The Direct Effects of Climate Change on Tench (Tinca tinca) Sperm Quality under a Real Heatwave Event Scenario
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animal Housing
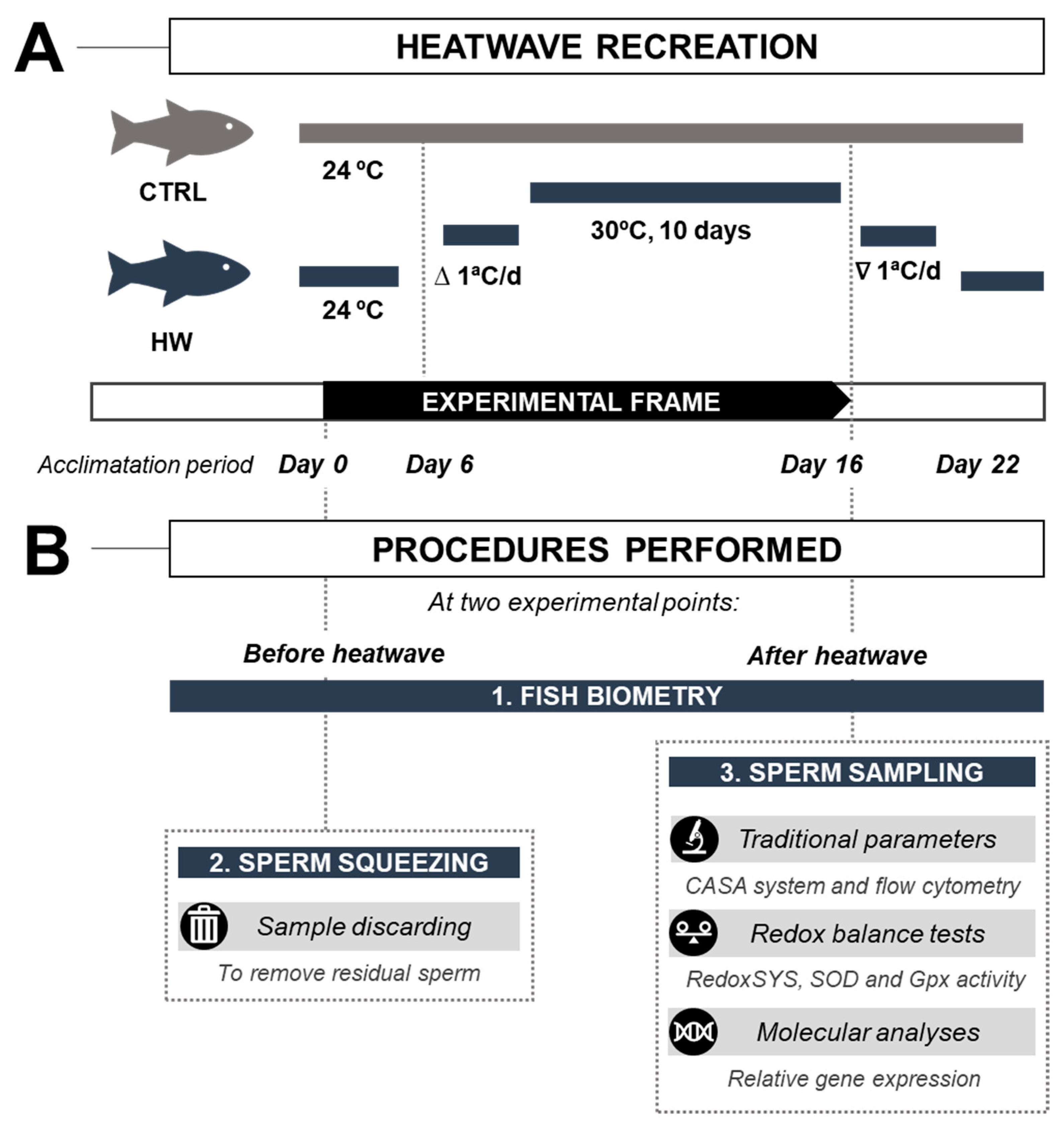
2.3. Heatwave Simulation
2.4. Fish Growth Performance Monitoring and Sperm Collection
2.5. Sperm Motility and Concentration Analyses
2.6. Sperm Flow Cytometry Analysis
2.7. Spermatozoa RedoxSYS Analysis
2.8. SOD and GPx Activities in Seminal Plasma
2.9. Gene Expression Analysis of Spermatozoa
2.10. Statistical Analyses
3. Results
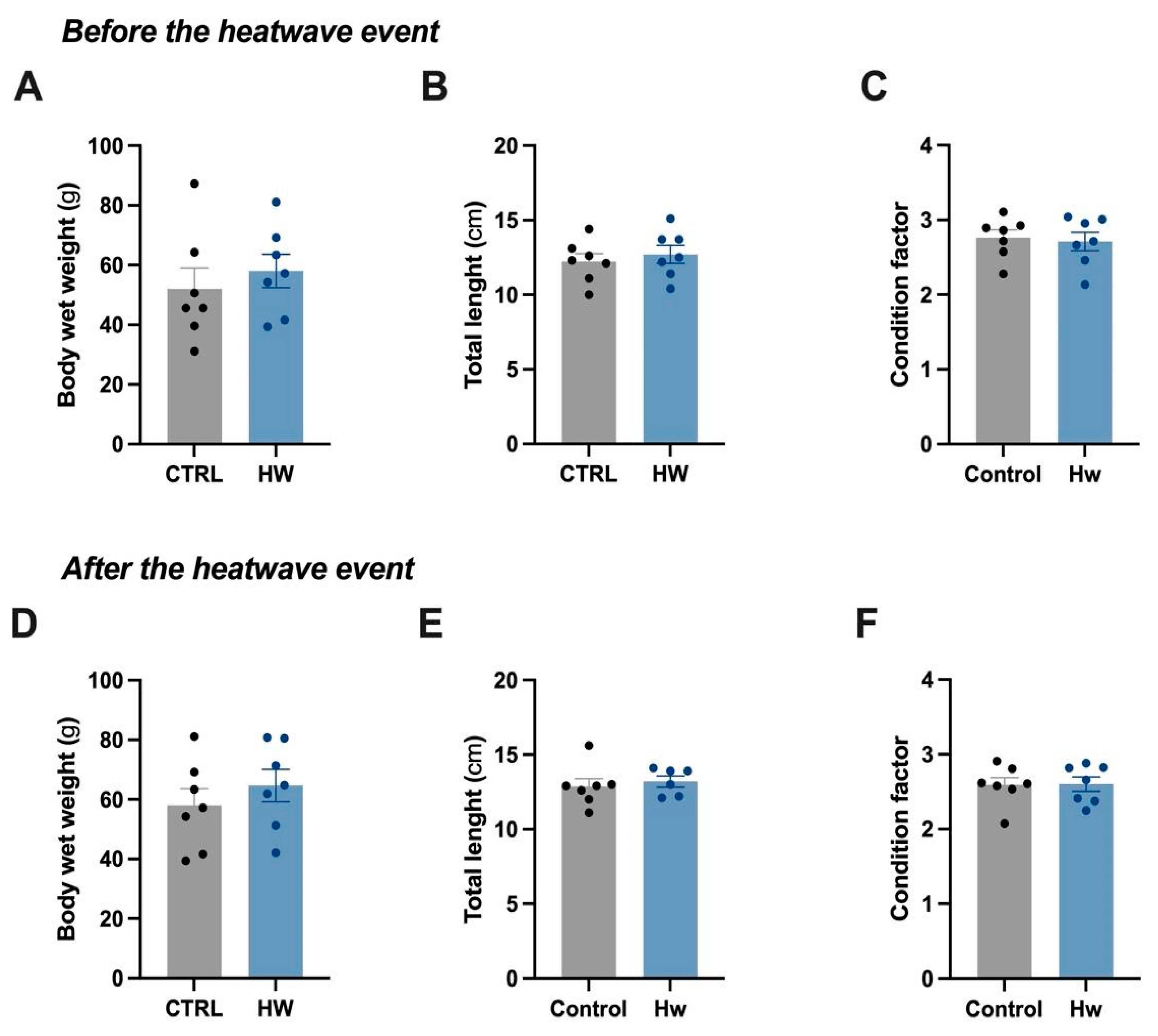
3.1. Biometric Analyses of the Control and Heatwave-Exposed Male Fish
3.2. Sperm Volume, Concentration, and Motility Parameters in Males Exposed to the Heatwave Event and the Control Group
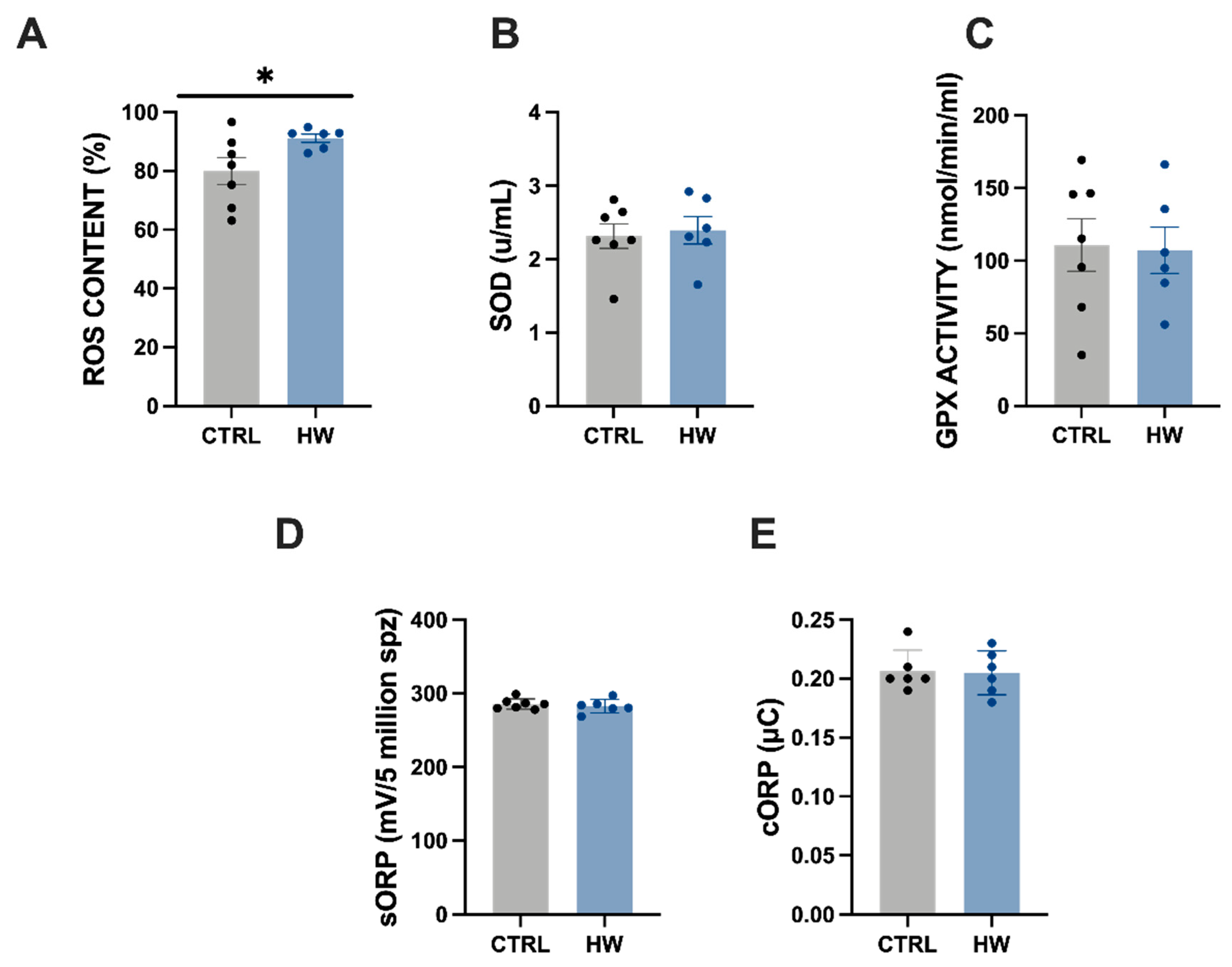
3.3. Seminal Plasma Redox Balance and Spermatozoa Intracellular ROS Content in Males Exposed to the Heatwave Event and the Control Group
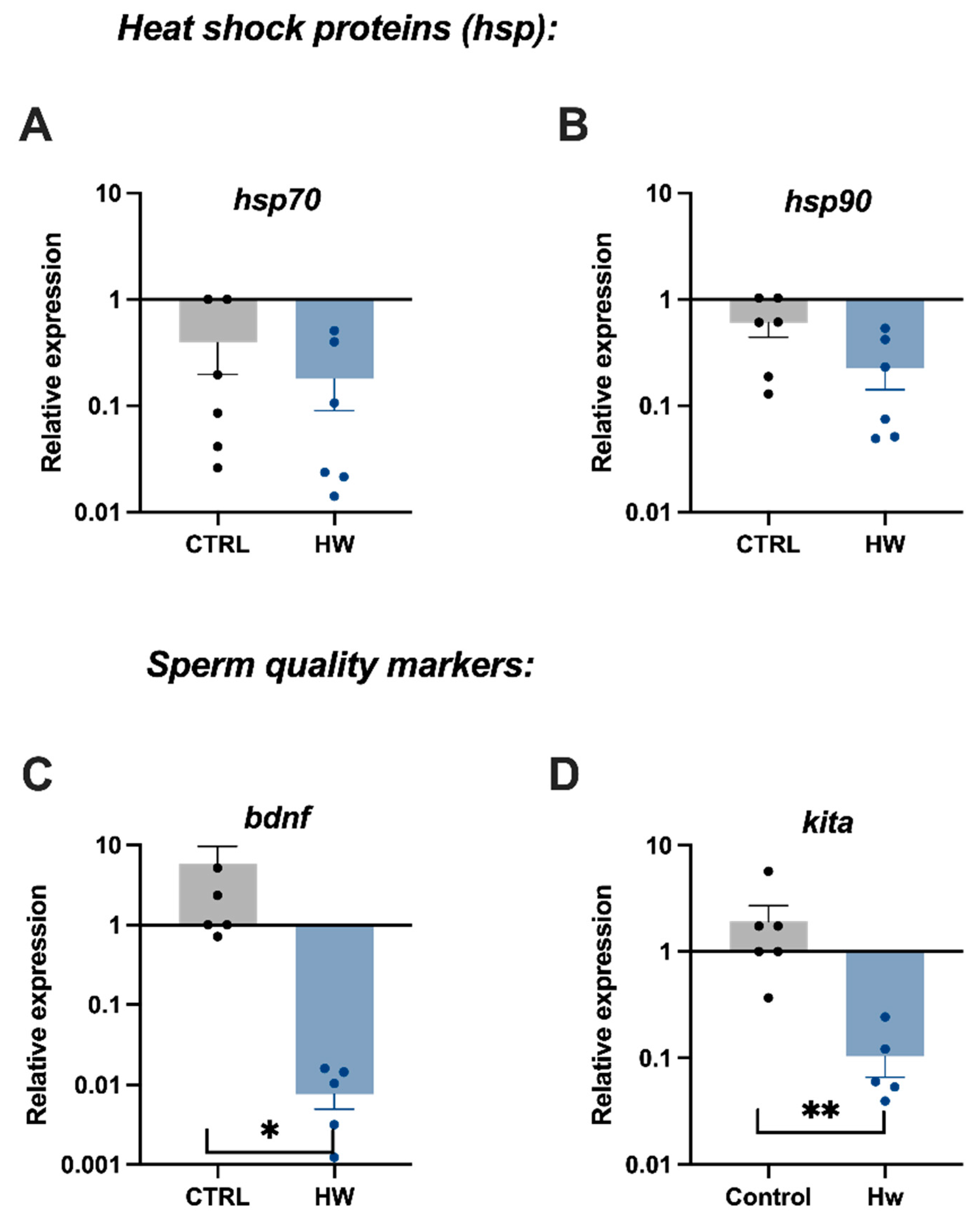
3.4. Gene Expression of Sperm Quality Markers in Males Exposed to the Heatwave Event and the Control Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mameri, D.; Branco, P.; Ferreira, M.T.; Santos, J.M. Heatwave effects on the swimming behaviour of a Mediterranean freshwater fish, the Iberian barbel Luciobarbus bocagei. Sci. Total Environ. 2020, 730, 139152. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2021—The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Sen Gupta, A.; Hobday, A.J.; et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018, 9, 1324. [Google Scholar] [CrossRef]
- Darmaraki, S.; Somot, S.; Sevault, F.; Nabat, P.; Cabos Narvaez, W.D.; Cavicchia, L.; Djurdjevic, V.; Li, L.; Sannino, G.; Sein, D.V. Future evolution of Marine Heatwaves in the Mediterranean Sea. Clim. Dyn. 2019, 53, 1371–1392. [Google Scholar] [CrossRef]
- O’Gorman, E.J.; Ólafsson, Ó.P.; Demars, B.O.L.; Friberg, N.; Guðbergsson, G.; Hannesdóttir, E.R.; Jackson, M.C.; Johansson, L.S.; McLaughlin, Ó.B.; Ólafsson, J.S.; et al. Temperature effects on fish production across a natural thermal gradient. Glob. Chang. Biol. 2016, 22, 3206–3220. [Google Scholar] [CrossRef]
- Hambrey, J. The 2030 Agenda and the Sustainable Development Goals: The challenge for aquaculture development and management. In FAO Fisheries and Aquaculture; Circular No. 1141; FAO: Rome, Italy, 2017. [Google Scholar]
- Reverter, M.; Sarter, S.; Caruso, D.; Avarre, J.C.; Combe, M.; Pepey, E.; Pouyaud, L.; Vega-Heredía, S.; de Verdal, H.; Gozlan, R.E. Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat. Commun. 2020, 11, 1870. [Google Scholar] [CrossRef]
- Crespel, A.; Zambonino-Infante, J.L.; Mazurais, D.; Koumoundouros, G.; Fragkoulis, S.; Quazuguel, P.; Huelvan, C.; Madec, L.; Servili, A.; Claireaux, G. The development of contemporary European sea bass larvae (Dicentrarchus labrax) is not affected by projected ocean acidification scenarios. Mar. Biol. 2017, 164, 155. [Google Scholar] [CrossRef] [PubMed]
