Simple Summary
Microplastics (MPs) are anthropogenic microscopic pieces of plastic in marine sediments and the water column that can originate from two different sources. Primary MPs are industrially produced microbeads, while secondary MPs result from the fragmentation of larger plastic debris by physical, chemical, and biological processes. Although not yet considered a food contaminant, they are readily ingested by marine organisms at several trophic levels, thus entering the food chain and representing a risk to consumers of fishery products. In this study, Donax trunculus (Truncate donax) specimens collected from Class A production areas in the Tyrrhenian Sea (Mediterranean Sea), and therefore destined directly for the final consumer, were analyzed for the presence of MPs. First, all items were morphologically classified and measured, and some of these were chemically identified. Then, the MPs’ mean abundance (MA) was calculated, and a risk assessment of human exposure to MPs was carried out. Both the MA of MPs and human exposure to MPs were found to be low. However, given the magnitude of the problem, the collection of further data using standardized methods is essential for a better risk assessment.
Abstract
Microplastics (MPs) (0.1 µm–5 mm particles) have been documented in oceans and seas. Bivalve molluscs (BMs) can accumulate MPs and transfer to humans through the food chain. BMs (especially mussels) are used to assess MPs’ contamination, but the genus Donax has not been thoroughly investigated. The aim of this study was to detect and characterize MPs in D. trunculus specimens collected along the Tuscan coast (Italy), and to assess the potential risk for consumers. The samples (~10 g of tissue and intervalval liquid from 35 specimens) were digested using a solution of 10% KOH, subjected to NaCl density separation, and filtered through 5 μm pore-size filters. All items were morphologically classified and measured, and their mean abundance (MA) was calculated. Furthermore, 20% of them were analyzed by Raman spectroscopy and, based on the obtained results, the MA was recalculated (corrected MA) and the annual human exposure was estimated. In the 39 samples analyzed, 85 items fibers (n = 45; 52.94%) and fragments (n = 40; 47.06%) were found. The MA was 0.23 ± 0.17 items/grww. Additionally, 83.33% of the items were confirmed as MPs (polyethylene and polyethylene terephthalate). Based on the correct MA (0.18 MPs/grww), D. trunculus consumers could be exposed to 19.2 MPs/per capita/year. The health risk level of MPs was classified as level III (moderate).
1. Introduction
The term microplastics (MPs) is used to describe a mixture of differently shaped plastic materials in the range of 0.1 µm–5 mm in marine sediments and in the water column [1,2]. MPs could have two different origins. Primary MPs are industrially manufactured microbeads (e.g., abrasive particles, powders for injection molding, resin pellets for bulk transportation of polymers between manufacturing sites), while secondary MPs derive from the fragmentation of larger plastic debris during their use (e.g., textile and rope fibers, weathering and the fragmentation of larger litter items, vehicle tire wear, paint flakes) or as a consequence of weathering degradation processes, especially in the marine environment [3,4]. MPs are therefore abundant in both the water column and in sediments. Depending on their density, MPs can float on the surface of the water (low density) or sink (high density) and accumulate [5,6]. Moreover, as degradation and physical breakdown proceeds, their total number magnifies [7]. MPs can also accumulate in different aquatic organisms by entering through multiple pathways, such as filter feeding, suspension feeding, direct ingestion, and trophic transfer through the consumption of exposed preys [8,9,10]. In this way, they can be transferred through food webs [11,12]. Thus, MPs should be considered as a food contaminant with a potential health threat for seafood consumers [8,13,14]. Despite the risk of exposure to MPs for human health being largely unknown, it is recognized that they can release additives and toxins that, once absorbed, can cause both physical and chemical stress to the human digestive system [15,16]. Further research to properly assess the human risks linked to MPs is therefore required [17,18].
Bivalve molluscs (BMs) are an important source of human food, and they are characterized by a high commercial interest worldwide [19]. Because of their filter-feeding activity [20], BMs are able both to accumulate and concentrate different kinds of contaminants [21,22]. Regarding MPs, part of them can be not egested by BMs, and they consequently accumulate in different organs based on their chemical and physical characteristics [23]. Therefore, considering that BMs are eaten whole, BMs can expose consumers to this kind of potential hazard [24].
Among BMs, mussels are the most analyzed for MPs detection, followed by oysters, clams, and scallops [25]. BMs of the Genus Donax (Ansell, 1893) (superfamily Tellinoidea, Family Donacidae) are distributed along sandy coastlines in tropical and temperate zones, including the Mediterranean Sea [26]. D. trunculus (Truncate donax) represents an important resource in France, Italy, Portugal, Spain, and Turkey [27]. This species is a constituent of the infra-littoral benthic fauna of well-graded fine sands, and it especially inhabits the wash zones of the beaches (at depths between 0 and about 2 m) where it feeds mainly off phytoplankton and organic material [28,29,30]. Therefore, MPs can be assimilated by D. trunculus, firstly, by gills, and then by the digestive gland where they seem to be accumulated [10,31]. While D. trunculus has been extensively used to monitor other types of marine pollution in Mediterranean areas [10], only five studies assessed the occurrence of MPs in this species (Table 1). Only three of them carried out polymer identification (see Table 2 for details). Of these, only two [32,33] analyzed specimens from the Mediterranean Sea finding fibers and fragments composed by different polymers (see Table 2 for details). Interesting to note, these two studies are rather recent, the first of them, by Olivieri et al. [33], being published in 2020. This study [33] was also the only one from the Tyrrhenian Sea along the Italian cost (Latium Region) (Table 1). In this study, the need to conduct further research to confirm the accumulation of specific MP shapes in D. trunculus from Mediterranean Sea was emphasized [33].

Table 1.
Studies available in the literature, investigating MP occurrence in D. trunculus.

