Identifying Relationships between Glutathione S-Transferase-2 Single Nucleotide Polymorphisms and Hypoxia Tolerance and Growth Traits in Macrobrachium nipponense
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. M. nipponense Origin and Experimantal Design
2.2. Genomic DNA Extraction and Cloning of the GST-2 Gene
2.3. SNPs Identification and Association Analysis
2.4. Linkage Disequilibrium (LD) Analysis of GST-2 SNPs
3. Result
3.1. Survival Comparisons between Different M. nipponense Populations
3.2. Gene Structure and GST-2 SNP Identification
3.3. SNPs Polymorphism of GST-2 Gene in M. nipponense
3.4. Correlation Analysis of GST-2 SNPs and Hypoxia Tolerance Traits in M. nipponense
3.5. Correlations between GST-2 SNPs and Growth Traits
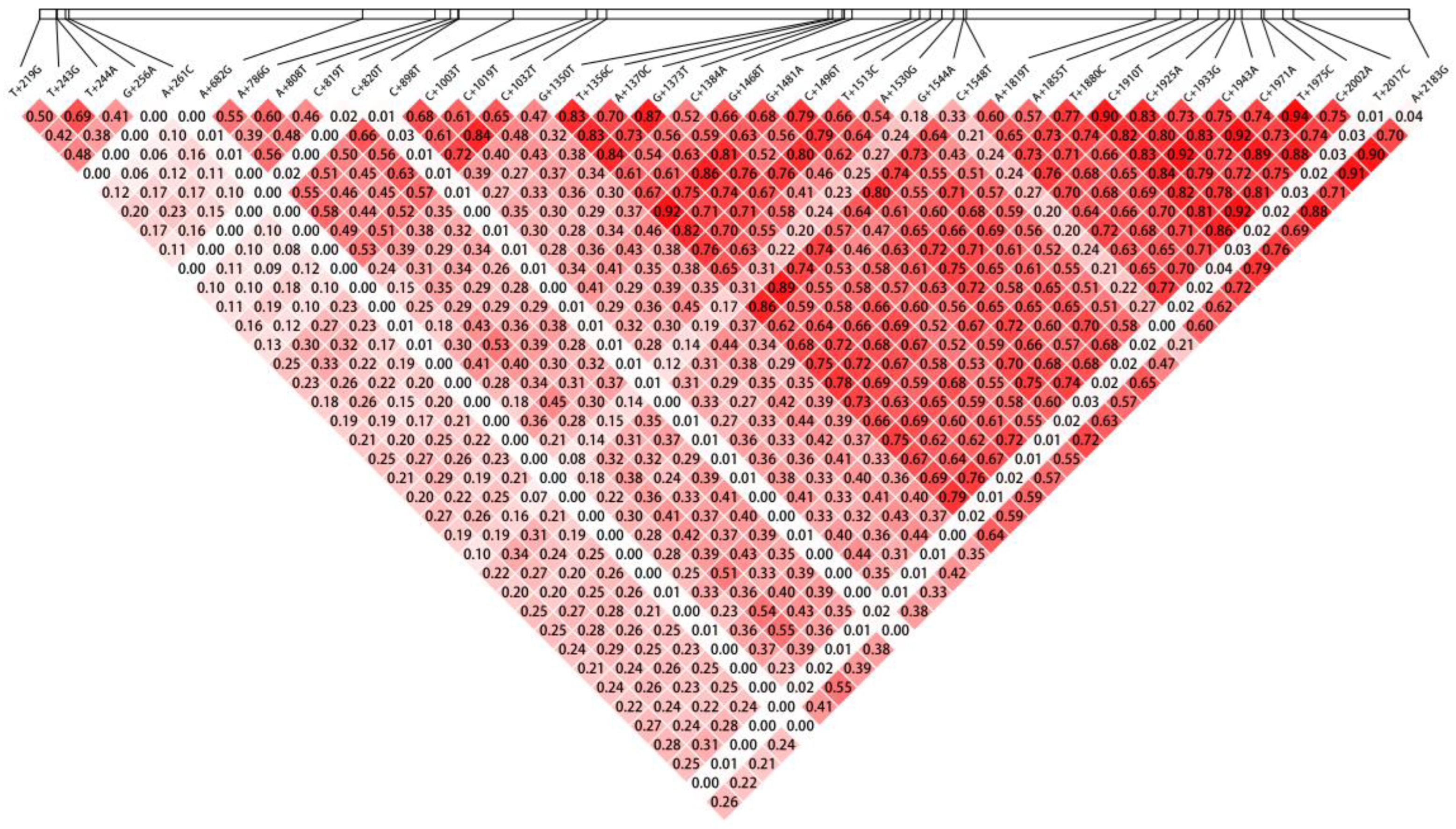
3.6. LD Analysis of GST-2 SNPs
4. Discussion
4.1. Hypoxia Tolerance and Growth Performance in M. nipponense Populations
4.2. The Identification and Polymorphism Analysis of GST-2 SNP Loci
4.3. Correlations between SNP Loci and Hypoxia Tolerance and Growth Traits
4.4. LD Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, S.; Fu, Y.; Hu, Y.; Fu, H.; Jiang, S.; Xiong, Y.; Qiao, H.; Zhang, W.; Gong, Y.; Wu, Y. Identification of candidate genes from androgenic gland in Macrobrachium nipponense regulated by eyestalk ablation. Sci. Rep. 2021, 11, 19855. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture, People’s Republic of China. 2013; pp. 133–135. Available online: http://www.moa.gov.cn/xw/bmdt/201904/t20190418_6194535.htm (accessed on 18 July 2023).
