Behaviour Indicators of Animal Welfare in Purebred and Crossbred Yearling Beef Reared in Optimal Environmental Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm Management
2.2. Species-Specific Behaviour Evaluations
2.2.1. Ex-Vivo Behaviour Observations
2.2.2. Behaviour Observations from Video-Recordings
2.3. Blood Oxytocin Determinations
2.4. Statistical Analysis
3. Results
3.1. Ex-Vivo Behaviour Observations by SCAN Sampling
3.2. Ex-Vivo Behavioural Observations by FOCUS Sampling
3.3. Behaviour Parameters from Video Recording Observations
3.4. Oxytocin Determinations in Yearling Beef Plasma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vigors, B.; Sandøe, P.; Lawrence, A.B. Positive welfare in science and society: Differences, similarities and synergies. Front. Anim. Sci. 2021, 2, 738193. [Google Scholar] [CrossRef]
- Pulina, G. Anthropocentrism, natural harmony, sentience, and animal rights: Are we allowed to use animals for our own purposes? Animals 2023, 13, 1083. [Google Scholar] [CrossRef]
- Broom, D.M. Can positive welfare counterbalance negative and can net welfare be assessed? Front. Anim. Sci. 2023, 4, 1101957. [Google Scholar] [CrossRef]
- Lawrence, A.B.; Vigors, B.; Sandøe, P. What is so positive about positive animal welfare?—A critical review of literature. Animals 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Kilgour, R.J. In pursuit of “normal”: A review of the behaviour of cattle at pasture. Appl. Anim. Behav. Sci. 2012, 138, 1–11. [Google Scholar] [CrossRef]
- Napolitano, F.; Knierim, U.; Grass, F.; De Rosa, G. Positive indicators of cattle welfare and their applicability to on-farm protocols. Ital. J. Anim. Sci. 2009, 8 (Suppl. S1), 355–365. [Google Scholar] [CrossRef]
- Mooring, M.S.; Blumstein, D.T.; Stoner, C.J. The evolution of parasite-defense grooming in ungulates. Biol. J. Linn. Soc. 2004, 81, 17–37. [Google Scholar] [CrossRef]
- Wierenga, H.K.; Metz, J.H.M.; Hopster, H. The effect of extra space on the behaviour of dairy cows kept in a cubic house. In Social Space for Domestic Animals; Zayan, R., Ed.; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1985; pp. 160–170. [Google Scholar]
- Altmann, J. Observational study of behaviour: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef] [PubMed]
- Lehner, P. Sampling methods in behavior research. Poult. Sci. 1992, 71, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Gilby, I.C.; Pokempner, A.A.; Wrangham, R.W. A direct comparison of scan and focal sampling methods for measuring wild chimpanzee feeding behaviour. Folia Primatol. 2010, 81, 254–264. [Google Scholar] [CrossRef]
- Pullin, A.N.; Pairis-Garcia, M.D.; Campler, M.R.; Proudfoot, K.L. Validation of scan sampling techniques for behavioural observations of pastured lambs. Anim. Welf. 2023, 26, 185–190. [Google Scholar] [CrossRef][Green Version]
- Miller-Cushonand, E.K.; DeVries, T.J. Technical note: Validation of methodology for characterization of feeding behavior in dairy calves. J. Dairy Sci. 2011, 94, 6103–6110. [Google Scholar] [CrossRef]
- Verdú, M.; Bach, A.; Devant, M. Effect of concentrate feeder design on performance, feeding and animal behaviour, welfare, ruminal health, and carcass quality in Holstein bulls fed high-concentrate diets. Anim. Sci. J. 2015, 93, 3018–3033. [Google Scholar] [CrossRef]
- Barrell, G.K. An appraisal of methods for measuring welfare of grazing ruminants. Front. Vet. Sci. 2019, 6, 289. [Google Scholar] [CrossRef]
- Carter, C. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology 1998, 23, 779–818. [Google Scholar] [CrossRef]
- Cesarani, A.; Pulina, G. Farm animals are long away from natural behaviour: Open questions and operative consequences on animal welfare. Animals 2021, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Horan, B.; Mee, J.F.; Rath, M.; O’ Connor, P.; Dillon, P. The effect of strain of Holstein-Friesian cow and feeding system on reproductive performance in seasonal-calving milk production systems. Anim. Sci. 2016, 79, 453–467. [Google Scholar] [CrossRef]
- Ministero della Salute, ClassyFarm® Animal Welfare Assessment in Cattle and Buffalo Species: Explanatory Manual Official Control. 2019. Available online: https://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=5174&area=sanitaAnimale&menu=VAeCF (accessed on 8 May 2023).