Table 2.
The abundance of MPS (range), dominant shape, color, size, and polymer of MPs detected in D. trunculus. PE = polyethylene; PP = polypropylene; PES = Polyester; PVDF = polyvinylidene fluoride; PET = polyethylene terephthalate; PVDC = polyvinylidene chloride; FT-IR: Fourier Transform Infrared Spectroscopy analysis; ATR: Attenuated Total Reflectance.
The Mediterranean Sea, with the highest concentrations of floating plastic particles worldwide, is one of the most impacted areas in terms of MPs [37,38]. The coasts of Latium and Tuscany (Tyrrhenian Sea) represent about 1000 of the 8000 km of the Italian coastline. Currently, the collection of D. trunculus along the Tuscany coast is allowed from natural banks located in sea areas, classified as Class A areas by the Local Health Authority (LHA) [39,40]. D. trunculus from these areas are therefore intended for direct human consumption without any treatment in a depuration center [41]. The aim of this study was to investigate the occurrence of MPs in D. trunculus specimens from the Mediterranean Sea (Tyrrhenian Sea) along the coasts of the Tuscany region and characterize them in terms of polymer composition. In addition, the potential risk for consumers was assessed. This study can therefore concur to better describe the MPs occurrence in poor areas (Tuscany coast).
2. Materials and Methods
2.1. Specimens’ Collection
According to the Community Guide to the Principles of Good Practice for the Microbiological Classification and Monitoring of Bivalve Molluscs Production and Relaying Areas, with regard to Implementing Regulation 2019/627 [42], LHA have to designate collection points in order to ensure that the results obtained from samples are representative of the degree of contamination of the entire area. In addition, a representative BMs sampling program in term of the number of samples, geographical distribution and frequency sampling (Commission Implementing Regulation (EU) No 2019/627, Article 56) must be defined [40]. Accordingly, D. trunculus specimens were collected in 2021 by the LHA of the Tuscany region from five collection points located within five production areas (Class A) (Viareggio Ponente (VP), Viareggio Levante (VL), Gombo San Giuliano Terme (G-SGT), Gombo Pisa (G-Pi), and Centro Coni and Tirrenia (CCT), as defined by the Guidelines provided by the Tuscany Regional Council Resolution 1401 of 11 December 2017 [43] (Figure 1). No collections were performed in January, due to the bad weather conditions, or in April, during which the harvesting of D. trunculus is prohibited for restocking purposes (Ministerial Decree of 22 December 2000 of the Ministry of Agriculture and Forestry (Fisheries and Aquaculture)) [44].
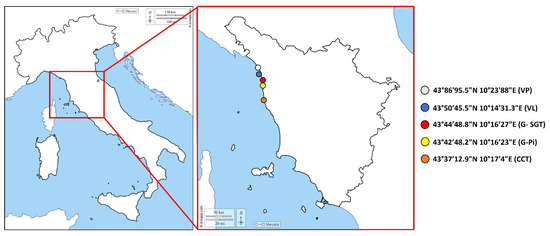
Figure 1.
Areas of D. trunculus harvesting along the Tuscany coast, and relative collection points with coordinates: Viareggio Ponente (VP), Viareggio Levante (VL), Gombo San Giuliano Terme (G-SGT), Gombo Pisa (G-Pi), and Centro Coni and Tirrenia (CCT).
The collection was performed from February to December 2021. All the collected specimens were stored in aluminum foil to avoid any types of contamination, and then transported at freezing temperature (−20 °C) to the Experimental Zooprophylactic Institute of Latium and Tuscany (IZSLT) (Pisa section). Each collection, composed of 50 specimens, was associated to an internal code (Table S1). Some specimens from the same collection points, but collected in 2020, were used as positive control (see Section 2.6).
2.2. Sample Preparation and Items Isolation (Digestion and Filtration)
All the collected specimens belonging to the samples collected in this study (Table S1) were defrosted and shelled using a metal scalpel to constitute a sample of ~10 g (accuracy of 0.001 mg) of tissue and intervalval liquid of 35 specimens. All the samples were processed using the protocol proposed by Ben-Haddad et al. [32] for the detection of MPs in D. trunculus, based on digestion with 10% KOH (Sigma-Aldrich, St. Louis, USA) solution, followed by a flotation with NaCl. The protocol was slightly modified with respect to the digestion time in 10% KOH which was reduced from the 72 h reported in the original protocol to 48 h by inserting an oscillator directly into the oven. Indeed, from the preliminary tests, it was observed that the digestion effectiveness was not influenced by decreasing the digestion times. Once digested, samples were removed from the oven and added with a concentrated saline solution in double-filtered deionized water (1.2 gr/mL NaCl > 99%; Sigma-Aldrich) to separate items from sediments, fecal casts, and D. trunculus matter. Furthermore, 250 mL of the NaCl solution was added to each sample (1:1 v/v), which was again covered with aluminum foil and transferred under a fume hood at room temperature overnight (ON). After the ON density separation, 200 mL of supernatant was collected using a 25 mL glass pipette through concentric movements from the walls to the center of the beaker. The supernatant was filtered using a device (BioSigma, Milan, Italy) connected to a vacuum pump through a cellulose-mixed ester membrane filters (Ø 47 mm; Whatman with 5 μm pore size). Each filter was then placed in a covered 90 mm Ø glass Petri dish (Biosigma)) and left to dry for a few hours, waiting for stereomicroscope observation. Overall, seven sessions (comprising five or six samples each) of digestion and filtering were performed.
