Modulation of Performance, Plasma Constituents, Small Intestinal Morphology, and Cecum Microbiota in Growing Geese by Dietary Citric Acid Supplementation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Experimental Design
2.2. Dietary pH Value and Acid-Binding Capacity
2.3. Growth Performance and Carcass Traits
2.4. Plasma Constituents
2.5. Small Intestinal pH and Morphology
2.6. Cecal Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. Dietary pH Value and Acid-Binding Capacity
3.2. Growth Performance
3.3. Carcass Traits
3.4. Plasma Constituents
3.5. Small Intestinal pH and Morphology
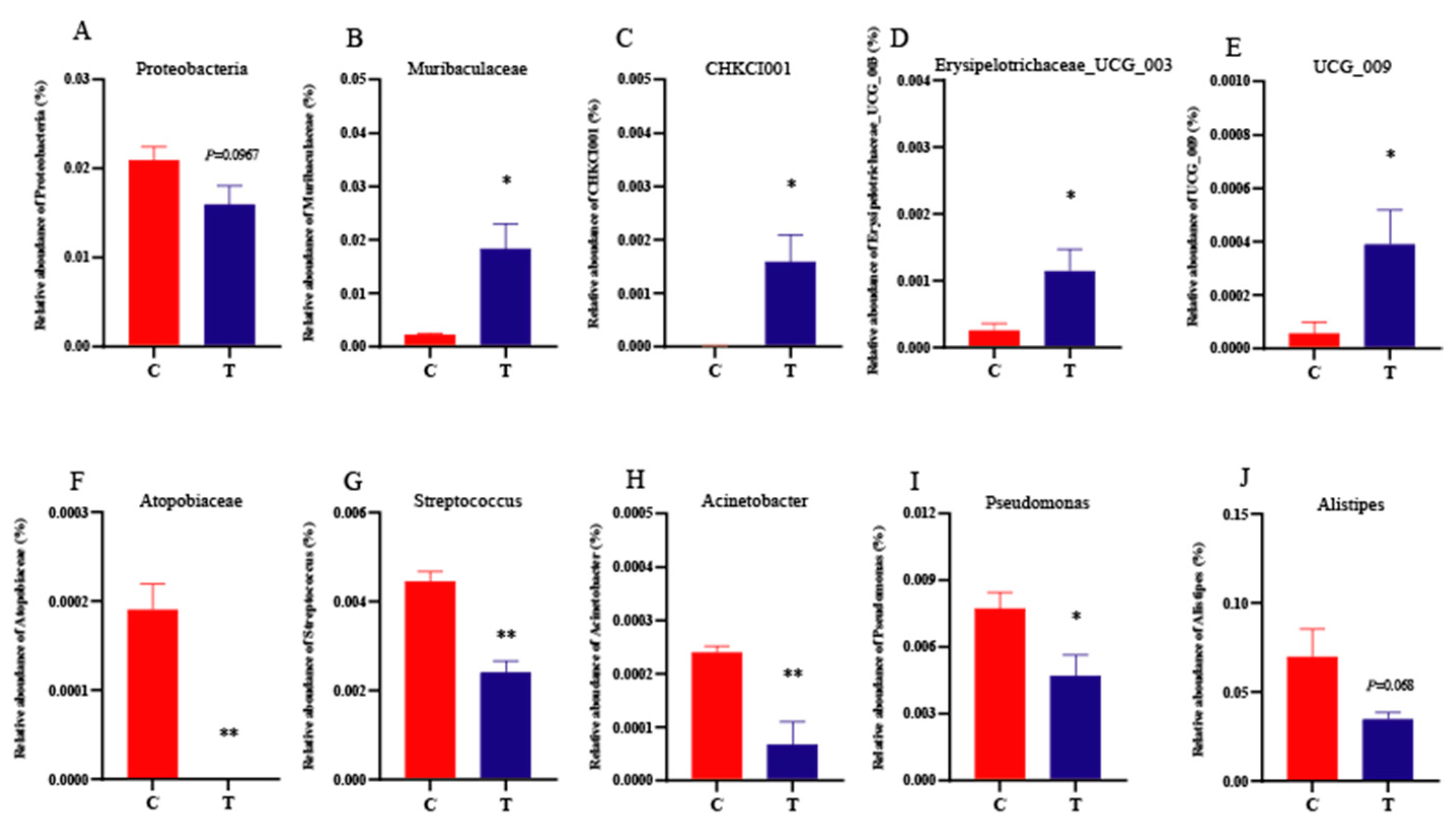
3.6. Cecum Microbiota Diversity and Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chowdhury, R.; Islam, K.M.S.; Khan, M.J.; Karim, M.R.; Haque, M.N.; Khatun, M.; Pesti, G.M. Effect of citric acid, avilamycin, and their combination on the performance, tibia ash, and immune status of broilers. Poult. Sci. 2009, 88, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.N.; Islam, K.M.; Akbar, M.; Chowdhury, R.; Khatun, M.; Karim, M.R.; Kemppainen, B.W. Effect of dietary citric acid, flavomycin and their combination on the performance, tibia ash and immune status of broiler. Can. J. Anim. Sci. 2010, 90, 57–63. [Google Scholar] [CrossRef]
- Liem, A.; Pesti, G.M.; Edwards, H.M., Jr. The effect of several organic acids on phytate phosphorus hydrolysis in broiler chicks. Poult. Sci. 2008, 87, 689–693. [Google Scholar] [CrossRef]
- Bagal, V.L.; Khatta, V.K.; Tewatia, B.S.; Sangwan, S.K.; Raut, S.S. Relative efficacy of organic acids and antibiotics as growth promoters in broiler chicken. Vet. World 2016, 9, 377–382. [Google Scholar] [CrossRef]
- Esmaeilipour, O.; Shivazad, M.; Moravej, H.; Aminzadeh, S.; Rezaian, M.; van Krimpen, M.M. Effects of xylanase and citric acid on the performance, nutrient retention, and characteristics of gastrointestinal tract of broilers fed low-phosphorus wheat-based diets. Poult. Sci. 2011, 90, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Fikry, A.M.; Attia, A.I.; Ismail, I.E.; Alagawany, M.; Reda, F.M. Dietary citric acid enhances growth performance, nutrient digestibility, intestinal microbiota, antioxidant status, and immunity of Japanese quails. Poult. Sci. 2021, 100, 101326. [Google Scholar] [CrossRef]
- Nourmohammadi, R.; Afzali, N. Effect of citric acid and microbial phytase on small intestinal morphology in broiler chicken. Ital. J. Anim. Sci. 2013, 12, e7. [Google Scholar] [CrossRef]
- Suiryanrayna, M.V.A.N.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef]
- Kogut, M.H. The gut microbiota and host innate immunity: Regulators of host metabolism and metabolic diseases in poultry? J. Appl. Poult. Res. 2013, 22, 637–646. [Google Scholar] [CrossRef]