- Sun, S.; Xuan, F.; Ge, X.; Fu, H.; Zhu, J.; Zhang, S. Identification of differentially expressed genes in hepatopancreas of oriental river prawn, Macrobrachium nipponense exposed to environmental hypoxia. Gene 2014, 534, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Mangum, C.P. Adaptation of the oxygen transport system to hypoxia in the blue crab, Callinectes sapidus. Am. Zool. 1997, 37, 604–611. [Google Scholar] [CrossRef]
- McMahon, B.R. Respiratory and circulatory compensation to hypoxia in crustaceans. Respir Physiol. 2001, 128, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Feng, J.; Lin, J.; Li, J. The complete mitochondrial genome of Macrobrachium nipponense. Gene 2011, 487, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liu, C.H.; Kuo, C.M.J.A. Effects of dissolved oxygen on hemolymph parameters of freshwater giant prawn, Macrobrachium rosenbergii (de Man). Aquaculture 2003, 220, 843–856. [Google Scholar] [CrossRef]
- Yang, M.; Sun, S.; Fu, H.; Qiao, H.; Zhang, W.; Gong, Y.; Jiang, S.; Xiong, Y.; Xu, L.; Zhao, C.; et al. Hypoxia and reoxygenation on antioxidant enzyme activities and histological structure of Macrobrachium nipponense. J. Fish. Sci. China 2019, 26, 493. [Google Scholar] [CrossRef]
- Xu, L.; Yang, M.; Fu, H.; Sun, S.; Wu, Y. Molecular Cloning and Expression of MnGST-1 and MnGST-2 from Oriental River Prawn, Macrobrachium nipponense, in Response to Hypoxia and Reoxygenation. Int. J. Mol. Sci. 2018, 19, 3102. [Google Scholar] [CrossRef]
- Sun, S.; Xuan, F.; Fu, H.; Zhu, J.; Ge, X.; Gu, Z. Transciptomic and histological analysis of hepatopancreas, muscle and gill tissues of oriental river prawn (Macrobrachium nipponense) in response to chronic hypoxia. BMC Genom. 2015, 16, 491. [Google Scholar] [CrossRef]
- Frova, C. Glutathione transferases in the genomics era: New insights and perspectives. Biomol. Eng. 2006, 23, 149–169. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, W.-N.; Wang, A.-L.; He, W.-Y.; Zhou, Q.-T.; Liu, Y.; Xu, J. Glutathione S-transferase in the white shrimp Litopenaeus vannamei: Characterization and regulation under pH stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Transport and metabolism of glutathione and γ-glutamyl amino acids. Biochem. Soc. Trans. 1983, 11, 793–794. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 17205–17208. [Google Scholar] [CrossRef] [PubMed]
- Vignal, A.; Milan, D.; SanCristobal, M.; Eggen, A. A review on SNP and other types of molecular markers and their use in animal genetics. Genet. Sel. Evol. 2002, 34, 275–305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.; Ji, X.; Zeng, Y.; Yang, P.; Ding, L. Isolation and characterization of 20 polymorphic microsatellite markers in Macrobrachium nipponense. Conserv. Genet. Resour. 2010, 2, 137–139. [Google Scholar] [CrossRef]
- Song, K.-H.; Kim, W.J. Isolation and characterization of polymorphic microsatellites from the oriental river prawn Macrobrachium nipponense (Caridea: Palaemonidae). J. Crustac. Biol. 2011, 31, 138–141. [Google Scholar] [CrossRef][Green Version]
