Effects of Seasonality and Pregnancy on Hair Loss and Regrowth in Rhesus Macaques
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Housing
2.2. Alopecia and Hair Regrowth Assessment
2.3. Reproductive Status
2.4. Data Analysis
3. Results
3.1. Summary of Data Collected
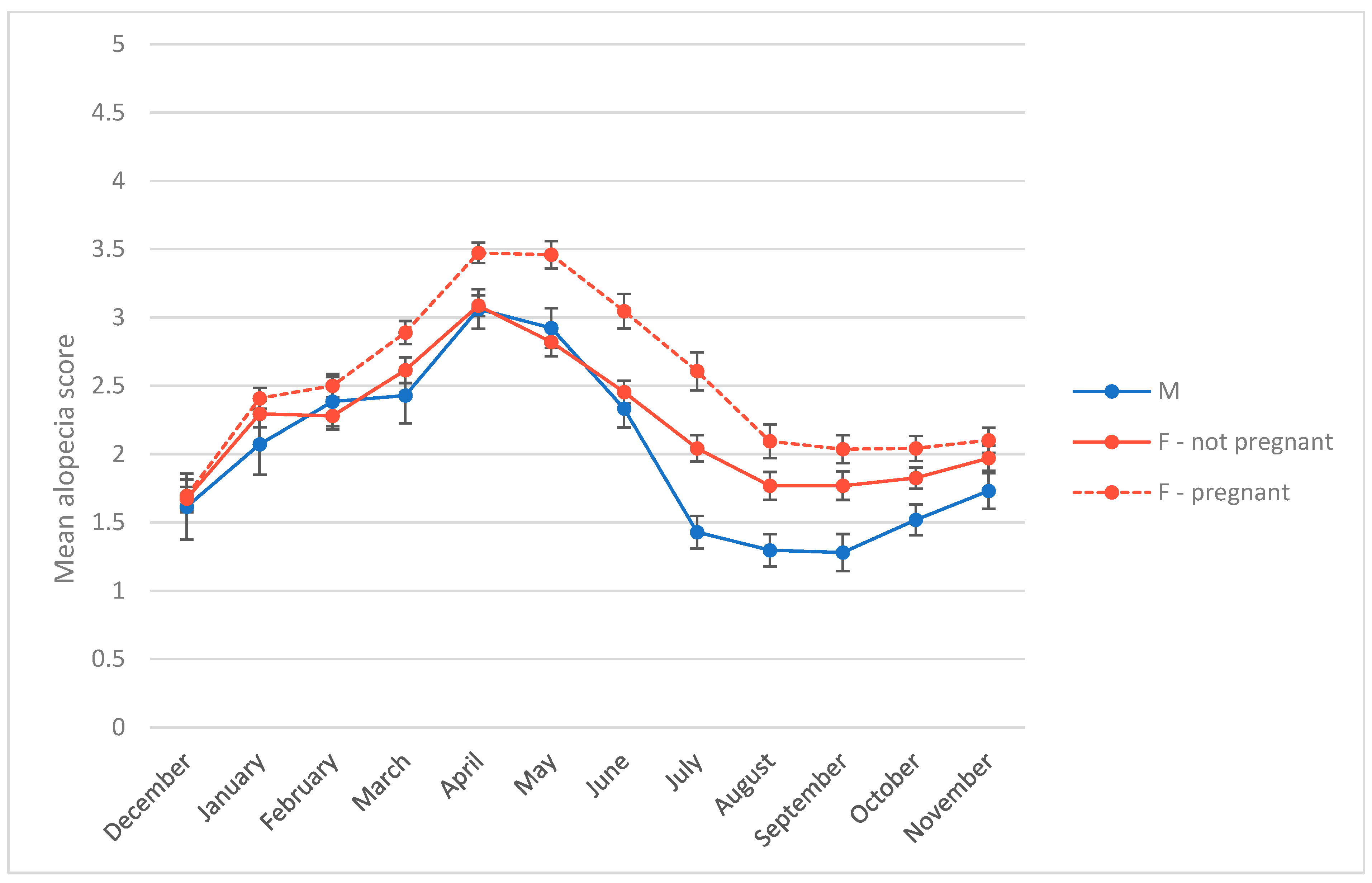
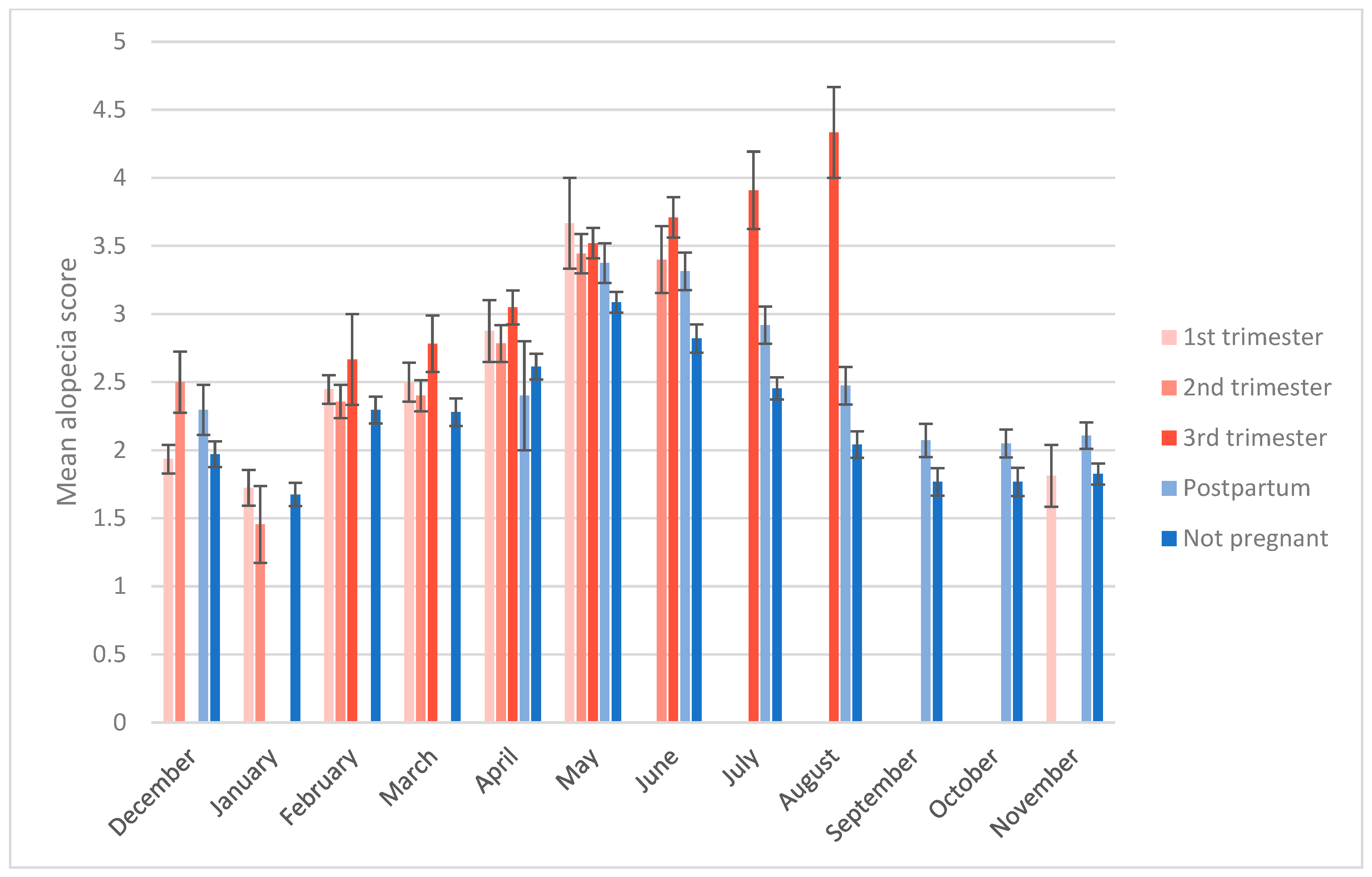
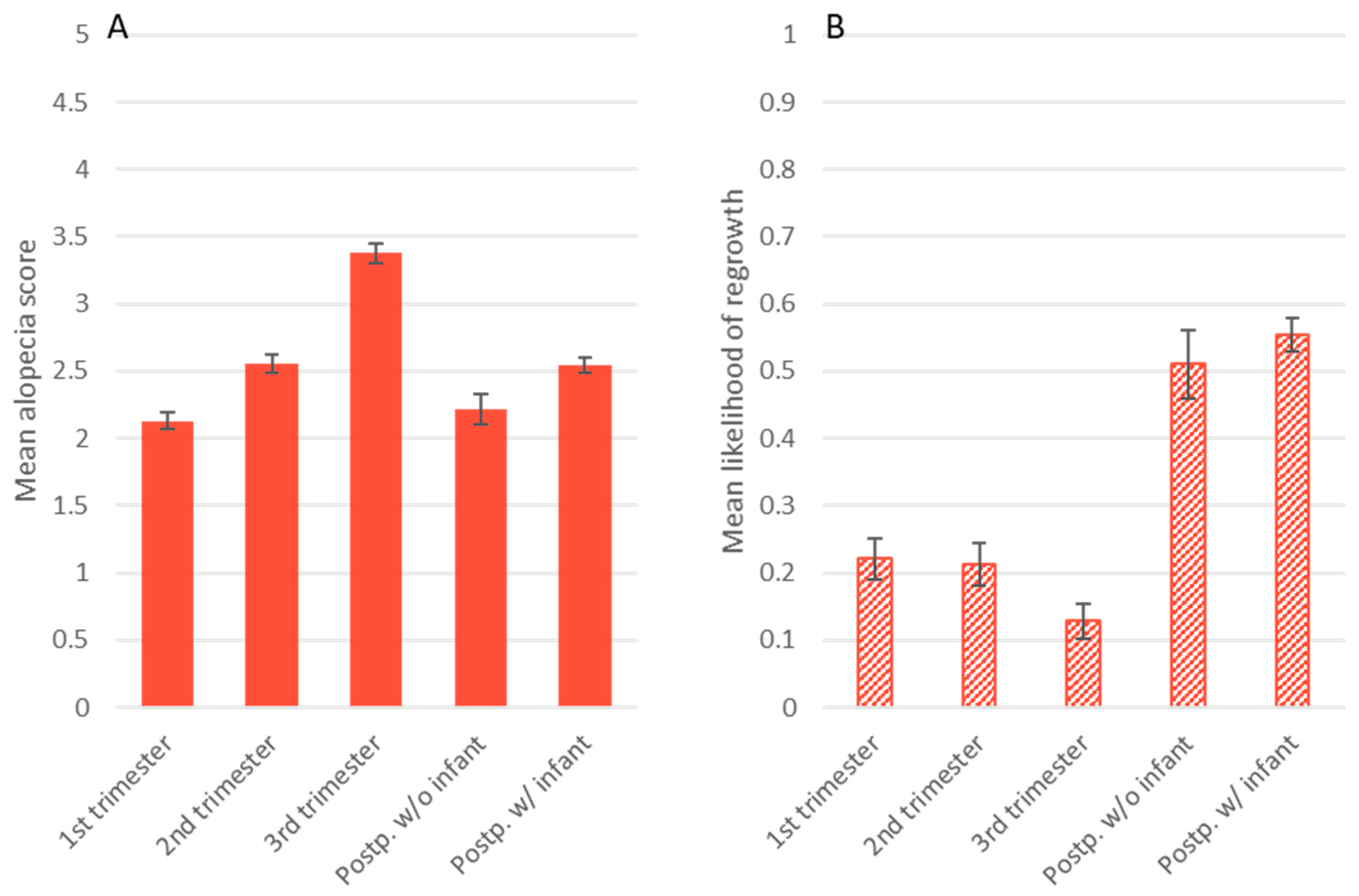
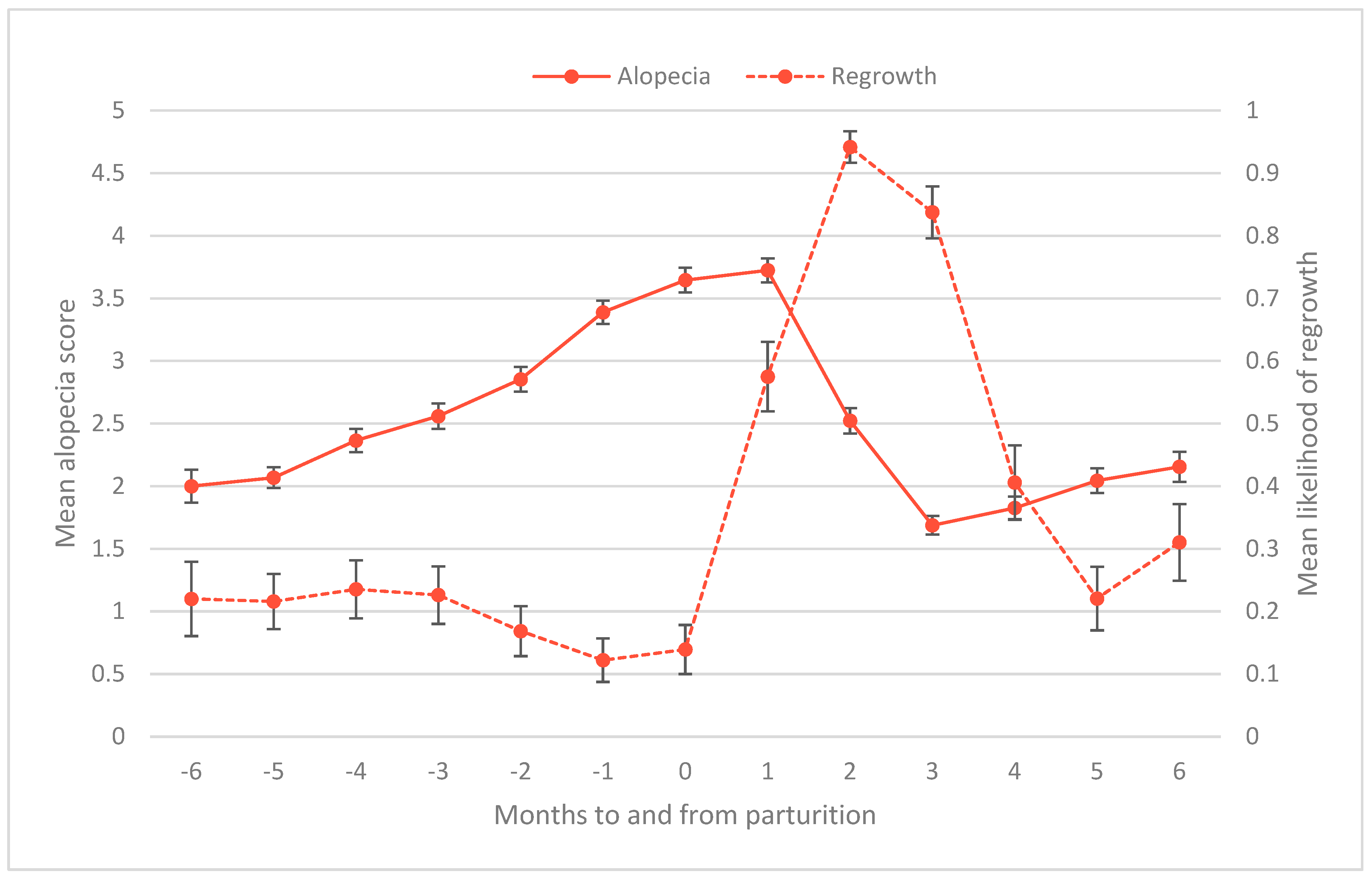
3.2. Alopecia Severity
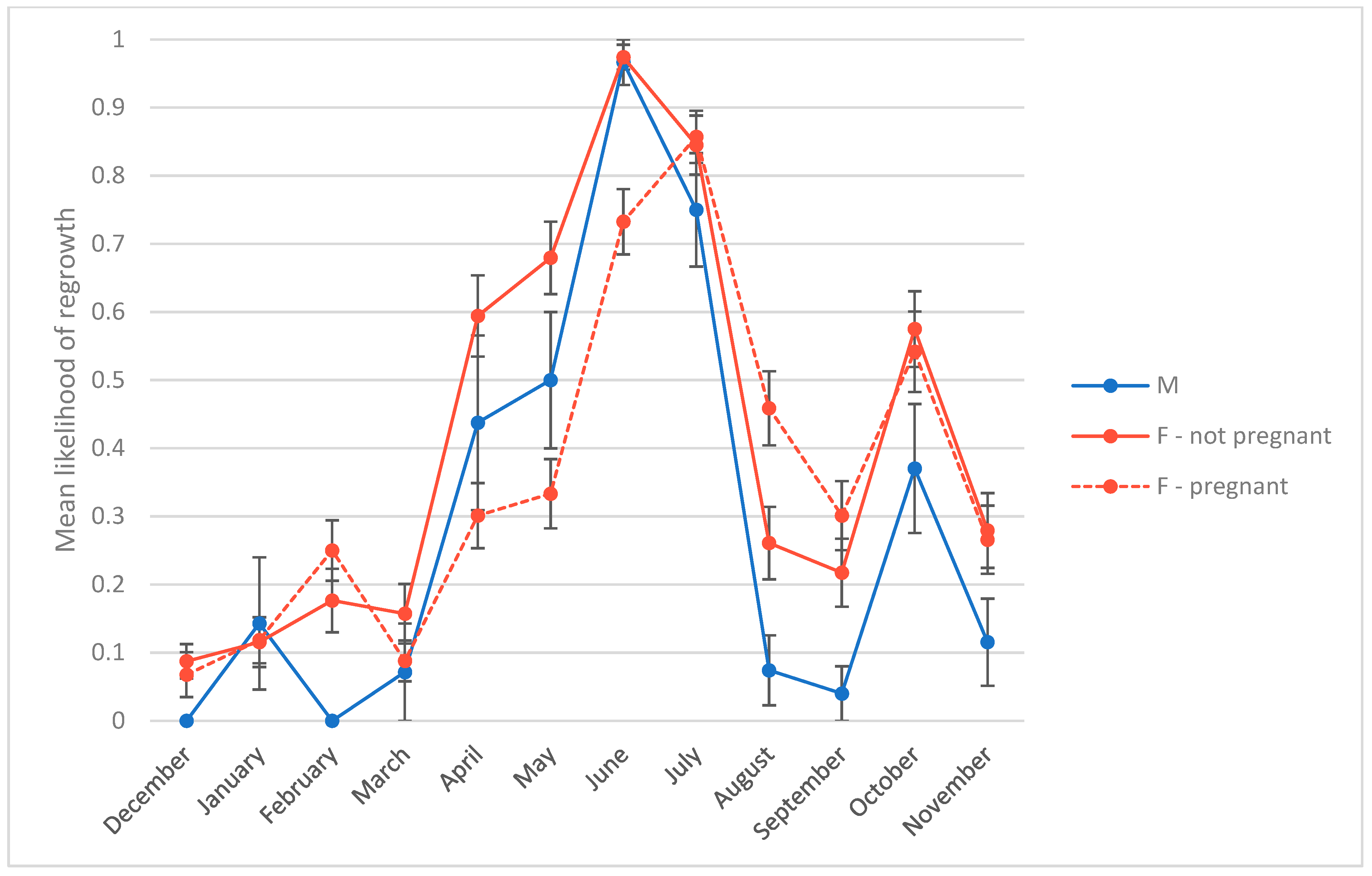
3.3. Presence of Hair Regrowth
3.4. Time to Regrow Hair Postpartum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.-C.; Chang, A.-M.; Tsai, M.-S.; Huang, Y.-H.; Pei, K.J.-C.; Li, Y.-C. Association between Stress and Bilateral Symmetrical Alopecia in Free-Ranging Formosan Macaques in Mt. Longevity, Taiwan. Sci. Rep. 2021, 11, 11189. [Google Scholar] [CrossRef]
- Lutz, C.K.; Coleman, K.; Worlein, J.M.; Kroeker, R.; Menard, M.T.; Rosenberg, K.; Meyer, J.S.; Novak, M.A. Factors Influencing Alopecia and Hair Cortisol in Rhesus Macaques (Macaca mulatta). J. Med. Primatol. 2016, 45, 180–188. [Google Scholar] [CrossRef]
- Lutz, C.K.; Menard, M.T.; Rosenberg, K.; Meyer, J.S.; Novak, M.A. Alopecia in Rhesus Macaques (Macaca mulatta): Association with Pregnancy and Chronic Stress. J. Med. Primatol. 2019, 48, 251–256. [Google Scholar] [CrossRef]
- Novak, M.A.; Menard, M.T.; El-Mallah, S.N.; Rosenberg, K.; Lutz, C.K.; Worlein, J.; Coleman, K.; Meyer, J.S. Assessing Significant (>30%) Alopecia as a Possible Biomarker for Stress in Captive Rhesus Monkeys (Macaca mulatta). Am. J. Primatol. 2017, 79, e22547. [Google Scholar] [CrossRef]