- Abdel-Fattah, S.; El-Sanhoury, M.H.; El-Medany, N.M.; Abdelazeem, F. Thyroid activity, some blood constituents, organs morphology and performance of broiler chicks fed supplemental organic acids. Int. J. Poult. Sci. 2008, 7, 215–222. [Google Scholar] [CrossRef]
- Elnaggar, A.S.; Abo El Maaty, H.M.A. Impact of using organic acids on growth performance, blood biochemical and hematological traits and immune response of ducks (Cairina moschata). Egypt. Poult. Sci. 2017, 37, 907–925. [Google Scholar] [CrossRef]
- Biesek, J.; Kuźniacka, J.; Banaszak, M.; Maiorano, G.; Grabowicz, M.; Adamski, M. The effect of various protein sources in goose diets on meat quality, fatty acid composition, and cholesterol and collagen content in breast muscles. Poult. Sci. 2020, 99, 6278–6286. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.S.; Liu, L.L. The current situation, prospect and suggestions on Waterfowl industry in 2022. Chin. J. Anim. Sci. 2023, 59, 274–280. [Google Scholar]
- Xue, J.J.; Huang, X.F.; Liu, Z.L.; Chen, Y.; Zhang, Y.K.; Luo, Y.; Wang, B.W.; Wang, Q.G.; Wang, C. Effects of citric acid supplementation on growth performance, intestinal morphology and microbiota, and blood parameters of geese from 1 to 28 days of age. Poult. Sci. 2023, 102, 102343. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Arlington, VA, USA, 2005. [Google Scholar]
- Bolduan, G.; Jung, H.; Schneider, R.; Block, J.; Klenke, B. Influence of fumaric acid and propanediol formate on piglets. J. Anim. Physiol. Anim. Nutr. 1988, 59, 143–149. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, Y.; Xue, J.J.; Huang, X.F.; Chen, Z.P.; Wang, Q.G.; Wang, C. Effects of ambient temperature on the growth performance, fat deposition, and intestinal morphology of geese from 28 to 49 days of age. Poult. Sci. 2022, 101, 101814. [Google Scholar] [CrossRef] [PubMed]
- Chaveerach, P.; Keuzenkamp, D.A.; Lipman, L.J.A.; Van Knapen, F. Effect of organic acids in drinking water for young broilers on Campylobacter infection, volatile fatty acid production, gut microflora and histological cell changes. Poult. Sci. 2004, 83, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Nourimoghadam, A.; Pourreza, J.; Samie, A.H. Effect of different levels of citric acid on calcium and phosphorus efficiencies in broiler chicks. Pak. J. Biol. Sci. 2006, 9, 1250–1256. [Google Scholar]
- Andrýs, R.; Klecker, D.; Zeman, L.; Mareček, E. The effect of changed pH values of feed in isophosphoric diets on chicken broiler performance. Czech. J. Anim. Sci. 2003, 48, 197–206. [Google Scholar]
- Afsharmanesh, M.; Pourreza, J. Effects of calcium, citric acid, ascorbic acid, Vitamin D3 on the efficacy of microbial phytase in broiler starters fed wheat-based diets I. Performance, bone mineralization and ileal digestibility. Int. J. Poult. Sci. 2005, 4, 418–424. [Google Scholar]
