Rudolf, V.H.W.; Lafferty, K.D. Stage structure alters how complexity affects stability of ecological networks. Ecol. Lett. 2011, 14, 75–79. [Google Scholar] [CrossRef]
- McClellan, C.M.; Read, A.J. Complexity and variation in loggerhead sea turtle life history. Biol. Lett. 2007, 3, 592–594. [Google Scholar] [CrossRef]
- Snover, M.L. Ontogenetic habitat sgifts in marine organisms:influencing factors and the impact of climate variability. Bull. Mar. Sci. 2008, 83, 53–67. [Google Scholar]
- Newsome, S.D.; Etnier, M.A.; Monson, D.H.; Fogel, M.L. Retrospective characterization of ontogenetic shifts in killer whale diets via δ13C and δ15N analysis of teeth. Mar. Ecol. Prog. Ser. 2009, 374, 229–242. [Google Scholar] [CrossRef]
- Labropoulou, M.; Machias, A.; Tsimenides, N.; Eleftheriou, A. Feeding habits and ontogenetic diet shift of the striped red mullet, Mullus surmuletus Linnaeus, 1758. Fish. Res. 1997, 31, 257–267. [Google Scholar] [CrossRef]
- Gerking, S.D. Feeding Ecology of Fish; Academic Press: Cambridge, MA, USA, 1994; ISBN 978-0-12-280780-0. [Google Scholar] [CrossRef]
- Werner, E.E.; Gilliam, J.F. The Ontogenetic Niche and Species Interactions in Size-Structured Populations. Annu. Rev. Ecol. Syst. 1984, 15, 393–425. [Google Scholar] [CrossRef]
- Grubbs, R.D. Ontogenetic Shifts in Movements and Habitat Use. In Sharks and Their Relatives II: Biodiversity, Adaptive Physiology, and Conservation; CRC Press: Boca Raton, FL, USA, 2010; pp. 319–350. [Google Scholar]
- Zhao, T.; Villéger, S.; Lek, S.; Cucherousset, J. High intraspecific variability in the functional niche of a predator is associated with ontogenetic shift and individual specialization. Ecol. Evol. 2014, 4, 4649–4657. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.; Eloranta, A.P.; Finstad, A.G.; Amundsen, P.A. Community structure affects trophic ontogeny in a predatory fish. Ecol. Evol. 2016, 7, 358–367. [Google Scholar] [CrossRef]
