Diversity of Anaplasma phagocytophilum Strains from Roe Deer (Capreolus capreolus) and Red Deer (Cervus elaphus) in Poland
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
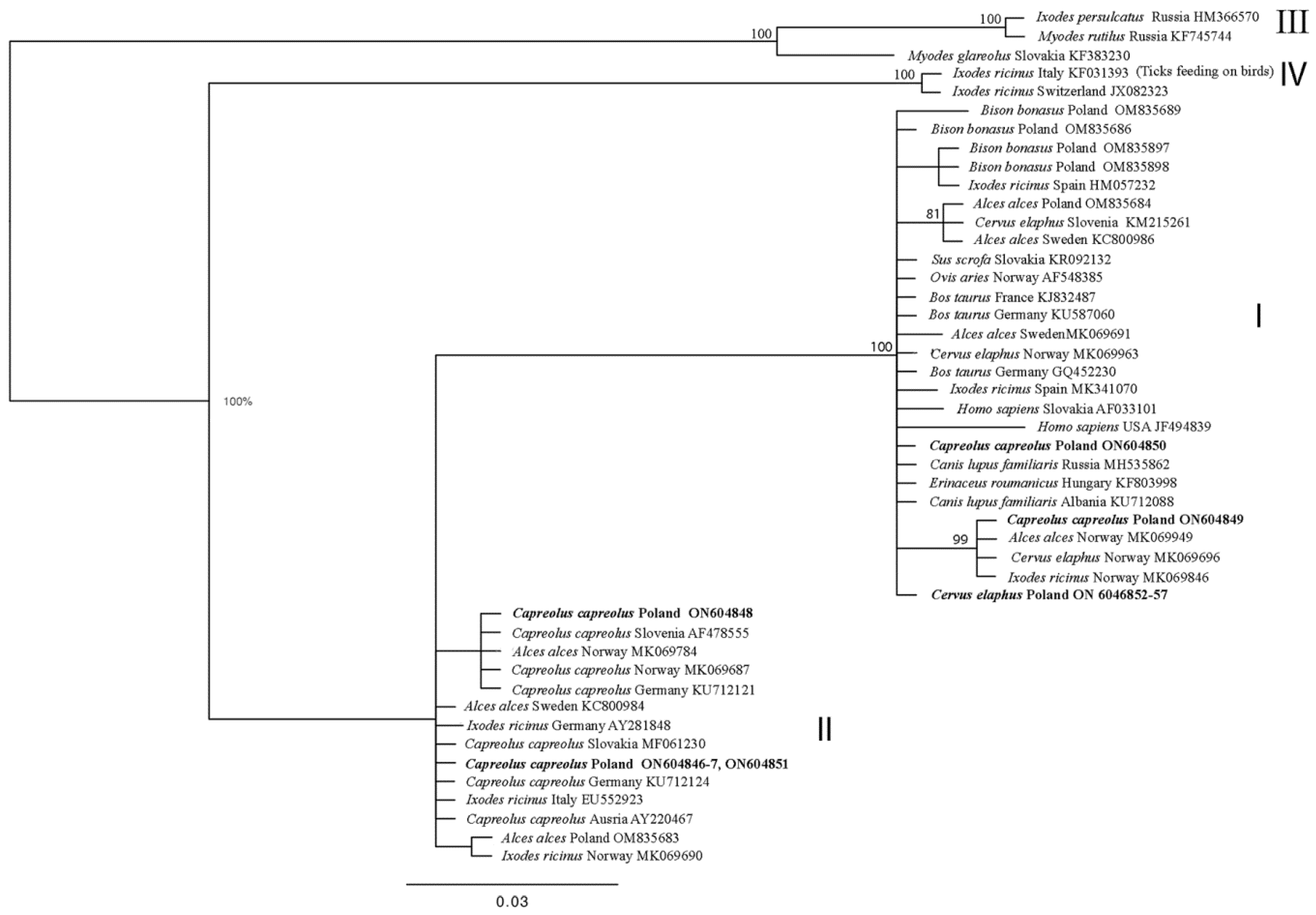
3.1. GroEL Diversity
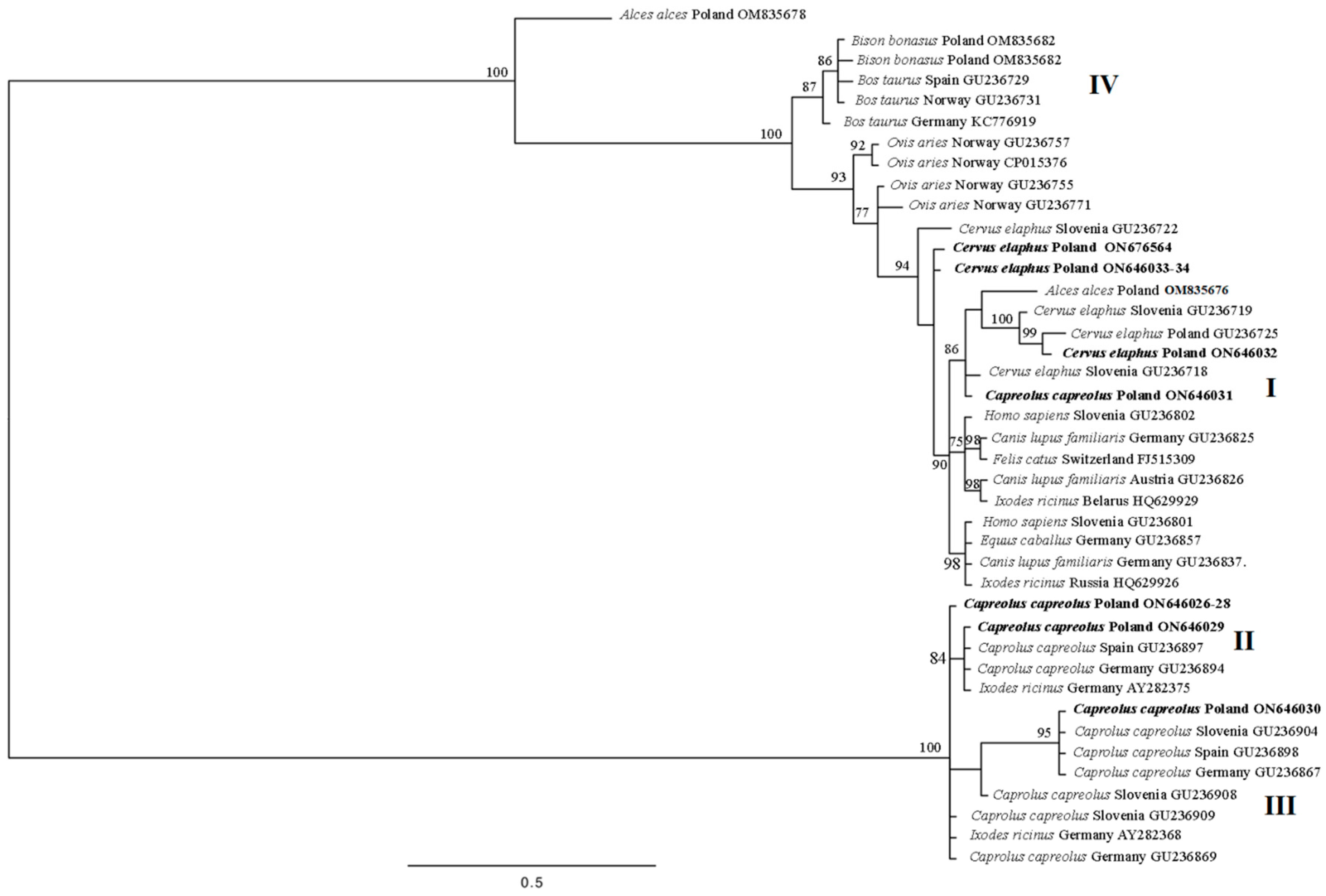
3.2. AnkA Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51 Pt. 6, 2145–2165. [Google Scholar] [CrossRef]
- Battilani, M.; De Arcangeli, S.; Balboni, A.; Dondi, F. Genetic diversity and molecular epidemiology of Anaplasma. Infect. Genet. Evol. 2017, 49, 195–211. [Google Scholar] [CrossRef]
- Gordon, W.S.; Brownlee, A.; Wilson, D.R.; MacLeod, J. Tick-borne fever (a hitherto undescribed disease of sheep). J. Comp. Pathol. 1932, 45, 301–307. [Google Scholar] [CrossRef]
- Pusterla, N.; Anderson, R.J.; House, J.K.; Pusterla, J.B.; DeRock, E.; Madigan, J.E. Susceptibility of cattle to infection with Ehrlichia equi and the agent of human granulocytic ehrlichiosis. J. Am. Vet. Med. Assoc. 2001, 218, 1160–1162. [Google Scholar] [CrossRef]
- Bown, K.J.; Begon, M.; Bennett, M.; Woldehiwet, Z.; Ogden, N.H. Seasonal dynamics of Anaplasma phagocytophila in a rodent-tick (Ixodes trianguliceps) system, United Kingdom. Emerg. Infect. Dis. 2003, 9, 63–70. [Google Scholar] [CrossRef]
- Aktas, M.; Altay, K.; Dumanli, N.; Kalkan, A. Molecular detection and identification of Ehrlichia and Anaplasma species in ixodid ticks. Parasitol. Res. 2009, 104, 1243–1248. [Google Scholar] [CrossRef]
- Santos, H.A.; Pires, M.S.; Vilela, J.A.; Santos, T.M.; Faccini, J.L.; Baldani, C.D.; Thomé, S.M.; Sanavria, A.; Massard, C.L. Detection of Anaplasma phagocytophilum in Brazilian dogs by real-time polymerase chain reaction. J. Vet. Diagn. Investig. 2011, 23, 770–774. [Google Scholar] [CrossRef]
- Barbet, A.F.; Al-Khedery, B.; Stuen, S.; Granquist, E.G.; Felsheim, R.F.; Munderloh, U.G. An emerging tick-borne disease of humans is caused by a subset of strains with conserved genome structure. Pathogens 2013, 2, 544–555. [Google Scholar] [CrossRef]
- Kim, K.H.; Yi, J.; Oh, W.S.; Kim, N.H.; Choi, S.J.; Choe, P.G.; Kim, N.J.; Lee, J.K.; Oh, M.D. Human granulocytic anaplasmosis, South Korea, 2013. Emerg. Infect. Dis. 2014, 20, 1708–1711. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Wagmann, N.; Meli, M.L.; Riond, B.; Novacco, M.; Joekel, D.; Gentilini, F.; Marsilio, F.; Pennisi, M.G.; Lloret, A.; et al. Detection of ‘Candidatus Neoehrlichia mikurensis’ and other Anaplasmataceae and Rickettsiaceae in Canidae in Switzerland and Mediterranean countries. Schweiz. Arch. Tierheilkd. 2016, 158, 691–700. [Google Scholar] [CrossRef]
- Sosa-Gutierrez, C.G.; Vargas-Sandoval, M.; Torres, J.; Gordillo-Pérez, G. Tick-borne rickettsial pathogens in questing ticks, removed from humans and animals in Mexico. J. Vet. Sci. 2016, 17, 353–360. [Google Scholar] [CrossRef]