- Alix, M.; Kjesbu, O.S.; Anderson, K.C. From gametogenesis to spawning: How climate-driven warming affects teleost reproductive biology. J. Fish Biol. 2020, 97, 607–632. [Google Scholar] [CrossRef]
- Geffroy, B.; Wedekind, C. Effects of global warming on sex ratios in fishes. J. Fish Biol. 2020, 97, 596–606. [Google Scholar] [CrossRef]
- Reid, G.K.; Gurney-Smith, H.J.; Flaherty, M.; Garber, A.F.; Forster, I.; Brewer-Dalton, K.; Knowler, D.; Marcogliese, D.J.; Chopin, T.; Moccia, R.D.; et al. Climate change and aquaculture: Considering adaptation potential. Aquac. Environ. Interact. 2019, 11, 603–624. [Google Scholar] [CrossRef]
- Kuczynski, L.; Chevalier, M.; Laffaille, P.; Legrand, M.; Grenouillet, G. Indirect effect of temperature on fish population abundances through phenological changes. PLoS ONE 2017, 12, e0175735. [Google Scholar] [CrossRef]
- Boudry, P.; Allal, F.; Aslam, M.L.; Bargelloni, L.; Bean, T.P.; Brard-Fudulea, S.; Brieuc, M.S.O.; Calboli, F.C.F.; Gilbey, J.; Haffray, P.; et al. Current status and potential of genomic selection to improve selective breeding in the main aquaculture species of International Council for the Exploration of the Sea (ICES) member countries. Aquac. Rep. 2021, 20, 100700. [Google Scholar] [CrossRef]
- Allal, F.; Nguyen, N.H. Genomic Selection in Aquaculture Species. Methods Mol. Biol. 2022, 2467, 469–491. [Google Scholar] [CrossRef]
- Teletchea, F.; Fontaine, P. Levels of domestication in fish: Implications for the sustainable future of aquaculture. Fish Fish. 2014, 15, 181–195. [Google Scholar] [CrossRef]
- Servili, A.; Canario, A.V.M.; Mouchel, O.; Muñoz-Cueto, J.A. Climate change impacts on fish reproduction are mediated at multiple levels of the brain-pituitary-gonad axis. Gen. Comp. Endocrinol. 2020, 291, 113439. [Google Scholar] [CrossRef]
- Ospina-Álvarez, N.; Piferrer, F. Temperature-Dependent Sex Determination in Fish Revisited: Prevalence, a Single Sex Ratio Response Pattern, and Possible Effects of Climate Change. PLoS ONE 2008, 3, e2837. [Google Scholar] [CrossRef]
- Martínez, P.; Viñas, A.M.; Sánchez, L.; Díaz, N.; Ribas, L.; Piferrer, F. Genetic architecture of sex determination in fish: Applications to sex ratio control in aquaculture. Front. Genet. 2014, 5, 340. [Google Scholar] [CrossRef]
- Penman, D.J.; Piferrer, F. Fish Gonadogenesis. Part I: Genetic and Environmental Mechanisms of Sex Determination. Rev. Fish. Sci. 2008, 16, 16–34. [Google Scholar] [CrossRef]
- Navarro-Martín, L.; Viñas, J.; Ribas, L.; Díaz, N.; Gutiérrez, A.; Di Croce, L.; Piferrer, F. DNA Methylation of the Gonadal Aromatase (cyp19a) Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass. PLoS Genet. 2011, 7, e1002447. [Google Scholar] [CrossRef]
- Bobe, J.; Labbé, C. Egg and sperm quality in fish. Gen. Comp. Endocrinol. 2010, 165, 535–548. [Google Scholar] [CrossRef]