- Podrabsky, J.E.; Hand, S.C. The bioenergetics of embryonic diapause in an annual killifish, Austrofundulus limnaeus. J. Exp. Biol. 1999, 202, 2567–2580. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, T.; Hou, G.; Lu, H.; Jin, X. Feeding habits and monthly and ontogenetic diet shifts of the greater lizardfish, Saurida Tumbil in the Beibu Gulf of the South China Sea. Fish. China 2010, 34, 1089–1098. [Google Scholar]
- Li, K.J.; Qiu, Y.S.; Wang, Y.J. Influence of natural environment variation on fishery resources in Beibu Gulf. South China Fish. Sci. 2007, 1, 7–13. [Google Scholar]
- Wang, X.-H.; Qiu, Y.; Du, F.-Y.; Lin, Z.-J.; Sun, D.-R.; Huang, S.-L. Fish community pattern and its relation to environmental factors in the Beibu Gulf. J. Fish. China 2011, 34, 1579–1586. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Y.; Zhu, S.; Li, J.; Li, S.; Li, X. Individual dietary specialization reduces intraspecific competition, rather than feeding activity, in black amur bream (Megalobrama terminalis). Sci. Rep. 2020, 10, 17961. [Google Scholar] [CrossRef]
- Estrada, J.; Rice, A.; Natanson, L.; Skomal, G. Use of isotopic analysis of vertebrae in reconstructing ontogenetic feeding ecology in White Sharks. Ecology 2006, 87, 829–834. [Google Scholar] [CrossRef]
- Du, J.; Ye, G.; Chen, B.; Zheng, X. Changes in the marine trophic index of Chinese marine area. Biodivers. Sci. 2014, 22, 532–538. [Google Scholar] [CrossRef]
- Zou, J.W.; Lin, P.W.; Wang, Q.Z. Evaluation of catch in Beibu Gulf of South China Sea in 2012. South China Fish. Sci. 2013, 9, 75–81. [Google Scholar] [CrossRef]
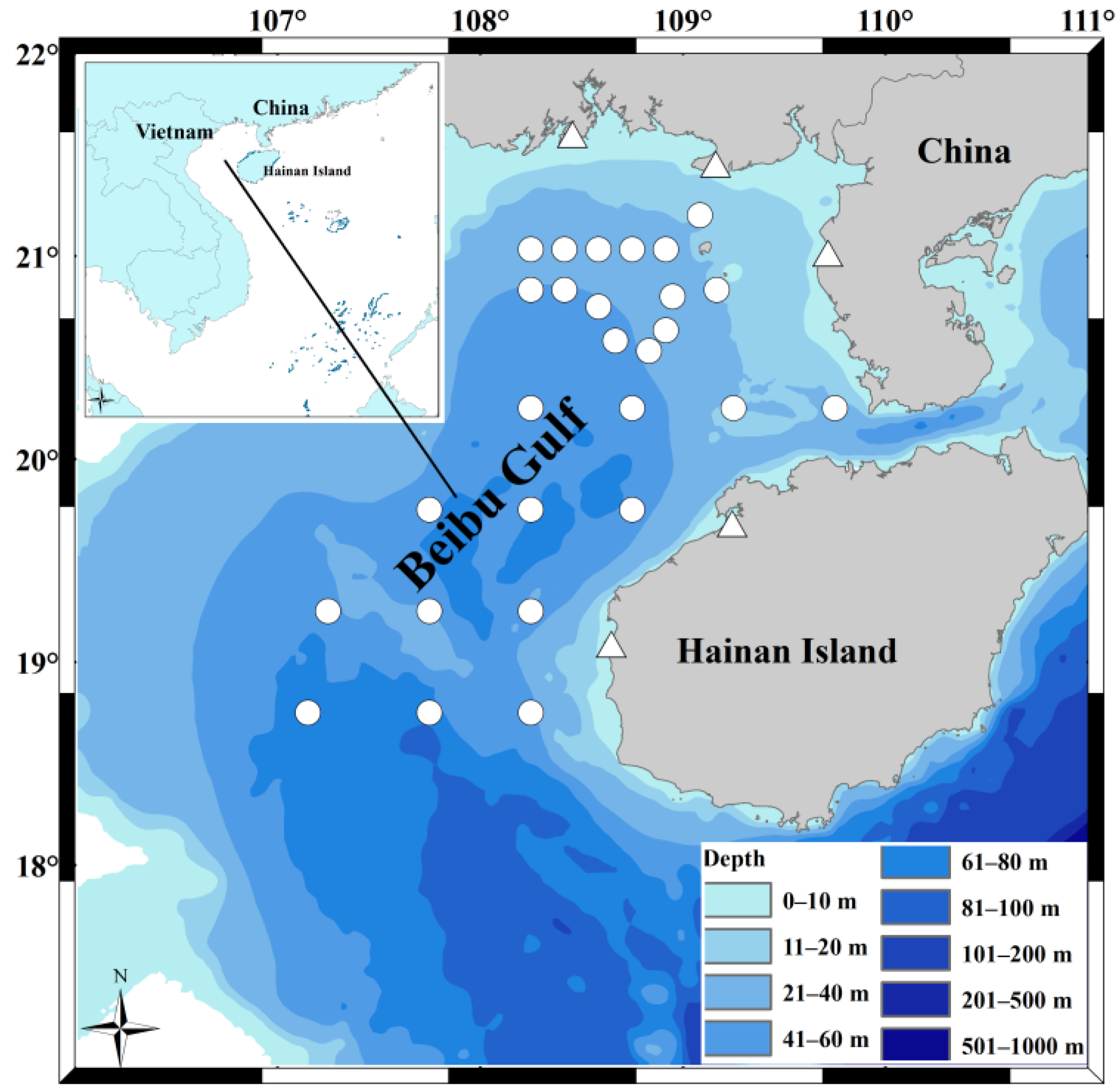
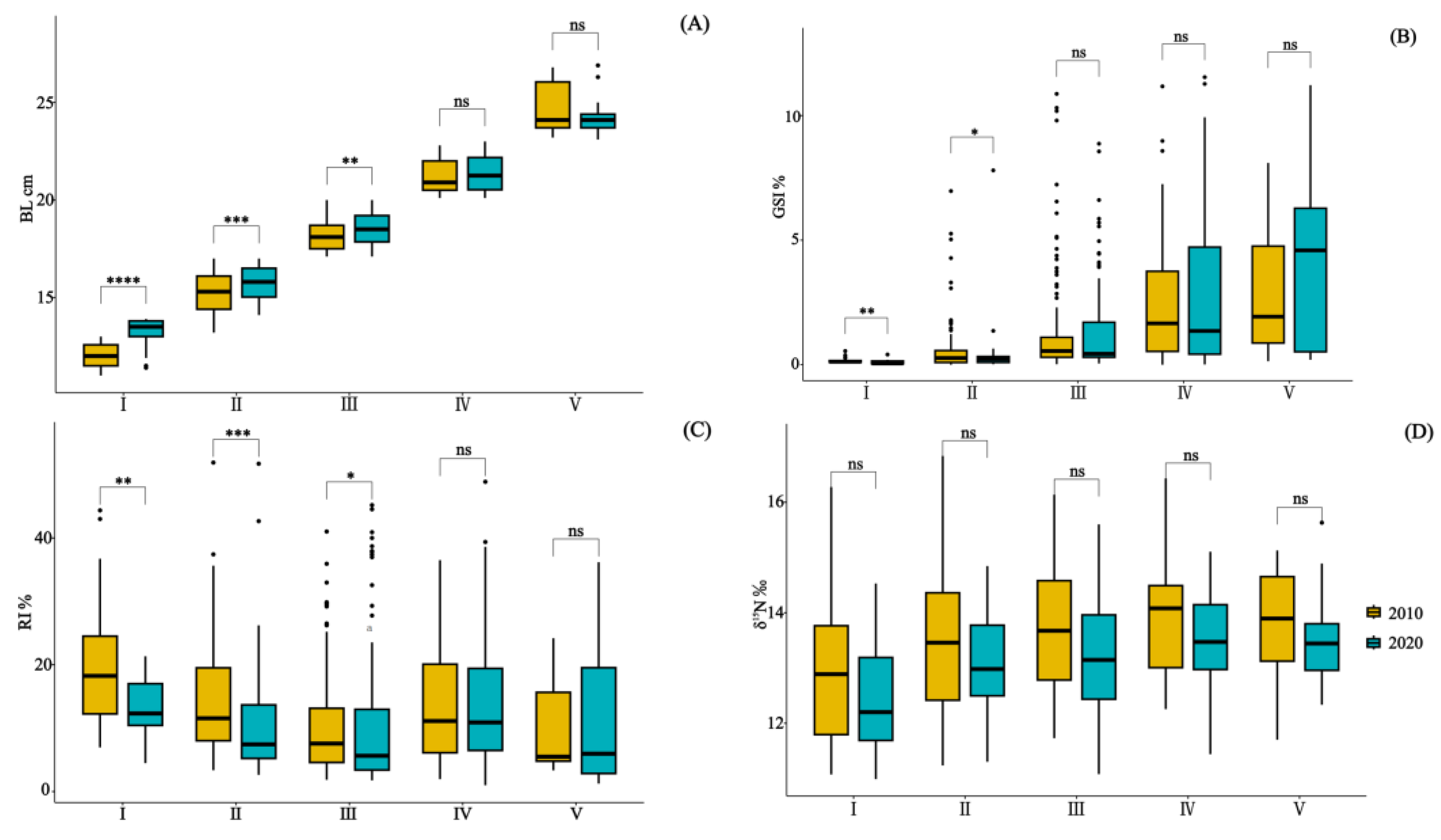
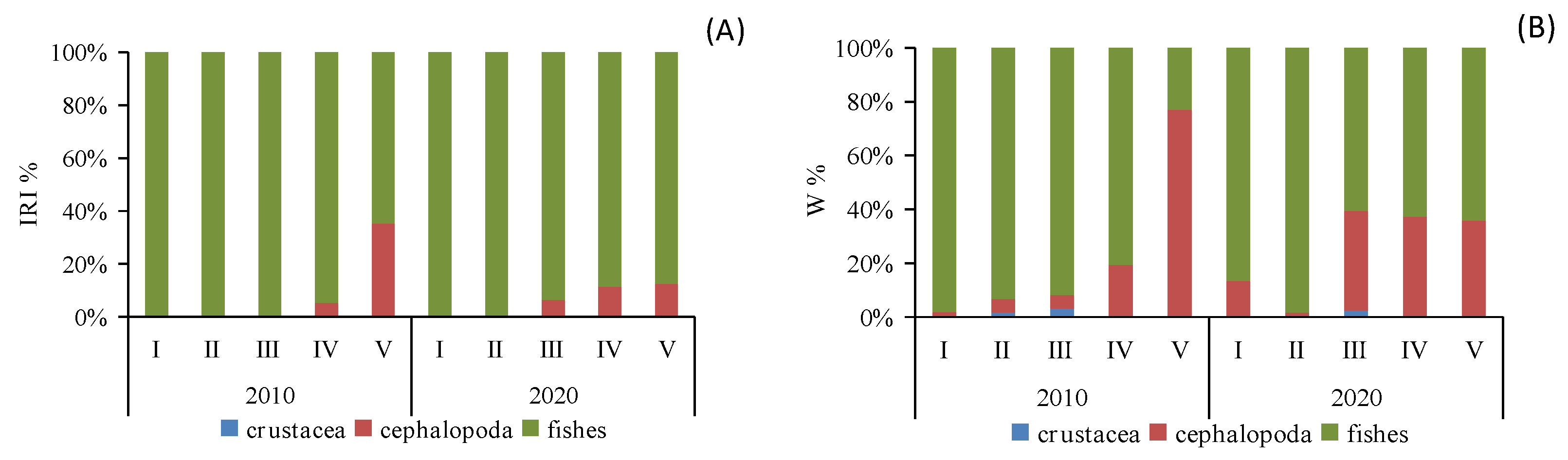


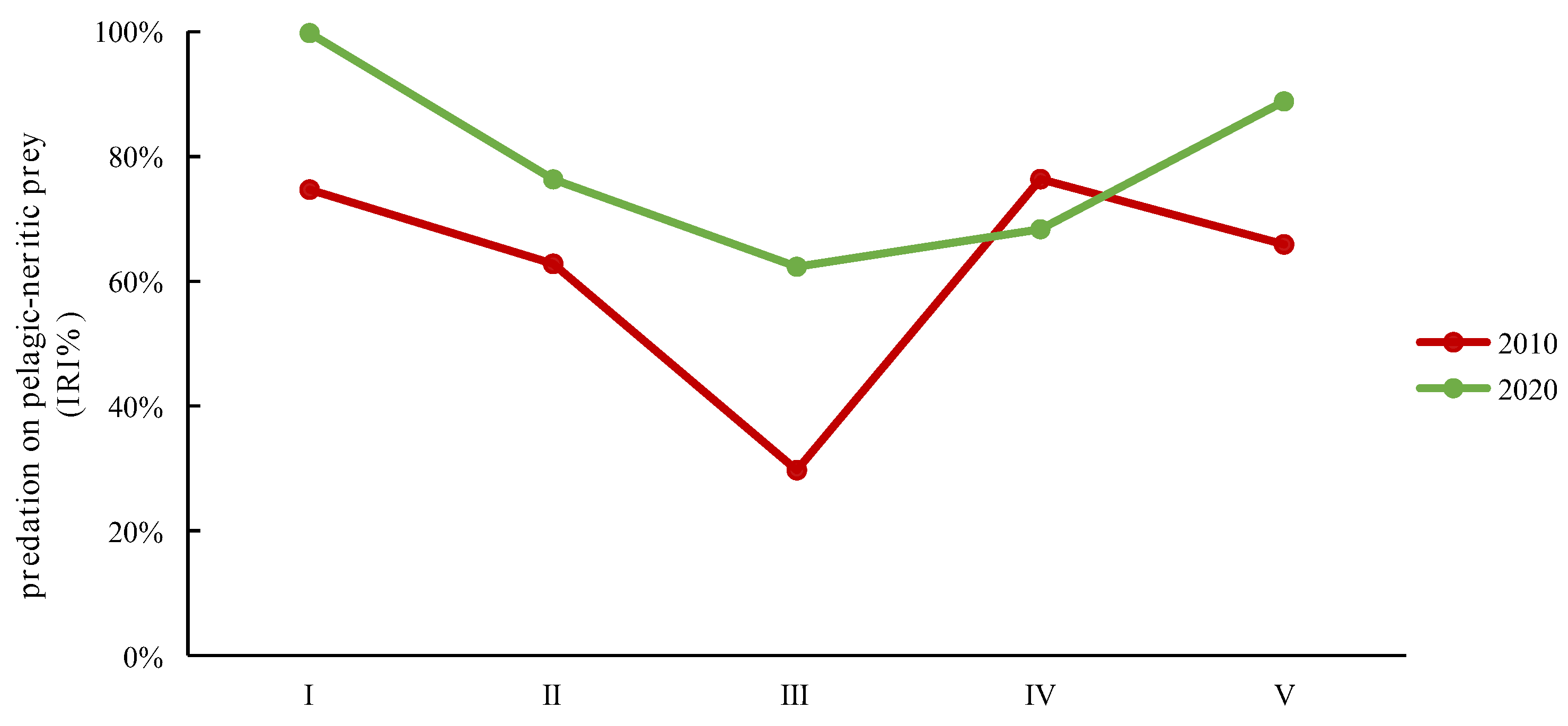