- Fukui, Y.; Ohkawa, S.; Inokuma, H. First Molecular Detection and Phylogenetic Analysis of Anaplasma phagocytophilum from a Clinical Case of Canine Granulocytic Anaplasmosis in Japan. Jpn. J. Infect. Dis. 2018, 71, 302–305. [Google Scholar] [CrossRef]
- Zaid, T.; Ereqat, S.; Nasereddin, A.; Al-Jawabreh, A.; Abdelkader, A.; Abdeen, Z. Molecular characterization of Anaplasma and Ehrlichia in ixodid ticks and reservoir hosts from Palestine: A pilot survey. Vet. Med. Sci. 2019, 5, 230–242. [Google Scholar] [CrossRef]
- Hurtado, C.; Torres, R.; Pérez-Macchi, S.; Sagredo, K.; Uberti, B.; de Souza Zanatto, D.C.; Machado, R.Z.; André, M.R.; Bittencourt, P.; Müller, A. Serological and molecular detection of Anaplasma phagocytophilum in Thoroughbred horses from Chilean racecourses. Ticks Tick. Borne Dis. 2020, 11, 101441. [Google Scholar] [CrossRef]
- Kolo, A.O.; Collins, N.E.; Brayton, K.A.; Chaisi, M.; Blumberg, L.; Frean, J.; Gall, C.A.; Wentzel, J.M.; Wills-Berriman, S.; Boni, L.; et al. Anaplasma phagocytophilum and Other Anaplasma spp. in Various Hosts in the Mnisi Community, Mpumalanga Province, South Africa. Microorganisms 2020, 8, 1812. [Google Scholar] [CrossRef]
- Myczka, A.W.; Kaczor, S.; Filip-Hutsch, K.; Czopowicz, M.; Plis-Kuprianowicz, E.; Laskowski, Z. Prevalence and Genotyping of Anaplasma phagocytophilum Strains from Wild Animals, European Bison (Bison bonasus) and Eurasian Moose (Alces alces) in Poland. Animals 2022, 12, 1222. [Google Scholar] [CrossRef]
- Karbowiak, G.; Biernat, B.; Stańczak, J.; Werszko, J.; Wróblewski, P.; Szewczyk, T.; Sytykiewicz, H. The role of particular ticks developmental stages in the circulation of tick-borne pathogens in Central Europe. 4. Anaplasmataceae. Ann. Parasitol. 2016, 62, 267–284. [Google Scholar] [CrossRef]
- Kowalec, M.; Szewczyk, T.; Welc-Falęciak, R.; Siński, E.; Karbowiak, G.; Bajer, A. Rickettsiales Occurrence and Co-occurrence in Ixodes ricinus Ticks in Natural and Urban Areas. Microb. Ecol. 2019, 77, 890–904. [Google Scholar] [CrossRef]
- Svitálková, Z.; Haruštiaková, D.; Mahríková, L.; Berthová, L.; Slovák, M.; Kocianová, E.; Kazimírová, M. Anaplasma phagocytophilum prevalence in ticks and rodents in an urban and natural habitat in South-Western Slovakia. Parasit. Vectors 2015, 8, 276. [Google Scholar] [CrossRef]
- Jaarsma, R.I.; Sprong, H.; Takumi, K.; Kazimirova, M.; Silaghi, C.; Mysterud, A.; Rudolf, I.; Beck, R.; Földvári, G.; Tomassone, L.; et al. Anaplasma phagocytophilum evolves in geographical and biotic niches of vertebrates and ticks. Parasit. Vectors 2019, 12, 328. [Google Scholar] [CrossRef]
