Quantifying the Impact of Different Dietary Rumen Modulating Strategies on Enteric Methane Emission and Productivity in Ruminant Livestock: A Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
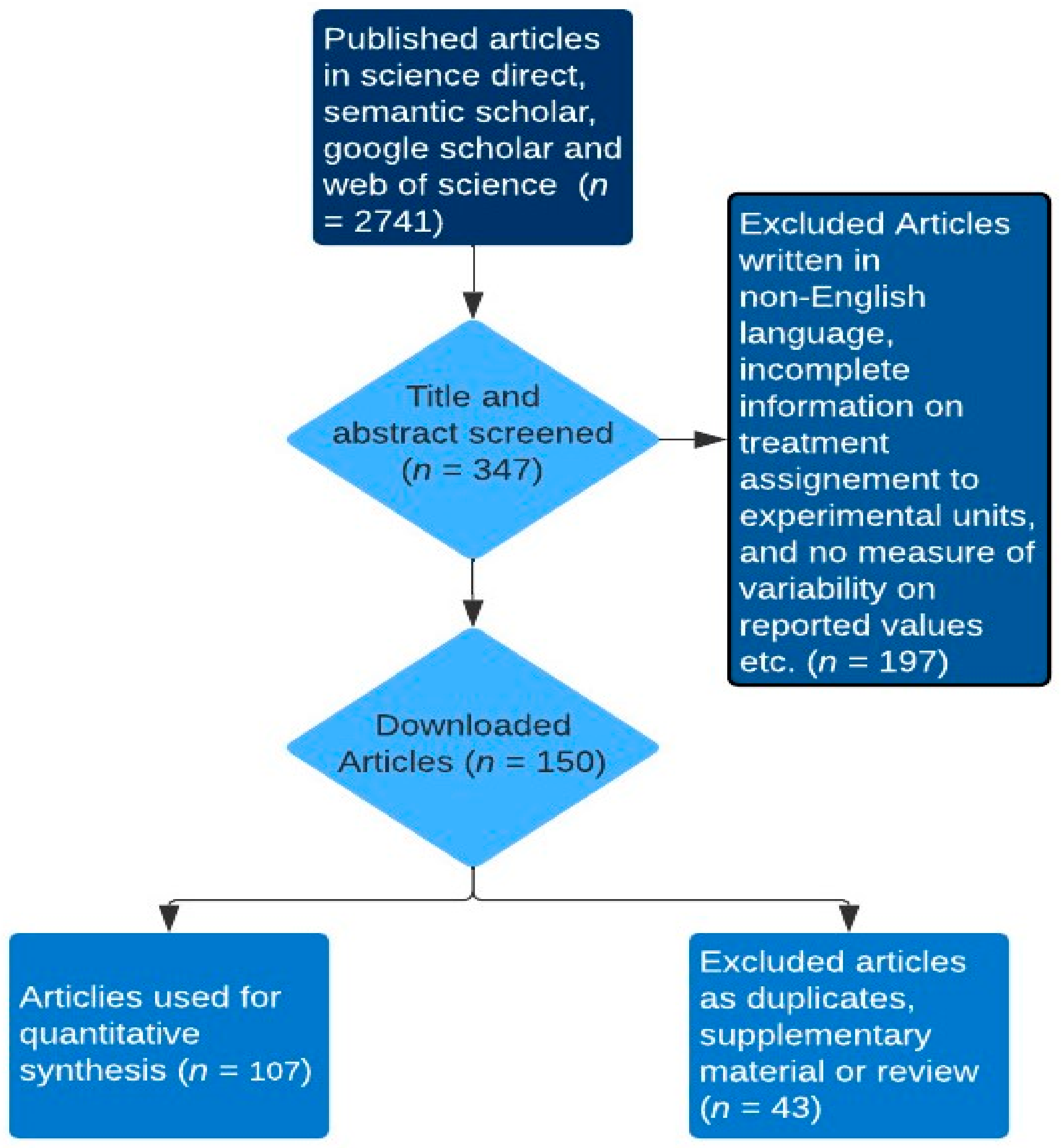
2. Materials and Methods
2.1. Dataset
2.2. Calculations and Statistical Analysis
3. Results
4. Discussion
4.1. Nitrate
4.2. Saponin
4.3. Tannin
4.4. Oils and Ether Extract
4.5. Concentrate Feeding
4.6. Seaweed
4.7. Nitroxypropanol
4.8. Biochar
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Study No. | Reference | Animal Species | Adaptation Period (days) | Experimental Design |
|---|---|---|---|---|
| 1 | Terry et al. [70] | Beef heifers | 14 | Latin square |
| 2 | Doreau et al. [71] | Beef bulls | 14 | RBD |
| 3 | Mirheidari et al. [72] | -She-ep | 14 | CRD |
| 4 | Leng et al. [67] | Beef heifers | 21 | Factorial |
| 5 | Lunsin et al. [73] | Dairy cows | 7 | Latin square |
| 6 | Singla et al. [74] | Buffaloes | 10 | CRD |
| 7 | Marino et al. [75] | Sheep | n.r | Factorial |
| 8 | Kim and Kim. [76] | Beef | n.r | Factorial |
| 9 | Ortiz-Chura et al. [77] | Dairy calves | 30 | Crossover |
| 10 | Al-kindi et al. [78] | Goats | 21 | CRD |
| 11 | Winders et al. [79] | Beef | 8 | CRD |
| 12 | Van Wyngaard et al. [44] | Dairy cows | 14 | Factorial |
| 13 | Valenti et al. [80] | Sheep | n.r | CRD |
| 14 | McAvoy et al. [81] | Sheep | 8 | CRD |
| 15 | Liu et al. [82] | Sheep | 14 | Factorial |
| 16 | Van et al. [83] | Goats | 14 | Latin square |
| 17 | Pen et al. [84] | Sheep | 14 | Latin square |
| 18 | Lind et al. [85] | Sheep | 14 | CRD |
| 19 | Wang et al. [86] | Sheep | 8 | CRD |
| 20 | Mirheidari et al. [74] | Sheep | 14 | CRD |
| 21 | Animut et al. [87] | Goats | 28 | CRD |
| 22 | Conlin. [69] | Beef | 14 | Latin square |
| 23 | Liu et al. [88] | Sheep | n.r | CRD |
| 24 | Poteko et al. [89] | Dairy cows | 21 | CRD |
| 25 | Mirzaei-Alamouti et al. [90] | Dairy cows | 14 | CRD |
| 26 | El-Essawy et al. [91] | Goats | 15 | Latin square |
| 27 | Gerlach et al. [92] | Sheep | 14 | CRD |
| 28 | Jimenez et al. [93] | Goats | 10 | CRD |
| 29 | Molina-Botero et al. [94] | Dairy heifers | 13 | Latin square |
| 30 | Adejoro et al. [95] | Sheep | 21 | Factorial |
| 31 | Nasehi et al. [96] | Sheep | 14 | CRD |
| 32 | Puchala et al. [97] | Goats | 14 | CRD |
| 33 | Zhang et al. [98] | Sheep | 14 | CRD |
| 34 | Vázquez-Carrillo et al. [99] | Beef | 28 | Latin square |
| 35 | Thirumalaisay et al. [100] | Sheep | 37 | CRD |
| 36 | Barbosa et al. [101] | Goats | 15 | CRD |
| 37 | Heidarian et al. [102] | Goats | 30 | CRBD |
| 38 | Hulsof et al. [103] | Beef | 16 | Crossover |
| 39 | Zhang et al. [104] | Sheep | 14 | CRD |
| 40 | Van Zijderveld et al. [105] | Dairy cows | 28 | Crossover |
| 41 | Yang et al. [106] | Goats | 15 | Latin square |
| 42 | Granja-Salcedo et al. [107] | Beef | n.r | Crossover |
| 43 | Hollman et al. [108] | Dairy | 21 | Latin square |
| 44 | Hundal et al. [109] | Goats | n.r | Crossover |
| 45 | Paengkoum et al. [110] | Goats | 14 | Factorial |
| 46 | Nguye et al. [111] | Sheep | 15 | Crossover |
| 47 | Na et al. [112] | Goats and deer | 7 | Latin square |
| 48 | Animut et al. [89] | Goats | 3 | CRD |
| 49 | Pilajun and Wanap [113] | Buffaloes | 7 | CRD |
| 50 | Anassori et al. [54] | Sheep | 10 | Crossover |
| 51 | Benchaar et al. [36] | Dairy cows | 18 | Latin square |
| 52 | Guyader et al. [114] | Dairy cows | 14 | Crossover |
| 53 | Klevenhusen et al. [115] | Sheep | 14 | Latin square |
| 54 | Malik et al. [116] | Sheep | 30 | RBD |
| 55 | Pilajun and Wanap [117] | Buffaloes | 7 | Latin square |
| 56 | Verma et al. [118] | Buffaloes | n.r | Crossover |
| 57 | Tomkins et al. [119] | Beef steers | 14 | Latin square |
| 58 | Polyorach et al. [120] | Dairy heifers | 14 | Latin square |
| 59 | Castro-Montoya et al. [121] | Dairy cows | 14 | Latin square |
| 60 | Hundalet et al. [122] | Buffaloes | 14 | CRD |
| 61 | Inamdar et al. [123] | Buffaloes | n.r | CRD |
| 62 | Yatoo et al. [124] | Buffaloes | n.r | CRD |
| 63 | Yang et al. [125] | Dairy cows | 11 | Latin square |
| 64 | Meale et al. [126] | Dairy cows | 11 | Latin square |
| 65 | Manasri et al. [127] | Beef steers | n.r | Latin square |
| 66 | Manh et al. [128] | Dairy cows | n.r | Latin square |
| 67 | Chaves et al. [129] | Sheep | n.r | CRD |
| 68 | Beauchemin and McGinn [130] | Beef steers | n.r | Latin square |
| 69 | Matloup et al. [131] | Dairy cows | n.r | CRD |
| 70 | Wall et al. [132] | Dairy cows | 7 | CRD |
| 71 | Hristov et al. [51] | Dairy cows | 14 | Latin square |
| 72 | Benchaar et al. [36] | Dairy cows | 14 | Latin square |
| 73 | Giannenas et al. [133] | Dairy Sheep | 7 | CRD |
| 74 | Malik and Singhal [134] | Buffaloes | 14 | Crossover |
| 75 | Chiofalo et al. [135] | Sheep | n.r | CRD |
| 76 | Munoz et al. [136] | Dairy cows | 21 | Crossover |
| 77 | Olijhoek et al. [137] | Dairy cows | 19 | Crossover |
| 78 | Wang et al. [138] | Sheep | 11 | Crossover |
| 79 | Alves et al. [139] | Dairy cows | 21 | CRD |
| 80 | Tekippe et al. [140] | Dairy cows | 21 | Crossover |
| 81 | Sun et al. [141] | Beef steers | 14 | Crossover |
| 82 | Abdalla et al. [142] | Sheep lambs | n.r | CRD |
| 83 | Chilliard et al. [143] | Dairy cows | n.r | Latin square |
| 84 | Patra et al. [144] | Sheep | n.r | RBD |
| 85 | Ramirez-Restrepo et al. [145] | Beef steers | 56 | Crossover |
| 86 | Mao et al. [146] | Sheep | n.r | Factorial |
| 87 | Bayat et al. [147] | Dairy cows | 14 | Latin square |
| 88 | Aguerre et al. [148] | Goats | 14 | Latin square |
| 89 | Olijhoek et al. [149] | Dairy cows | 19 | Crossover |
| 90 | Jiao et al. [46] | Dairy cows | n.r | Crossover |
| 91 | Seremula. [150] | Sheep | 14 | Latin square |
| 92 | Haisan et al. [151] | Dairy cows | 20 | Latin square |
| 93 | Vyas et al. [152] | Beef heifers | 13 | Latin square |
| 94 | Romero-Perez et al. [153] | Beef heifers | 14 | Latin square |
| 95 | Alemu et al. [154] | Beef | 16 | Crossover |
| 96 | Zhang et al. [155] | Beef heifers | 13 | Latin square |
| 97 | Kinley et al. [56] | Beef steers | n.r | RIBD |
| 98 | Stefenoni et al. [57] | Dairy cows | 21 | Latin square |
| 99 | Singh et al. [156] | Dairy cows | 56 | CRD |
| 100 | Roque et al. [54] | Dairy cowa | n.r | Latin square |
| 101 | Barbosa et al. [103] | Goats | 15 | CRD |
| 102 | Phongphanith and Preston [157] | Beef cattle | 15 | Factorial |
| 103 | Terler et al. [158] | Dairy cows | n.r | Latin square |
References
- Gerber, P.; Opio, C. Food and Agriculture Organization of the United Nations. Animal Production and Health Division. In Greenhouse Gas Emmission from Ruminant Supply Chains: A Global Life Cycle Assessment; FAO: Rome, Italy, 2013; ISBN 9789251079454. [Google Scholar]
- MacLeod, M.J.; Vellinga, T.; Opio, C.; Falcucci, A.; Tempio, G.; Henderson, B.; Makkar, H.; Mottet, A.; Robinson, T.; Steinfeld, H.; et al. Invited Review: A Position on the Global Livestock Environmental Assessment Model (GLEAM). Animal 2018, 12, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Kebreab, E.; Hristov, A.N.; Oh, J.; Arndt, C.; Bannink, A.; Bayat, A.R.; Brito, A.F.; Boland, T.; Casper, D.; et al. Prediction of Enteric Methane Production, Yield, and Intensity in Dairy Cattle Using an Intercontinental Database. Glob. Chang. Biol. 2018, 24, 3368–3389. [Google Scholar] [CrossRef] [PubMed]