- Novak, M.A.; Meyer, J.S. Alopecia: Possible Causes and Treatments, Particularly in Captive Nonhuman Primates. Comp. Med. 2009, 59, 18–26. [Google Scholar]
- Steinmetz, H.W.; Kaumanns, W.; Dix, I.; Heistermann, M.; Fox, M.; Kaup, F.-J. Coat Condition, Housing Condition and Measurement of Faecal Cortisol Metabolites—A Non-Invasive Study about Alopecia in Captive Rhesus Macaques (Macaca mulatta). J. Med. Primatol. 2006, 35, 3–11. [Google Scholar] [CrossRef]
- Hamel, A.F.; Menard, M.T.; Novak, M.A. Fatty Acid Supplements Improve Hair Coat Condition in Rhesus Macaques. J. Med. Primatol. 2017, 46, 248–251. [Google Scholar] [CrossRef]
- Kramer, J.; Fahey, M.; Santos, R.; Carville, A.; Wachtman, L.; Mansfield, K. Alopecia in Rhesus Macaques Correlates with Immunophenotypic Alterations in Dermal Inflammatory Infiltrates Consistent with Hypersensitivity Etiology. J. Med. Primatol. 2010, 39, 112–122. [Google Scholar] [CrossRef]
- Heagerty, A.; Wales, R.A.; Prongay, K.; Gottlieb, D.H.; Coleman, K. Social Hair Pulling in Captive Rhesus Macaques (Macaca mulatta). Am. J. Primatol. 2017, 79, e22720. [Google Scholar] [CrossRef]
- Kramer, J.A.; Mansfield, K.G.; Simmons, J.H.; Bernstein, J.A. Psychogenic Alopecia in Rhesus Macaques Presenting as Focally Extensive Alopecia of the Distal Limb. Comp. Med. 2011, 61, 263–268. [Google Scholar]