- Lesiczka, P.M.; Hrazdilová, K.; Majerová, K.; Fonville, M.; Sprong, H.; Hönig, V.; Hofmannová, L.; Papežík, P.; Růžek, D.; Zurek, L.; et al. The Role of Peridomestic Animals in the Eco-Epidemiology of Anaplasma phagocytophilum. Microb. Ecol. 2021, 82, 602–612. [Google Scholar] [CrossRef]
- Rikihisa, Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin. Microbiol. Rev. 2011, 24, 469–489. [Google Scholar] [CrossRef]
- Bakken, J.S.; Dumler, S. Human granulocytic anaplasmosis. Infect. Dis. Clin. N. Am. 2008, 22, 433–448. [Google Scholar] [CrossRef]
- Tylewska-Wierzbanowska, S.; Chmielewski, T. Zoonozy przenoszone przez kleszcze na terenie Polski. Post. Mikrobiol. 2010, 49, 191–197. (In Polish) [Google Scholar]
- Matei, I.A.; Estrada-Peña, A.; Cutler, S.J.; Vayssier-Taussat, M.; Varela-Castro, L.; Potkonjak, A.; Zeller, H.; Mihalca, A.D. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasit. Vectors 2019, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Scharf, W.; Schauer, S.; Freyburger, F.; Petrovec, M.; Schaarschmidt-Kiener, D.; Liebisch, G.; Runge, M.; Ganter, M.; Kehl, A.; Dumler, J.S.; et al. Distinct host species correlate with Anaplasma phagocytophilum ankA gene clusters. J. Clin. Microbiol. 2011, 49, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Razanske, I.; Rosef, O.; Radzijevskaja, J.; Krikstolaitis, R.; Paulauskas, A. Impact of tick-borne Anaplasma phagocytophilum infections in calves of moose (Alces alces) in southern Norway. Folia Parasitol. 2021, 68, 2021.023. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Cao, W.C.; Jiang, J.F.; Zhang, X.A.; Liu, Y.X.; Wu, X.M.; Zhang, W.Y.; Zhang, P.H.; Bian, C.L.; Dumler, J.S.; et al. Anaplasma phagocytophilum from Rodents and Sheep, China. Emerg. Infect. Dis. 2010, 16, 764–768. [Google Scholar] [CrossRef]
- Aktas, M.; Özübek, S. Bovine anaplasmosis in Turkey: First laboratory confirmed clinical cases caused by Anaplasma phagocytophilum. Vet. Microbiol. 2015, 178, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Djiba, M.L.; Mediannikov, O.; Mbengue, M.; Thiongane, Y.; Molez, J.F.; Seck, M.T.; Fenollar, F.; Raoult, D.; Ndiaye, M. Survey of Anaplasmataceae bacteria in sheep from Senegal. Trop. Anim. Health Prod. 2013, 45, 1557–1561. [Google Scholar] [CrossRef]
- Ben Said, M.; Belkahia, H.; Messadi, L. Anaplasma spp. in North Africa: A review on molecular epidemiology, associated risk factors and genetic characteristics. Ticks Tick Borne Dis. 2018, 9, 543–555. [Google Scholar] [CrossRef]
- Woldehiwet, Z. The natural history of Anaplasma phagocytophilum. Vet. Parasitol. 2010, 167, 108–122. [Google Scholar] [CrossRef]