- Tseten, T.; Sanjorjo, R.A.; Kwon, M.; Kim, S.W. Strategies to Mitigate Enteric Methane Emissions from Ruminant Animals. J. Microbiol. Biotechnol. 2022, 32, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Melgar, A.; Wasson, D.; Arndt, C. Symposium Review: Effective Nutritional Strategies to Mitigate Enteric Methane in Dairy Cattle. J. Dairy Sci. 2022, 105, 8543–8557. [Google Scholar] [CrossRef]
- Jayanegara, A.; Sarwono, K.A.; Kondo, M.; Matsui, H.; Ridla, M.; Laconi, E.B.; Nahrowi. Use of 3-Nitrooxypropanol as Feed Additive for Mitigating Enteric Methane Emissions from Ruminants: A Meta-Analysis. Ital. J. Anim. Sci. 2018, 17, 650–656. [Google Scholar] [CrossRef]
- Belanche, A.; Newbold, C.J.; Morgavi, D.P.; Bach, A.; Zweifel, B.; Yáñez-Ruiz, D.R. A Meta-Analysis Describing the Effects of the Essential Oils Blend Agolin Ruminant on Performance, Rumen Fermentation and Methane Emissions in Dairy Cows. Animals 2020, 10, 620. [Google Scholar] [CrossRef]
- Angeles-Hernandez, J.C.; Vieyra Alberto, R.; Kebreab, E.; Appuhamy, J.A.D.R.N.; Dougherty, H.C.; Castelan-Ortega, O.; Gonzalez-Ronquillo, M. Effect of Forage to Concentrate Ratio and Fat Supplementation on Milk Composition in Dairy Sheep: A Meta-Analysis. Livest. Sci. 2020, 238, 104069. [Google Scholar] [CrossRef]
- van Gastelen, S.; Dijkstra, J.; Bannink, A. Are Dietary Strategies to Mitigate Enteric Methane Emission Equally Effective across Dairy Cattle, Beef Cattle, and Sheep? J. Dairy Sci. 2019, 102, 6109–6130. [Google Scholar] [CrossRef]
- de Souza Congio, G.F.; Bannink, A.; Mayorga Mogollón, O.L.; Jaurena, G.; Gonda, H.; Gere, J.I.; Cerón-Cucchi, M.E.; Ortiz-Chura, A.; Tieri, M.P.; Hernández, O.; et al. Enteric Methane Mitigation Strategies for Ruminant Livestock Systems in the Latin America and Caribbean Region: A Meta-Analysis. J. Clean. Prod. 2021, 312, 127693. [Google Scholar] [CrossRef]
- Arndt, C.; Hristov, A.N.; Price, W.J.; McClelland, S.C.; Pelaez, A.M.; Cueva, S.F.; Oh, J.; Dijkstra, J.; Bannink, A.; Bayat, A.R.; et al. Full Adoption of the Most Effective Strategies to Mitigate Methane Emissions by Ruminants Can Help Meet the 1.5 °C Target by 2030 but Not 2050. Proc. Natl. Acad. Sci. USA 2023, 119, e2111294119. [Google Scholar] [CrossRef]
- Benaouda, M.; Martin, C.; Li, X.; Kebreab, E.; Hristov, A.N.; Yu, Z.; Yáñez-Ruiz, D.R.; Reynolds, C.K.; Crompton, L.A.; Dijkstra, J.; et al. Evaluation of the Performance of Existing Mathematical Models Predicting Enteric Methane Emissions from Ruminants: Animal Categories and Dietary Mitigation Strategies. Anim. Feed Sci. Technol. 2019, 255, 114207. [Google Scholar] [CrossRef]
- Congio, G.F.S.; Bannink, A.; Mayorga, O.L.; Rodrigues, J.P.P.; Bougouin, A.; Kebreab, E.; Silva, R.R.; Maurício, R.M.; da Silva, S.C.; Oliveira, P.P.A.; et al. Prediction of Enteric Methane Production and Yield in Dairy Cattle Using a Latin America and Caribbean Database. Sci. Total Environ. 2022, 825, 153982. [Google Scholar] [CrossRef]
- St-Pierre, N.R. Invited Review. Integrating Quantitative Findings from Multiple Studies Using Mixed Model Methodology. J. Dairy Sci. 2001, 84, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive Statistics and Normality Tests for Statistical Data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K. A Meta-Analysis of the Effect of Dietary Fat on Enteric Methane Production, Digestibility and Rumen Fermentation in Sheep, and a Comparison of These Responses between Cattle and Sheep. Livest. Sci. 2014, 162, 97–103. [Google Scholar] [CrossRef]
- Gurevitch, J.; Hedges, L.V. Statistical Issues in Ecological Meta-Analyses. Ecology 1999, 80, 1142–1149. [Google Scholar] [CrossRef]
- Almeida, A.K.; Hegarty, R.S.; Cowie, A. Meta-Analysis Quantifying the Potential of Dietary Additives and Rumen Modifiers for Methane Mitigation in Ruminant Production Systems. Anim. Nutr. 2021, 7, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Latham, E.A.; Anderson, R.C.; Pinchak, W.E.; Nisbet, D.J. Insights on Alterations to the Rumen Ecosystem by Nitrate and Nitrocompounds. Front. Microbiol. 2016, 7, 228. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.; Hegarty, R.; Van Tol, M.; Godwin, I.; Nolan, J. Dietary Nitrate Metabolism and Enteric Methane Mitigation in Sheep Consuming a Protein-Deficient Diet. Anim. Prod. Sci. 2019, 60, 232–241. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.S. Methane Mitigation from Ruminants Using Tannins and Saponins. Trop. Anim. Health Prod. 2012, 44, 729–739. [Google Scholar] [CrossRef]
- Abarghuei, M.J.; Rouzbehan, Y.; Salem, A.Z.M.; Zamiri, M.J. Nutrient Digestion, Ruminal Fermentation and Performance of Dairy Cows Fed Pomegranate Peel Extract. Livest. Sci. 2013, 157, 452–461. [Google Scholar] [CrossRef]
- Anantasook, N.; Wanapat, M.; Cherdthong, A. Manipulation of Ruminal Fermentation and Methane Production by Supplementation of Rain Tree Pod Meal Containing Tannins and Saponins in Growing Dairy Steers. J. Anim. Physiol. Anim. Nutr. 2014, 98, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, M.P.; Diao, Q.Y.; Tu, Y. Saponin-Induced Shifts in the Rumen Microbiome and Metabolome of Young Cattle. Front. Microbiol. 2019, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.R.; Apdini, T.; Freire, A.S.; Santana, A.S.; Moura, L.M.L.; Nascimento, J.C.S.; Rodrigues, R.T.S.; Dijkstra, J.; Garcez Neto, A.F.; Queiroz, M.A.Á.; et al. Dietary Supplementation with Tannin and Soybean Oil on Intake, Digestibility, Feeding Behavior, Ruminal Protozoa and Methane Emission in Sheep. Anim. Feed. Sci. Technol. 2019, 249, 10–17. [Google Scholar] [CrossRef]
- Silanikove, N.; Perevolotsky, A.; Provenza, F.D. Use of Tannin-Binding Chemicals to Assay for Tannins and Their Negative Postingestive Effects in Ruminants. Anim. Feed. Sci. Technol. 2001, 91, 69–81. [Google Scholar] [CrossRef]
- Woodward, S.; Waghorn, G.; Laboyrie, P. Condensed Tannins in Birdsfoot Trefoil (Lotus Corniculatus) Reduce Methane Emissions from Dairy Cows. In Proceedings of the New Zealand Society of Animal Production 64th Conference, Hamilton, New Zealand, 28 June–1 July 2004. [Google Scholar]
- Stewart, E.K.; Beauchemin, K.A.; Dai, X.; MacAdam, J.W.; Christensen, R.G.; Villalba, J.J. Effect of Tannin-Containing Hays on Enteric Methane Emissions and Nitrogen Partitioning in Beef Cattle. J. Anim. Sci. 2019, 97, 3286–3299. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of Dietary Tannins to Improve Rumen Metabolism and Ruminant Nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Pepeta, B.N.; Moyo, M.; Adejoro, F.A.; Hassen, A.; Nsahlai, I.V. Techniques Used to Determine Botanical Composition, Intake, and Digestibility of Forages by Ruminants. Agronomy 2022, 12, 2456. [Google Scholar] [CrossRef]
- Mahdavi, A.; Mahdavi, A.; Darabighane, B.; Mead, A.; Lee, M.R.F. Effects of Soybean Oil Supplement to Diets of Lactating Dairy Cows, on Productive Performance, and Milk Fat Acids Profile: A Meta-Analysis. Ital. J. Anim. Sci. 2019, 18, 809–819. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Cantalapiedra-Hijar, G.; Eugène, M.; Martin, C.; Noziere, P.; Popova, M.; Ortigues-Marty, I.; Muñoz-Tamayo, R.; Ungerfeld, E.M. Review: Reducing Enteric Methane Emissions Improves Energy Metabolism in Livestock: Is the Tenet Right? Animal 2023, 17, 100830. [Google Scholar] [CrossRef]
- Bes, A.; Nozière, P.; Renand, G.; Rochette, Y.; Guarnido-Lopez, P.; Cantalapiedra-Hijar, G.; Martin, C. Individual Methane Emissions (and Other Gas Flows) Are Repeatable and Their Relationships with Feed Efficiency Are Similar across Two Contrasting Diets in Growing Bulls. Animal 2022, 16, 100583. [Google Scholar] [CrossRef]