- Nourmohammadi, R.; Hosseini, S.M.; Farhangfar, H. Influence of citric acid and microbial phytase on growth performance and carcass characteristics of broiler chickens. Am. J. Anim. Vet. Sci. 2010, 5, 282–288. [Google Scholar] [CrossRef]
- Esmaeilipour, O.; Moravej, H.; Shivazad, M.; Rezaian, M.; Aminzadeh, S.; van Krimpen, M.M. Effects of diet acidification and xylanase supplementation on performance, nutrient digestibility, duodenal histology and gut microflora of broilers fed wheat based diet. Br. Poult. Sci. 2012, 53, 235–244. [Google Scholar] [CrossRef]
- Yin, F.; Yu, H.; Lepp, D.; Shi, X.; Yang, X.; Hu, J.; Leeson, S.; Yang, C.; Nie, S.; Hou, Y.; et al. Transcriptome analysis reveals regulation of gene expression for lipid catabolism in young broilers by butyrate glycerides. PLoS ONE 2016, 11, e0160751. [Google Scholar]
- Haq, A.; Chaudhry, M.; Ahmad, F.; Shafi, J.; Ashraf, M.; Javed, M.; Ur-Rehman, S. Effect of dietary acidification with citric acid on carcass characteristics, haemogram and serum metabolite values of broiler chicken. Pak. J. Life Soc. Sci. 2014, 12, 36–41. [Google Scholar]
- Dehghani-Tafti, N.; Jahanian, R. Effect of supplemental organic acids on performance, carcass characteristics, and serum biochemical metabolites in broilers fed diets containing different crude protein levels. Anim. Feed. Sci. Technol. 2016, 211, 109–116. [Google Scholar] [CrossRef]
- Brzóska, F.; Śliwiński, B.; Michalik-Rutkowska, O. Effect of dietary acidifier on growth, mortality, post-slaughter parameters and meat composition of broiler chickens. Ann. Anim. Sci. 2013, 13, 85–96. [Google Scholar] [CrossRef][Green Version]
- Ghazalah, A.A.; Atta, A.M.; Elkloub, K.; Moustafa, M.E.; Shata, R.F.H. Effect of dietary supplementation of organic acids on performance, nutrients digestibility and health of broiler chicks. Int. J. Poult. Sci. 2011, 10, 176–184. [Google Scholar] [CrossRef]
- Reda, F.M.; Ismail, I.E.; Attia, A.I.; Fikry, A.M.; Khalifa, E.; Alagawany, M. Use of fumaric acid as a feed additive in quail’s nutrition: Its effect on growth rate, carcass, nutrient digestibility, digestive enzymes, blood metabolites, and intestinal microbiota. Poult. Sci. 2021, 100, 101493. [Google Scholar] [CrossRef]
- Rock, K.L.; Kataoka, H.; Lai, J.-J. Uric acid as a danger signal in gout and its comorbidities. Nat. Rev. Rheumatol. 2013, 9, 13–23. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, B.; Wang, B.; Zhang, M.; Fan, W.; Li, W. Effects of dietary arginine supplementation on production performance, serum biochemicals, antioxidant capacity, and immunity of laying Wulong geese. Poult. Sci. 2023, 102, 102727. [Google Scholar] [CrossRef]