- Dugat, T.; Lagrée, A.C.; Maillard, R.; Boulouis, H.J.; Haddad, N. Opening the black box of Anaplasma phagocytophilum diversity: Current situation and future perspectives. Front. Cell. Infect. Microbiol. 2015, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Langenwalder, D.B.; Silaghi, C.; Nieder, M.; Pfeffer, M.; von Loewenich, F.D. Co-infection, reinfection and superinfection with Anaplasma phagocytophilum strains in a cattle herd based on ankA gene and multilocus sequence typing. Parasit. Vectors 2020, 13, 157. [Google Scholar] [CrossRef]
- Jenkins, A.; Handeland, K.; Stuen, S.; Schouls, L.; van de Pol, I.; Meen, R.T.; Kristiansen, B.E. Ehrlichiosis in a moose calf in Norway. J. Wildl. Dis. 2001, 37, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Pettersen, K.S.; Granquist, E.G.; Bergström, K.; Bown, K.J.; Birtles, R.J. Anaplasma phagocytophilum variants in sympatric red deer (Cervus elaphus) and sheep in southern Norway. Ticks Tick Borne Dis. 2013, 4, 197–201. [Google Scholar] [CrossRef]
- Jahfari, S.; Coipan, E.C.; Fonville, M.; van Leeuwen, A.D.; Hengeveld, P.; Heylen, D.; Heyman, P.; van Maanen, C.; Butler, C.M.; Földvári, G.; et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit. Vectors 2014, 7, 365. [Google Scholar] [CrossRef] [PubMed]
- Adamska, M. The role of different species of wild ungulates and Ixodes ricinus ticks in the circulation of genetic variants of Anaplasma phagocytophilum in a forest biotope in north-western Poland. Ticks Tick Borne Dis. 2020, 11, 101465. [Google Scholar] [CrossRef]
- Jouglin, M.; Chagneau, S.; Faille, F.; Verheyden, H.; Bastian, S.; Malandrin, L. Detecting and characterizing mixed infections with genetic variants of Anaplasma phagocytophilum in roe deer (Capreolus capreolus) by developing an ankA cluster-specific nested PCR. Parasit. Vectors 2017, 10, 377. [Google Scholar] [CrossRef]
- Grassi, L.; Franzo, G.; Martini, M.; Mondin, A.; Cassini, R.; Drigo, M.; Pasotto, D.; Vidorin, E.; Menandro, M.L. Ecotyping of Anaplasma phagocytophilum from Wild Ungulates and Ticks Shows Circulation of Zoonotic Strains in Northeastern Italy. Animals 2021, 11, 310. [Google Scholar] [CrossRef]
- Petrovec, M.; Sixl, W.; Schweiger, R.; Mikulasek, S.; Elke, L.; Wüst, G.; Marth, E.; Strasek, K.; Stünzner, D.; Avsic-Zupanc, T. Infections of wild animals with Anaplasma phagocytophila in Austria and the Czech Republic. Ann. N. Y. Acad. Sci. 2003, 990, 103–106. [Google Scholar] [CrossRef]
- Silaghi, C.; Fröhlich, J.; Reindl, H.; Hamel, D.; Rehbein, S. Anaplasma phagocytophilum and Babesia Species of Sympatric Roe Deer (Capreolus capreolus), Fallow Deer (Dama dama), Sika Deer (Cervus nippon) and Red Deer (Cervus elaphus) in Germany. Pathogens 2020, 9, 968. [Google Scholar] [CrossRef]
- Hornok, S.; Sugár, L.; Fernández de Mera, I.G.; de la Fuente, J.; Horváth, G.; Kovács, T.; Micsutka, A.; Gönczi, E.; Flaisz, B.; Takács, N.; et al. Tick- and fly-borne bacteria in ungulates: The prevalence of Anaplasma phagocytophilum, haemoplasmas and rickettsiae in water buffalo and deer species in Central Europe, Hungary. BMC Vet. Res. 2018, 14, 98. [Google Scholar] [CrossRef]