- Lema, S.C.; Luckenbach, J.A.; Yamamoto, Y.; Housh, M.J. Fish reproduction in a warming world: Vulnerable points in hormone regulation from sex determination to spawning. Phil. Trans. R. Soc. B 2024, 379, 20220516. [Google Scholar] [CrossRef] [PubMed]
- Bobe, J. Egg quality in fish: Present and future challenges. Anim. Front. 2015, 5, 66–72. [Google Scholar] [CrossRef]
- Kandalski, P.K.; de Souza, M.R.D.P.; Herrerias, T.; Machado, C.; Zaleski, T.; Forgati, M.; Guillen, A.C.; Viana, D.; Moura, M.O.; Donatti, L. Effects of short-term thermal stress on the plasma biochemical profiles of two Antarctic nototheniid species. Rev. Fish Biol. Fish. 2018, 28, 925–940. [Google Scholar] [CrossRef]
- Sharma, U. Paternal contributions to offspring health: Role of sperm small RNAs in intergenerational transmission of epigenetic information. Front. Cell Dev. Biol. 2019, 7, 215. [Google Scholar] [CrossRef]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014, 17, 667. [Google Scholar] [CrossRef]
- Parisi, G.; Terova, G.; Gasco, L.; Piccolo, G.; Roncarati, A.; Moretti, V.M.; Centoducati, G.; Gatta, P.P.; Pais, A. Current status and future perspectives of Italian finfish aquaculture. Rev. Fish Biol. Fish. 2014, 24, 15–73. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br. J. Pharmacol. 2020, 177, 3617. [Google Scholar] [CrossRef]
- Álvarez-Cobelas, M.; Fernández-López, J. A Primer on the Limnology of Some Castilian Wetlands (Central Spain); LimnoIberia nº 1; Grupo de Investigación del Agua: Madrid, Spain, 2013; 239p, Available online: https://bibdigital.rjb.csic.es/idurl/1/16350 (accessed on 14 February 2024).
- Ferreira-Rodríguez, N.; Fernández, I.; Cancela, M.L.; Pardo, I. Multibiomarker response shows how native and non-native freshwater bivalves differentially cope with heat-wave events. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 934–943. [Google Scholar] [CrossRef]
- Infobae. Available online: https://www.infobae.com/espana/2023/08/09/la-ola-de-calor-mas-larga-de-la-historia-de-espana-26-dias-de-temperaturas-disparadas/ (accessed on 14 February 2024).
- Jesenšek, D.; Horváth, A.; Bernáth, G. Application of sperm cryopreservation to hatchery practice and species conservation: A case of the Adriatic grayling (Thymallus thymallus). Aquaculture 2012, 358–359, 213–215. [Google Scholar] [CrossRef]
- Riesco, M.F.; Anel-Lopez, L.; Neila-Montero, M.; Palacin-Martinez, C.; Montes-Garrido, R.; Alvarez, M.; de Paz, P.; Anel, L. Proakap4 as novel molecular marker of sperm quality in ram: An integrative study in fresh, cooled and cryopreserved sperm. Biomolecules 2020, 10, 1046. [Google Scholar] [CrossRef]
- Arthur, J.R.; Boyne, R. Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci. 1985, 36, 1569–1575. [Google Scholar] [CrossRef]