- Luo, Q.; Cui, X.; Yan, J.; Yang, M.; Liu, J.; Jiang, Y.; Li, J.; Zhou, Y. Antagonistic effects of Lycium barbarum polysaccharides on the impaired reproductive system of male rats induced by local subchronic exposure to 60Co-γ irradiation. Phytother. Res. 2011, 25, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Bai, S.; Zeng, Q.; Ding, X.; Wang, J.; Xuan, Y.; Su, Z.; Zhang, K. Effect of organic acids on growth performance, intestinal morphology, and immunity of broiler chickens with and without coccidial challenge. AMB Express 2021, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Abudabos, A.; Al-Mufarrej, S. Effects of organic acid supplementation on antioxidant capacity and immune responses of broilers challenged orally with Salmonella enterica subsp. enterica Typhimurium. S. Afr. J. Anim. Sci. 2014, 44, 342. [Google Scholar] [CrossRef]
- Wang, F.; Chen, J.; Yin, Y.; Yang, M.; Xiao, Y.; Cheng, Y.; Yin, L.; Fu, C. The effects of dietary ellagic acid supplementation on growth performance, immune response, antioxidant activity, digestive enzyme activities, and intestinal functions in yellow-feathered broilers. J. Anim. Sci. 2022, 100, skac301. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.V.; Figueiredo, J.R.; Van Den Hurk, R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 2009, 71, 1193–1208. [Google Scholar] [CrossRef]
- Martins, F.S.; Saraiva, M.V.; Magalhães-Padilha, D.M.; Almeida, A.P.; Celestino, J.J.; Padilha, R.T.; Cunha, R.M.; Silva, J.R.; Campello, C.C.; Figueiredo, J.R. Presence of growth hormone receptor (GH-R) mRNA and protein in goat ovarian follicles and improvement of in vitro preantral follicle survival and development with GH. Theriogenology 2014, 82, 27–35. [Google Scholar] [CrossRef]
- O’donnell, D.; Wang, L.; Xu, J.; Ridgway, D.; Gu, T.; Moo-Young, M. Enhanced heterologous protein production in Aspergillus niger through pH control of extracellular protease activity. Biochem. Eng. J. 2001, 8, 187–193. [Google Scholar] [CrossRef]
- Pesti, G.; Bakalli, R.I.; Vendrell, P.F.; Chen, H.Y. Effects of organic acid on control of bacteria growth in drinking water for broilers. Poult. Sci. 2004, 83, 75–76. [Google Scholar]
- Guo, Y.J.; Wang, Z.Y.; Wang, Y.S.; Chen, B.; Huang, Y.Q.; Li, P.; Tan, Q.; Zhang, H.Y.; Chen, W. Impact of drinking water supplemented 2-hydroxy-4-methylthiobutyric acid in combination with acidifier on performance, intestinal development, and microflora in broilers. Poult. Sci. 2022, 101, 101661. [Google Scholar] [CrossRef]
- Khosravinia, H.; Nourmohammadi, R.; Afzali, N. Productive performance, gut morphometry, and nutrient digestibility of broiler chicken in response to low and high dietary levels of citric acid. J. Appl. Poult. Res. 2015, 24, 470–480. [Google Scholar] [CrossRef]