- Guarnido-Lopez, P.; Ortigues-Marty, I.; Salis, L.; Chantelauze, C.; Bes, A.; Nozière, P.; Cantalapiedra-Hijar, G. Protein Metabolism, Body Composition and Oxygen Consumption in Young Bulls Divergent in Residual Feed Intake Offered Two Contrasting Forage-Based Diets. Animal 2022, 16, 100558. [Google Scholar] [CrossRef]
- Villar, M.L.; Hegarty, R.S.; Nolan, J.V.; Godwin, I.R.; McPhee, M. The Effect of Dietary Nitrate and Canola Oil Alone or in Combination on Fermentation, Digesta Kinetics and Methane Emissions from Cattle. Anim. Feed Sci. Technol. 2020, 259, 114294. [Google Scholar] [CrossRef]
- Benchaar, C.; Hassanat, F.; Petit, H.V. Dose-Response to Eugenol Supplementation to Dairy Cow Diets: Methane Production, N Excretion, Ruminal Fermentation, Nutrient Digestibility, Milk Production, and Milk Fatty Acid Profile. Anim. Feed Sci. Technol. 2015, 209, 51–59. [Google Scholar] [CrossRef]
- Martin, C.; Rouel, J.; Jouany, J.P.; Doreau, M.; Chilliard, Y. Methane Output and Diet Digestibility in Response to Feeding Dairy Cows Crude Linseed, Extruded Linseed, or Linseed Oil. J. Anim. Sci. 2008, 86, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty Years of Research on Rumen Methanogenesis: Lessons Learned and Future Challenges for Mitigation. Animal 2020, 14, S2–S16. [Google Scholar] [CrossRef] [PubMed]
- Kliem, K.E.; Humphries, D.J.; Kirton, P.; Givens, D.I.; Reynolds, C.K. Differential Effects of Oilseed Supplements on Methane Production and Milk Fatty Acid Concentrations in Dairy Cows. Animal 2019, 13, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Giger-Reverdin, S.; Morand-Fehr, P.; Tran, G. Literature Survey of the Influence of Dietary Fat Composition on Methane Production in Dairy Cattle. Livest. Prod. Sci. 2003, 82, 73–79. [Google Scholar] [CrossRef]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited Review: Enteric Methane in Dairy Cattle Production: Quantifying the Opportunities and Impact of Reducing Emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef]
- Boadi, D.; Benchaar, C.; Chiquette, J.; Massé, D.; Massé, J. Mitigation Strategies to Reduce Enteric Methane Emissions from Dairy Cows: Update Review. J. Microbiol. Biotechnol. 2022, 32, 269–277. [Google Scholar] [CrossRef]
- Lovett, D.; Lovell, S.; Stack, L.; Callan, J.; Finlay, M.; Conolly, J.; O’Mara, F.P. Effect of Forage/Concentrate Ratio and Dietary Coconut Oil Level on Methane Output and Performance of Finishing Beef Heifers. Livest. Prod. Sci. 2003, 84, 135–146. [Google Scholar] [CrossRef]
- van Wyngaard, J.D.V.; Meeske, R.; Erasmus, L.J. Effect of Concentrate feeding on Methane Emissions, Production Performance and Rumen Fermentation of Jersey Cows Grazing Ryegrass Pasture during Spring. Anim. Feed Sci. Technol. 2018, 241, 121–132. [Google Scholar] [CrossRef]
- Hristov, A.N.; Oh, J.; Firkins, J.L.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.P.S.; Adesogan, A.T.; Yang, W.; Lee, C.; et al. SPECIAL TOPICS-Mitigation of Methane and Nitrous Oxide Emissions from Animal Operations: I. A Review of Enteric Methane Mitigation Options 1. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef]
- Jiao, H.P.; Dale, A.J.; Carson, A.F.; Murray, S.; Gordon, A.W.; Ferris, C.P. Effect of Concentrate Feed Level on Methane Emissions from Grazing Dairy Cows. J. Dairy Sci. 2014, 97, 7043–7053. [Google Scholar] [CrossRef] [PubMed]
- Schilde, M.; von Soosten, D.; Hüther, L.; Meyer, U.; Zeyner, A.; Dänicke, S. Effects of 3-Nitrooxypropanol and Varying Concentrate Feed Proportions in the Ration on Methane Emission, Rumen Fermentation and Performance of Periparturient Dairy Cows. Arch. Anim. Nutr. 2021, 75, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Huhtanen, P.; Hetta, M. Comparison of Feed Intake and Milk Production Responses in Continuous and Change-over Design Dairy Cow Experiments. Livest. Sci. 2012, 143, 184–194. [Google Scholar] [CrossRef]
- Maekawa, M.; Beauchemin, K.A.; Christensen, D.A. Effect of Concentrate Level and Feeding Management on Chewing Activities, Saliva Production, and Ruminal PH of Lactating Dairy Cows. J. Dairy Sci. 2002, 85, 1165–1175. [Google Scholar] [CrossRef]
- Ferraretto, L.F.; Shaver, R.D.; Bertics, S.J. Effect of Dietary Supplementation with Live-Cell Yeast at Two Dosages on Lactation Performance, Ruminal Fermentation, and Total-Tract Nutrient Digestibility in Dairy Cows. J. Dairy Sci. 2012, 95, 4017–4028. [Google Scholar] [CrossRef]
- Hristov, A.N.; Lee, C.; Cassidy, T.; Heyler, K.; Tekippe, J.A.; Varga, G.A.; Corl, B.; Brandt, R.C. Effect of Origanum Vulgare L. Leaves on Rumen Fermentation, Production, and Milk Fatty Acid Composition in Lactating Dairy Cows. J. Dairy Sci. 2013, 96, 1189–1202. [Google Scholar] [CrossRef]
- Machado, L.; Magnusson, M.; Paul, N.A.; Kinley, R.; de Nys, R.; Tomkins, N. Identification of Bioactives from the Red Seaweed Asparagopsis Taxiformis that Promote Antimethanogenic Activity In Vitro. J. Appl. Phycol. 2016, 28, 3117–3126. [Google Scholar] [CrossRef]
- Magnusson, M.; Vucko, M.J.; Neoh, T.L.; de Nys, R. Using Oil Immersion to Deliver a Naturally-Derived, Stable Bromoform Product from the Red Seaweed Asparagopsis Taxiformis. Algal Res. 2020, 51, 102065. [Google Scholar] [CrossRef]
- Roque, B.M.; Brooke, C.G.; Ladau, J.; Polley, T.; Marsh, L.J.; Najafi, N.; Pandey, P.; Singh, L.; Kinley, R.; Salwen, J.K.; et al. Effect of the Macroalgae Asparagopsis Taxiformis on Methane Production and Rumen Microbiome Assemblage. Anim. Microbiome 2019, 1, 3. [Google Scholar] [CrossRef]
- El-Waziry, A.; Al-Haidary, A.; Okab, A.; Samara, E.; Abdoun, K. Effect of Dietary Seaweed (Ulva Lactuca) Supplementation on Growth Performance of Sheep and on In Vitro Gas Production Kinetics. Turk. J. Vet. Anim. Sci. 2015, 39, 81–86. [Google Scholar] [CrossRef]
- Kinley, R.D.; Martinez-Fernandez, G.; Matthews, M.K.; de Nys, R.; Magnusson, M.; Tomkins, N.W. Mitigating the Carbon Footprint and Improving Productivity of Ruminant Livestock Agriculture Using a Red Seaweed. J. Clean. Prod. 2020, 259, 120836. [Google Scholar] [CrossRef]
- Stefenoni, H.A.; Räisänen, S.E.; Cueva, S.F.; Wasson, D.E.; Lage, C.F.A.; Melgar, A.; Fetter, M.E.; Smith, P.; Hennessy, M.; Vecchiarelli, B.; et al. Effects of the Macroalga Asparagopsis Taxiformis and Oregano Leaves on Methane Emission, Rumen Fermentation, and Lactational Performance of Dairy Cows. J. Dairy Sci. 2021, 104, 4157–4173. [Google Scholar] [CrossRef] [PubMed]
- Lean, I.J.; Golder, H.M.; Grant, T.M.D.; Moate, P.J. A Meta-Analysis of Effects of Dietary Seaweed on Beef and Dairy Cattle Performance and Methane Yield. PLoS ONE 2021, 16, e0249053. [Google Scholar] [CrossRef]
- Li, X.; Norman, H.C.; Kinley, R.D.; Laurence, M.; Wilmot, M.; Bender, H.; de Nys, R.; Tomkins, N. Asparagopsis Taxiformis Decreases Enteric Methane Production from Sheep. Anim. Prod. Sci. 2018, 58, 681–688. [Google Scholar] [CrossRef]
- Melgar, A.; Nedelkov, K.; Martins, C.M.M.R.; Welter, K.C.; Chen, X.; Räisänen, S.E.; Harper, M.T.; Oh, J.; Duval, S.; Hristov, A.N. Short Communication: Short-Term Effect of 3-Nitrooxypropanol on Feed Dry Matter Intake in Lactating Dairy Cows. J. Dairy Sci. 2020, 103, 11496–11502. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.G.; Baek, Y.C.; Lee, S.; Seo, J. The Effects of Dietary Supplementation with 3-Nitrooxypropanol on Enteric Methane Emissions, Rumen Fermentation, and Production Performance in Ruminants: A Meta-Analysis. J. Anim. Sci. Technol. 2020, 62, 31–42. [Google Scholar] [CrossRef]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-Analysis of the Relationship between Dietary Tannin Level and Methane Formation in Ruminants from In Vivo and In Vitro Experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, L.; Jonker, A.; Munidasa, S.; Pacheco, D. A Review: Plant Carbohydrate Types—The Potential Impact on Ruminant Methane Emissions. Front. Vet. Sci. 2022, 9, 880115. [Google Scholar] [CrossRef]