- Remesar, S.; Díaz, P.; Prieto, A.; García-Dios, D.; Fernández, G.; López, C.M.; Panadero, R.; Díez-Baños, P.; Morrondo, P. Prevalence and molecular characterization of Anaplasma phagocytophilum in roe deer (Capreolus capreolus) from Spain. Ticks Tick. Borne Dis. 2020, 11, 101351. [Google Scholar] [CrossRef]
- Stefanidesova, K.; Kocianova, E.; Boldis, V.; Kostanova, Z.; Kanka, P.; Nemethova, D.; Spitalska, E. Ecidence of Anaplasma phagocytophilum and Ricktettsia helvetica infection in free-ranging ungulates in central Slovakia. Eur. J. Wildl. Res. 2008, 54, 519–524. [Google Scholar] [CrossRef]
- Bown, K.J.; Lambin, X.; Ogden, N.H.; Begon, M.; Telford, G.; Woldehiwet, Z.; Birtles, R.J. Delineating Anaplasma phagocytophilum ecotypes in coexisting, discrete enzootic cycles. Emerg. Infect. Dis. 2009, 15, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- Stigum, V.M.; Jaarsma, R.I.; Sprong, H.; Rolandsen, C.M.; Mysterud, A. Infection prevalence and ecotypes of Anaplasma phagocytophilum in moose Alces alces, red deer Cervus elaphus, roe deer Capreolus capreolus and Ixodes ricinus ticks from Norway. Parasit. Vectors 2019, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Massung, R.F.; Owens, J.H.; Ross, D.; Reed, K.D.; Petrovec, M.; Bjoersdorff, A.; Coughlin, R.T.; Beltz, G.A.; Murphy, C.I. Sequence analysis of the ank gene of granulocytic ehrlichiae. J. Clin. Microbiol. 2000, 38, 2917–2922. [Google Scholar] [CrossRef] [PubMed]
- von Loewenich, F.D.; Baumgarten, B.U.; Schröppel, K.; Geissdörfer, W.; Röllinghoff, M.; Bogdan, C. High diversity of ankA sequences of Anaplasma phagocytophilum among Ixodes ricinus ticks in Germany. J. Clin. Microbiol. 2003, 41, 5033–5040. [Google Scholar] [CrossRef]
- Majazki, J.; Wüppenhorst, N.; Hartelt, K.; Birtles, R.; von Loewenich, F.D. Anaplasma phagocytophilum strains from voles and shrews exhibit specific ankA gene sequences. BMC Vet. Res. 2013, 9, 235. [Google Scholar] [CrossRef]
- Myczka, A.W.; Steiner-Bogdaszewska, Z.; Filip-Hutsch, K.; Olos´, G.; Czopowicz, M.; Laskowski, Z. Detection of Anaplasma phagocytophilum in Wild and Farmed Cervids in Poland. Pathogens 2021, 10, 1190. [Google Scholar] [CrossRef]
- Alberti, A.; Zobba, R.; Chessa, B.; Addis, M.F.; Sparagano, O.; Pinna Parpaglia, M.L.; Cubeddu, T.; Pintori, G.; Pittau, M. Equine and canine Anaplasma phagocytophilum strains isolated on the island of Sardinia (Italy) are phylogenetically related to pathogenic strains from the United States. Appl. Environ. Microbiol. 2005, 71, 6418–6422. [Google Scholar] [CrossRef]