2.3. Filter Observation
The filters obtained from each sample were observed to detect potentially plastic items differently from organic materials and sand [45,46]. Each filter was observed three times by two operators (under the same conditions) to confirm the number of detected items. Only items < 5 mm in size with a uniform color and thickness (in the case of fibers) were considered during the observation [47]. Moreover, items were checked to assess their nature (plastics or organic). If items disintegrate when touched with a needle, they were considered organic residues ‘surviving’ digestion [48]. The items observed in the filters of the samples were compared (in terms of size, color, and shape) with those detected on the filters of the procedural blanks and blank filters of the same analysis session (see Section 2.6). Items resemblant to those observed in blanks were excluded from the count. The retained items were subsequently classified morphologically as proposed by the Group of Experts on the Scientific Aspects of Marine Environmental Protection for MPs [14,47].
To measure items, images obtained by observing filters at a 40X magnification were captured with the aid of a camera (Apple iPhone 11; 12 MPX ƒ/1.8). Subsequently, the major axis of each photographed item was measured using ImageJ software (version 1.52t; https://imagej.nih.gov/ij/download.html, accessed on 25 November 2023). In addition, the items were also classified according to their length using the following intervals: (a) 25–50 µm; (b) 50–100 µm; (c)101–200 µm; (d) 201–500 µm; (e) 501–1000 µm; (f) 1001–1500 µm; (g) 1501–2000 µm; (h) 2001–3000 µm; (i) >3001 µm [47]. The morphology, color, and size of each item were recorded.
2.4. Items Abundance and Statistical Analysis
First, the item abundance per sample (pool = 10 g) was calculated as follows:
Then, the overall mean abundance (MA) (mean ± standard deviation) of the item was calculated. Moreover, each sample was assigned to categories based on the number of items found: (a) no items (0); (b) 1 or 2 items; (c) >2 items. Finally, the percentage of positive samples (samples in which at least one item was found) per site and per season was calculated.
2.5. Polymer Identification
2.5.1. Chemical Identification of the Items
Approximately 20% of the items found were analyzed to determine their chemical composition, identifying the constituent polymers by Raman spectroscopy. The items to be tested were randomly selected among those extracted from the samples, based on their morphological prevalence. In detail, twelve MPs’ fibers (ranging in size between ~150 μm and ~1500 μm) and six MPs’ fragments (ranging in size between ~50 μm and ~1000 μm) were analyzed. Figure 2 shows some of the items analyzed.

Figure 2.
Items extracted from samples of D. trunculus, analyzed for MP detection. The items were observed under a stereomicroscope and selected for the chemical identification of their constituent polymers.
Items were transferred from the filters’ surface onto scotch tape previously attached to glass slides, and subsequently covered with a glass coverslip to prevent further contamination. Each item was first spotted under the stereomicroscope, and then the correspondent area was analyzed with the microspectrometer for the Raman characterization.
2.5.2. Raman Analysis
Raman characterizations were carried out using an XploRA Plus microspectrometer (Horiba Scientific, Kyoto, Japan), equipped with a diode laser emitting at 785 nm. The acquisition time was set at 20 s, and the laser power was set at 37 mV. The measurements were performed at room temperature using either a 100× or 50× long working distance microscope objective (Evident corporation-Olympus, Tokyo, Japan),which helped focus the laser beam onto the sample surface. The scattered light was collected in a backscattered configuration through the same objective, and subsequently was dispersed by a diffraction grating (1200 lines/mm) onto a CCD detector (Syncerity, Horiba Scientific, Rome, Italy). The vibrational fingerprint of each item was compared with a database of reference polymer spectra using commercial software (SpectraGenius v1.3.p—S.T. Japan-Europe GmbH, Cologne, Germany). Only a match of at least 70% was considered reliable [49,50,51]. The Raman spectrum of each item was analyzed after the subtraction of the glass contribution and the removal of the fluorescence background (see Figure S1). The Raman spectrum of the scotch tape was also acquired to avoid any misleading identifications of the items.
2.6. Quality Control Measures
To avoid accidental contamination with MPs of environmental origin, the analysis was performed in a restricted access room. All procedures, except for the sample preparation (see Section 2.2) and filter observation, were carried out under a clear airflow cabinet, wearing nitrile gloves and white 100% cotton gowns [48,52]. All materials, equipment, and laboratory surfaces were washed and rinsed with double-filtered deionized water, obtained using sterile syringe filters (cellulose acetate) with 0.22 μm pore size (Ø 25 mm; Millipore, Sigma-Aldrich). All the solutions used in this study were produced using the same water. Only glassware was used. The samples were covered with aluminum foil when at risk of being exposed to airborne particles, and all the experiments were completed as quickly as possible.
One procedural blank (sample without tissue prepared as described in Section 2.3) was included during each session of digestion and filtering. In addition, blank filters, consisting of filters laid on open Petri dishes and placed next to the sample or to the operator during procedures conducted outside the cabinet, were used (one for each observation session). The blank filters were subsequently observed under a stereomicroscope (Olympus SZX9) at a 40× magnification to detect the occurrence of items to monitor environmental contamination during sample processing.
Three positive controls were performed to evaluate both the recovery rate of the method, and to determine the minimum observable particle size with the used stereomicroscope. They were prepared by spiking 20 MPs made of blue polystyrene (bPS) (1.050 g/cm3) of a known size range (106–500 µm) into 10 g of D. trunculus samples (see Section 2.3), collected in 2020. MPs were obtained by fragmenting bPS plastic plates with an ultra-centrifugal mill (ZM 200, Retsch GmbH, Haan, Germany), equipped with a 500 μm steel mesh sieve. The obtained particles were further sieved with a 106 μm steel mesh column sieve obtaining a batch of MPs ranging in the known size between 106 μm and 500 μm. The positive controls were processed using the protocol described in Section 2.2, and the MPs recovery rate was calculated by counting the number of retrieved particles on the filters (as described in Section 2.6). The minimum detectable particle size was established by spiking white low-density polyethylene (LDPE) (0.924 g/cm3; Sigma-Aldrich, USA) MPs of different size ranges (40–48 μm, 63–90 μm; 91–125 μm; 126–180 μm; 181–355 μm; and 356–510 μm) onto white filters, which were then observed under the stereomicroscope at a 40× magnification. In detail, particles were tested in decreasing order of size by spiking 20 particles of each size batch onto a white filter. The batch size of the smallest particles that were easily observable on the filter surface under the stereomicroscope was the minimum detectable particle size of our method.