- Derveaux, S.; Vandesompele, J.; Hellemans, J. How to do successful gene expression analysis using real-time PCR. Methods 2010, 50, 227–230. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009; pp. 1–302. [Google Scholar]
- Abram, P.K.; Boivin, G.; Moiroux, J.; Brodeur, J. Behavioural effects of temperature on ectothermic animals: Unifying thermal physiology and behavioural plasticity. Biol. Rev. 2017, 92, 1859–1876. [Google Scholar] [CrossRef]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef]
- James, R.S. A review of the thermal sensitivity of the mechanics of vertebrate skeletal muscle. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2013, 183, 723–733. [Google Scholar] [CrossRef]
- Scharsack, J.P.; Franke, F. Temperature effects on teleost immunity in the light of climate change. J. Fish Biol. 2022, 101, 780–796. [Google Scholar] [CrossRef]
- van Denderen, D.; Gislason, H.; van den Heuvel, J.; Andersen, K.H. Global analysis of fish growth rates shows weaker responses to temperature than metabolic predictions. Glob. Ecol. Biogeogr. 2020, 29, 2203–2213. [Google Scholar] [CrossRef]
- Réalis-Doyelle, E.; Pasquet, A.; De Charleroy, D.; Fontaine, P.; Teletchea, F. Strong Effects of Temperature on the Early Life Stages of a Cold Stenothermal Fish Species, Brown Trout (Salmo trutta L.). PLoS ONE 2016, 11, e0155487. [Google Scholar] [CrossRef]
- Cordova-De la Cruz, S.E.; Riesco, M.F.; Martínez-Bautista, G.; Calzada-Ruiz, D.; Martínez-Burguete, T.; Peña-Marín, E.S.; Álvarez-Gonzalez, C.A.; Fernández, I. Larval Development in Tropical Gar (Atractosteus tropicus) Is Dependent on the Embryonic Thermal Regime: Ecological Implications under a Climate Change Context. Fishes 2022, 7, 16. [Google Scholar] [CrossRef]
- Fenkes, M.; Fitzpatrick, J.L.; Shiels, H.A.; Nudds, R.L. Acclimation temperature changes spermatozoa flagella length relative to head size in brown trout. Biol. Open 2019, 8, 039461. [Google Scholar] [CrossRef]
- Fenkes, M.; Fitzpatrick, J.L.; Ozolina, K.; Shiels, H.A.; Nudds, R.L. Sperm in hot water: Direct and indirect thermal challenges interact to impact on brown trout sperm quality. J. Exp. Biol. 2017, 220, 2513–2520. [Google Scholar] [CrossRef]
- Miranda, L.A.; Chalde, T.; Elisio, M.; Strüssmann, C.A. Effects of global warming on fish reproductive endocrine axis, with special emphasis in pejerrey Odontesthes bonariensis. Gen. Comp. Endocrinol. 2013, 192, 45–54. [Google Scholar] [CrossRef]
- Hani, Y.M.I.; Turies, C.; Palluel, O.; Delahaut, L.; Bado-Nilles, A.; Geffard, A.; Dedourge-Geffard, O.; Porcher, J.M. Effects of a chronic exposure to different water temperatures and/or to an environmental cadmium concentration on the reproduction of the threespine stickleback (Gasterosteus aculeatus). Ecotoxicol. Environ. Saf. 2019, 174, 48–57. [Google Scholar] [CrossRef]