- Qiao, H.; Lv, D.; Jiang, S.; Sun, S.; Gong, Y.; Xiong, Y.; Jin, S.; Fu, H. Genetic diversity analysis of oriental river prawn, Macrobrachium nipponense, in Yellow River using microsatellite marker. Evolution 2013, 12, 5694–5703. [Google Scholar] [CrossRef]
- Jombart, T.; Ahmed, I. adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef]
- Muhammad, S. Association mapping for salinity tolerance in cotton (Gossypium hirsutum L.) germplasm from US and diverse regions of China. Aust. J. Crop Sci. 2014, 8, 338–346. [Google Scholar]
- Lalitha, S. Primer premier 5. Biotech Softw. Internet Rep. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Jiang, S.; Xiong, Y.; Xia, Z.; Wang, J.; Zhang, W.; Cheng, D.; Gong, Y.; Wu, Y.; Qiao, H.; Fu, H. Identification SNPs in vitellogenin gene and their association with ovarian development and growth of Macrobrachium nipponense. Aquac. Res. 2022, 53, 6478–6486. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Janzen, F.J. Genetic effects of a persistent bottleneck on a natural population of ornate box turtles (Terrapene ornata). Conserv. Genet. 2004, 5, 425–437. [Google Scholar] [CrossRef]
- Nagy, S.; Poczai, P.; Cernák, I.; Gorji, A.M.; Hegedűs, G.; Taller, J. PICcalc: An online program to calculate polymorphic information content for molecular genetic studies. Biochem. Genet. 2012, 50, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.; Bennett, K.; Heritage, B. SPSS Statistics Version 22: A Practical Guide; Cengage Learning Australia: Melbourne, Australia, 2014. [Google Scholar]
- Slate, J.; Pemberton, J.M. Admixture and patterns of linkage disequilibrium in a free-living vertebrate population. J. Evol. Biol. 2010, 20, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- García-Guerrero, M.; Orduña-Rojas, J.; Cortés-Jacinto, E. Oxygen Consumption of the Prawn Macrobrachium americanum over the Temperature Range of its Native Environment and in Relation to its Weight. N. Am. J. Aquac. 2011, 73, 320–326. [Google Scholar] [CrossRef]
- Yin, S.-W.; Huang, H.; Lei, C.-G.; Chen, G.-H.; Zhang, B. Application and Prospect of DNA Molecular Markers in Genetics and Breeding of Marine Fish. Hai Nan Da Xue Xue Bao 2007, 2, 194–199. [Google Scholar]
- Xu, Z.; Taylor, J.A. SNPinfo: Integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 2009, 37 (Suppl. S2), W600–W605. [Google Scholar] [CrossRef]
- Wang, Z.; Moult, J. SNPs, protein structure, and disease. Hum. Mutat. 2001, 17, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Su, Y.; Mao, Y.; Liu, M.; Wang, J.; Zhang, Q. Development and characterization of 24 SNP markers in Kuruma shrimp (Marsupenaeus japonicus) by illumina sequencing. Conserv. Genet. Resour. 2018, 10, 727–730. [Google Scholar] [CrossRef]