| Year | Size Class | Body Length (BL cm) | Individuals Sampled | Stomachs Analyzed | Vacuity Index (%) | Stable Isotopes Analysis |
|---|---|---|---|---|---|---|
| 2010 | I | <14.1 | 82 | 38 | 36.67 | 35 |
| II | 14.1–17.0 | 221 | 97 | 38.99 | 42 | |
| III | 17.1–20.0 | 255 | 149 | 26.24 | 43 | |
| IV | 20.1–23.0 | 92 | 47 | 32.37 | 26 | |
| V | >23.0 | 20 | 7 | 48.15 | 18 | |
| Total specimens | 670 | 338 | 49.55 | 164 | ||
| 2020 | I | <14.1 | 36 | 21 | 26.32 | 23 |
| II | 14.1–17.0 | 182 | 86 | 35.82 | 66 | |
| III | 17.1–20.0 | 266 | 130 | 34.34 | 54 | |
| IV | 20.1–23.0 | 166 | 78 | 36.07 | 43 | |
| V | >23.0 | 58 | 21 | 46.84 | 24 | |
| Total specimens | 708 | 336 | 52.54 | 210 |
| Year | Size Class | BL Mean ± s.d. | GSI% Mean ± s.d. | RI% Mean ± s.d. | δ15N Mean ± s.d. |
|---|---|---|---|---|---|
| 2010 | I | 12.03 ± 0.64 | 0.16 ± 0.10 d | 19.82 ± 9.82 c | 12.94 ± 1.31 b |
| II | 15.23 ± 1.07 | 0.66 ± 1.16 cd | 14.54 ± 9.22 b | 13.52 ± 1.53 ab | |
| III | 18.18 ± 0.79 | 1.25 ± 1.99 bc | 10.09 ± 7.57 a | 13.74 ± 1.30 ab | |
| IV | 21.23 ± 0.80 | 2.65 ± 2.71 a | 13.57 ± 9.64 ab | 13.96 ± 1.08 a | |
| V | 24.80 ± 1.52 | 3.06 ± 2.93 ab | 10.55 ± 8.41 abc | 13.69 ± 1.11 ab | |
| 2020 | I | 13.18 ± 0.79 | 0.10 ± 0.10 BC | 13.17 ± 4.63 AB | 12.44 ± 1.03 B |
| II | 15.77 ± 0.85 | 0.33 ± 0.84 B | 10.36 ± 7.93 AB | 13.08 ± 0.91 A | |
| III | 18.52 ± 0.87 | 1.28 ± 1.71 C | 10.06 ± 10.18 A | 13.15 ± 0.98 A | |
| IV | 21.35 ± 0.86 | 2.78 ± 2.98 A | 13.98 ± 10.73 B | 13.43 ± 0.78 A | |
| V | 24.23 ± 0.93 | 4.00 ± 3.30 A | 11.71 ± 11.18 AB | 13.46 ± 0.76 A |
| Taxa | Habitat | Behavior | 2010 | 2020 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N% | F% | W% | IRI | IRI% | N% | F% | W% | IRI | IRI% | |||
| Crustacea | 5.96 | 3.53 | 1.61 | 26.77 | 0.17 | 3.22 | 3.80 | 0.96 | 15.88 | 0.12 | ||
| Scyllaridae | ||||||||||||