- Tovo-Neto, A.; Martinez, E.R.M.; Melo, A.G.; Doretto, L.B.; Butzge, A.J.; Rodrigues, M.S.; Nakajima, R.T.; Habibi, H.R.; Nóbrega, R.H. Cortisol directly stimulates spermatogonial differentiation, meiosis, and spermiogenesis in zebrafish (Danio rerio) testicular explants. Biomolecules 2020, 10, 429. [Google Scholar] [CrossRef]
- Al Fatle, F.A.; Meleg, E.E.; Sallai, Z.; Szabó, G.; Várkonyi, E.; Urbányi, B.; Kovács, B.; Molnár, T.; Lehoczky, I. Genetic Structure and Diversity of Native Tench (Tinca tinca L. 1758) Populations in Hungary—Establishment of Basic Knowledge Base for a Breeding Program. Diversity 2022, 14, 336. [Google Scholar] [CrossRef]
- Butzge, A.J.; Yoshinaga, T.T.; Acosta, O.D.M.; Fernandino, J.I.; Sanches, E.A.; Tabata, Y.A.; de Oliveira, C.; Takahashi, N.S.; Hattori, R.S. Early warming stress on rainbow trout juveniles impairs male reproduction but contrastingly elicits intergenerational thermotolerance. Sci. Rep. 2021, 11, 17053. [Google Scholar] [CrossRef]
- Haverinen, J.; Vornanen, M. Responses of action potential and K+ currents to temperature acclimation in fish hearts: Phylogeny or thermal preferences? Physiol. Biochem. Zool. 2009, 82, 468–482. [Google Scholar] [CrossRef]
- Vinagre, C.; Narciso, L. Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol. Indic. 2012, 23, 274–279. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Devret, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Kaludercic, N.; Soni, D.; Di Lisa, F. Reactive Oxygen Species and Redox Compartmentalization. Front. Physiol. 2014, 5, 285. [Google Scholar] [CrossRef]
- Pahune, P.P.; Choudhari, A.R.; Muley, P.A. The Total Antioxidant Power of Semen and Its Correlation with the Fertility Potential of Human Male Subjects. J. Clin. Diagn. Res. 2013, 7, 991. [Google Scholar] [CrossRef]
- Cabrita, E.; Martínez-Páramo, S.; Gavaia, P.J.J.; Riesco, M.F.F.; Valcarce, D.G.G.; Sarasquete, C.; Herráez, M.P.P.; Robles, V.; Herraez, M.P.; Robles, V.; et al. Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture 2014, 432, 389–401. [Google Scholar] [CrossRef]
- Słowińska, M.; Nynca, J.; Cejko, B.I.; Dietrich, M.A.; Horváth, Á.; Urbányi, B.; Kotrik, L.; Ciereszko, A. Total antioxidant capacity of fish seminal plasma. Aquaculture 2013, 400–401, 101–104. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef]
- Kowalczyk, A. The Role of the Natural Antioxidant Mechanism in Sperm Cells. Reprod. Sci. 2022, 29, 1387–1394. [Google Scholar] [CrossRef]
- Félix, F.; Oliveira, C.C.V.; Cabrita, E. Antioxidants in Fish Sperm and the Potential Role of Melatonin. Antioxidants 2021, 10, 36. [Google Scholar] [CrossRef]
- Soren, S.; Singh, S.V.; Singh, P. Influence of season on seminal antioxidant enzymes in Karan Friesbulls under tropical climatic conditions. Turkish J. Vet. Anim. Sci. 2016, 40, 797–802. [Google Scholar] [CrossRef]
- El-Zeftawy, M.; Mahmoud, G.B.; Hassan, M. Impact of thermal stress exposure on seminal quality, antioxidant defence system, TNF-α and TIMP-3 in Ossimi ram. Reprod. Domest. Anim. 2020, 55, 870–881. [Google Scholar] [CrossRef]