- Jung, H.; Lyons, R.E.; Li, Y.; Thanh, N.M.; Dinh, H.; Hurwood, D.A.; Salin, K.R.; Mather, P.B. A candidate gene association study for growth performance in an improved giant freshwater prawn (Macrobrachium rosenbergii) culture line. Mar. Biotechnol. 2014, 16, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Chen, X.; Li, Y.; Peng, M.; Ma, N.; Jiang, W.; Yang, C.; Li, M.J. Analysis of Hsp70 in Litopenaeus vannamei and detection of SNPs. J. Crustac. Biol. 2008, 28, 727–730. [Google Scholar] [CrossRef]
- Sun, S.; Fu, H.; Zhu, J.; Ge, X.; Wu, X.; Qiao, H.; Jin, S.; Zhang, W. Molecular cloning and expression analysis of lactate dehydrogenase from the oriental river prawn Macrobrachium nipponense in response to hypoxia. Int. J. Mol. Sci. 2018, 19, 1990. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, M.; Fu, H.; Sun, S.; Qiao, H.; Zhang, W.; Gong, Y.; Jiang, S.; Xiong, Y.; Jin, S. Molecular cloning, expression, and in situ hybridization analysis of MnGPx-3 and MnGPx-4 from oriental river prawn, Macrobrachium nipponense, in response to hypoxia and reoxygenation. PLoS ONE 2020, 15, e0229171. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Gu, Z.; Fu, H.; Zhu, J.; Ge, X.; Xuan, F. Molecular cloning, characterization, and expression analysis of p53 from the oriental river prawn, Macrobrachium nipponense, in response to hypoxia. Fish Shellfish. Immunol. 2016, 54, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xuan, F.; Fu, H.; Zhu, J.; Ge, X.; Wu, X. Molecular cloning, characterization and expression analysis of caspase-3 from the oriental river prawn, Macrobrachium nipponense when exposed to acute hypoxia and reoxygenation. Fish Shellfish Immunol. 2017, 62, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Y.; Niu, C.-J.; Chen, B.-J.; Storey, K.B. Digital Gene Expression Profiling reveals transcriptional responses to acute cold stress in Chinese soft-shelled turtle Pelodiscus sinensis juveniles. Cryobiology 2018, 81, 43–56. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, D.; Lv, J.; Gao, B.; Li, J.; Liu, P. Identification of SNP markers correlated with the tolerance of low-salinity challenge in swimming crab (Portunus trituberculatus). Acta Oceanol. Sin. 2019, 38, 41–47. [Google Scholar] [CrossRef]
- Morin, P.A.; Aitken, N.C.; Rubio-Cisneros, N.; Dizon, A.E.; Mesnick, S. Characterization of 18 SNP markers for sperm whale (Physeter macrocephalus). Mol. Ecol. Notes 2007, 7, 626–630. [Google Scholar] [CrossRef]
- Wang, W.; Yi, Q.; Ma, L.; Zhou, X.; Zhao, H.; Wang, X.; Qi, J.; Yu, H.; Wang, Z.; Zhang, Q. Sequencing and characterization of the transcriptome of half-smooth tongue sole (Cynoglossus semilaevis). BMC Genom. 2014, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Bai, J.; Yu, L.; Fan, J.J. Polymorphisms of SNPs in ALDO B gene and association analysis with growth traits in grass carp (Ctenopharyngodon idellus). J. Fish. China 2012, 36, 481–488. [Google Scholar] [CrossRef]