- Ragaa, N.M.; Korany, R.M.S. Studying the effect of formic acid and potassium diformate on performance, immunity and gut health of broiler chickens. Anim. Nutr. 2016, 2, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Liu, J.; Wei, S.; Yang, G.; Chen, X.; Tong, Y.; Guo, P. The integrated analysis of digestive physiology and gastrointestinal microbiota structure in Changle goose. Poult. Sci. 2023, 102, 102588. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin improves the homeostasis of mice gut microbiota rhythm caused by sleep restriction. Microbes Infect. 2023, 25, 105121. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liang, S.; Zhang, Y.; Sun, X.; Li, Y.; Diao, J.; Dong, L.; Ni, H.; Yin, Y.; Ren, J.; et al. Effects of compound Chinese herbal medicine additive on growth performance and gut microbiota diversity of Zi goose. Animals 2022, 12, 2942. [Google Scholar] [CrossRef] [PubMed]
- Majidian, P.; Tabatabaei, M.; Zeinolabedini, M.; Naghshbandi, M.P.; Chisti, Y. Metabolic engineering of microorganisms for biofuel production. Renew. Sustain. Energy Rev. 2018, 82, 3863–3885. [Google Scholar] [CrossRef]
- Liu, G.; Ren, W.; Fang, J.; Hu, C.-A.A.; Guan, G.; Al-Dhabi, N.A.; Yin, J.; Duraipandiyan, V.; Chen, S.; Peng, Y.; et al. L-Glutamine and L-arginine protect against enterotoxigenic Escherichia coli infection via intestinal innate immunity in mice. Amino Acids 2017, 49, 1945–1954. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic effect of Quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Yan, L.; An, S.; Lv, Z.Z.; Choct, M.; Zhou, G.L.; Li, Y.; Zhuo, J.S.; Wang, Z.G.; Lai, J.L.; Lv, M.B.; et al. Effects of corn particle size on growth performance, gastrointestinal development, carcass indices and intestinal microbiota of broilers. Poult. Sci. 2022, 101, 102205. [Google Scholar] [CrossRef]
- Cobo, F.; Foronda, C.; Pérez-Carrasco, V.; Martin-Hita, L.; García-Salcedo, J.A.; Navarro-Marí, J.M. First description of abdominal infection due to Alistipes onderdonkii. Anaerobe 2020, 66, 102283. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]


| Items | % |
|---|---|
| Ingredients | |
| Maize | 54.00 |
| Soybean meal | 20.50 |
| Wheat bran | 15.00 |
| Paddy rice | 6.80 |
| Limestone | 1.10 |
| Calcium hydrogen phosphate | 1.50 |
| Sodium chloride | 0.40 |
| DL-Methionine | 0.30 |
| Choline chloride | 0.10 |
| Mineral and vitamin premix 1 | 0.30 |
| Total | 100 |
| Calculated nutrient levels | |
| Metabolizable energy (MJ/kg) | 12.06 |
| Crude protein | 16.22 |
| Calcium | 0.80 |
| Total phosphorus | 0.65 |
| Lysine | 0.90 |
| Methionine | 0.45 |
| Analyzed values | |
| Crude protein | 16.00 |
| Ether extract | 2.80 |
| Lysine | 0.92 |
| Methionine | 0.40 |