- Ngoula, F.; Lontio, F.A.; Tchoffo, H.; Manfo Tsague, F.P.; Djeunang, R.M.; Vemo, B.N.; Moffo, F.; Djuissi Motchewo, N. Heat Induces Oxidative Stress: Reproductive Organ Weights and Serum Metabolite Profile, Testes Structure, and Function Impairment in Male Cavy (Cavia porcellus). Front. Vet. Sci. 2020, 7, 471335. [Google Scholar] [CrossRef]
- Barradas, V.; Antoniassi, M.P.; Intasqui, P.; Nichi, M.; Bertolla, R.P.; Spaine, D.M. Evaluation of oxidative stress in seminal plasma of adolescents with varicocele. Reprod. Fertil. 2021, 2, 141. [Google Scholar] [CrossRef]
- Peña, F.J.; O’Flaherty, C.; Ortiz Rodríguez, J.M.; Martín Cano, F.E.; Gaitskell-Phillips, G.L.; Gil, M.C.; Ferrusola, C.O. Redox Regulation and Oxidative Stress: The Particular Case of the Stallion Spermatozoa. Antioxidants 2019, 8, 567. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Ostermeier, G.C.; Dix, D.J.; Miller, D.; Khatri, P.; Krawetz, S.A. Spermatozoal RNA profiles of normal fertile men. Lancet 2002, 360, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, S.; Paasch, U.; Glander, H.-J.; Anderegg, U. Mature human spermatozoa do not transcribe novel RNA. Andrologia 2005, 37, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, M.; Garrido, N.; Simón, C.; Pellicer, A.; Remohí, J. Concentration of glutathione and expression of glutathione peroxidases 1 and 4 in fresh sperm provide a forecast of the outcome of cryopreservation of human spermatozoa. J. Androl. 2004, 25, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Riesco, M.F.; Valcarce, D.G.; Martínez-Vázquez, J.M.; Robles, V. Effect of low sperm quality on progeny: A study on zebrafish as model species. Sci. Rep. 2019, 9, 11192. [Google Scholar] [CrossRef]
- Riesco, M.F.; Valcarce, D.G.; Martínez-Vázquez, J.M.; Martín, I.; Calderón-García, A.Á.; Gonzalez-Nunez, V.; Robles, V. Male reproductive dysfunction in Solea senegalensis: New insights into an unsolved question. Reprod. Fertil. Dev. 2019, 31, 1104–1115. [Google Scholar] [CrossRef]
- Guerra, S.M.; Valcarce, D.G.; Cabrita, E.; Robles, V. Analysis of transcripts in gilthead seabream sperm and zebrafish testicular cells: mRNA profile as a predictor of gamete quality. Aquaculture 2013, 406, 28–33. [Google Scholar] [CrossRef]
- Altuvia, Y.; Landgraf, P.; Lithwick, G.; Elefant, N.; Pfeffer, S.; Aravin, A.; Brownstein, M.J.; Tuschl, T.; Margalit, H. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005, 33, 2697–2706. [Google Scholar] [CrossRef]
- Purandhar, K.; Jena, P.K.; Prajapati, B.; Rajput, P.; Seshadri, S. Understanding the role of heat shock protein isoforms in male fertility, aging and apoptosis. World J. Mens. Health 2014, 32, 123–132. [Google Scholar] [CrossRef]