2.7. MP Abundance and Annual Human Exposure
Once the true nature of the items was verified, the MA was calculated again based on the percentage of items identified as MPs. For literature data comparison, considering the extremely high number of studies investigating MPs abundance in BMs, we exclusively considered the data provided by two recent systematic reviews on this topic [25,53]. In addition, the five studies investigating D. trunculus (Table 1), or other co-generic species, were considered in the comparison. The MPs consumers’ annual exposure was then calculated. In this case, since no data on the per capita consumption of D. trunculus are available, we considered data provided for clams by EUMOFA (2022) [54], corresponding to 320 g of the total body weight (including shell). Since the soft tissue (edible part) of BMs is reported to be almost one-third of the whole-body weight [49], we calculated the annual human exposure to MPs as corresponding to 106.6 g (320/3).
3. Results and Discussion
3.1. Specimens Collection
Sampling parameters, including collection point and collection date, are important factors in the characterization of MPs contamination, and they should therefore be reported. In addition, considering that the MP concentration is highly dependent on the area, human presence, and the season, with the highest near-heavily populated urban areas [55], detailed sampling information are essential for data interpretation and comparison. However, such information is not always provided in studies published to date [25]. The samples investigated in this study were collected from collection points identified by the LHA of the Tuscany region, as representative of the pollution of the five production areas in which BMs are harvested (see Section 2.1). In particular, the specimens analyzed in this study were collected in the context of official monitoring activities. To the best of our knowledge, no study conducted in the EU for the detection of MPs in BMs mentions the class of the production or relaying area, from which the BMs were collected. In the available studies on MPs in D. trunculus (Table 1), the specimens were collected from collection points (from 1 up to 9) selected by researchers, based on the harvesting and marketing areas of this species.
Regarding the sample size, at least 50 specimens per research unit is recommended to represent the population, according to the Marine Strategy Framework Directive [56]. Indeed, more than 50 BM specimens were collected in most of the studies published between 2011 and 2020 [25]. Accordingly, in the five studies conducted on D. trunculus, the number of collected specimens (reported only in four) ranged from 51 to 1632 (Table 1). However, it should be considered that the number of collected specimens does not always correspond to those analyzed (Table 1). Also, in this study, 50 specimens of D. trunculus per 39 collection date (1950 specimens) were collected and ~1365 specimens were analyzed. This, therefore, represents the second study in terms of the number of D. trunculus specimens analyzed for MP detection (Table 1). In detail, nine samplings were performed at G-Pi (23.08%) and G-GT (23.08%), eight (20.51%) at CCT, seven (17.95%) at VL, and the last six at VP (15.38%) (Figure 3). The collection was also distributed along seasons with eighteen (46.15%; n = 900) samplings carried out during summer, eight (20.51%; n = 400) in spring, seven (17.95%; n = 350) in autumn, and six (15.38%; n = 300) in winter. The highest number of samples were collected in June (n = 9; 450 specimens), followed by March (n = 6; 300 specimens), and July/August (n = 5; 250 specimens). Literature studies analyzing D. trunculus (Table 1) did not cover all the season periods (Table 1). Accordingly, as reported in the review by Ding et al. [25], only in six of the sixty-one analyzed studies regarding the detection of MPs in BMs was the sampling performed in more than one season.
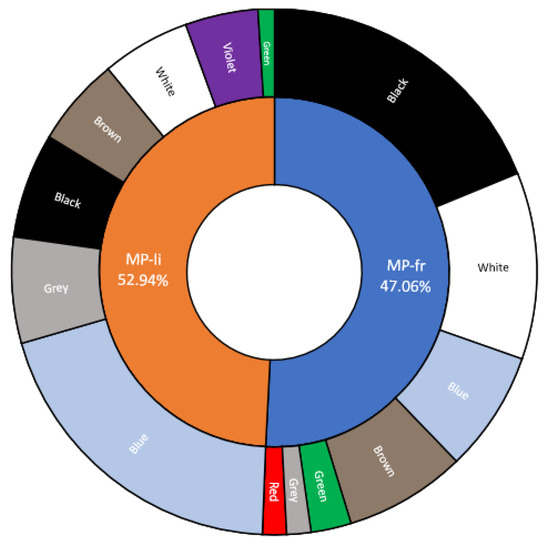
Figure 3.
Sunburst diagram showing the shape (fibers (MP-li); fragments (MP-fr)) and color of the extracted items and their relative percentages.
3.2. Quality Control Measure
Quality control measures should always be conducted and described for the assessment of the reliability of the results [22]. In this study, strict measures to avoid contamination, which could lead to an overestimation of the number of MPs in the sample, were implemented, starting from the specimen collection. In fact, given their structural heterogeneity, MPs can also be traced in the atmosphere, as well as in the equipment, reagents, and materials used for the extraction process itself, such as gloves, gowns, and solvents [57]. Background contamination from the surrounding environment was avoided throughout sample treatments and analysis. As a result, the procedural blanks only contained 0.29 ± 0.49 items per filter. Seven procedural blanks (one for each digestion session) were produced and analyzed together with the samples to monitor secondary contamination (environment and air) [25,58]. Procedural blanks are effectively widely used as quality control in studies aimed at detecting MPs in BMs. According to the review by Ding et al. [25], 93.4% of the studies on this topic included this kind of quality control. In contrast, only 28% included positive controls to determine the recovery rate [25]. Positive controls were instead included in this study. Among the different size classes tested, the 40–48 μm range was the smallest in which potential LDPE items could be clearly identified under the 40x stereomicroscope magnification. An MP recovery rate of 95% (57 particles found on a total of 60) was defined, allowing an evaluation of the results obtained with a good level of confidence. Indeed, recovery rates between 80 and 100% allow for the obtaining of comparable results [25,48]. In addition, the high recovery rate confirmed the low degradation impact on the MPs of the protocol digestion, in line with the literature [59].