- Lutz, C.K.; Coleman, K.; Worlein, J.; Novak, M.A. Hair Loss and Hair-Pulling in Rhesus Macaques (Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 454–457. [Google Scholar]
- Coleman, K.; Lutz, C.K.; Worlein, J.M.; Gottlieb, D.H.; Peterson, E.; Lee, G.H.; Robertson, N.D.; Rosenberg, K.; Menard, M.T.; Novak, M.A. The Correlation between Alopecia and Temperament in Rhesus Macaques (Macaca mulatta) at Four Primate Facilities. Am. J. Primatol. 2017, 79, e22504. [Google Scholar] [CrossRef]
- Kroeker, R.; Lee, G.H.; Bellanca, R.U.; Thom, J.P.; Worlein, J.M. Prior Facility Affects Alopecia in Adulthood for Rhesus Macaques. Am. J. Primatol. 2017, 79, e22551. [Google Scholar] [CrossRef]
- Gauvin, D.; Cooper, D. Hair Loss in Laboratory Bred Macaques: An Idiopathic Disorder of Major Consequence. Arch. Vet. Sci. Technol. AVST-146 2018, 10. [Google Scholar] [CrossRef]
- Beisner, B.A.; Isbell, L.A. Factors Influencing Hair Loss among Female Captive Rhesus Macaques (Macaca mulatta). Appl. Anim. Behav. Sci. 2009, 119, 91–100. [Google Scholar] [CrossRef]
- Davis, E.; Suomi, S. Hair Loss and Replacement Cycles in Socially Housed, Pregnant, Rhesus Macaques. In American Journal of Primatology; DIV JOHN WILEY & SONS INC.: Hoboken, NJ, USA, 2006; Volume 68, p. 58. [Google Scholar]
- Dettmer, A.M.; Rosenberg, K.; Menard, M.T.; El-Mallah, S.N.; Woodward, R.A.; Suomi, S.J.; Meyer, J.S. Differential Relationships between Chronic Hormone Profiles in Pregnancy and Maternal Investment in Rhesus Monkey Mothers with Hair Loss in the Neonatal Period. Am. J. Primatol. 2017, 79, e22489. [Google Scholar] [CrossRef]