- De Maio, A. Heat shock proteins: Facts, thoughts, and dreams. Shock 1999, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.A.; Buckley, B.A.; Podrabsky, J.E.; Stillman, J.H.; Tomanek, L. Transcriptomic responses to environmental temperature in eurythermal and stenothermal fishes. J. Exp. Biol. 2015, 218, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liang, X.; Zhang, Y.; Li, Y.; Cao, X.; Gao, J. Cloning of three heat shock protein genes (HSP70, HSP90α and HSP90β) and their expressions in response to thermal stress in loach (Misgurnus anguillicaudatus) fed with different levels of vitamin C. Fish Shellfish Immunol. 2017, 66, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I.S. A Review on the Involvement of Heat Shock Proteins (Extrinsic Chaperones) in Response to Stress Conditions in Aquatic Organisms. Antioxidants 2023, 12, 1444. [Google Scholar] [CrossRef] [PubMed]
- Madeira, D.; Mendonça, V.; Dias, M.; Roma, J.; Costa, P.M.; Larguinho, M.; Vinagre, C.; Diniz, M.S. Physiological, cellular and biochemical thermal stress response of intertidal shrimps with different vertical distributions: Palaemon elegans and Palaemon serratus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 183, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Günther, O.P.; Houde, A.L.; Li, S.; Ming, T.J.; Jeffries, K.M.; Hinch, S.G.; Miller, K.M. Developing specific molecular biomarkers for thermal stress in salmonids. BMC Genom. 2018, 19, 749. [Google Scholar] [CrossRef]
- Qin, H.; Long, Z.; Huang, Z.; Ma, J.; Kong, L.; Lin, Y.; Lin, H.; Zhou, S.; Li, Z. A Comparison of the Physiological Responses to Heat Stress of Two Sizes of Juvenile Spotted Seabass (Lateolabrax maculatus). Fishes 2023, 8, 340. [Google Scholar] [CrossRef]
- Pandey, A.; Rajesh, M.; Baral, P.; Sarma, D.; Tripathi, P.H.; Akhtar, M.S.; Ciji, A.; Dubey, M.K.; Pande, V.; Sharma, P.; et al. Concurrent changes in thermal tolerance thresholds and cellular heat stress response reveals novel molecular signatures and markers of high temperature acclimation in rainbow trout. J. Therm. Biol. 2021, 102, 103124. [Google Scholar] [CrossRef]
- Najafi, A.; Amidi, F.; Sedighi Gilani, M.A.; Moawad, A.R.; Asadi, E.; Khanlarkhni, N.; Fallah, P.; Rezaiian, Z.; Sobhani, A. Effect of brain-derived neurotrophic factor on sperm function, oxidative stress and membrane integrity in human. Andrologia 2017, 49, e12601. [Google Scholar] [CrossRef]
- Valvassori, S.S.; Arent, C.O.; Steckert, A.V.; Varela, R.B.; Jornada, L.K.; Tonin, P.T.; Budni, J.; Mariot, E.; Kapczinski, F.; Quevedo, J. Intracerebral Administration of BDNF Protects Rat Brain Against Oxidative Stress Induced by Ouabain in an Animal Model of Mania. Mol. Neurobiol. 2015, 52, 353–362. [Google Scholar] [CrossRef]