| Items | Citric Acid Level % | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.8 | 1.6 | 2.4 | 3.2 | 4 | |||
| pH value | 6.43 a | 5.90 b | 5.53 c | 5.28 d | 5.11 e | 5.08 f | 0.116 | 0.0001 |
| Acid-binding capacity (mL/100 g feed) | 64.7 a | 60.8 b | 58.6 b | 48.1 c | 35.2 d | 25.3 e | 3.477 | 0.0001 |
| Items | Citric Acid Level % | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.8 | 1.6 | 2.4 | 3.2 | 4 | |||
| IBW (g/bird) | 1295.8 | 1296.6 | 1298.6 | 1294.1 | 1295.6 | 1297.0 | 3.06 | 0.999 |
| FBW (g/bird) | 3012.8 b | 3019.0 b | 3060.6 b | 3171.7a b | 3241.9 a | 3015.9 b | 21.91 | 0.001 |
| ADG (g/bird per day) | 40.88 c | 40.94 c | 42.06 bc | 44.70 ab | 46.45 a | 40.88 c | 0.52 | 0.000 |
| ADFI (g/bird per day) | 196.69 b | 199.95 b | 200.80 ab | 213.15 a | 212.71 a | 193.53 b | 1.83 | 0.000 |
| F/G (g/g) | 4.82 | 4.89 | 4.77 | 4.77 | 4.58 | 4.73 | 0.03 | 0.083 |
| Items | Citric Acid Level % | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.8 | 1.6 | 2.4 | 3.2 | 4 | |||
| DP | 89.28 | 89.32 | 88.41 | 87.84 | 88.82 | 88.52 | 0.24 | 0.500 |
| EP | 75.60 | 75.79 | 74.64 | 75.16 | 75.60 | 75.33 | 0.36 | 0.963 |
| BMP | 8.47 | 8.01 | 8.25 | 9.08 | 8.46 | 8.75 | 0.24 | 0.846 |
| TMP | 10.92 b | 12.89 ab | 13.30 a | 12.54 ab | 12.05 ab | 13.35 a | 0.24 | 0.045 |
| SFP | 20.61 a | 18.96 ab | 18.08 ab | 18.30 ab | 16.97 ab | 16.50 b | 0.42 | 0.025 |
| AFP | 4.51 a | 3.10 ab | 3.16 ab | 2.75 b | 3.31 ab | 2.23 b | 0.20 | 0.004 |
| HP | 0.67 | 0.68 | 0.61 | 0.68 | 0.68 | 0.66 | 0.01 | 0.832 |
| LP | 2.53 | 2.50 | 2.71 | 3.19 | 2.68 | 3.08 | 0.10 | 0.251 |
| PGP | 3.03 | 2.94 | 2.99 | 3.12 | 2.80 | 2.90 | 0.09 | 0.964 |
| Items | Citric Acid Level % | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.8 | 1.6 | 2.4 | 3.2 | 4 | |||
| Metabolites | ||||||||
| TP (g/L) | 43.10 ab | 44.34 ab | 45.40 ab | 45.90 a | 44.50 ab | 41.68 b | 0.430 | 0.037 |
| ALB (g/L) | 12.08 | 12.24 | 12.38 | 12.46 | 12.38 | 11.84 | 0.096 | 0.447 |
| GLO (g/L) | 31.02 ab | 32.10 ab | 33.02 ab | 33.44 a | 32.12 ab | 29.84 b | 0.361 | 0.030 |
| Urea (mmol/L) | 0.33 a | 0.17 b | 0.22 ab | 0.25 ab | 0.17 b | 0.21 ab | 0.015 | 0.012 |
| CREA (μmol/L) | 15.50 | 13.50 | 16.00 | 15.44 | 15.16 | 15.80 | 0.287 | 0.136 |
| UA (μmol/L) | 224.4 a | 206.4 a | 140.5 b | 180.6 ab | 180.5 ab | 173.0 ab | 6.720 | 0.002 |
| Immune indices | ||||||||
| IgA (g/L) | 3.12 | 3.26 | 3.25 | 3.11 | 3.07 | 2.98 | 0.070 | 0.885 |
| IgG (g/L) | 8.16 | 10.25 | 9.39 | 9.30 | 9.30 | 9.08 | 0.224 | 0.183 |
| IgM (g/L) | 0.85 | 0.86 | 0.86 | 0.87 | 0.85 | 0.79 | 0.022 | 0.913 |
| Antioxidant capacity | ||||||||
| T-AOC (U/mL) | 0.75 | 0.75 | 0.75 | 0.76 | 0.78 | 0.70 | 0.008 | 0.091 |
| GSH-Px (U/mL) | 569.6 ab | 537.1 ab | 540.8 ab | 616.8 ab | 653.2 a | 529.3 b | 13.37 | 0.019 |
| SOD (U/mL) | 776.6 | 790.7 | 816.5 | 824.4 | 804.2 | 768.6 | 16.96 | 0.937 |