- Duin, E.C.; Wagner, T.; Shima, S.; Prakash, D.; Cronin, B.; Yáñez-Ruiz, D.R.; Duval, S.; Rümbeli, R.; Stemmler, R.T.; Thauer, R.K.; et al. Mode of Action Uncovered for the Specific Reduction of Methane Emissions from Ruminants by the Small Molecule 3-Nitrooxypropanol. Proc. Natl. Acad. Sci. USA 2016, 113, 6172–6177. [Google Scholar] [CrossRef]
- Dadhich, A. Engineered Biochar as Feed Supplement and Other Husbandry Applications. In Engineered Biochar: Fundamentals, Preparation, Characterization and Applications; Ramola, S., Mohan, D., Masek, O., Méndez, A., Tsubota, T., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 319–329. ISBN 978-981-19-2488-0. [Google Scholar]
- Leng, R.A.; Preston, T.R.; Inthapanya, S. Biochar Reduces Enteric Methane and Improves Growth and Feed Conversion in Local “Yellow” Cattle Fed Cassava Root Chips and Fresh Cassava Foliage. Livest. Res. Rural. Dev. 2012, 24, 1–7. [Google Scholar]
- Bartzanas, T.; Calvet, S.; Zhang, G. Technology for Environmentally Friendly Livestock Production. In Technol for Environ Friend Livest Product; Bartzanas, T., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–9. ISBN 978-3-031-19730-7. [Google Scholar]
- Conlin, E.E. Performance and Environmental Benefits from Biochar Supplementation in Beef Cattle Grazing Systems. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2021. [Google Scholar]
- Hegarty, R. An Evaluation of Evidence for Efficacy and Applicability of Methane Inhibiting Feed Additives for Livestock; New Zealand Agricultural Greenhouse Gas Research Centre: Palmerston North, New Zealand, 2021. [Google Scholar]
- Terry, S.A.; Ribeiro, G.O.; Gruninger, R.J.; Chaves, A.V.; Beauchemin, K.A.; Okine, E.; McAllister, T.A. A Pine Enhanced Biochar Does Not Decrease Enteric CH4 Emissions, but Alters the Rumen Microbiota. Front. Vet. Sci. 2019, 6, 308. [Google Scholar] [CrossRef]
- Doreau, M.; Arbre, M.; Popova, M.; Rochette, Y.; Martin, C. Linseed plus Nitrate in the Diet for Fattening Bulls: Effects on Methane Emission, Animal Health and Residues in Offal. Animal 2018, 12, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Mirheidari, A.; Torbatinejad, N.M.; Shakeri, P.; Mokhtarpour, A. Effects of Biochar Produced from Different Biomass Sources on Digestibility, Ruminal Fermentation, Microbial Protein Synthesis and Growth Performance of Male Lambs. Small Rumin. Res. 2020, 183, 106042. [Google Scholar] [CrossRef]
- Lunsin, R. Effect of Oil Palm Meal on Nutrient Utilization and Milk Production in Lactating Dairy Cows Fed with Urea-Treated Rice Straw. Agric. Nat. Resour. 2018, 52, 285–289. [Google Scholar] [CrossRef]
- Singla, A.; Singh Hundal, J.; Kumar Patra, A.; Wadhwa, M.; Nagarajappa, V.; Malhotra, P. Effect of Dietary Supplementation of Emblica officinalis Fruit Pomace on Methane Emission, Ruminal Fermentation, Nutrient Utilization, and Milk Production Performance in Buffaloes. Environ. Sci. Pollut. Res. 2021, 28, 18120–18133. [Google Scholar] [CrossRef]
- Marino, R.; Caroprese, M.; Annicchiarico, G.; Ciampi, F.; Ciliberti, M.G.; della Malva, A.; Santillo, A.; Sevi, A.; Albenzio, M. Effect of Diet Supplementation with Quinoa Seed and/or Linseed on Immune Response, Productivity and Meat Quality in Merinos Derived Lambs. Animals 2018, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Kim, Y.J. Effects of Feeding Charcoal Powder and Vitamin A on Growth Performance, Serum Profile and Carcass Characteristics of Fattening Hanwoo Steers. J. Anim. Sci. Technol. 2005, 47, 233–242. [Google Scholar]
- Ortiz-Chura, A.; Gere, J.; Marcoppido, G.; Depetris, G.; Cravero, S.; Faverín, C.; Pinares-Patiño, C.; Cataldi, A.; Cerón-Cucchi, M.E. Dynamics of the Ruminal Microbial Ecosystem, and Inhibition of Methanogenesis and Propiogenesis in Response to Nitrate Feeding to Holstein Calves. Anim. Nutr. 2021, 7, 1205–1218. [Google Scholar] [CrossRef]
- Al-Kindi, A.; Schlecht, E.; Schiborra, A.; Joergensen, R.G. Effects of Quebracho Tannin Extract (Schinopsis Balansae) and Activated Charcoal on Feed Intake and Digestibility by Goats and Their Faecal Microbial Biomass. Biol. Agric. Hortic. 2016, 32, 159–169. [Google Scholar] [CrossRef]
- Winders, T.M.; Jolly-Breithaupt, M.L.; Wilson, H.C.; MacDonald, J.C.; Erickson, G.E.; Watson, A.K. Evaluation of the Effects of Biochar on Diet Digestibility and Methane Production from Growing and Finishing Steers. Transl. Anim. Sci. 2019, 3, 775–783. [Google Scholar] [CrossRef]
- Valenti, B.; Natalello, A.; Vasta, V.; Campidonico, L.; Roscini, V.; Mattioli, S.; Pauselli, M.; Priolo, A.; Lanza, M.; Luciano, G. Effect of Different Dietary Tannin Extracts on Lamb Growth Performances and Meat Oxidative Stability: Comparison between Mimosa, Chestnut and Tara. Animal 2019, 13, 435–443. [Google Scholar] [CrossRef]
- McAvoy, D.J.; Burritt, B.; Villalba, J.J. Use of Biochar by Sheep: Impacts on Diet Selection, Digestibility, and Performance. J. Anim. Sci. 2020, 98, skaa380. [Google Scholar] [CrossRef]
- Liu, H.; Vaddella, V.; Zhou, D. Effects of Chestnut Tannins and Coconut Oil on Growth Performance, Methane Emission, Ruminal Fermentation, and Microbial Populations in Sheep. J. Dairy Sci. 2011, 94, 6069–6077. [Google Scholar] [CrossRef]
- Van, D.T.T.; Mui, N.T.; Ledin, I. Effect of Method of Processing Foliage of Acacia Mangium and Inclusion of Bamboo Charcoal in the Diet on Performance of Growing Goats. Anim. Feed. Sci. Technol. 2006, 130, 242–256. [Google Scholar] [CrossRef]
- Pen, B.; Takaura, K.; Yamaguchi, S.; Asa, R.; Takahashi, J. Effects of Yucca schidigera and Quillaja saponaria with or without β 1-4 Galacto-Oligosaccharides on Ruminal Fermentation, Methane Production and Nitrogen Utilization in Sheep. Anim. Feed. Sci. Technol. 2007, 138, 75–88. [Google Scholar] [CrossRef]
- Lind, L.; Sizmaz, S.; Weldon, D.; Dragan, D.; Jørgensen, G.M. Enteric Methane Emissions from Sheep Fed Diets Including Biochar. In Proceedings of the 28th General Meeting of the European Grassland Federation, Helsinki, Finland, 19–22 October 2020; p. 306. [Google Scholar]
- Wang, C.J.; Wang, S.P.; Zhou, H. Influences of Flavomycin, Ropadiar, and Saponin on Nutrient Digestibility, Rumen Fermentation, and Methane Emission from Sheep. Anim. Feed. Sci. Technol. 2009, 148, 157–166. [Google Scholar] [CrossRef]
- Animut, G.; Puchala, R.; Goetsch, A.L.; Patra, A.K.; Sahlu, T.; Varel, V.H.; Wells, J. Methane Emission by Goats Consuming Different Sources of Condensed Tannins. Anim. Feed. Sci. Technol. 2008, 144, 228–241. [Google Scholar] [CrossRef]
- Liu, H.; Puchala, R.; Leshure, S.; Gipson, T.A.; Flythe, M.D.; Goetsch, A.L. Effects of Lespedeza Condensed Tannins Alone or with Monensin, Soybean Oil, and Coconut Oil on Feed Intake, Growth, Digestion, Ruminal Methane Emission, and Heat Energy by Yearling Alpine Doelings. J. Anim. Sci. 2019, 97, 885–899. [Google Scholar] [CrossRef]
- Poteko, J.; Schrade, S.; Zeyer, K.; Mohn, J.; Zaehner, M.; Zeitz, J.O.; Kreuzer, M.; Schwarm, A. Methane Emissions and Milk Fatty Acid Profiles in Dairy Cows Fed Linseed, Measured at the Group Level in a Naturally Ventilated Housing and Individually in Respiration Chambers. Animals 2020, 10, 1091. [Google Scholar] [CrossRef]