3.3. Items Detected in D. trunculus
3.3.1. Items Isolation Protocol
Considering that treatments (like depuration) might affect the true estimation of MPs in the samples, the specimens were subjected to MPs extraction without preliminary washing steps [53]. For the MPs extraction, the protocol of Ben-Haddad et al. [32] was chosen as a starting point, because it was designed for the detection of MPs in D. trunculus, and it also used a 10% KOH solution (at 40 °C), which is considered to provide the most efficient removal of soft tissue, while protecting MPs [59]. In fact, digestion with 10% KOH is the most suitable technique for digesting BMs tissues, even when the target particles are only one micrometer in size. In addition, it is a cheaper and less time-consuming method [59]. In the review of Ding et al. [25], 10% KOH is reported as the most-used solution, followed by H2O2, HNO3, and enzyme digestion for MP detection in BMs. About the five studies in which D. trunculus was analyzed, only two used the 10% KOH protocol [25,34]. Then, a flotation step with NaCl was also carried out. Accordingly, the saturated NaCl solution was the most used for the flotation step in previous studies [25], due to its low cost and low chemical risk [60]. In addition, it has also been proven efficient for the detection of low-density polymers, such as polystyrene (PS), polyethylene (PE), polypropylene (PP), polyamide (PA), ethylene vinyl acetate (EVA), and polymethyl methacrylate (PMMA) [61,62]. These represent the main polymers found in the environment including marine sediments [63]. However, denser polymers such as polyethylene terephthalate (PET) and polyvinylchloride (PVC) could be extracted due to the change induced by weathering or other processes [64]. Even though the flotation step is not a must when using 10% KOH, this method allowed for the best separation of MPs [65]. It is reported that the MPs recovery rate in protocols involving a basic digestion with 10% KOH combined with a NaCl flotation reaches 90–95% [63]. Finally, 5 µm filters were used because they are the most widely used in the literature for MP detection in bivalves, even if very different pore sizes, ranging from 0.2 µm to 80 µm, have been used [25,66].
3.3.2. Item Abundance
Items found in the procedural blanks and in the blank filters were morphologically analyzed and compared with those found in the samples. In this way, the items of similar appearances were eliminated from the total counting. Out of the 39 samples, 35 (89.74%) were positive, with a total of 85 items found. Moreover, in 53.85% (n = 21) of the samples, one or two items were found, while, in 35.90% (n = 14), more than two items were found (maximum eight items). In the other samples (10.26%; n = 4), no items were found. Overall, the MA of the items was 0.23 ± 0.17 items/g ww with a maximum number of 0.8 items/g ww, and 2.18 ± 1.78 items/35 individuals (0.06 ± 0.05 items/individual) (Table 3). In the literature, most studies reported an abundance of items/g or of items/individual. Abundance expressed as items/g is usually reported as wet weight (ww) (as in this study), although in a minority of cases, it has also been reported as dry weight (dw) [67]. The lack of standardization in reporting MP abundance values is an issue also highlighted by the EFSA (2016) [13,25,53]. Due to this, a comparison between the results obtained from different studies should be made carefully, and the use of different analytical methods, the units of measurement, and the number of samples should be especially considered. In studies in which D. trunculus specimens were analyzed, the relative MAs are reported as items/individual (60%; n = 3), items/g ww (20%, n = 1), and in both ways (20%; n = 1) (Table 4). In the study of Ben-Haddad et al. [32], the MA, expressed in items/g ww, is higher than that found in our study. However, the analyzed specimens of D. trunculus were not harvested in the Mediterranean Sea, but rather along the Atlantic coast of Morocco, so that the level of MP contamination is not the same. Also, both the MA values found in the studies conducted in the Black Sea are higher than this study, in terms of items/individuals (Table 2). Interestingly, our MA falls within the reported MA range of the unique study conducted in the Tyrrhenian Sea [33] (Table 2). Instead, in the other study conducted in Mediterranean Sea (Catalan coast), the MA values found were higher [36] (Table 2). Interestingly, in the same study, no significant difference between 48 h purified and non-purified D. trunculus samples were found [36]. Higher MAs were observed in another co-generic species (D. cutaneus) [68], and, more generally, in the entire Donax genus [69]. Of course, even in these cases, high MA values could be related to the collection site.

Table 3.
MA values for the four BM (bivalve mollusc) categories (clam, mussel, oyster, and scallop), reported in the two reviews used for the comparison.

Table 4.
Positive samples found according to collection point and season, with the relative percentage.
The literature considered for the comparison [25,53] reported the MP abundances according to four BM categories, namely clams, mussels, oysters, and scallops. To note, in both the reviews, the MA was reported as MPs/g ww, confirming how this unit is most suitable for comparing MAs. In particular, the calculated MAs are reported in Table 3.
Although the MAs reported by Danopoulus et al. [53] were lower overall in all the categories, with respect to Ding et al. [25], clams were found as the category with the highest MA in both the reviews (Table 3). In detail, Danopoulus et al. [53] reported 1.25 ± 0.54 MPs/g ww, and Ding et al. [25] reported 3.2 ± 4.2 MPs/g ww.