- Lutz, C.K. Effect of Pregnancy and Age on Alopecia in Adult Female Baboons (Papio hamadryas spp.). J. Am. Assoc. Lab. Anim. Sci. 2021, 60, 484–488. [Google Scholar] [CrossRef]
- Kroeker, R.; Chichester, L.; Lee, G.H.; Worlien, J.M. Effects of Pregnancy, Outdoor Access, and Antifungal Medication on Hair Loss in Breeding-Age Female Pigtailed Macaques (Macaca nemestrina). Comp. Med. 2019, 69, 221–239. [Google Scholar] [CrossRef]
- Kroeker, R.; Bellanca, R.U.; Lee, G.H.; Thom, J.P.; Worlein, J.M. Alopecia in Three Macaque Species Housed in a Laboratory Environment. Am. J. Primatol. 2014, 76, 325–334. [Google Scholar] [CrossRef]
- Lutz, C.K.; Sharp, R.M. Alopecia in Outdoor Group- and Corral-Housed Baboons (Papio hamadryas spp.). J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 384–388. [Google Scholar]
- Zhang, P. A Non-Invasive Study of Alopecia in Japanese Macaques Macaca fuscata. Curr. Zool. 2011, 57, 26–35. [Google Scholar] [CrossRef]
- Vessey, S.H.; Morrison, J.A. Molt in Free-Ranging Rhesus Monkeys, Macaca mulatta. J. Mammal. 1970, 51, 89–93. [Google Scholar] [CrossRef]
- Chien Yin, G.O.; Siong-See, J.L.; Wang, E.C.E. Telogen Effluvium—A Review of the Science and Current Obstacles. J. Dermatol. Sci. 2021, 101, 156–163. [Google Scholar] [CrossRef]
- Piérard-Franchimont, C.; Piérard, G.E. Teloptosis, a Turning Point in Hair Shedding Biorhythms. Dermatology 2001, 203, 115–117. [Google Scholar] [CrossRef]