- Mirzaei-Alamouti, H.; Akbari-Pabandi, K.; Mansouryar, M.; Sirjani, M.A.; Cieslak, A.; Szumacher-Strabel, M.; Patra, A.K.; Vazirigohar, M. Effects of Feeding Frequency and Oil Supplementation on Feeding Behavior, Ruminal Fermentation, Digestibility, Blood Metabolites, and Milk Performance in Late-Lactation Cows Fed a High-Forage Diet. J. Dairy Sci. 2020, 103, 11424–11438. [Google Scholar] [CrossRef] [PubMed]
- El-Essawy, A.M.; Anele, U.Y.; Abdel-Wahed, A.M.; Abdou, A.R.; Khattab, I.M. Effects of Anise, Clove and Thyme Essential Oils Supplementation on Rumen Fermentation, Blood Metabolites, Milk Yield and Milk Composition in Lactating Goats. Anim. Feed. Sci. Technol. 2021, 271, 114760. [Google Scholar] [CrossRef]
- Gerlach, K.; Pries, M.; Südekum, K.H. Effect of Condensed Tannin Supplementation on In Vivo Nutrient Digestibilities and Energy Values of Concentrates in Sheep. Small Rumin. Res. 2018, 161, 57–62. [Google Scholar] [CrossRef]
- Robles Jimenez, L.E.; Ruiz Perez, J.A.; Nicolas, D.L.; Chay Canul, A.J.; Ramirez-Rivera, J.C.; Villegas-Estrada, D.; Vargas-Bello-Pérez, E.; Gonzalez-Ronquillo, M. Productive Behavior in Growing Kid Goats and Methane Production with the Inclusion of Chokecherry Leaf (Prunus Salicifolia). Trop. Anim. Health Prod. 2020, 52, 1257–1267. [Google Scholar] [CrossRef]
- Molina-Botero, I.C.; Arroyave-Jaramillo, J.; Valencia-Salazar, S.; Barahona-Rosales, R.; Aguilar-Pérez, C.F.; Ayala Burgos, A.; Arango, J.; Ku-Vera, J.C. Effects of Tannins and Saponins Contained in Foliage of Gliricidia sepium and Pods of Enterolobium cyclocarpum on Fermentation, Methane Emissions and Rumen Microbial Population in Crossbred Heifers. Anim. Feed. Sci. Technol. 2019, 251, 1–11. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A.; Akanmu, A.M.; Morgavi, D.P. Replacing Urea with Nitrate as a Non-Protein Nitrogen Source Increases Lambs’ Growth and Reduces Methane Production, Whereas Acacia Tannin Has No Effect. Anim. Feed. Sci. Technol. 2020, 259, 114360. [Google Scholar] [CrossRef]
- Nasehi, M.; Torbatinejad, N.M.; Rezaie, M.; Ghoorchi, T. Effects of Partial Substitution of Alfalfa Hay with Green Tea Waste on Growth Performance and In Vitro Methane Emission of Fat-Tailed Lambs. Small Rumin. Res. 2018, 168, 52–59. [Google Scholar] [CrossRef]
- Puchala, R.; Leshure, S.; Gipson, T.A.; Tesfai, K.; Flythe, M.D.; Goetsch, A.L. Effects of Different Levels of Lespedeza and Supplementation with Monensin, Coconut Oil, or Soybean Oil on Ruminal Methane Emission by Mature Boer Goat Wethers after Different Lengths of Feeding. J. Appl. Anim. Res. 2018, 46, 1127–1136. [Google Scholar] [CrossRef]
- Zhang, F.; Li, B.; Ban, Z.; Liang, H.; Li, L.; Zhao, W.; Yan, X. Evaluation of Origanum Oil, Hydrolysable Tannins and Tea Saponin in Mitigating Ruminant Methane: In Vitro and In Vivo Methods. J. Anim. Physiol. Anim. Nutr. 2021, 105, 630–638. [Google Scholar] [CrossRef]
- Vázquez-Carrillo, M.F.; Montelongo-Pérez, H.D.; González-Ronquillo, M.; Castillo-Gallegos, E.; Castelán-Ortega, O.A. Effects of Three Herbs on Methane Emissions from Beef Cattle. Animals 2020, 10, 1671. [Google Scholar] [CrossRef]
- Thirumalaisamy, G.; Malik, P.K.; Kolte, A.P.; Trivedi, S.; Dhali, A.; Bhatta, R. Effect of Silkworm (Bombyx mori) Pupae Oil Supplementation on Enteric Methane Emission and Methanogens Diversity in Sheep. Anim. Biotechnol. 2022, 33, 128–140. [Google Scholar] [CrossRef]
- Barbosa, A.L.; Voltolini, T.V.; Menezes, D.R.; de Moraes, S.A.; Nascimento, J.C.S.; de Souza Rodrigues, R.T. Intake, Digestibility, Growth Performance, and Enteric Methane Emission of Brazilian Semiarid Non-Descript Breed Goats Fed Diets with Different Forage to Concentrate Ratios. Trop. Anim. Health Prod. 2018, 50, 283–289. [Google Scholar] [CrossRef]
- Heidarian Miri, V.; Tyagi, A.K.; Ebrahimi, S.H.; Mohini, M. Effect of Cumin (Cuminum cyminum) Seed Extract on Milk Fatty Acid Profile and Methane Emission in Lactating Goat. Small Rumin. Res. 2013, 113, 66–72. [Google Scholar] [CrossRef]
- Hulshof, R.B.A.; Berndt, A.; Gerrits, W.J.J.; Dijkstra, J.; Van Zijderveld, S.M.; Newbold, J.R.; Perdok, H.B. Dietary Nitrate Supplementation Reduces Methane Emission in Beef Cattle Fed Sugarcane-Based Diets 1. J. Anim. Sci 2012, 90, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Medrano, R.F.; Wang, M.; Beauchemin, K.A.; Ma, Z.; Wang, R.; Wen, J.; Bernard, L.A.; Tan, Z. Effects of Urea plus Nitrate Pretreated Rice Straw and Corn Oil Supplementation on Fiber Digestibility, Nitrogen Balance, Rumen Fermentation, Microbiota and Methane Emissions in Goats. J. Anim. Sci. Biotechnol 2019, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Van Zijderveld, S.M.; Gerrits, W.J.J.; Dijkstra, J.; Newbold, J.R.; Hulshof, R.B.A.; Perdok, H.B. Persistency of Methane Mitigation by Dietary Nitrate Supplementation in Dairy Cows. J. Dairy Sci. 2011, 94, 4028–4038. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Mao, S.Y.; Long, L.M.; Zhu, W.Y. Effect of Disodium Fumarate on Microbial Abundance, Ruminal Fermentation and Methane Emission in Goats under Different Forage: Concentrate Ratios. Animal 2012, 6, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Granja-Salcedo, Y.T.; Fernandes, R.M.I.; De Araujo, R.C.; Kishi, L.T.; Berchielli, T.T.; De Resende, F.D.; Berndt, A.; Siqueira, G.R. Long-Term Encapsulated Nitrate Supplementation Modulates Rumen Microbial Diversity and Rumen Fermentation to Reduce Methane Emission in Grazing Steers. Front. Microbiol. 2019, 10, 614. [Google Scholar] [CrossRef]
- Hollmann, M.; Powers, W.J.; Fogiel, A.C.; Liesman, J.S.; Bello, N.M.; Beede, D.K. Enteric Methane Emissions and Lactational Performance of Holstein Cows Fed Different Concentrations of Coconut Oil. J. Dairy Sci. 2012, 95, 2602–2615. [Google Scholar] [CrossRef]
- Hundal, J.S.; Wadhwa, M.; Bakshi, M.P.S.; Chatli, M.K. Effect of Herbal Feed Additive Containing Saponins on the Performance of Goat Kids. Indian J. Anim. Sci. 2020, 90, 229–236. [Google Scholar] [CrossRef]
- Paengkoum, S.; Khotsakdee, J.; Paengkoum, P.; Schonewille, T.; Yuangklang, C. Nitrate Supplementation of Rations Based on Rice Straw but Not Pangola Hay, Improves Growth Performance in Meat Goats. Anim. Biosci. 2021, 34, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.H.; Barnett, M.C.; Hegarty, R.S. Use of Dietary Nitrate to Increase Productivity and Reduce Methane Production of Defaunated and Faunated Lambs Consuming Protein-Deficient Chaff. Anim. Prod. Sci. 2016, 56, 290–297. [Google Scholar] [CrossRef]
- Na, Y.; Li, D.H.; Lee, S.R. Effects of Dietary Forage-to-Concentrate Ratio on Nutrient Digestibility and Enteric Methane Production in Growing Goats (Capra hircus hircus) and Sika Deer (Cervus nippon hortulorum). Asian-Australas. J. Anim. Sci. 2017, 30, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Pilajun, R.; Wanapat, M. Effect of Coconut Oil and Mangosteen Peel Supplementation on Ruminal Fermentation, Microbial Population, and Microbial Protein Synthesis in Swamp Buffaloes. Livest. Sci. 2011, 141, 148–154. [Google Scholar] [CrossRef]
- Anassori, E.; Dalir-Naghadeh, B.; Pirmohammadi, R.; Taghizadeh, A.; Asri-Rezaei, S.; Maham, M.; Farahmand-Azar, S.; Farhoomand, P. Garlic: A Potential Alternative for Monensin as a Rumen Modifier. Livest. Sci. 2011, 142, 276–287. [Google Scholar] [CrossRef]