| Scyllarus sp. | B | - | - | - | - | - | 0.23 | 0.27 | 0.08 | 0.08 | 0.01 | |
| Penaeidae | ||||||||||||
| Metapenaeopsis barbata | B | 1.65 | 1.90 | 1.08 | 5.19 | 0.34 | 0.23 | 0.27 | 0.03 | 0.07 | 0.01 | |
| Metapenaeopsis acclivis | B | - | - | - | - | - | 0.46 | 0.54 | 0.24 | 0.38 | 0.03 | |
| Trachypenaeus curvirostris | B | 0.95 | 1.09 | 0.48 | 1.55 | 0.10 | 0.69 | 0.82 | 0.11 | 0.66 | 0.06 | |
| Solenoceridae | ||||||||||||
| Solenocera sp. | B | - | - | - | - | - | 0.46 | 0.54 | 0.04 | 0.27 | 0.02 | |
| Solenocera crassicornis | B | - | - | - | - | - | 0.23 | 0.27 | 0.14 | 0.10 | 0.01 | |
| Squillidae | ||||||||||||
| Oratosquilla.sp. | B | 0.47 | 0.54 | 0.06 | 0.29 | 0.02 | 0.69 | 0.82 | 0.12 | 0.66 | 0.06 | |
| Oratosquilla oratoria | B | - | - | - | - | - | 0.23 | 0.27 | 0.19 | 0.11 | 0.01 | |
| Cephalopoda | 7.57 | 8.42 | 12.13 | 165.97 | 1.08 | 11.95 | 13.59 | 32.75 | 607.43 | 4.63 | ||
| Loliginidae | ||||||||||||
| Loligo sp. | P | C | 5.20 | 5.71 | 5.63 | 61.81 | 4.04 | 6.90 | 7.88 | 15.82 | 179.04 | 15.23 |
| Uroteuthis chinensis | P | C | 2.36 | 2.45 | 6.34 | 21.30 | 1.39 | 2.30 | 2.45 | 7.02 | 22.79 | 1.94 |
| Uroteuthis duvauceli | P | C | 0.24 | 0.27 | 0.16 | 0.11 | 0.01 | 2.76 | 3.26 | 9.91 | 41.31 | 3.51 |
| Fishes | 86.47 | 88.04 | 86.25 | 15,206.96 | 98.75 | 84.83 | 82.61 | 66.29 | 12,483.70 | 95.24 | ||
| Clupeidae | ||||||||||||
| Sardinella jussieu | P | C, M | 4.26 | 4.35 | 7.44 | 50.84 | 3.32 | 0.23 | 0.27 | 0.29 | 0.14 | 0.01 |
| Sardinella lemuru | P | C, M | - | - | - | - | - | 3.68 | 4.35 | 12.40 | 69.91 | 5.95 |
| Engraulidae | ||||||||||||
| Stolephorus indicus | P | C | 7.57 | 7.34 | 5.19 | 93.60 | 6.12 | 5.52 | 3.53 | 3.35 | 31.32 | 2.67 |
| Stolephorus commersonnii | P | C | 0.24 | 0.27 | 0.04 | 0.08 | 0.00 | 1.38 | 1.36 | 0.65 | 2.76 | 0.23 |