The higher MA of MPs in clams could be related to the fact that they are classified as infaunal bivalves living within the substrate, whereas mussels, oysters, and scallops are all epifaunal bivalves living above the substrate. Therefore, the differences in item numbers could be attributed to the different MPs present in the two habitats (water and substrate, respectively) [70,71]. D. trunculus belongs to the order of Veneroida as clams, and lives on the sandy seabed, being exposed not only to the suspended MP water column, but also to the plastic material that is deposited. However, in this study, a lower MA with respect to clams was found. Interestingly, in the study of Exposito et al. [36], in which six species of BMs among infaunal (D. trunculus, Ensis siliqua, Ruditapes decussatus) and epifaunal (Mytilus galloprovincialis, Crassostrea gigas; Bolinus brandaris) bivalves were analyzed, D. trunculus presented significantly lower levels of items per individual with respect to the other species analyzed; this is likely due to its low ability to ingest large MPs. Moreover, in the same study, clams (R. decussatus) and mussels (M. galloprovincialis) showed higher MAs in terms of MPs/g ww. Also, in another study conducted on specimens of these two species collected in the bay of Izmire (Turkey), similar MA values, in terms of MPs/individual, were found [72]. Regarding clams, these may accumulate microfibers and microfragments which may be more difficult to expel, and, because they are infaunal, they are also affected by sediment MP contaminant [70,71,73,74]. On the contrary, mussels can accumulate more MPs due to their size, high pumping, and filtration rates [58,70,75]. Thus, the MPs’ filtering efficiency and accumulation probably depends on size, filtering selection, and environmental conditions [36]. Due to these differences, more than one species, selected among epifaunal and infaunal, should be analyzed to produce a better monitoring of MPs [25].
3.3.3. Positive Samples per Collection Point and per Season
In this study, the low total number of collected samples, associated with the differences in their number per collection point and season, allowed for the calculation of the percentage of positive samples per site and per season. Therefore, we provide only a comparison of the positivity rates to eliminate differences due to the different sample numbers per collection point and per season.
With respect to the collection points, 100% of the samples collected in VL were found to be positive regarding items, followed by those of Gombo SGT and Gombo Pi (88.88%), CCT (87.50%), and VP (83.33%) (Table 3). Interestingly, VL was the only site where more than two items were found in most samples (71.00%).
With respect to the season, 100% (n = 8) of the samples were found to be positive in spring, followed by summer, autumn, and winter, in which 88.24%, 85.71%, and 85.71% of the samples, respectively, were positive (Table 3).
With respect to the season trend in BMs, the review by Ding et al. [25] reported higher MAs in autumn, while the MAs in spring/summer only ranked third and second, respectively, among season [25]. Contrariwise, the lower MA observed in winter is in line with our findings. The MA seasonal trend observed in the literature for D. trunculus are different, according to the analyzed study [32,35]. For instance, the highest MA of items in specimens collected during the summer season is explained by some authors in relation to the greater MP accumulation, which was caused by the increase in tourist activities, fishing, and industrial processes, and also by the intensification of the metabolic and reproductive activities of D. trunculus during this period [33,35,76]. Previous studies found that BMs collected from areas with intensive human activities contained a higher number of items [77] and highlighted a correlation between the abundance of items in BMs and the surrounding environment [78,79]. Therefore, the highest positivity found in the spring/summer season in our study can be linked to the increase of the anthropic presence, related to tourist activities in this part of the Tuscan coast. This thesis could be supported by the fact that most of the items chemically identified in this study were PET polyester fibers that are widely used in textile industries (see Section 3.5). Moreover, during this period, items are more available for these BMs as they tend to deposit, whereas, in autumn and winter, waves and winds can cause the water to stir, thus causing items to migrate [80].
3.3.4. Items Shape, Size, and Color
Based on GESAMP (2019) [14], 45 items were classified as MP-li (52.94%), and 40(47.06%) were identified as MP-fr (Figure 4). The items were of various colors, including black (29%) and blue (28%), followed by white (16%), brown (8%), grey (7%), purple (5%), green (4%), and red (1%) (Figure 3).
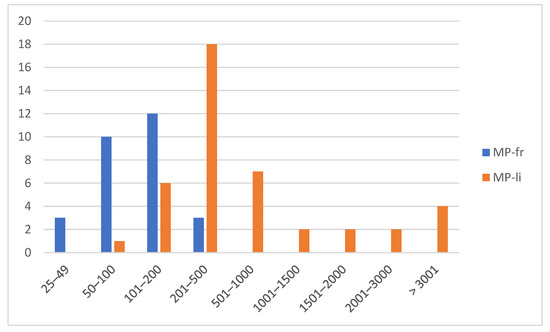
Figure 4.
Distribution of items in relation to size and shape (MP-fr; MP-li).