- Welfare Quality®. 5.1.4.3. Good human-animal relationship—Avoidance distance. In Welfare Quality® Assessment Protocol for Cattle; Welfare Quality® Consortium: Lelystad, The Netherlands, 2009. [Google Scholar]
- Brambell, F.W.R. Report of the Technical Committee to Enquire into the Welfare of Animals Kept under Intensive; Political Science Her Majesty’s Stationary Office: London, UK, 1965. [Google Scholar]
- Buoio, E.; Cialini, C.; Costa, A. Air Quality Assessment in Pig Farming: The Italian Classyfarm. Animals 2023, 13, 2297. [Google Scholar] [CrossRef] [PubMed]
- Mariottini, F.; Giuliotti, L.; Gracci, M.; Benvenuti, M.N.; Salari, F.; Arzilli, L.; Martini, M.; Roncoroni, C.; Brajon, G. The Classyfarm system in tuscan beef cattle farms and the association between animal welfare level and productive performance. Animals 2022, 12, 1924. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.L.; Sandøe, P.; Sonntag, Q.; Stuardo, L. Positive animal welfare: Bridging the gap or raising inequalities worldwide? Front. Anim. Sci. 2022, 3, 825379. [Google Scholar] [CrossRef]
- DelCurto-Wyffels, H.M.; Dafoe, J.M.; Parsons, C.T.; Boss, D.L.; DelCurto, T.; Wyffels, S.A.; Van Emon, M.L.; Bowman, J.G.P. The influence of environmental conditions on intake behavior and activity by feedlot steers fed corn or barley-based diets. Animals 2021, 11, 1261. [Google Scholar] [CrossRef]
- Jensen, M.B.; Kyhn, R. Play behaviour in group-housed dairy calves, the effect of space allowance. Appl. Anim. Behav. Sci. 2000, 67, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Haley, D.B.; Rushen, J.; de Passillé, A.M. Behavioural indicators of cow comfort: Activity and resting behaviour of dairy cows in two types of housing. Can. J. Anim. Sci. 2000, 80, 257–263. [Google Scholar] [CrossRef]
- Horvath, K.C.; Miller-Cushon, E.K. Characterizing grooming behaviour patterns and the influence of brush access on the behaviour of group-housed dairy calves. J. Dairy Sci. 2019, 102, 3421–3430. [Google Scholar] [CrossRef]
- Dallemagne, M.; Ghys, E.; De Schrevel, C.; Mwema, A.; De Troy, D.; Rasse, C.; Donnay, I. Oxidative stress differentially impacts male and female bovine embryos depending on the culture medium and the stress condition. Theriogenology 2018, 117, 49–56. [Google Scholar] [CrossRef]
- Kelly, D.N.; Sleator, R.D.; Murphy, C.P.; Conroy, S.B.; Judge, M.M.; Berry, D.P. Large variability in feeding behaviour among crossbred growing cattle. J. Anim. Sci 2020, 98, skaa216. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Hussain, T.; Dai, C.; Li, J.; Huang, P. Effects of dietary energy level on growth performance, blood parameters and meat quality in fattening male Hu lambs. J. Anim. Physiol. Anim. Nutr. 2020, 104, 418–430. [Google Scholar] [CrossRef]