- Montalban, G.; Bonache, S.; Moles-Fernández, A.; Gisbert-Beamud, A.; Tenés, A.; Bach, V.; Carrasco, E.; López-Fernández, A.; Stjepanovic, N.; Balmaña, J.; et al. Screening of BRCA1/2 deep intronic regions by targeted gene sequencing identifies the first germline BRCA1 variant causing pseudoexon activation in a patient with breast/ovarian cancer. J. Med. Genet. 2019, 56, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Rhine, C.L.; Cygan, K.J.; Soemedi, R.; Maguire, S.; Murray, M.F.; Monaghan, S.F.; Fairbrother, W.G. Hereditary cancer genes are highly susceptible to splicing mutations. PLoS Genet. 2018, 14, e1007231. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Ni, H.; Yang, Z.; Chen, J.; Li, Y.; Ding, S.; Jiang, X.; Wang, M.; Li, L. A novel splice-site mutation in MSH2 is associated with the development of lynch syndrome. Front. Oncol. 2020, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bernhardy, A.J.; Nacson, J.; Krais, J.J.; Tan, Y.-F.; Nicolas, E.; Radke, M.R.; Handorf, E.; Llop-Guevara, A.; Balmaña, J.; et al. BRCA1 intronic Alu elements drive gene rearrangements and PARP inhibitor resistance. Nat. Commun. 2019, 10, 5661. [Google Scholar] [CrossRef] [PubMed]
- Reumers, J.; Conde, L.; Medina, I.; Maurer-Stroh, S.; Van Durme, J.; Dopazo, J.; Rousseau, F.; Schymkowitz, J. Joint annotation of coding and non-coding single nucleotide polymorphisms and mutations in the SNPeffect and PupaSuite databases. Nucleic Acids Res. 2008, 36 (Suppl. S1), D825–D829. [Google Scholar] [CrossRef]
- Slatkin, M. Linkage disequilibrium—Understanding the evolutionary past and mapping the medical future. Nat. Rev. Genet. 2008, 9, 477–485. [Google Scholar] [CrossRef]
- Jorde, L.B. Linkage disequilibrium as a gene-mapping tool. Am. J. Hum. Genet. 1995, 56, 11. [Google Scholar]
| Primer | Primer Sequence (5′-3′) | Amplification Length (bp) |
|---|---|---|
| GST-1F | ATGCCCCTCGATCTCTACTAC | 287 |
| GST-1R | GTTGTCACTTGTTGTTAATAAG | |
| GST-2F | CAACTCACTGAATTCTTTCAGG | 581 |
| GST-2R | GAATCGACCTGAATTGTCCG | |
| GST-3F | GTTCTGCGTCATCCTATATTCG | 443 |
| GST-3R | GTCTCTTAGATGAACTTCAAGC | |
| GST-4F | ATCCAAGGACGATAACTTTGAGA | 526 |
| GST-4R | TGATCATCGACCTCACGAAATCA |
| Population | 0–2 h | 2–4 h | 4–6 h | 6–8 h | Total |
|---|---|---|---|---|---|
| “Taihu Lake No. 3” | 2 | 11 | 22 | 28 | 63 |
| “Taihu Lake No. 2” | 6 | 5 | 8 | 8 | 27 |
| “Pearl River” | 3 | 5 | 8 | 2 | 18 |
| Serial Number | SNP Locus | Effective Number of Alleles (Ne) | Observed Heterozygosity (Ho) | Expected Heterozygosity (He) | Genetic Diversity Index (Nei) | Polymorphic Information Content (PIC) |
|---|---|---|---|---|---|---|
| 1 | T+219G | 1.9246 | 0.1929 | 0.4816 | 0.4804 | 0.37 |
| 2 | T+243G | 1.9900 | 0.2183 | 0.4987 | 0.4975 | 0.37 |
| 3 | T+244A | 1.9938 | 0.2538 | 0.4997 | 0.4984 | 0.37 |
| 4 | G+256A | 1.9938 | 0.2538 | 0.4997 | 0.4984 | 0.37 |
| 5 | A+261C | 1.8198 | 0.5939 | 0.4516 | 0.4505 | 0.35 |
| 6 | A+682G | 1.5967 | 0.2335 | 0.3747 | 0.3737 | 0.30 |
| 7 | A+786G | 1.7871 | 0.3299 | 0.4415 | 0.4404 | 0.34 |
| 8 | A+808T | 1.7643 | 0.2487 | 0.4343 | 0.4332 | 0.34 |
| 9 | C+819T | 1.4587 | 0.1980 | 0.3153 | 0.3145 | 0.27 |
| 10 | C+820T | 1.3162 | 0.1878 | 0.2408 | 0.2402 | 0.21 |
| 11 | C+898T | 1.5772 | 0.2386 | 0.3669 | 0.3660 | 0.30 |
| 12 | C+1003T | 1.5115 | 0.2284 | 0.3392 | 0.3384 | 0.28 |