- Piérard-Franchimont, C.; Piérard, G.E. Alterations in Hair Follicle Dynamics in Women. BioMed Res. Int. 2013, 2013, 957432. [Google Scholar] [CrossRef]
- Rebora, A. Proposing a Simpler Classification of Telogen Effluvium. Skin Appendage Disord. 2016, 2, 35–38. [Google Scholar] [CrossRef]
- Kligman, A.M. Pathologic Dynamics of Human Hair Loss: I. Telogen Effluvium. Arch. Dermatol. 1961, 83, 175–198. [Google Scholar] [CrossRef]
- Gizlenti, S.; Ekmekci, T.R. The Changes in the Hair Cycle during Gestation and the Post-Partum Period. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 878–881. [Google Scholar] [CrossRef]
- Lynfield, Y.L. Effect of Pregnancy on the Human Hair Cycle. J. Investig. Dermatol. 1960, 35, 323–327. [Google Scholar] [CrossRef]
- Aoki, E.; Shibasaki, T.; Kawana, S. Intermittent Foot Shock Stress Prolongs the Telogen Stage in the Hair Cycle of Mice. Exp. Dermatol. 2003, 12, 371–377. [Google Scholar] [CrossRef]
- Katayama, M.; Aoki, E.; Suzuki, H.; Kawana, S. Foot Shock Stress Prolongs the Telogen Stage of the Spontaneous Hair Cycle in a Non-Depilated Mouse Model. Exp. Dermatol. 2007, 16, 553–560. [Google Scholar] [CrossRef]
- Arck, P.C.; Handjiski, B.; Peters, E.M.J.; Peter, A.S.; Hagen, E.; Fischer, A.; Klapp, B.F.; Paus, R. Stress Inhibits Hair Growth in Mice by Induction of Premature Catagen Development and Deleterious Perifollicular Inflammatory Events via Neuropeptide Substance P-Dependent Pathways. Am. J. Pathol. 2003, 162, 803–814. [Google Scholar] [CrossRef]