In particular, the most frequently encountered color for MP-fr was black (43%), while for MP-li, it was blue (40%) (Figure 4). This result is in line with previous studies conducted in the same species (see Table 3). The percentage of fibers (MP-li) was also statistically higher in most of the studies selected in the review by Ding et al. [25], analyzing different BM species. Fibers represent the most-found MP type in seawater, freshwater, and sediment samples [81,82,83,84]. Anthropogenic fibers are mainly derived from textile industries and can be divided into three categories: natural fibers, such as cotton and wool; semi-synthetic fibers, such as rayon, which are reconstituted from the dissolved cellulose of plant materials and shaped into fibers via extrusion; and synthetic fibers from petrochemical-based compounds [85]. Nevertheless, the principals’ sources of fibrous material in the marine ecosystem might be untreated sewage and debris, such as torn fishing nets and broken ropes [86,87,88]. Fiber predominated in BMs collected from China, Thailand, and many European countries, while fragments are more reported in bivalves from Vietnam, France, Greece, and Brazil, reflecting the different types of plastics used [89]. Our results are also in line with those reported in the review of Santini et al. [90], in which microfibers released from synthetic fabrics represent about 40% (1.6–84.9%) of the MPs in the water column and sediments of the Mediterranean Sea, followed by fragments (34.5%; 1.6–72.7%). However, it must be considered that filaments tend to be overestimated, being more easily detected than other types of items [2,49]. In fact, Song et al. [50] reported that using the stereomicroscope can determine an overestimation of fibers and an underestimation of fragments. On the contrary, using the Fourier transformed infrared (FTIR) spectroscopy, more fragments are detected. Concerning the colors, dark items (black, blue, green, red) are more ingested by BMs. In fact, dark items may attract predators and increase the likelihood of ingestion, due to prey item resemblance [91]. In addition, a possible underestimation of transparent items could happens considering that colored items are more easily visible. However, light items (pink, transparent) were also found in the soft tissue of many BM species [32,89,91,92]. In fact, in some studies, non-colored and light-colored MPs (transparent, white, light blue), that could be the result of an alteration or loss of their original colors caused by environmental weathering processes, were the most common, followed by black and blue [66,89,90,93]. Finally, it must be considered that, although measures to avoid contamination were adopted in this study, it is possible that some fibers may be of environmental origin.
Using the ImageJ software version 1.52t, 70 out of the 85 items (82.35%) were measured. Of these, 28 were classified as MPs-fr (40%) and 42 as MPs-li (60%). Items with sizes between ~201 µm and ~500 µm (n = 21; 30%) were the most found, followed by those with sizes between ~101 µm and ~200 µm (n = 18; 25.71%), and those with size between ~50 µm and ~100 µm µm (n = 11; 15.71%) (Figure 3). Specifically, MPs-li showed sizes between ~100 µm and ~4000 µm in length, but about half (n = 18; 42.85%) were in the ~201 µm to ~500 µm range. The length of the MPs-fr ranged between ~30 µm and ~500 µm, with a predominance of those between ~101 µm and ~200 µm (Figure 4).
The item sizes were like those found in the studies reported in Table 3, ranging from ~25 to ~2000 µm. Accordingly, also in the studies selected in the review by Ding et al. 2022, the percentage of items < 1000 µm was higher than those between 1000 and 5000 µm. This suggests that the smaller items are more easily ingested by BMs, being like its natural food items [68,94]. In this regard, it is known that particles > 100 µm are unlikely to be ingested by BMs due to the anatomical constraints of the gills and mouth. Particles of this size, corresponding to the most part of those detected in the present study, once captured by the gills, are usually transported towards the mantle and rejected as pseudofeces. This process reduces the risk of adverse effects for BMs, due to mechanical blockages of the digestive tract and the absorption of contaminants released by the particles [23]. However, in some areas, including Italy, South Africa, and China, MPs between 1000 and 5000 µm were the most abundant in mussels and clams [47,87,95]. Nevertheless, the wide size range could also be related to the pore size of the filters used for item extraction and the limitations of the technique used for polymer identification [53]. However, comparing data from the various studies is difficult, as there is no standard for selecting items and measuring particles [25].
3.4. Polymer Identification of Items
Item identification via light microscopy serves as the first step in MP detection, but it is susceptible to human error [85] and requires analysis to reliably distinguish plastic materials from other compounds, such as natural fibers or organic material [90,96,97]. Spectroscopy techniques are effective in item polymer identification, but they are laborious, time-consuming, and expensive, making them difficult to use for routine investigations [98]. Accordingly, only three out of the five studies that investigated MPs in D. trunculus performed a chemical identification of the items (Table 2). Studies using spectroscopy for chemical identification usually test only a small percentage of particles [48,98], as in the present study. According to Ding et al. [25], the most frequently used methods for polymer identification were FT-IR and Raman spectroscopy. In this study, 18 items randomly selected from those extracted based on their morphological prevalence were analyzed by Raman spectroscopy. Out of the 18 items analyzed (21.17% of the total), 15 (83.33%) were confirmed to be MPs, and two were not made of plastic polymers. For the final one, it was not possible to make any identification due to the huge fluorescence background which prevented any Raman characterization, despite the excitation source being in the NIR spectral region (λ_exc = 785 nm), where the fluorescence emission is mostly absent or weak. The chemical identifications revealed that most of the tested items were made of PE followed by PET. Some items were instead identified as copolymers. Figure 5 shows an example of polymer identification. In detail, eight MP-lis and one MP-fr (red line in Figure 5) were identified as PE (60%). The reference spectrum acquired on scotch tape has a completely different signal, which matches with the Raman fingerprint of PP (Figure S4). Four (30%) MPs-frs were identified as PET, while one MP-li and one MP-fr were copolymers partially identified as Poly(styrene-ethylene-butylene) and Poly[(2,3-epoxypropyl methacrylate):Styrene:Ethylene Dimethacrylate)], respectively (Figure S2). The two MPs-li non-plastic items were identified as cotton and microcrystalline cellulose (Figure S3).
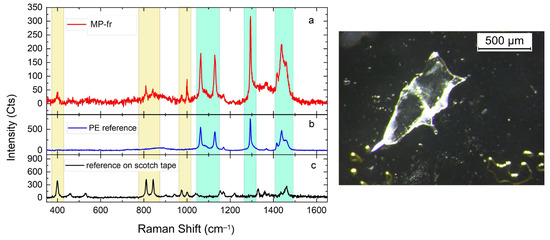
Figure 5.