| Stolephorus chinensis | P | C | 0.47 | 0.54 | 0.36 | 0.45 | 0.03 | - | - | - | - | - |
| Stolephorus sp. | P | 6.38 | 6.52 | 5.95 | 80.41 | 5.26 | 20.69 | 21.74 | 6.56 | 592.47 | 50.41 | |
| Thryssa dussumieri | P | C | 0.95 | 1.09 | 0.43 | 1.49 | 0.10 | 3.22 | 3.80 | 2.78 | 22.80 | 1.94 |
| Thryssa setirostris | P | - | - | - | - | - | 0.23 | 0.27 | 0.37 | 0.16 | 0.01 | |
| Thryssa sp. | P | 0.95 | 1.09 | 0.53 | 1.60 | 0.10 | 0.46 | 0.54 | 0.21 | 0.36 | 0.03 | |
| Bregmacerotida | ||||||||||||
| Bregmaceros rarisquamosus | P | C, M | 1.42 | 1.63 | 0.99 | 3.93 | 0.26 | 5.75 | 3.26 | 0.89 | 21.64 | 1.84 |
| Bregmaceros mcclellandii | P | C, M | - | - | - | - | - | 0.23 | 0.27 | 0.19 | 0.11 | 0.01 |
| Bregmaceros sp. | P | - | - | - | - | - | 2.99 | 3.26 | 0.49 | 11.35 | 0.97 | |
| Leiognathidae | ||||||||||||
| Photopectoralis bindus | D | C | 12.29 | 12.50 | 6.98 | 240.89 | 15.74 | 1.84 | 1.90 | 0.55 | 4.55 | 0.39 |
| Secutor ruconius | D | C | - | - | - | - | - | 5.06 | 5.43 | 4.27 | 50.69 | 4.31 |
| leiognathus lineolatus | D | C | 20.57 | 16.30 | 4.66 | 411.39 | 26.88 | - | - | - | - | - |
| Nuchequula nuchalis | D | C | - | - | - | - | - | 1.15 | 0.82 | 0.21 | 1.11 | 0.09 |
| Leiognathus berbis | D | C | - | - | - | - | - | 2.76 | 2.45 | 0.48 | 7.92 | 0.67 |
| Gazza minuta | D | - | - | - | - | - | 0.23 | 0.27 | 0.04 | 0.07 | 0.01 | |
| Leiognathus sp. | D | - | - | - | - | - | 6.67 | 5.98 | 1.53 | 48.97 | 4.17 | |
| Acropomatidae | ||||||||||||
| Acropoma japonicum | D | 7.80 | 7.88 | 6.91 | 115.93 | 7.58 | 3.22 | 2.72 | 1.60 | 13.10 | 1.11 | |
| Apogonidae | ||||||||||||
| Ostorhinchus pleuron | D | 3.07 | 3.53 | 5.17 | 29.10 | 1.90 | 1.38 | 1.63 | 1.26 | 4.30 | 0.37 | |
| Jaydia lineata | D | 0.24 | 0.27 | 0.09 | 0.09 | 0.01 | 0.23 | 0.27 | 0.37 | 0.16 | 0.01 | |
| Ostorhinchus gularis | D | - | - | - | - | - | 0.46 | 0.54 | 0.19 | 0.36 | 0.03 | |