- Brajac, I.; Tkalčić, M.; Dragojević, D.M.; Gruber, F. Roles of Stress, Stress Perception and Trait-Anxiety in the Onset and Course of Alopecia Areata. J. Dermatol. 2003, 30, 871–878. [Google Scholar] [CrossRef]
- Baker, K.C.; Bloomsmith, M.A.; Coleman, K.; Crockett, C.M.; Worlein, J.; Lutz, C.K.; McCowan, B.; Pierre, P.; Weed, J. The Behavioral Management Consortium. In Handbook of Primate Behavioral Management; CRC Press: Boca Raton, FL, USA, 2017; pp. 9–23. [Google Scholar]
- Silk, J.; Short, J.; Roberts, J.; Kusnitz, J. Gestation Length in Rhesus Macaques (Macaca mulatta). Int. J. Primatol. 1993, 14, 95–104. [Google Scholar] [CrossRef]
- Akaike, H. Factor Analysis and AIC. Psychometrika 1987, 52, 317–332. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Botchkarev, V.A. Stress and the Hair Follicle: Exploring the Connections. Am. J. Pathol. 2003, 162, 709–712. [Google Scholar] [CrossRef]
- Gittleman, J.L.; Thompson, S.D. Energy Allocation in Mammalian Reproduction. Am. Zool. 1988, 28, 863–875. [Google Scholar] [CrossRef]
- Hinde, K.; Power, M.L.; Oftedal, O.T. Rhesus Macaque Milk: Magnitude, Sources, and Consequences of Individual Variation over Lactation. Am. J. Phys. Anthropol. 2009, 138, 148–157. [Google Scholar] [CrossRef]
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Males > 3 years | 3 | 6 | 7 | 1 | 3 | 2 | 6 | 5 | 33 |
| Females > 3 years | 25 | 29 | 17 | 34 | 23 | 23 | 20 | 37 | 208 |
| Males & females < 3 years | 20 | 26 | 19 | 32 | 29 | 26 | 24 | 21 | 197 |
| Total | 48 | 61 | 43 | 67 | 55 | 51 | 50 | 63 | 438 |