- Massung, R.F.; Levin, M.L.; Munderloh, U.G.; Silverman, D.J.; Lynch, M.J.; Gaywee, J.K.; Kurtti, T.J. Isolation and propagation of the Ap-Variant 1 strain of Anaplasma phagocytophilum in a tick cell line. J. Clin. Microbiol. 2007, 45, 2138–2143. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Lu, M.; Li, F.; Liao, Y.; Shen, J.J.; Xu, J.M.; Chen, Y.Z.; Li, J.H.; Holmes, E.C.; Zhang, Y.Z. Epidemiology and Diversity of Rickettsiales Bacteria in Humans and Animals in Jiangsu and Jiangxi provinces, China. Sci. Rep. 2019, 9, 13176. [Google Scholar] [CrossRef] [PubMed]
- Liz, J.S.; Sumner, J.W.; Pfister, K.; Brossard, M. PCR detection and serological evidence of granulocytic ehrlichial infection in roe deer (Capreolus capreolus) and chamois (Rupicapra rupicapra). J. Clin. Microbiol. 2002, 40, 892–897. [Google Scholar] [CrossRef]
- Liddell, A.M.; Stockham, S.L.; Scott, M.A.; Sumner, J.W.; Paddock, C.D.; Gaudreault-Keener, M.; Arens, M.Q.; Storch, G.A. Predominance of Ehrlichia ewingii in Missouri dogs. J. Clin. Microbiol. 2003, 41, 4617–4622. [Google Scholar] [CrossRef]
- Rejmanek, D.; Bradburd, G.; Foley, J. Molecular characterization reveals distinct genospecies of Anaplasma phagocytophilum from diverse North American hosts. J. Med. Microbiol. 2012, 61 Pt 2, 204–212. [Google Scholar] [CrossRef]
- Bermúdez, C.S.E.; Félix, M.L.; Domínguez, A.L.; Kadoch, N.; Muñoz-Leal, S.; Venzal, J.M. Molecular screening for tick-borne bacteria and hematozoa in Ixodes cf. boliviensis and Ixodes tapirus (Ixodida: Ixodidae) from western highlands of Panama. Curr. Res. Parasitol. Vector Borne Dis. 2021, 1, 100034. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, J.; Fischer, S.; Bauer, B.; Hamel, D.; Kohn, B.; Ahlers, M.; Obiegala, A.; Overzier, E.; Pfeffer, M.; Pfister, K.; et al. Host-pathogen associations revealed by genotyping of European strains of Anaplasma phagocytophilum to describe natural endemic cycles. Parasit. Vectors 2023, 16, 289. [Google Scholar] [CrossRef]
- Chastagner, A.; Dugat, T.; Vourc’h, G.; Verheyden, H.; Legrand, L.; Bachy, V.; Chabanne, L.; Joncour, G.; Maillard, R.; Boulouis, H.J.; et al. Multilocus sequence analysis of Anaplasma phagocytophilum reveals three distinct lineages with different host ranges in clinically ill French cattle. Vet. Res. 2014, 45, 114. [Google Scholar] [CrossRef]
- Bauer, B.U.; Răileanu, C.; Tauchmann, O.; Fischer, S.; Ambros, C.; Silaghi, C.; Ganter, M. Anaplasma phagocytophilum and Anaplasma ovis-Emerging Pathogens in the German Sheep Population. Pathogens 2021, 10, 1298. [Google Scholar] [CrossRef]
- Welc-Falęciak, R.; Kowalec, M.; Karbowiak, G.; Bajer, A.; Behnke, J.M.; Siński, E. Rickettsiaceae and Anaplasmataceae infections in Ixodes ricinus ticks from urban and natural forested areas of Poland. Parasit. Vectors 2014, 7, 121. [Google Scholar] [CrossRef]