| Jaydia carinatus | D | - | - | - | - | - | 0.46 | 0.54 | 0.97 | 0.78 | 0.07 | |
| Rhabdamia gracilis | D | - | - | - | - | - | 0.23 | 0.27 | 0.07 | 0.08 | 0.01 | |
| Jaydia striata | D | 0.47 | 0.54 | 0.80 | 0.69 | 0.05 | 0.23 | 0.27 | 0.05 | 0.08 | 0.01 | |
| Jaydia poecilopterus | D | 0.24 | 0.27 | 0.28 | 0.14 | 0.01 | 1.15 | 1.36 | 1.24 | 3.24 | 0.28 | |
| Synodontidae | ||||||||||||
| Saurida tumbil | B | 0.71 | 0.82 | 1.92 | 2.14 | 0.14 | 0.46 | 0.54 | 2.58 | 1.65 | 0.14 | |
| Saurida sp. | B | - | - | - | - | - | 1.38 | 1.63 | 2.95 | 7.06 | 0.60 | |
| Carangidae | ||||||||||||
| Decapterus maruadsi | P | C, M | 5.20 | 4.35 | 10.96 | 70.27 | 4.59 | 0.46 | 0.54 | 3.16 | 1.97 | 0.17 |
| Trachurus japonicus | P | C | 10.40 | 11.96 | 16.11 | 316.98 | 20.72 | - | - | - | - | - |
| Siganidae | ||||||||||||
| Siganus fuscescens | D | - | - | - | - | - | 0.23 | 0.27 | 0.67 | 0.25 | 0.02 | |
| Siganus sp. | D | 0.47 | 0.54 | 1.12 | 0.87 | 0.06 | 0.23 | 0.27 | 0.68 | 0.25 | 0.02 | |
| Nemipteridae | ||||||||||||
| Nemipterus nematophorus | D | - | - | - | - | - | 0.23 | 0.27 | 1.71 | 0.53 | 0.04 | |
| Mullidae | ||||||||||||
| Upeneus sulphureus | D | 0.24 | 0.27 | 1.03 | 0.34 | 0.02 | 0.46 | 0.54 | 0.50 | 0.52 | 0.04 | |
| Upeneus bensari | D | 0.24 | 0.27 | 1.59 | 0.50 | 0.03 | 0.46 | 0.27 | 0.28 | 0.20 | 0.02 | |
| Champsodontidae | ||||||||||||
| Champsodon snyderi | B | - | - | - | - | - | 2.53 | 0.82 | 0.43 | 2.41 | 0.21 | |
| Champsodon atridorsalis | B | - | - | - | - | - | 0.69 | 0.82 | 0.20 | 0.73 | 0.06 | |
| Trichiuridae | ||||||||||||
| Trichiurus japonicus | B | - | - | - | - | - | 0.46 | 0.54 | 0.36 | 0.44 | 0.04 | |
| Trichiurus sp. | B | - | - | - | - | - | 2.53 | 2.72 | 3.28 | 15.79 | 1.34 | |
| Sciaenidae | ||||||||||||
| Pennahia pawak | D | - | - | - | - | - | 0.23 | 0.27 | 0.17 | 0.11 | 0.01 | |