| Percent of Body Affected by Alopecia | Alopecia Score |
|---|---|
| 0 | 0 |
| 1–9 | 1 |
| 10–24 | 2 |
| 25–50 | 3 |
| 51–74 | 4 |
| 75–100 | 5 |
| Females | |||||||
|---|---|---|---|---|---|---|---|
| Month | Male | Nonpregnant | 1st Trimester | 2nd Trimester | 3rd Trimester | Postpartum w/o Infant | Postpartum w/Infant |
| December | 13 | 126 | 47 | 11 | 1 | ||
| January | 14 | 78 | 47 | 42 | 3 | 1 | |
| February | 13 | 68 | 22 | 50 | 23 | 1 | |
| March | 14 | 70 | 8 | 37 | 41 | 5 | |
| April | 16 | 69 | 3 | 18 | 48 | 3 | 21 |
| May | 26 | 78 | 5 | 31 | 13 | 38 | |
| June | 30 | 77 | 1 | 11 | 16 | 58 | |
| July | 28 | 71 | 6 | 16 | 62 | ||
| August | 27 | 69 | 1 | 18 | 66 | ||
| September | 25 | 69 | 1 | 18 | 64 | ||
| October | 27 | 80 | 16 | 10 | 46 | ||
| November | 26 | 68 | 46 | 6 | 2 | 25 | |
| Estimate | Std. Error | p-Value (* Indicates Value < 0.05) | |
|---|---|---|---|
| Intercept | −0.31 | 0.10 | <0.01 * |
| Study month | 0.24 | 0.02 | <0.001 * |
| Study month squared | −0.02 | <0.001 | <0.001 * |
| 1st trimester pregnant | −0.04 | 0.10 | 0.71 |
| 2nd trimester pregnant | 0.07 | 0.13 | 0.57 |
| 3rd trimester pregnant | 0.30 | 0.23 | 0.18 |
| Male | 0.18 | 0.16 | 0.26 |
| Postpartum without infant | 1.75 | 0.37 | <0.001 * |
| Postpartum with infant | 1.38 | 0.19 | <0.001 * |
| Study month × 1st trimester pregnant | 0.03 | 0.01 | 0.04 * |
| Study month × 2nd trimester pregnant | 0.06 | 0.03 | 0.03 * |
| Study month × 3rd trimester pregnant | 0.12 | 0.04 | <0.01 * |