| 13 | C+1019T | 1.5115 | 0.2589 | 0.3392 | 0.3384 | 0.28 |
| 14 | C+1032T | 1.5181 | 0.2538 | 0.3421 | 0.3413 | 0.28 |
| 15 | G+1350T | 1.7527 | 0.4315 | 0.4305 | 0.4294 | 0.34 |
| 16 | T+1356C | 1.7229 | 0.4264 | 0.4207 | 0.4196 | 0.33 |
| 17 | A+1370C | 1.7468 | 0.4162 | 0.4286 | 0.4275 | 0.34 |
| 18 | G+1373T | 1.6923 | 0.3909 | 0.4101 | 0.4091 | 0.33 |
| 19 | C+1384A | 1.8405 | 0.3604 | 0.4578 | 0.4567 | 0.35 |
| 20 | G+1468T | 1.9050 | 0.4721 | 0.4763 | 0.4751 | 0.36 |
| 21 | G+1481A | 1.7585 | 0.4162 | 0.4324 | 0.4313 | 0.34 |
| 22 | C+1496T | 1.6673 | 0.3807 | 0.4012 | 0.4002 | 0.32 |
| 23 | T+1513C | 1.8554 | 0.4365 | 0.4622 | 0.4610 | 0.35 |
| 24 | A+1530G | 1.5772 | 0.3096 | 0.3669 | 0.3660 | 0.30 |
| 25 | G+1544A | 1.3480 | 0.2030 | 0.2588 | 0.2582 | 0.22 |
| 26 | C+1548T | 1.7290 | 0.4518 | 0.4227 | 0.4216 | 0.33 |
| 27 | A+1819T | 1.7169 | 0.3299 | 0.4186 | 0.4175 | 0.33 |
| 28 | A+1855T | 1.8650 | 0.3858 | 0.4650 | 0.4638 | 0.36 |
| 29 | T+1880C | 1.7527 | 0.3807 | 0.4305 | 0.4294 | 0.34 |
| 30 | C+1910T | 1.7349 | 0.3959 | 0.4247 | 0.4236 | 0.33 |
| 31 | C+1925A | 1.6799 | 0.3503 | 0.4057 | 0.4047 | 0.32 |
| 32 | C+1933G | 1.7926 | 0.4264 | 0.4433 | 0.4422 | 0.34 |
| 33 | C+1943A | 1.7169 | 0.3909 | 0.4186 | 0.4175 | 0.33 |
| 34 | C+1971A | 1.7982 | 0.4315 | 0.4450 | 0.4439 | 0.35 |
| 35 | T+1975C | 1.8144 | 0.4264 | 0.4500 | 0.4489 | 0.35 |
| 36 | C+2002A | 1.7290 | 0.4112 | 0.4227 | 0.4216 | 0.33 |
| 37 | T+2017C | 1.3035 | 0.1675 | 0.2334 | 0.2328 | 0.21 |
| 38 | A+2183G | 1.8302 | 0.4416 | 0.4548 | 0.4536 | 0.35 |
| Average | 1.7130 | 0.3401 | 0.4107 | 0.4096 | 0.32 |
| Locus | Genotype | Genotype Frequency Ratio of Deceased M. nipponense | Genotype Frequency Ratio of Surviving M. nipponense |
|---|---|---|---|
| T+219G | GG: GT: TT | 31 (0.301): 15 (0.146): 57 (0.553) | 29 (0.309): 23 (0.245): 42 (0.447) |
| T+243G | GG: GT: TT | 40 (0.388): 17 (0.165): 46 (0.447) | 30 (0.319): 26 (0.277): 38 (0.404) |
| T+244A | AA: AT: TT | 40 (0.388): 25 (0.243): 38 (0.369) | 39 (0.415): 25 (0.266): 30 (0.319) |
| G+256A | AA: AG: GG | 50 (0.485): 24 (0.233): 29 (0.282) | 29 (0.309): 26 (0.277): 39 (0.415) |
| A+261C | AA: AC: CC | 8 (0.078): 62 (0.602): 33 (0.32) | 1 (0.011): 55 (0.585): 38 (0.404) |
| A+682G | AA: AG: GG | 13 (0.126): 26 (0.252): 64 (0.621) | 13 (0.138): 20 (0.213): 61 (0.649) |
| A+786G | AA: AG: GG | 18 (0.175): 31 (0.301): 54 (0.524) | 14 (0.149): 34 (0.362): 46 (0.489) |
| A+808T | AA: AT: TT | 20 (0.194): 26 (0.252): 57 (0.553) | 18 (0.191): 23 (0.245): 53 (0.564) |
| C+819T | CC: CT: TT | 8 (0.078): 21 (0.204): 74 (0.718) | 11 (0.117): 18 (0.191): 65 (0.691) |
| C+820T | CC: CT: TT | 4 (0.039): 21 (0.204): 78 (0.757) | 5 (0.053): 16 (0.17): 73 (0.777) |
| C+898T | CC: CT: TT | 11 (0.107): 27 (0.262): 65 (0.631) | 13 (0.138): 20 (0.213): 61 (0.649) |
| C+1003T | CC: CT: TT | 10 (0.097): 26 (0.252): 67 (0.65) | 10 (0.106): 19 (0.202): 65 (0.691) |
| C+1019T | CC: CT: TT | 7 (0.068): 31 (0.301): 65 (0.631) | 10 (0.106): 20 (0.213): 64 (0.681) |