- Guyader, J.; Eugène, M.; Doreau, M.; Morgavi, D.P.; Gérard, C.; Loncke, C.; Martin, C. Nitrate but Not Tea Saponin Feed Additives Reduce Methane in Dairy Cows. J. Anim. Sci. 2015, 93, 5367–5377. [Google Scholar] [CrossRef]
- Klevenhusen, F.; Zeitz, J.O.; Duval, S.; Kreuzer, M.; Soliva, C.R. Garlic Oil and Its Principal Component Diallyl Disulfide Fail to Mitigate Methane, but Improve Digestibility in Sheep. Anim. Feed. Sci. Technol. 2011, 166–167, 356–363. [Google Scholar] [CrossRef]
- Malik, P.K.; Kolte, A.P.; Baruah, L.; Saravanan, M.; Bakshi, B.; Bhatta, R. Enteric Methane Mitigation in Sheep through Leaves of Selected Tanniniferous Tropical Tree Species. Livest. Sci. 2017, 200, 29–34. [Google Scholar] [CrossRef]
- Verma, V.; Chaudhary, L.C.; Agarwal, N.; Bhar, R.; Kamra, D.N. Effect of Feeding Mixture of Garlic Bulb and Peppermint Oil on Methane Emission, Rumen Fermentation and Microbial Profile in Buffaloes. Anim. Nutr. Feed. Technol. 2012, 12, 157–164. [Google Scholar]
- Tomkins, N.W.; Denman, S.E.; Pilajun, R.; Wanapat, M.; McSweeney, C.S.; Elliott, R. Manipulating Rumen Fermentation and Methanogenesis Using an Essential Oil and Monensin in Beef Cattle Fed a Tropical Grass Hay. Anim. Feed. Sci. Technol. 2015, 200, 25–34. [Google Scholar] [CrossRef]
- Polyorach, S.; Wanapat, M.; Phesatcha, K.; Kang, S. Effect of Different Levels of Mangosteen Peel Powder Supplement on the Performance of Dairy Cows Fed Concentrate Containing Yeast Fermented Cassava Chip Protein. Trop. Anim. Health Prod. 2015, 47, 1473–1480. [Google Scholar] [CrossRef]
- Castro-Montoya, J.; Peiren, N.; Cone, J.W.; Zweifel, B.; Fievez, V.; De Campeneere, S. In Vivo and In Vitro Effects of a Blend of Essential Oils on Rumen Methane Mitigation. Livest. Sci. 2015, 180, 134–142. [Google Scholar] [CrossRef]
- Hundal, J.S.; Singh, I.; Wadhwa, M.; Singh, C.; Uppa, C.; Kaur, G. Effect of Punica granatum and Tecomella undulata supplementation on nutrient utilization, enteric methane emission and growth performance of Murrah male buffaloes. J. Anim. Feed Sci. 2019, 28, 110–119. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chaudhary, L.C.; Agarwal, N.; Kamra, D.N. Effect of Madhuca longifolia and Terminalia chebula on Methane Production and Nutrient Utilization in Buffaloes. Anim. Feed. Sci. Technol. 2015, 201, 38–45. [Google Scholar] [CrossRef]
- Yatoo, M.A.; Chaudhary, L.C.; Agarwal, N.; Chaturvedi, V.B.; Kamra, D.N. Effect of Feeding of Blend of Essential Oils on Methane Production, Growth, and Nutrient Utilization in Growing Buffaloes. Asian-Australas. J. Anim. Sci. 2018, 31, 672–676. [Google Scholar] [CrossRef]
- Yang, W.Z.; Benchaar, C.; Ametaj, B.N.; Chaves, A.V.; He, M.L.; McAllister, T.A. Effects of Garlic and Juniper Berry Essential Oils on Ruminal Fermentation and on the Site and Extent of Digestion in Lactating Cows. J. Dairy Sci. 2007, 90, 5671–5681. [Google Scholar] [CrossRef] [PubMed]
- Meale, S.J.; Chaves, A.V.; Mcallister, T.A.; Iwaasa, A.D.; Yang, W.Z.; Benchaar, C. Including Essential Oils in Lactating Dairy Cow Diets: Effects on Methane Emissions1. Anim. Prod. Sci. 2014, 54, 1215–1218. [Google Scholar] [CrossRef]
- Manasri, N.; Wanapat, M.; Navanukraw, C. Improving Rumen Fermentation and Feed Digestibility in Cattle by Mangosteen Peel and Garlic Pellet Supplementation. Livest. Sci. 2012, 148, 291–295. [Google Scholar] [CrossRef]
- ManhN, N.S.; Wanapat, M.; Uriyapongson, S.; Khejornsart, P.; Chanthakhoun, V. Effect of Eucalyptus (Camaldulensis) Leaf Meal Powder on Rumen Fermentation Characteristics in Cattle Fed on Rice Straw. Afr. J. Agric. Res. 2012, 7, 614. [Google Scholar] [CrossRef]
- Chaves, A.V.; Stanford, K.; Dugan, M.E.R.; Gibson, L.L.; McAllister, T.A.; Van Herk, F.; Benchaar, C. Effects of Cinnamaldehyde, Garlic and Juniper Berry Essential Oils on Rumen Fermentation, Blood Metabolites, Growth Performance, and Carcass Characteristics of Growing Lambs. Livest. Sci. 2008, 117, 215–224. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Mcginn, S.M. Methane Emissions from Beef Cattle: Effects of Fumaric Acid, Essential Oil, and Canola Oil 1. J. Anim. Sci. 2006, 84, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Matloup, O.H.; Abd El Tawab, A.M.; Hassan, A.A.; Hadhoud, F.I.; Khattab, M.S.A.; Khalel, M.S.; Sallam, S.M.A.; Kholif, A.E. Performance of Lactating Friesian Cows Fed a Diet Supplemented with Coriander Oil: Feed Intake, Nutrient Digestibility, Ruminal Fermentation, Blood Chemistry, and Milk Production. Anim. Feed. Sci. Technol. 2017, 226, 88–97. [Google Scholar] [CrossRef]
- Wall, E.H.; Doane, P.H.; Donkin, S.S.; Bravo, D. The Effects of Supplementation with a Blend of Cinnamaldehyde and Eugenol on Feed Intake and Milk Production of Dairy Cows. J. Dairy Sci. 2014, 97, 5709–5717. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Skoufos, J.; Giannakopoulos, C.; Wiemann, M.; Gortzi, O.; Lalas, S.; Kyriazakis, I. Effects of Essential Oils on Milk Production, Milk Composition, and Rumen Microbiota in Chios Dairy Ewes. J. Dairy Sci. 2011, 94, 5569–5577. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Singhal, K. Effect of Alfalfa Fodder Supplementation on Enteric Methane Emission Measured by Sulfur Hexafluoride Technique in Murrah Buffaloes. Buffalo Bull. 2016, 35, 125–134. [Google Scholar]
- Chiofalo, V.; Liotta, L.; Fiumanò, R.; Riolo, E.B.; Chiofalo, B. Influence of Dietary Supplementation of Rosmarinus officinalis L. on Performances of Dairy Ewes Organically Managed. Small Rumin. Res. 2012, 104, 122–128. [Google Scholar] [CrossRef]
- Muñoz, C.; Herrera, D.; Hube, S.; Morales, J.; Ungerfeld, E.M. Effects of Dietary Concentrate Supplementation on Enteric Methane Emissions and Performance of Late Lactation Dairy Cows. Chil. J. Agric. Res. 2018, 78, 429–437. [Google Scholar] [CrossRef]
- Olijhoek, D.W.; Løvendahl, P.; Lassen, J.; Hellwing, A.L.F.; Höglund, J.K.; Weisbjerg, M.R.; Noel, S.J.; McLean, F.; Højberg, O.; Lund, P. Methane Production, Rumen Fermentation, and Diet Digestibility of Holstein and Jersey Dairy Cows Being Divergent in Residual Feed Intake and Fed at 2 Forage-to-Concentrate Ratios. J. Dairy Sci. 2018, 101, 9926–9940. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Terranova, M.; Kreuzer, M.; Marquardt, S.; Eggerschwiler, L.; Schwarm, A. Supplementation of Pelleted Hazel (Corylus avellana) Leaves Decreases Methane and Urinary Nitrogen Emissions by Sheep at Unchanged Forage Intake. Sci. Rep. 2018, 8, 5427. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.P.; Dall-Orsoletta, A.C.; Ribeiro-Filho, H.M.N. The Effects of Supplementing Acacia mearnsii Tannin Extract on Dairy Cow Dry Matter Intake, Milk Production, and Methane Emission in a Tropical Pasture. Trop. Anim. Health Prod. 2017, 49, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Tekippe, J.A.; Tacoma, R.; Hristov, A.N.; Lee, C.; Oh, J.; Heyler, K.S.; Cassidy, T.W.; Varga, G.A.; Bravo, D. Effect of Essential Oils on Ruminal Fermentation and Lactation Performance of Dairy Cows. J. Dairy Sci. 2013, 96, 7892–7903. [Google Scholar] [CrossRef]
- Sun, Y.K.; Yan, X.G.; Ban, Z.B.; Yang, H.M.; Hegarty, R.S.; Zhao, Y.M. The Effect of Cysteamine Hydrochloride and Nitrate Supplementation on In-Vitro and In-Vivo Methane Production and Productivity of Cattle. Anim. Feed. Sci. Technol. 2017, 232, 49–56. [Google Scholar] [CrossRef]