| Study month × male | −0.05 | 0.02 | <0.01 * |
| Study month × postpartum without infant | −0.21 | 0.04 | <0.001 * |
| Study month × postpartum rearing infant | −0.13 | 0.02 | <0.001 * |
| Estimate | Std. Error | p-Value (* Indicates Value < 0.05) | |
|---|---|---|---|
| Intercept | −4.52 | 0.31 | <0.001 * |
| Study month | 1.43 | 0.09 | <0.001 * |
| Study month squared | −0.10 | 0.07 | <0.001 * |
| 1st trimester pregnant | 0.23 | 0.22 | 0.31 |
| 2nd trimester pregnant | −0.53 | 0.24 | 0.02 * |
| 3rd trimester pregnant | −2.15 | 0.27 | <0.001 * |
| Male | −0.63 | 0.19 | <0.001 * |
| Postpartum without infant | −0.36 | 0.25 | 0.15 |
| Postpartum with infant | −0.06 | 0.15 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heagerty, A.; Wales, R.A.; Coleman, K. Effects of Seasonality and Pregnancy on Hair Loss and Regrowth in Rhesus Macaques. Animals 2024, 14, 747. https://doi.org/10.3390/ani14050747
Heagerty A, Wales RA, Coleman K. Effects of Seasonality and Pregnancy on Hair Loss and Regrowth in Rhesus Macaques. Animals. 2024; 14(5):747. https://doi.org/10.3390/ani14050747
Chicago/Turabian StyleHeagerty, Allison, Rebecca A. Wales, and Kristine Coleman. 2024. "Effects of Seasonality and Pregnancy on Hair Loss and Regrowth in Rhesus Macaques" Animals 14, no. 5: 747. https://doi.org/10.3390/ani14050747
APA StyleHeagerty, A., Wales, R. A., & Coleman, K. (2024). Effects of Seasonality and Pregnancy on Hair Loss and Regrowth in Rhesus Macaques. Animals, 14(5), 747. https://doi.org/10.3390/ani14050747