- Chen, S.; Tanaka, S.; Oyakawa, C.; Roh, S.; Sato, S. Individual difference in serum oxytocin concentrations of calves and the correlation with those in dams. Anim. Sci. J. 2014, 85, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tanaka, S.; Ogura, S.; Roh, S.; Sato, S. Effect of suckling systems on serum oxytocin and cortisol concentrations and behaviour to a novel object in beef calves. Asian-Aus. J Anim. Sci 2015, 28, 1662–1668. [Google Scholar] [CrossRef]
- Chen, S.; Sanggun, R.; Shusuke, S.; Ogura, S. Relationship of serum oxytocin concentration to positive social behaviour in cattle. J. Integr. Field Sci. 2017, 14, 15–21. [Google Scholar]
- Chen, S.; Sato, S. Role of oxytocin in improving the welfare of farm animals-A review. Asian-Aus. J. Anim. Sci. 2017, 30, 449–454. [Google Scholar] [CrossRef] [PubMed]

| Item 1 | Definition |
|---|---|
| SCAN sampling, each pen instantaneous for 5 min, every 45 min for general activities | |
| Sternal resting | When an animal was lying down on the sternum |
| Lateral resting | When an animal was lying down laterally along the body |
| Peripheral Position | When an animal occupied a peripheral position in the pen |
| Central position | When an animal occupied a central position in the pen |
| Standing | When an animal was standing on 4 legs |
| Walking | When an animal walked around the pen |
| Feeding | When an animal approached the feeder and consumed the meal |
| Drinking | When an animal approached the trough and consumed water |
| FOCUS sampling, each pen continuous for 15 min, every 45 min for social activities | |
| Head shots | When an animal has hit the wall or fence with its head |
| Displacement F/W | When an animal competed with another animal for a meal or water |
| Displacement space | When an animal would sneak between 2 animals to gain space |
| Play-fighting | When two animals engaged in fast galloping, interrupted by sudden changes of direction, arching, kicking the hind legs, rotations, and twists of the body, without an intent to defend or fight |
| Self-grooming | When an animal licked its own body in a non-stereotyped way, scratching with a hind limb or against fixture |
| Allo-grooming | When an animal licked a neighbor along the body |
| Stereotipy | Tongue rolling or biting on bars or other sites in the barn |
| Mounts | When an animal jumped on the back of another animal |
| Item | Genetic Type | Sex Mean | SEM 1 | p-Value 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | LMS | LMS × SRB | SRB | GT | Sex | GT × Sex | Time | |||
| Posture, % of animals | ||||||||||