| Pennahia argentata | D | - | - | - | - | - | 0.46 | 0.54 | 0.33 | 0.43 | 0.04 | |
| Johnius sp. | D | - | - | - | - | - | 0.46 | 0.54 | 0.77 | 0.67 | 0.06 | |
| Pennahia sp. | D | - | - | - | - | - | 0.23 | 0.27 | 0.05 | 0.08 | 0.01 | |
| Sparidae | ||||||||||||
| Evynnis cardinalis | D | 0.24 | 0.27 | 0.19 | 0.12 | 0.01 | - | - | - | - | - | |
| Gobiidae | ||||||||||||
| Oxyurichthys tentacularis | B | - | - | - | - | - | 0.23 | 0.27 | 0.35 | 0.16 | 0.01 | |
| Trypauchen vagina | B | - | - | - | - | - | 0.23 | 0.27 | 0.02 | 0.07 | 0.01 | |
| Oxyurichthys sp. | B | - | - | - | - | - | 0.23 | 0.27 | 0.34 | 0.15 | 0.01 | |
| Gobiidae sp. | B | 1.89 | 2.17 | 2.82 | 10.25 | 0.67 | 0.69 | 0.82 | 0.44 | 0.92 | 0.08 | |
| Citharidae | ||||||||||||
| Brachypleura novaezeelandiae | B | 1.42 | 1.63 | 2.28 | 6.03 | 0.39 | - | - | - | - | - | |
| Triglidae | ||||||||||||
| Pterygotrigla hemisticta | B | 0.24 | 0.27 | 0.12 | 0.10 | 0.01 | - | - | - | - | - | |
| Soleidae | ||||||||||||
| Solea ovata | B | 0.47 | 0.54 | 1.12 | 0.86 | 0.06 | - | - | - | - | - | |
| Cynoglossidae | ||||||||||||
| Cynoglossus sp. | B | - | - | - | - | - | 0.69 | 0.82 | 0.97 | 1.35 | 0.11 | |
| Callionymidae | ||||||||||||
| Callionymidae sp. | B | - | - | - | - | - | 0.23 | 0.27 | 0.02 | 0.07 | 0.01 | |
| Bothidae | ||||||||||||
| Arnoglossus sp. | B | - | - | - | - | - | 0.23 | 0.27 | 0.12 | 0.09 | 0.01 | |
| Tetraodontidae | ||||||||||||
| Lagocephalus spadiceus | B | - | - | - | - | - | 0.46 | 0.54 | 2.02 | 1.35 | 0.11 | |
| Platycephalidae | ||||||||||||
| Thysanophrys chiltonae | B | 0.47 | 0.54 | 0.72 | 0.65 | 0.04 | - | - | - | - | - | |
| Samaridae | ||||||||||||
| Samaris cristatus | B | 0.24 | 0.27 | 0.47 | 0.19 | 0.01 | - | - | - | - | - | |
| 2010 | 2020 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indices | Total Specimens | I | II | III | IV | V | Total Specimens | I | II | III | IV | V |