| C+1032T | CC: CT: TT | 9 (0.087): 28 (0.272): 66 (0.641) | 9 (0.096): 22 (0.234): 63 (0.67) |
| G+1350T | GG: GT: TT | 9 (0.087): 46 (0.447): 48 (0.466) | 10 (0.106): 39 (0.415): 45 (0.479) |
| T+1356C | CC: CT: TT | 51 (0.495): 43 (0.417): 9 (0.087) | 45 (0.479): 41 (0.436): 8 (0.085) |
| A+1370C | AA: AC: CC | 11 (0.107): 40 (0.388): 52 (0.505) | 9 (0.096): 42 (0.447): 43 (0.457) |
| G+1373T | GG: GT: TT | 9 (0.087): 39 (0.379): 55 (0.534) | 9 (0.096): 38 (0.404): 47 (0.5) |
| C+1384A | AA: AC: CC | 48 (0.466): 36 (0.35): 19 (0.184) | 44 (0.468): 35 (0.372): 15 (0.16) |
| G+1468T | GG: GT: TT | 16 (0.155): 46 (0.447): 41 (0.398) | 14 (0.149): 47 (0.5): 33 (0.351) |
| G+1481A | AA: AG: GG | 50 (0.485): 42 (0.408): 11 (0.107) | 44 (0.468): 40 (0.426): 10 (0.106) |
| C+1496T | CC: CT: TT | 8 (0.078): 38 (0.369): 57 (0.553) | 9 (0.096): 37 (0.394): 48 (0.511) |
| T+1513C | CC: CT: TT | 44 (0.427): 43 (0.417): 16 (0.155) | 39 (0.415): 43 (0.457): 12 (0.128) |
| A+1530G | AA: AG: GG | 8 (0.078): 39 (0.379): 56 (0.544) | 9 (0.096): 22 (0.234): 63 (0.67) |
| G+1544A | AA: AG: GG | 77 (0.748): 21 (0.204): 5 (0.049) | 70 (0.745): 19 (0.202): 5 (0.053) |
| C+1548T | CC: CT: TT | 7 (0.068): 46 (0.447): 50 (0.485) | 8 (0.085): 43 (0.457): 43 (0.457) |
| A+1819T | AA: AT: TT | 15 (0.146): 30 (0.291): 58 (0.563) | 11 (0.117): 35 (0.372): 48 (0.511) |
| A+1855T | AA: AT: TT | 48 (0.466): 42 (0.408): 13 (0.126) | 39 (0.415): 34 (0.362): 21 (0.223) |
| T+1880C | CC: CT: TT | 49 (0.476): 41 (0.398): 13 (0.126) | 49 (0.521): 34 (0.362): 11 (0.117) |
| C+1910T | CC: CT: TT | 11 (0.107): 43 (0.417): 49 (0.476) | 10 (0.106): 35 (0.372): 49 (0.521) |
| C+1925A | AA: AC: CC | 59 (0.573): 34 (0.33): 10 (0.097) | 48 (0.511): 35 (0.372): 11 (0.117) |
| C+1933G | CC: CG: GG | 12 (0.117): 44 (0.427): 47 (0.456) | 11 (0.117): 40 (0.426): 43 (0.457) |
| C+1943A | AA: AC: CC | 52 (0.505): 41 (0.398): 10 (0.097) | 48 (0.511): 36 (0.383): 10 (0.106) |
| C+1971A | AA: AC: CC | 48 (0.466): 43 (0.417): 12 (0.117) | 41 (0.436): 42 (0.447): 11 (0.117) |
| T+1975C | CC: CT: TT | 47 (0.456): 44 (0.427): 12 (0.117) | 41 (0.436): 40 (0.426): 13 (0.138) |
| C+2002A | AA: AC: CC | 49 (0.476): 44 (0.427): 10 (0.097) | 48 (0.511): 37 (0.394): 9 (0.096) |
| T+2017C | CC: CT: TT | 78 (0.757): 19 (0.184): 6 (0.058) | 76 (0.809): 14 (0.149): 4 (0.043) |
| A+2183G | AA: AG: GG | 12 (0.117): 43 (0.417): 48 (0.466) | 13 (0.138): 44 (0.468): 37 (0.394) |
| SNP Locus | Genotype | Sample Number | Genotype Frequency | Allele Frequency | Average Survival Time/Min |
|---|---|---|---|---|---|
| G+256A | AA | 79 | 0.401 | A/0.528 | 725.90 ± 62.25 a |
| AG | 50 | 0.254 | G/0.472 | 887.84 ± 81.95 ab | |
| GG | 68 | 0.345 | 964.40 ± 67.75 b |
| SNP Locus | Genotype | Sample Number | Genotype Frequency | Allele Frequency | Average Survival Time/Min |
|---|---|---|---|---|---|
| T+2017C | CC | 45 | 0.865 | C/0.90 | 975.60 ± 85.68 b |
| CT | 4 | 0.077 | T/0.10 | 468.75 ± 281.12 a | |
| TT | 3 | 0.058 | 396.67 ± 3.33 a |