| Sternal Resting | Females | 58.0 a | 40.7 ab | 27.3 b | 3.13 | 0.044 | 0.015 | 0.020 | <0.0001 | |
| Males | 50.7 ab | 59.3 a | 52.0 a | |||||||
| GT mean | ||||||||||
| Lateral Resting | Females | 2.0 b | 2.7 b | 0.0 b | 0.75 | <0.0001 | 0.105 | 0.003 | 0.003 | |
| Males | 0.0 b | 10.7 a | 0.0 b | |||||||
| GT mean | ||||||||||
| Peripheral position | Females | 54.0 b | 36.7 b | 47.3 b | 3.26 | 0.202 | 0.016 | <0.0001 | <0.0001 | |
| Males | 43.3 b | 78.0 a | 50.0 b | |||||||
| GT mean | ||||||||||
| Central position | Females | 40.0 ab | 56.7 a | 54.0 a | 3.34 | 0.128 | 0.020 | 0.001 | <0.0001 | |
| Males | 50.0 a | 20.0 b | 46.7 a | |||||||
| GT mean | ||||||||||
| Activities, % of animals | ||||||||||
| Standing | Females | 38.7 | 50.0 | 61.3 | 50.0 a | 3.31 | 0.035 | 0.044 | 0.219 | <0.0001 |
| Males | 40.7 | 35.3 | 46.0 | 40.7 b | ||||||
| GT mean | 39.7 b | 42.7 ab | 53.7 a | |||||||
| Walking | Females | 1.3 | 2.7 | 5.3 | 3.1 | 0.75 | 0.225 | 0.778 | 0.358 | <0.0001 |
| Males | 4.0 | 0.0 | 4.0 | 2.7 | ||||||
| GT mean | 2.7 | 1.3 | 4.7 | |||||||
| Feeding | Females | 18.7 | 23.3 | 24.0 | 22.0 | 1.85 | 0.546 | 0.293 | 0.144 | 0.039 |
| Males | 24.0 | 11.3 | 19.3 | 18.2 | ||||||
| GT mean | 21.3 | 17.3 | 21.7 | |||||||
| Drinking | Females | 4.0 | 2.0 | 4.0 | 3.3 | 0.65 | 0.075 | 0.852 | 0.377 | 0.001 |
| Males | 6.0 | 1.3 | 2.0 | 3.1 | ||||||
| GT mean | 5.0 | 1.7 | 3.0 | |||||||
| Ruminating | Females | 16.0 | 15.3 | 8.0 | 13.1 a | 1.42 | 0.261 | 0.022 | 0.601 | 0.301 |
| Males | 6.7 | 8.0 | 5.3 | 6.7 b | ||||||
| GT mean | 11.3 | 11.7 | 6.7 | |||||||
| Item | Genetic Type | Sex | SEM 1 | p-Value 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LMS | LMS × SRB | SRB | Females | Males | GT | Sex | GT × Sex | Time | ||
| Head shots | 0.38 | 0.25 | 0.50 | 0.08 | 0.67 | 0.191 | 0.839 | 0.109 | 0.480 | 0.067 |
| Displacement for water/feed | 2.06 | 1.00 | 1.81 | 2.04 | 1.21 | 0.599 | 0.241 | 0.125 | 0.955 | <0.0001 |
| Displacement for space | 0.06 | 0.25 | 0.25 | 0.21 | 0.17 | 0.079 | 0.299 | 0.708 | 0.185 | 0.001 |
| Play-fighting | 1.13 | 0.50 | 0.50 | 0.17 b | 1.25 a | 0.229 | 0.354 | 0.015 | 0.282 | 0.364 |
| Allo-grooming | 0.94 b | 0.81 b | 2.44 a | 1.38 | 1.42 | 0.316 | 0.032 | 0.935 | 0.600 | 0.029 |
| Self-grooming | 3.06 | 2.00 | 4.56 | 2.83 | 3.58 | 0.727 | 0.165 | 0.482 | 0.242 | 0.003 |
| Stereotyping | 0.06 | 0.19 | 0.25 | 0.13 | 0.21 | 0.065 | 0.374 | 0.451 | 0.048 | 0.076 |
| Mounting | 0.06 | 0.00 | 0.81 | 0.42 | 0.17 | 0.210 | 0.240 | 0.557 | 0.577 | 0.312 |
| Item | Genetic Type | Sex | SEM 1 | p-Value 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LMS | LMS × SRB | SRB | Females | Males | GT | Sex | GT × Sex | Time | ||