- Luu, L.; Palomar, A.M.; Farrington, G.; Schilling, A.K.; Premchand-Branker, S.; McGarry, J.; Makepeace, B.L.; Meredith, A.; Bell-Sakyi, L. Bacterial Pathogens and Symbionts Harboured by Ixodes ricinus Ticks Parasitising Red Squirrels in the United Kingdom. Pathogens 2021, 10, 458. [Google Scholar] [CrossRef]
- Rymaszewska, A. Genotyping of Anaplasma phagocytophilum strains from Poland for selected genes. Folia Biol. 2014, 62, 37–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Myczka, A.W.; Szewczyk, T.; Laskowski, Z. The Occurrence of Zoonotic Anaplasma phagocytophilum Strains, in the Spleen and Liver of Wild Boars from North-West and Central Parts of Poland. Acta Parasitol. 2021, 66, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J.; Buńkowska-Gawlik, K.; Adamczyk, M.; Gajda, E.; Merta, D.; Popiołek, M.; Perec-Matysiak, A. The occurrence of Anaplasmataceae in European populations of invasive carnivores. Ticks Tick Borne Dis. 2018, 9, 934–937. [Google Scholar] [CrossRef]
- Katargina, O.; Geller, J.; Alekseev, A.; Dubinina, H.; Efremova, G.; Mishaeva, N.; Vasilenko, V.; Kuznetsova, T.; Järvekülg, L.; Vene, S.; et al. Identification of Anaplasma phagocytophilum in tick populations in Estonia, the European part of Russia and Belarus. Clin. Microbiol. Infect. 2012, 18, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Michalik, J.; Stańczak, J.; Cieniuch, S.; Racewicz, M.; Sikora, B.; Dabert, M. Wild boars as hosts of human-pathogenic Anaplasma phagocytophilum variants. Emerg. Infect. Dis. 2012, 18, 998–1001. [Google Scholar] [CrossRef] [PubMed]
| Species | Adults | Juvenile | Total | |
|---|---|---|---|---|
| Males | Females | |||
| Red deer | 8 (0) | 49 (15) | 33 (0) | 90 (15) |
| Roe deer | 7 | 49 | 14 | 70 |
| In total | 15 | 98 | 47 | 160 |
| Reaction | Primers | Tm (°C) | Reference |
|---|---|---|---|
| PCR | ankAF1a 5′-TGCTGTAAATGAAGAAATTACAACTTC-3′ ankAF1b 5′-TGGTGTAAATGAAGAAATTACAACTC-3′ ankARC 5′-GCCTTTAGTAGTACTCTACATGC-3′ | 53 °C 52 °C 54 °C | this study |
| Nested—PCR | ankAF2a 5′-CTGACCGCTGAAGCACTAA-3′ ankAR1a 5′-GAAGCCAGATGCAGTAACGA-3′ ankAR1b 5′-GAAGCAAGATGCAGTAACGA-3′ | 51 °C | |
| 52 °C | |||
| 50 °C |
| Ecotype | Host | No. GenBank Sequence (This Study) | Sequences with 100% Similarity | Host | Country | ||
|---|---|---|---|---|---|---|---|
| I | red deer (Cervus elaphus) | ON604852-87 | MK069963 | red deer | Norway | ||
| KU712106 | Austria | ||||||
| KJ832471 | horse | France | |||||
| MZ348280 | sheep | Germany | |||||
| MK069889 | Ixodes ricinus | Norway | |||||
| MW732493 | UK | ||||||
| KF312358 | Poland | ||||||
| roe deer (Capreolus capreolus) | ON604849 | MK069949 | moose | Norway | |||
| MK069696 | red deer | ||||||
| MK069797 | I. ricinus | ||||||