- Abdalla, A.L.; Louvandini, H.; Sallam, S.M.A.H.; da Bueno, I.C.S.; Tsai, S.M.; de Figueira, A.V.O. In Vitro Evaluation, In Vivo Quantification, and Microbial Diversity Studies of Nutritional Strategies for Reducing Enteric Methane Production. Trop. Anim. Health Prod. 2012, 44, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Martin, C.; Rouel, J.; Doreau, M. Milk Fatty Acids in Dairy Cows Fed Whole Crude Linseed, Extruded Linseed, or Linseed Oil, and Their Relationship with Methane Output. J. Dairy Sci. 2009, 92, 5199–5211. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Kamra, D.N.; Bhar, R.; Kumar, R.; Agarwal, N. Effect of Terminalia chebula and Allium sativum on In Vivo Methane Emission by Sheep. J. Anim. Physiol. Anim. Nutr. 2011, 95, 187–191. [Google Scholar] [CrossRef]
- Ramírez-Restrepo, C.A.; Tan, C.; O’Neill, C.J.; López-Villalobos, N.; Padmanabha, J.; Wang, J.; McSweeney, C.S. Methane Production, Fermentation Characteristics, and Microbial Profiles in the Rumen of Tropical Cattle Fed Tea Seed Saponin Supplementation. Anim. Feed. Sci. Technol. 2016, 216, 58–67. [Google Scholar] [CrossRef]
- Mao, H.L.; Wang, J.K.; Zhou, Y.Y.; Liu, J.X. Effects of Addition of Tea Saponins and Soybean Oil on Methane Production, Fermentation and Microbial Population in the Rumen of Growing Lambs. Livest. Sci. 2010, 129, 56–62. [Google Scholar] [CrossRef]
- Bayat, A.R.; Tapio, I.; Vilkki, J.; Shingfield, K.J.; Leskinen, H. Plant Oil Supplements Reduce Methane Emissions and Improve Milk Fatty Acid Composition in Dairy Cows Fed Grass Silage-Based Diets without Affecting Milk Yield. J. Dairy Sci. 2018, 101, 1136–1151. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Wattiaux, M.A.; Powell, J.M.; Broderick, G.A.; Arndt, C. Effect of Forage-to-Concentrate Ratio in Dairy Cow Diets on Emission of Methane, Carbon Dioxide, and Ammonia, Lactation Performance, and Manure Excretion. J. Dairy Sci. 2011, 94, 3081–3093. [Google Scholar] [CrossRef]
- Olijhoek, D.W.; Hellwing, A.L.F.; Brask, M.; Weisbjerg, M.R.; Højberg, O.; Larsen, M.K.; Dijkstra, J.; Erlandsen, E.J.; Lund, P. Effect of Dietary Nitrate Level on Enteric Methane Production, Hydrogen Emission, Rumen Fermentation, and Nutrient Digestibility in Dairy Cows. J. Dairy Sci. 2016, 99, 6191–6205. [Google Scholar] [CrossRef] [PubMed]
- Serumula, D.M. In Vitro Fermentation and Growth Performance of Merino Lambs Fed on Umbrella Thorn (Vachellia tortilis) Leaf Meal and Sunflower Oil. Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2020. [Google Scholar]
- Haisan, J.; Sun, Y.; Guan, L.; Beauchemin, K.A.; Iwaasa, A.; Duval, S.; Kindermann, M.; Barreda, D.R.; Oba, M. The Effects of Feeding 3-Nitrooxypropanol at Two Doses on Milk Production, Rumen Fermentation, Plasma Metabolites, Nutrient Digestibility, and Methane Emissions in Lactating Holstein Cows. Anim. Prod. Sci. 2017, 57, 282–289. [Google Scholar] [CrossRef]
- Vyas, D.; McGinn, S.M.; Duval, S.M.; Kindermann, M.K.; Beauchemin, K.A. Optimal Dose of 3-Nitrooxypropanol for Decreasing Enteric Methane Emissions from Beef Cattle Fed High-Forage and High-Grain Diets. Anim. Prod. Sci. 2018, 58, 1049–1055. [Google Scholar] [CrossRef]
- Romero-Perez, A.; Okine, E.K.; McGinn, S.M.; Guan, L.L.; Oba, M.; Duval, S.M.; Kindermann, M.; Beauchemin, K.A. The Potential of 3-Nitrooxypropanol to Lower Enteric Methane Emissions from Beef Cattle. J. Anim. Sci. 2014, 92, 4682–4693. [Google Scholar] [CrossRef]
- Alemu, A.W.; Pekrul, L.K.D.; Shreck, A.L.; Booker, C.W.; McGinn, S.M.; Kindermann, M.; Beauchemin, K.A. 3-Nitrooxypropanol Decreased Enteric Methane Production from Growing Beef Cattle in a Commercial Feedlot: Implications for Sustainable Beef Cattle Production. Front. Anim. Sci. 2021, 2, 4. [Google Scholar] [CrossRef]
- Zhang, X.M.; Smith, M.L.; Gruninger, R.J.; Kung, L.; Vyas, D.; Mcginn, S.M.; Kindermann, M.; Wang, M.; Tan, Z.L.; Beauchemin, K.A. Combined Effects of 3-Nitrooxypropanol and Canola Oil Supplementation on Methane Emissions, Rumen Fermentation and Biohydrogenation, and Total Tract Digestibility in Beef Cattle. J. Anim. Sci. 2021, 99, skab081. [Google Scholar] [CrossRef]
- Singh, B.K.; Chopra, R.C.; Rai, S.N.; Verma, M.P.; Mohanta, R.K. Nutritional Evaluation of Seaweed on Nutrient Digestibility, Nitrogen Balance, Milk Production and Composition in Sahiwal Cows. Proc. Natl. Acad. Sci. India Sect. B—Biol. Sci. 2017, 87, 437–443. [Google Scholar] [CrossRef]
- Phongphanith, S.; Preston, T.R. Effect of Rice-Wine Distillers’ Byproduct and Biochar on Growth Performance and Methane Emissions in Local “Yellow” Cattle Fed Ensiled Cassava Root, Urea, Cassava Foliage and Rice Straw. Livest. Res. Rural. Dev. 2016, 28, 178. [Google Scholar]
- Terler, G.; Winter, M.; Mandl, M.; Sweeney, J.; Steinwidder, A. Effect of Biochar or Biochar and Urea Supplementation on Feed Intake, Milk Yield, Feed Conversion and Methane Production of Dairy Cows. Czech J. Anim. Sci. 2023, 68, 245–254. [Google Scholar] [CrossRef]
| Parameter | n | Mean | Minimum | Maximum | Standard Error | CV | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|---|
| Dietary rumen modulating strategies (g/kg) | ||||||||
| Nitrate | 39 | 19.5 | 0.30 | 223 | 5.59 | 179.48 | 5.46 | 32.28 |
| Saponin | 49 | 21 | 3.30 | 170 | 5.10 | 169.64 | 3.48 | 12.06 |
| Tannins | 112 | 54.3 | 0.10 | 272 | 6.15 | 119.95 | 1.24 | 1.03 |
| Oils | 104 | 20.3 | 0.05 | 50 | 1.49 | 74.59 | 0.15 | −0.90 |
| Ether extract | 24 | 36.3 | 4.82 | 174 | 1.17 | 54.95 | 1.56 | 6.88 |
| Concentrate feeding | 28 | 467.3 | 33 | 800 | 36.74 | 41.61 | −2.00 | −0.46 |
| Biochar | 18 | 181 | 50 | 460 | 26.40 | 61.71 | 0.92 | 0.52 |
| Seaweed | 14 | 73 | 0.50 | 300 | 16.70 | 97 | 2.04 | 5.42 |
| 3-Nitroxypropanol | 21 | 98.5 | 2.01 | 200 | 18.90 | 71.79 | 0.57 | −1.26 |
| Initial body weight (kg) | 521 | 202 | 11.20 | 1405 | 10.32 | 116.83 | 1.29 | 0.67 |
| Dietary chemical composition (g/kg) | ||||||||
| DM | 231 | 766.3 | 173 | 956 | 13.44 | 26.65 | −1.21 | 0.27 |
| OM | 292 | 919.6 | 753 | 970 | 1.86 | 3.45 | −2.17 | 8.86 |
| CP | 473 | 157.6 | 27 | 850 | 2.69 | 37.07 | 4.26 | 41.97 |
| NDF | 438 | 414 | 86 | 781 | 6.49 | 32.80 | 0.50 | −0.17 |
| ADF | 427 | 261.2 | 15.97 | 644 | 5.82 | 46.06 | 0.82 | 0.98 |
| Intake (kg/d) | ||||||||
| DM | 531 | 6.6 | 0.418 | 28.60 | 0.33 | 113.66 | 1.18 | 0.076 |
| OM | 276 | 0.9 | 0.435 | 18.20 | 0.09 | 165.11 | 8.83 | 87.02 |
| CP | 427 | 1.2 | 0.026 | 5.16 | 0.07 | 118.41 | 1.17 | 0.04 |
| NDF | 402 | 2.7 | 0.154 | 12.50 | 0.15 | 109.57 | 1.25 | 0.75 |
| ADF | 385 | 1.6 | 0.019 | 8.64 | 0.09 | 115.61 | 1.55 | 1.98 |
| Total tract digestibility (g/kg) | ||||||||
| DM | 324 | 632 | 410 | 866 | 4.23 | 12.05 | 0.04 | 0.06 |
| CP | 226 | 611 | 203 | 901 | 10.23 | 25.16 | −0.98 | 1.10 |
| NDF | 355 | 509 | 211 | 854 | 6.11 | 22.61 | −0.17 | 0.413 |
| ADF | 266 | 466 | 126 | 639 | 5.65 | 19.76 | −0.41 | 0.64 |
| EE | 140 | 682 | 302 | 970 | 14 | 24.30 | −0.45 | −0.37 |
| ADG (g/day) | 254 | 172 | −32 | 1600 | 14.14 | 130.94 | 3.06 | 11.44 |
| Milk production (kg/day) | 135 | 14.5 | 1.15 | 45.20 | 1.10 | 69.34 | 0.18 | −0.94 |
| Feed conversion ratio (g/kg DMI) | 125 | 3.4 | −15.77 | 145.72 | 1.24 | 404 | 8.85 | 90.53 |
| Milk yield (g/kg DMI) | 26 | 10.4 | 0.66 | 27.13 | 1.965 | 96.43 | 0.57 | −1.38 |
| Milk quality | ||||||||
| MFp (g/kg) | 124 | 3.67 | 0.30 | 7.76 | 6.20 | 53.42 | 3.75 | 1.87 |
| MFy (kg/d) | 103 | 3.2 | 0.001 | 7.33 | 0.23 | 7.69 | −1.5 | −0.5 |
| MPp (g/kg) | 91 | 0.5 | 0.01 | 8.0 | 0.026 | 4.44 | −0.16 | −1.36 |
| MPy (kg/d) | 124 | 3.7 | 0.30 | 7.76 | 0.18 | 6.85 | 1.29 | 1.13 |
| Methane | ||||||||
| g/kg LWG | 40 | 117.7 | 1.52 | 482 | 18.54 | 99.66 | 1.15 | 0.97 |
| g/day | 252 | 74.7 | 2.81 | 635 | 8.29 | 176.21 | 2.59 | 6.04 |