| n-events | ||||||||||
| Eating concentrate | 30.17 ab | 18.67 b | 38.17 a | 30.56 | 27.44 | 3.734 | 0.048 | 0.587 | 0.265 | 0.039 |
| Eating hay | 35.67 | 21.33 | 40.00 | 33.00 | 31.67 | 3.244 | 0.054 | 0.819 | 0.761 | 0.206 |
| Competition at feeder | 8.67 | 3.17 | 5.67 | 6.11 | 5.56 | 1.258 | 0.051 | 0.732 | 0.175 | 0.004 |
| Resting | 12.67 | 4.83 | 8.67 | 11.22 | 6.22 | 2.402 | 0.142 | 0.119 | 0.201 | 0.002 |
| Rumination | 4.83 | 0.67 | 3.17 | 3.22 | 2.56 | 0.816 | 0.171 | 0.697 | 0.911 | 0.442 |
| Duration, minutes | ||||||||||
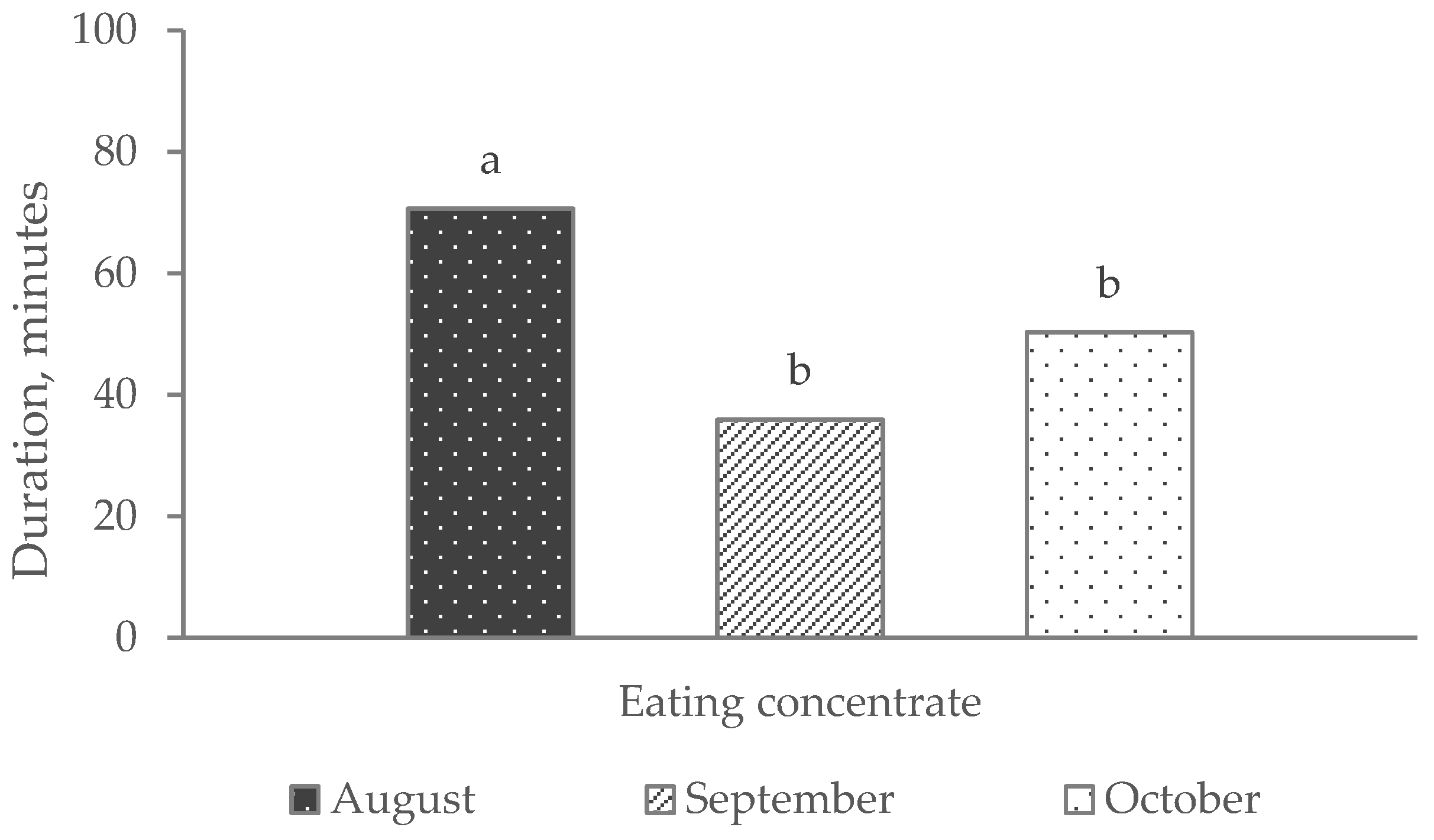
| Eating concentrate | 42.99 | 57.60 | 56.28 | 58.15 a | 46.43 b | 4.507 | 0.070 | 0.041 | 0.846 | 0.001 |
| Eating hay | 72.69 | 65.28 | 60.30 | 72.58 | 59.60 | 4.232 | 0.509 | 0.157 | 0.254 | 0.985 |
| Competition at feeder | 160.87 | 122.89 | 103.63 | 127.01 | 131.26 | 18.786 | 0.487 | 0.914 | 0.323 | 0.352 |
| Frequency, n-events/minutes | ||||||||||
| At feeder | 0.50 | 0.33 | 1.06 | 0.50 | 0.76 | 0.165 | 0.197 | 0.426 | 0.358 | 0.484 |
| Eating concentrate | 0.75 | 0.38 | 0.66 | 0.55 | 0.65 | 0.070 | 0.068 | 0.402 | 0.200 | 0.420 |
| Eating hay | 0.49 ab | 0.33 b | 0.74 a | 0.46 | 0.58 | 0.070 | 0.030 | 0.265 | 0.184 | 0.194 |
| Oxytocin, pg/mL | Genetic Type | Sex | SEM 1 | p Value 2 | ||||
|---|---|---|---|---|---|---|---|---|
| LMS | LMS × SRB | SRB | Females | Males | GT | Sex | ||
| at farm | 249.5 | 201.6 | 657.6 | 319.9 | 419.2 | 94.78 | 0.374 | 0.468 |
| at slaughering | 367.9 | 186.3 | 368.9 | 135.0 b | 480.4 a | 82.40 | 0.546 | 0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzano, A.; Correddu, F.; Lunesu, M.F.; Zgheib, E.; Nudda, A.; Pulina, G. Behaviour Indicators of Animal Welfare in Purebred and Crossbred Yearling Beef Reared in Optimal Environmental Conditions. Animals 2024, 14, 712. https://doi.org/10.3390/ani14050712
Marzano A, Correddu F, Lunesu MF, Zgheib E, Nudda A, Pulina G. Behaviour Indicators of Animal Welfare in Purebred and Crossbred Yearling Beef Reared in Optimal Environmental Conditions. Animals. 2024; 14(5):712. https://doi.org/10.3390/ani14050712
Chicago/Turabian StyleMarzano, Alessandra, Fabio Correddu, Mondina Francesca Lunesu, Elias Zgheib, Anna Nudda, and Giuseppe Pulina. 2024. "Behaviour Indicators of Animal Welfare in Purebred and Crossbred Yearling Beef Reared in Optimal Environmental Conditions" Animals 14, no. 5: 712. https://doi.org/10.3390/ani14050712
APA StyleMarzano, A., Correddu, F., Lunesu, M. F., Zgheib, E., Nudda, A., & Pulina, G. (2024). Behaviour Indicators of Animal Welfare in Purebred and Crossbred Yearling Beef Reared in Optimal Environmental Conditions. Animals, 14(5), 712. https://doi.org/10.3390/ani14050712