| WIC | 0.107 | 0.068 | 0.066 | 0.115 | 0.162 | 0.252 | 0.117 | 0 | 0.026 | 0.094 | 0.224 | 0.187 |
| BIC | 2.630 | 2.225 | 2.455 | 2.384 | 2.599 | 1.516 | 3.151 | 0.730 | 2.340 | 3.193 | 2.662 | 2.414 |
| TNW | 2.737 | 2.293 | 2.521 | 2.498 | 2.761 | 1.768 | 3.267 | 0.730 | 2.366 | 3.287 | 2.887 | 2.602 |
| WIC/TNW | 0.039 | 0.029 | 0.026 | 0.046 | 0.059 | 0.143 | 0.036 | 0 | 0.011 | 0.029 | 0.078 | 0.072 |
| PSi | 0.117 | 0.166 | 0.127 | 0.163 | 0.115 | 0.361 | 0.088 | 0.649 | 0.211 | 0.076 | 0.117 | 0.138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Deng, Y.; Qin, J.; Luo, K.; Kang, B.; He, X.; Yan, Y. Dietary Shifts in the Adaptation to Changing Marine Resources: Insights from a Decadal Study on Greater Lizardfish (Saurida tumbil) in the Beibu Gulf, South China Sea. Animals 2024, 14, 798. https://doi.org/10.3390/ani14050798
Yang X, Deng Y, Qin J, Luo K, Kang B, He X, Yan Y. Dietary Shifts in the Adaptation to Changing Marine Resources: Insights from a Decadal Study on Greater Lizardfish (Saurida tumbil) in the Beibu Gulf, South China Sea. Animals. 2024; 14(5):798. https://doi.org/10.3390/ani14050798
Chicago/Turabian StyleYang, Xiaodong, Yujian Deng, Jiao Qin, Konglan Luo, Bin Kang, Xiongbo He, and Yunrong Yan. 2024. "Dietary Shifts in the Adaptation to Changing Marine Resources: Insights from a Decadal Study on Greater Lizardfish (Saurida tumbil) in the Beibu Gulf, South China Sea" Animals 14, no. 5: 798. https://doi.org/10.3390/ani14050798
APA StyleYang, X., Deng, Y., Qin, J., Luo, K., Kang, B., He, X., & Yan, Y. (2024). Dietary Shifts in the Adaptation to Changing Marine Resources: Insights from a Decadal Study on Greater Lizardfish (Saurida tumbil) in the Beibu Gulf, South China Sea. Animals, 14(5), 798. https://doi.org/10.3390/ani14050798