- Safari, H.; Khanlarkhani, N.; Sobhani, A.; Najafi, A.; Amidi, F. Effect of brain-derived neurotrophic factor (BDNF) on sperm quality of normozoospermic men. Hum. Fertil. 2018, 21, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Somero, G.N. The physiology of climate change: How potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 2010, 213, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Chust, G.; Taboada, F.G.; Alvarez, P.; Ibaibarriaga, L. Species acclimatization pathways: Latitudinal shifts and timing adjustments to track ocean warming. Ecol. Indic. 2023, 146, 109752. [Google Scholar] [CrossRef]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E.; Sini, M.; Gerovasileiou, V.; et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob. Chang. Biol. 2022, 28, 5708–5725. [Google Scholar] [CrossRef]

| Gene Name | Accession Number | 5′ to 3′ Sequence | Amplicon Size (bp) |
|---|---|---|---|
| Small ribosomal subunit protein uS17—rps11 | KX082695.1 | F-CATCAGGCGGGACTACTTGC | 86 |
| R- ATGGGGAGAGGTGGACAGACA | |||
| Tubulin alpha chain—tuba | KX082693.1 | F-GGCTGTGTTTGTAGACCTGGA | 123 |
| R-CGGGCGTAGTTATTGGCT | |||
| Large ribosomal subunit protein uL2—rpl8 | KX082694.1 | F-CTCCCACAACCCCGAGACCA | 300 |
| R-AGACCGACCTTGCGTCCAGC | |||
| Elongation factor 1-alpha 1—ef1a | KX082697.1 | F-TGGAGACAGCAAGAACGACCC | 186 |
| R-CGAGCACTGGAGCGTAGCCC | |||
| Glyceraldehyde-3-phosphate dehydrogenase—gapdh | KX082698.1 | F-CGTGTCCCCACCCCCAATGT | 191 |
| R-GCAGCCTTGACCACTTTCTTGATGT | |||
| Heat shock protein HSP 90-alpha 1—hsp90a | GFZX01157613.1; FZX01157620.1; GFZX01157619.1; FZX01157615.1 | F-ACCCCCTCCATCCCATCGTC | 264 |
| R-TGTCCTCATCGCCCTCCAGA | |||
| Heat shock 70 kDa protein—hsp70 | GFZX01034562.1; FZX01034564.1; FZX01034563.1; GFZX01034565.1; GFZX01252235.1; FZX01252238.1; GFZX01252236.1 | F-ACGCAACACCACCATCCCCA | 99 |
| R-GCCCTCTCTCCCTCATACACCTG | |||
| Mast/stem cell growth factor receptor kita—kita | GFZX01104841.1; GFZX01104842.1; GFZX01056577.1; GFZX01056627.1 | F-TTGGAAAGGTGGTTGAGGC | 91 |
| R-TGGGCACTTGGTTTGAGCAT | |||
| Brain-derived neurotrophic factor—bdnf | GFZX01217235.1 | F-CACAGAGTGGGGGCGGGC | 260 |
| R-GGCGGCGTCCAGGTAGTTTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, I.; Larrán, A.M.; de Paz, P.; Riesco, M.F. The Direct Effects of Climate Change on Tench (Tinca tinca) Sperm Quality under a Real Heatwave Event Scenario. Animals 2024, 14, 778. https://doi.org/10.3390/ani14050778
Fernández I, Larrán AM, de Paz P, Riesco MF. The Direct Effects of Climate Change on Tench (Tinca tinca) Sperm Quality under a Real Heatwave Event Scenario. Animals. 2024; 14(5):778. https://doi.org/10.3390/ani14050778
Chicago/Turabian StyleFernández, Ignacio, Ana M. Larrán, Paulino de Paz, and Marta F. Riesco. 2024. "The Direct Effects of Climate Change on Tench (Tinca tinca) Sperm Quality under a Real Heatwave Event Scenario" Animals 14, no. 5: 778. https://doi.org/10.3390/ani14050778
APA StyleFernández, I., Larrán, A. M., de Paz, P., & Riesco, M. F. (2024). The Direct Effects of Climate Change on Tench (Tinca tinca) Sperm Quality under a Real Heatwave Event Scenario. Animals, 14(5), 778. https://doi.org/10.3390/ani14050778