| g/kg DMI | 220 | 22.4 | 4.70 | 58.80 | 0.503 | 33.33 | 1.10 | 4.35 |
| g/kg milk | 35 | 12.3 | 0.33 | 60.41 | 29.3 | 145.77 | 1.58 | 1.52 |
| Fermentation parameters | ||||||||
| Acetate | 345 | 41.5 | 7.10 | 94.48 | 1.11 | 49.71 | 0.383 | −1.07 |
| Butyrate | 345 | 13 | 1.35 | 35.20 | 0.39 | 54.96 | 0.85 | −0.11 |
| Propionate | 345 | 7.6 | 1.12 | 30.71 | 0.266 | 64.90 | 1.78 | 4.52 |
| Acetate: propionate ratio | 321 | 3.5 | 0.99 | 7.32 | 0.066 | 33.63 | 0.86 | 0.79 |
| pH | 140 | 6.5 | 5.9 | 7.11 | 0.02 | 4.32 | −0.026 | −1.06 |
| Dietary Rumen Modulating Strategies | Enteric Methane | Fermentation Parameters | Ruminant Type | Feeding System | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH4liveweight | CH4milk | CH4yield | CH4production | Acetate | Propionate | Butyrate | Acetate/Propionate Ratio | pH | ||||
| Nitrate | NE | −16.63% | −10.11% | NE | ND | ND | ND | ND | ND | Beef, dairy, goats, and sheep | Confined and grazing | |
| Saponin | NE | ND | NE | −37.27% | ND | ND | ND | ND | ND | Beef, buffaloes, goats and sheep | Confined and grazing | |
| Tannin | NE | ND | −37% | −40% | NE | +10.90% | +9.80% | −5.70% | NE | Beef, buffaloes, deer, goats and sheep | Confined and grazing | |
| Oils | ND | −38.96 | −7.13% | NE | NE | +2% | −2.90% | −3.80% | NE | Beef, buffaloes, deer, goats and sheep | Confined and grazing | |
| Ether extract | NE | NE | ND | NE | NE | NE | NE | NE | NE | Dairy, deer and goats | Confined | |
| Concentrate feeding | ND | NE | NE | NE | NE | NE | NE | NE | NE | NE | Confined and grazing | |
| Biochar | ND | ND | NE | −5.45% | NE | NE | NE | NE | NE | Beef, goats and sheep | Confined | |
| Seaweed | ND | ND | NE | −35.34% | −21.80% | ND | ND | ND | ND | Beef, goats and sheep | Confined | |
| 3-Nitroxy propanol | ND | −30.46 | −27.36% | NE | NE | +13.10 | +17.26% | −20.26% | NE | Dairy, deer, and goats | Confined | |
| Variables | Production Performance | Milk Quality | Ruminant Livestock | Feeding System | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMI | TTDIG | ADG | MP | FCR | MY | MFp | MFy | MPp | MPy | ||||
| Nitrate | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | Beef, dairy, goats, and sheep | Confined and Grazing, | |
| Saponin | NE | NE | NE | NE | NE | NE | ND | ND | ND | ND | Beef, buffaloes, goats, and sheep | Confined and grazing | |
| Tannin | NE | −12 | NE | NE | NE | NE | NE | NE | NE | NE | Beef, buffaloes, dairy, goats, and sheep | Confined and grazing | |
| Oils | NE | NE | NE | ND | NE | NE | NE | +16 | NE | +20 | Beef, buffaloes, dairy, goats, and sheep | Confined | |
| Ether extract | NE | ND | NE | ND | NE | ND | NE | NE | NE | NE | Beef, buffaloes, dairy, goats, and sheep | Confined | |
| Concentrate feeding | +23.41% | ND | NE | NE | NE | +19% | NE | NE | +16.25% | NE | Dairy, deer, and goats | Confined and grazing | |
| Biochar | NE | NE | NE | ND | NE | NE | ND | ND | ND | ND | Beef, goats and sheep | Confined | |
| Seaweed | NE | NE | −3.75 | ND | ND | NE | NE | NE | NE | NE | Beef and dairy | Confined | |
| 3-Nitroxy propanol | NE | NE | NE | ND | NE | NE | +15 | NE | NE | NE | Beef and dairy | Grazing and confined | |
| Parameters | Methane Emission | Rumen Fermentation Parameters | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH4 g/kg LWG | CH4 g/kg DMI | CH4 g/d | CH4 g/kg Milk | Acetate | Propionate | Butyrate | A/P Ratio | pH | ||||||||||
| Dietary Rumen Modulating Strategies | % | p-Value | % | p-Value | % | p-Value | % | p-Value | % | p-Value | % | p-Value | % | p-Value | % | p-Value | % | p-Value |
| Nitrate | −28 | 0.169 | −10 | 0.007 | 1.80 | 0.91 | −16.63 | 0.10 | ND | ND | ND | ND | ND | ND | ND | ND | −17 | 0.1 |
| Saponin | −2.52 | 0.87 | −4.29 | 0.32 | −37 | 0.04 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Tannin | −6.30 | 0.77 | −37 | <0.001 | −40 | 0.003 | ND | ND | −6.1 | 0.34 | 10.91 | <0.001 | −9.83 | 0.07 | −5.66 | 0.01 | ND | ND |
| Oils | ND | ND | −7.13 | 0.003 | −28 | 0.12 | −38 | 0.01 | −0.4 | 0.96 | 2 | 0.02 | −2.92 | 0.07 | −3.81 | 0.04 | −39 | 0.01 |
| Ether extract | −17 | 0.28 | ND | ND | −0.07 | 0.99 | −15.66 | 0.09 | 0.17 | 1.0 | 0.66 | 0.64 | −0.19 | 0.94 | 4.68 | 0.15 | −15.6 | 0.09 |
| Concentrate | ND | ND | −3.96 | 0.32 | 7.07 | 0.45 | −11 | 0.27 | −1.7 | 0.94 | 3.98 | 0.25 | 1.69 | 0.78 | −3.41 | 0.74 | −11 | 0.27 |
| Biochar | ND | ND | −4.24 | 0.14 | −5.45 | 0.05 | ND | ND | 1.03 | 0.94 | 2 | 0.35 | 1.94 | 0.87 | 1.58 | 0.59 | ND | ND |
| Seaweed | ND | ND | −35 | 0.005 | −22 | 0.08 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 3-Nitroxy propanol | −4.8 | 0.79 | −27 | 0.002 | ND | ND | −30 | 0.001 | −8.5 | 0.64 | +13.1 | <0.001 | 17.26 | <0.001 | −20 | 0.001 | −30 | 0.18 |
| Parameters | DMI | TTDIG | MP | FCR | MY | ADG | MF g/kg | MF kg/d | MP g/kg | MP kg/d | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Rumen Modulating Strategies | % | p-Value | % | p-Value | % | p-Value | % | p-Value | % | p-Value | % | p-Value | % | p-Value | % | p-Value | % | p-Value | % | p-Value |
| Nitrate | 11.70 | 0.20 | −0.16 | 0.97 | 1.45 | 0.78 | −7.01 | 0.77 | 2.74 | 0.85 | −8.24 | 0.94 | 2.74 | 0.70 | 2.74 | 0.75 | 2.74 | 0.44 | 2.74 | 0.86 |
| Saponin | −0.10 | 1.00 | −0.11 | 0.96 | −7.23 | 0.81 | 2.25 | 0.86 | ND | ND | −16 | 0.86 | ND | ND | ND | ND | ND | ND | ND | ND |
| Tannin | −1.07 | 0.99 | −12 | 0.01 | −0.03 | 1.00 | −7.87 | 0.44 | 3.84 | 0.77 | 0.88 | 0.99 | 6 | 0.36 | 2.51 | 0.88 | −0.61 | 0.72 | ND | ND |
| Oils | 1.42 | 1.00 | −2.25 | 0.20 | ND | ND | −8.21 | 0.31 | 6.57 | 0.17 | 69.34 | 0.26 | 0.17 | 0.98 | 16.3 | <0.001 | ND | ND | 20 | 0.008 |
| Ether extract | 7.45 | 0.25 | ND | ND | ND | ND | −0.71 | 0.94 | ND | ND | 0.02 | 1.00 | −8 | 0.14 | 1.46 | 0.77 | −0.45 | 0.83 | −3 | 0.73 |
| Concentrate | +23.41 | 0.10 | ND | ND | 2.68 | 0.68 | 11.83 | 0.45 | +19.90 | <0.001 | 60.58 | 0.43 | −3 | 0.66 | 3.65 | 0.77 | −16 | 0.01 | 9.28 | 0.58 |
| Biochar | −3.72 | 0.20 | 0.380 | 0.88 | ND | ND | −3.72 | 0.83 | 18.41 | 0.21 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Seaweed | −2.16 | 0.23 | 1.38 | 0.33 | ND | ND | ND | ND | 6.90 | 0.30 | −3.75 | 0.004 | 0.14 | 0.98 | 0.65 | 0.82 | 0.69 | 0.86 | ND | ND |
| 3-Nitroxy propanol | −0.21 | 0.97 | 1.80 | 0.87 | ND | ND | 2.64 | 0.9 | −0.94 | 0.87 | −7.42 | 0.85 | 1.17 | 0.86 | 15 | 0.007 | −0.16 | 0.97 | 0.64 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepeta, B.N.; Hassen, A.; Tesfamariam, E.H. Quantifying the Impact of Different Dietary Rumen Modulating Strategies on Enteric Methane Emission and Productivity in Ruminant Livestock: A Meta-Analysis. Animals 2024, 14, 763. https://doi.org/10.3390/ani14050763
Pepeta BN, Hassen A, Tesfamariam EH. Quantifying the Impact of Different Dietary Rumen Modulating Strategies on Enteric Methane Emission and Productivity in Ruminant Livestock: A Meta-Analysis. Animals. 2024; 14(5):763. https://doi.org/10.3390/ani14050763
Chicago/Turabian StylePepeta, Bulelani N., Abubeker Hassen, and Eyob H. Tesfamariam. 2024. "Quantifying the Impact of Different Dietary Rumen Modulating Strategies on Enteric Methane Emission and Productivity in Ruminant Livestock: A Meta-Analysis" Animals 14, no. 5: 763. https://doi.org/10.3390/ani14050763
APA StylePepeta, B. N., Hassen, A., & Tesfamariam, E. H. (2024). Quantifying the Impact of Different Dietary Rumen Modulating Strategies on Enteric Methane Emission and Productivity in Ruminant Livestock: A Meta-Analysis. Animals, 14(5), 763. https://doi.org/10.3390/ani14050763