| MDA (nmol/mL) | 5.33 | 5.47 | 5.51 | 5.28 | 5.13 | 5.25 | 0.136 | 0.977 |
| CAT (μmol/mL) | 0.79 | 0.79 | 0.77 | 0.80 | 0.79 | 0.64 | 0.038 | 0.887 |
| Hormones | ||||||||
| GH (ng/mL) | 2.13 | 2.02 | 2.10 | 2.12 | 2.45 | 2.02 | 0.065 | 0.410 |
| IGF-1 (ng/mL) | 58.20 b | 74.05 ab | 68.93 ab | 70.58 ab | 84.00 a | 62.92 ab | 2.570 | 0.039 |
| Items | Citric Acid Level % | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.8 | 1.6 | 2.4 | 3.2 | 4 | |||
| Duodenum | ||||||||
| pH | 6.47 | 6.29 | 6.34 | 6.41 | 6.38 | 6.15 | 0.032 | 0.092 |
| VH (μm) | 902.0 | 882.7 | 910.1 | 960.7 | 920.3 | 988.2 | 13.25 | 0.173 |
| CD (μm) | 140.4 | 126.6 | 133.2 | 129.2 | 119.0 | 136.0 | 2.556 | 0.218 |
| VH/CD | 6.46 | 7.01 | 6.83 | 7.71 | 7.79 | 7.31 | 0.153 | 0.087 |
| Jejunum | ||||||||
| pH | 6.89 | 6.91 | 6.84 | 6.65 | 6.54 | 6.42 | 0.058 | 0.087 |
| VH (μm) | 1079.1 | 1154.4 | 1187.1 | 1207.7 | 1196.8 | 1264.6 | 24.77 | 0.397 |
| CD (μm) | 101.3 | 104.3 | 101.5 | 102.7 | 95.5 | 104.0 | 1.400 | 0.521 |
| VH/CD | 10.50 | 11.06 | 11.71 | 12.32 | 12.69 | 12.21 | 0.251 | 0.080 |
| Ileum | ||||||||
| pH | 6.82 | 6.72 | 6.75 | 6.90 | 6.97 | 6.96 | 0.063 | 0.829 |
| VH (μm) | 772.9 | 732.2 | 847.4 | 843.4 | 816.1 | 784.0 | 16.81 | 0.349 |
| CD (μm) | 110.8 | 102.4 | 116.6 | 113.3 | 111.4 | 106.3 | 2.351 | 0.592 |
| VH/CD | 7.03 | 7.16 | 7.16 | 7.48 | 7.43 | 7.46 | 0.148 | 0.937 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xue, J.; Chen, Y.; Huang, X.; Liu, Z.; Zhong, H.; Xie, Q.; Luo, Y.; Wang, Q.; Wang, C. Modulation of Performance, Plasma Constituents, Small Intestinal Morphology, and Cecum Microbiota in Growing Geese by Dietary Citric Acid Supplementation. Animals 2024, 14, 660. https://doi.org/10.3390/ani14050660
Zhang Y, Xue J, Chen Y, Huang X, Liu Z, Zhong H, Xie Q, Luo Y, Wang Q, Wang C. Modulation of Performance, Plasma Constituents, Small Intestinal Morphology, and Cecum Microbiota in Growing Geese by Dietary Citric Acid Supplementation. Animals. 2024; 14(5):660. https://doi.org/10.3390/ani14050660
Chicago/Turabian StyleZhang, Yongkang, Jiajia Xue, Ying Chen, Xiaofeng Huang, Zuolan Liu, Hang Zhong, Qun Xie, Yi Luo, Qigui Wang, and Chao Wang. 2024. "Modulation of Performance, Plasma Constituents, Small Intestinal Morphology, and Cecum Microbiota in Growing Geese by Dietary Citric Acid Supplementation" Animals 14, no. 5: 660. https://doi.org/10.3390/ani14050660
APA StyleZhang, Y., Xue, J., Chen, Y., Huang, X., Liu, Z., Zhong, H., Xie, Q., Luo, Y., Wang, Q., & Wang, C. (2024). Modulation of Performance, Plasma Constituents, Small Intestinal Morphology, and Cecum Microbiota in Growing Geese by Dietary Citric Acid Supplementation. Animals, 14(5), 660. https://doi.org/10.3390/ani14050660