| SNP Locus | Genotype | Sample Number | Genotype Frequency | Allele Frequency | Average Survival Time/Min |
|---|---|---|---|---|---|
| G+256A | AA | 45 | 0.455 | A/0.551 | 562.82 ± 67.02 a |
| AG | 19 | 0.192 | G/0.449 | 832.16 ± 133.90 ab | |
| GG | 35 | 0.354 | 948.54 ± 91.69 b | ||
| A+808T | AA | 17 | 0.172 | A/0.253 | 605.29 ± 114.86 ab |
| AT | 16 | 0.162 | T/0.747 | 473.31 ± 96.25 a | |
| TT | 66 | 0.667 | 855.67 ± 68.52 b | ||
| C+1032T | CC | 9 | 0.091 | C/0.182 | 570.78 ± 158.95 a |
| CT | 18 | 0.182 | T/0.818 | 520.50 ± 100.21 a | |
| TT | 72 | 0.727 | 830.99 ± 65.06 b | ||
| A+1530G | AA | 8 | 0.081 | A/0.182 | 730.88 ± 197.19 b |
| AG | 20 | 0.202 | G/0.818 | 360.30 ± 29.38 a | |
| GG | 71 | 0.717 | 863.16 ± 66.34 b |
| SNP Locus | Genotype | Sample Number | Total Length/mm | Weight/g | Body Length/mm |
|---|---|---|---|---|---|
| A+261C | AA | 9 | 46.73 ± 15.91 ab | 1.90 ± 1.51 ab | 20.15 ± 6.99 |
| AC | 117 | 49.90 ± 12.45 b | 2.11 ± 1.23 b | 22.25 ± 5.36 | |
| CC | 71 | 44.81 ± 11.87 a | 1.52 ± 1.05 a | 20.26 ± 5.35 | |
| C+898T | CC | 24 | 47.06 ± 11.08 ab | 1.67 ± 0.93 ab | 21.13 ± 4.99 ab |
| CT | 47 | 43.67 ± 12.31 a | 1.51 ± 1.17 a | 19.69 ± 5.50 a | |
| TT | 126 | 49.66 ± 12.64 b | 2.07 ± 1.23 b | 22.15 ± 5.47 b | |
| A+1370C | AA | 20 | 49.19 ± 10.03 ab | 1.88 ± 0.84 ab | 22.02 ± 4.89 |
| AC | 82 | 44.62 ± 12.34 a | 1.53 ± 1.08 a | 20.26 ± 5.39 | |
| CC | 95 | 50.50 ± 12.73 b | 2.19 ± 1.30 b | 22.34 ± 5.56 | |
| G+1373T | GG | 18 | 48.74 ± 10.47 ab | 1.77 ± 0.81 ab | 21.51 ± 4.86 |
| GT | 77 | 44.57 ± 12.41 a | 1.54 ± 1.09 a | 20.27 ± 5.42 | |
| TT | 102 | 50.30 ± 12.59 b | 2.17 ± 1.28 b | 22.31 ± 5.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Gao, Z.; Zhang, M.; Qiao, H.; Jiang, S.; Zhang, W.; Xiong, Y.; Jin, S.; Fu, H. Identifying Relationships between Glutathione S-Transferase-2 Single Nucleotide Polymorphisms and Hypoxia Tolerance and Growth Traits in Macrobrachium nipponense. Animals 2024, 14, 666. https://doi.org/10.3390/ani14050666
Gao X, Gao Z, Zhang M, Qiao H, Jiang S, Zhang W, Xiong Y, Jin S, Fu H. Identifying Relationships between Glutathione S-Transferase-2 Single Nucleotide Polymorphisms and Hypoxia Tolerance and Growth Traits in Macrobrachium nipponense. Animals. 2024; 14(5):666. https://doi.org/10.3390/ani14050666
Chicago/Turabian StyleGao, Xuanbin, Zijian Gao, Minglei Zhang, Hui Qiao, Sufei Jiang, Wenyi Zhang, Yiwei Xiong, Shubo Jin, and Hongtuo Fu. 2024. "Identifying Relationships between Glutathione S-Transferase-2 Single Nucleotide Polymorphisms and Hypoxia Tolerance and Growth Traits in Macrobrachium nipponense" Animals 14, no. 5: 666. https://doi.org/10.3390/ani14050666
APA StyleGao, X., Gao, Z., Zhang, M., Qiao, H., Jiang, S., Zhang, W., Xiong, Y., Jin, S., & Fu, H. (2024). Identifying Relationships between Glutathione S-Transferase-2 Single Nucleotide Polymorphisms and Hypoxia Tolerance and Growth Traits in Macrobrachium nipponense. Animals, 14(5), 666. https://doi.org/10.3390/ani14050666