| ON604850 | AY281823 | I. ricinus | Germany | ||||
| KJ832474 | cow | France | |||||
| GQ452227 | goat | Switzerland | |||||
| MW013536 | hedgehog | Czech Republic | |||||
| II | roe deer (Capreolus capreolus) | ON604846-47 ON6048451 | MN093177 | I. ricinus | Nederland | ||
| KU712112 | roe deer | Germany | |||||
| KC800984 | moose | ||||||
| AY220467 | roe deer | Austria | |||||
| ON604848 | KF031380 | I. ricinus | Italy | ||||
| HQ629905 | Estonia | ||||||
| MK069774 | moose | Norway | |||||
| GQ988754 | roe deer | Austria | |||||
| KU712121 | Germany | ||||||
| Cluster | Host | No. GenBank Sequence (This Study) | Sequences with Highest Identity | Host | Country |
|---|---|---|---|---|---|
| I | red deer (Cervus elaphus) | ON646032 | GU236718 (100%) | red deer | Slovenia |
| ON646033-34 | KJ832286 (99.69%) | dog | France | ||
| HQ629928 (99.69%) | Ixodes ricinus | Estonia | |||
| ON676564 | GU236718 (99.68%) | red deer | Slovenia | ||
| roe deer (Capreolus capreolus) | ON646031 | GU236718 (99.83%) | red deer | Slovenia | |
| KJ832286 (99.66%) | dog | France | |||
| HQ629928 (99.66%) | Ixodes ricinus | Estonia | |||
| II | roe deer (Capreolus capreolus) | ON646026-28 | GU236909 (100%) | roe deer | Slovenia |
| GU236894 (100%) | Germany | ||||
| GU236900 (100%) | Spain | ||||
| AY282386 (100%) | Ixodes ricinus | Germany | |||
| ON646029 | GU236897 (100%) | roe deer | Spain | ||
| GU236874 (100%) | Germany | ||||
| AY282375 (100%) | Ixodes ricinus | ||||
| III | roe deer (Capreolus capreolus) | ON646030 | GU236904 (100%) | roe deer | Slovenia |
| GU236898 (100%) | Spain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myczka, A.W.; Steiner-Bogdaszewska, Ż.; Oloś, G.; Bajer, A.; Laskowski, Z. Diversity of Anaplasma phagocytophilum Strains from Roe Deer (Capreolus capreolus) and Red Deer (Cervus elaphus) in Poland. Animals 2024, 14, 637. https://doi.org/10.3390/ani14040637
Myczka AW, Steiner-Bogdaszewska Ż, Oloś G, Bajer A, Laskowski Z. Diversity of Anaplasma phagocytophilum Strains from Roe Deer (Capreolus capreolus) and Red Deer (Cervus elaphus) in Poland. Animals. 2024; 14(4):637. https://doi.org/10.3390/ani14040637
Chicago/Turabian StyleMyczka, Anna W., Żaneta Steiner-Bogdaszewska, Grzegorz Oloś, Anna Bajer, and Zdzisław Laskowski. 2024. "Diversity of Anaplasma phagocytophilum Strains from Roe Deer (Capreolus capreolus) and Red Deer (Cervus elaphus) in Poland" Animals 14, no. 4: 637. https://doi.org/10.3390/ani14040637
APA StyleMyczka, A. W., Steiner-Bogdaszewska, Ż., Oloś, G., Bajer, A., & Laskowski, Z. (2024). Diversity of Anaplasma phagocytophilum Strains from Roe Deer (Capreolus capreolus) and Red Deer (Cervus elaphus) in Poland. Animals, 14(4), 637. https://doi.org/10.3390/ani14040637