Comparison between the Raman spectra (a) of a micro fragment extracted from samples of Donax trunculus, analyzed for microplastics detection, the reference spectrum of PE (b), and the spectrum of the scotch tape (c) used during the analysis for transporting the extracted items.
On the one hand, the chemical identification of items allows for assessment of the real level of MP contamination; on the other hand, it provides useful information for tracing MPs’ sources [99]. From a risk assessment perspective, polymer identification allows the evaluation of possible overestimates in the number of MPs identified by using the stereomicroscope [48]. In this regard, in the present study, two MPs-lis, considered as potential MPs under the stereomicroscope, were later identified as cotton and cellulose. The occurrence of cotton fiber could be indicative of airborne contamination, a recurring phenomenon in the microplastic study [100], while the cellulose fiber could be algae captured by the D. trunculus from the marine environment.
PE, the polymer most detected in this study, was also reported as the predominant polymer in the study of Ben-Haddad et al. [32], while in Olivieri et al. [33], a predominance of PET, polyvinylidene chloride (PVDC), and nylon was reported. In Exposito et al. [36], a predominance of polyester (PES), polyvinylidene fluoride (PVDF), and then PE was reported (Table 2). The other two studies that analyzed Trucate donax did not perform any chemical identification of items (Table 2). PE was also the prevalent polymer found in another species belonging to the Donax genus (D. cutaneus) collected in India, while in a study conducted on the Donax genus (China), the prevalent polymers were cellophane (CP) and PET [68,69]. Additionally, in another study conducted in South Korea, PE was also found among the prevailing polymers of clam species belonging to a different genus [89]. In the review conducted by Ding et al. [25], PET was the most frequently reported polymer, followed by PE, rayon, PP, cellophane, PES, PA, PVC, PS, and acrylic [25]. These authors, therefore, reported PE and PET polymers to be the two main contributors to global MP pollution in BMs. Their reviews showed that PET was the dominant polymer in BMs from China, South Africa, Mexico, and Portugal, whereas PE dominated a large proportion of MP composition in bivalves from Greece, France, New Zealand, and Italy (according with our study) [71]. An earlier review, conducted by Cavalca Bom and Sà [101], also reported PE and PET as prevalent polymers in MBs, also showing a correlation between these polymers and those found in the marine environment [102]. Indeed, polymers such as PE, PP, PET, PVC, and PA, used for packaging materials, are the common sources of plastic pollution [62,101]. Moreover, polymers such as PE, PP, PET, and PA are largely used to produce fishery tools like ropes, nets, buoys, and tubes [103]; this could produce a large amount of MPs because of long-term exposure to sunlight and mechanical damage [49]. Additionally, MPs in PETs discharged into the marine environment with sewage can originate from the breakdown of clothes and carpets. Indeed, PET polyester fibers have been widely used in textile industries since the 1990s [104,105]. All these findings are in line with the global data on plastic production, with PE representing 26.9%, and PET representing 6.2% [106]. However, it must be emphasized that the differences in polymer MAs observed between BM species also depend on their natural habitat (and thus on the density of the particles), their method of production, and their feeding characteristics [36]. Finally, regarding the styrene-ethylene-butylene copolymer identified in the present study, this is used in road materials to make the asphalt more elastic [107].
3.5. MP Abundance and Human Exposure
The MA calculated based on the item numbers was then recalculated after the polymer identification (corrected MA). In fact, considering that only 83.33% of the items were identified as MPs, the correct MA would be 0.18 MPs/g ww. Our results regarding the MPs could be considered as representative of the current MP contamination in the investigated area. In fact, samples derived from five harvesting areas (G-SGT, G-PI, CCT, VL, VP) all classified as Class A in 2021, according to Reg. (UE) 625/17 [39]. BMs harvested in these areas are therefore directly intended for human consumption without having to undergo prior depuration treatment (as in the case of BMs harvested in Class B or Class C areas) [39]. Several studies reported that the depuration process, which BMs undergo, is an important variable in defining the MA [5,108]. However, no significant differences in MAs between depurated and non-depurated BMs were found, although this could be due to insufficient time to remove the MPs from organisms [36].
Based on the corrected MA, D. trunculus consumers could be exposed to 19.2 MPs/per capita/year, according to the annual consumption of clams (106.6 g). This value is lower than that provided by Ben-Haddad et al. [32] (1072 MPs/capita/year ranging from 560 to 1897.6). On the contrary, in the study of Exposito et al. [36], they estimate a mean annual consumption of MPs per capita for the adult population of Catalonia to be 8103 (MPs/per capita/year), suggesting how BMs consumption could be a crucial cause of exposure [36]. In a study on risk assessment related to the consumption of BMs at a global level, an intake of 715 (ranging from 15 to 7333) MPs/capita/year was estimated on the basis of the global average consumption of molluscs of 367 g of pulp (considering the data from FAOSTAT and the ratio (value: 3) of the whole body weight–the soft tissue weight, as reported by Cho et al. [89] [73,89]). Nevertheless, the human exposure to MPs is very different between countries due to geographical and cultural differences in BM consumption [73]. Considering the global mean consumption established in the study of Ding et al. [73] (367 g of soft tissue/capita/year), the MP intake found in our study would still be much lower (66.1 MPs/capita/year). A high risk of exposure, calculated based on annual BM consumption and the MAs of MPs per gram, was found in countries such as China and South Korea, while at the European level, high risks was found in France and Greece [49].
Although we did not identify all items (only the 21.17%), we also performed the MP risk assessment on human health using the model proposed by Lithner et al. [109]. In particular, the risk was calculated based on Xu et al. [104], who considers the percentage of MP polymer types detected in D. trunculus and their relative hazard scores [109]. Then, the health risk level of MPs based on the polymer risk index was evaluated according to the hazard grades (1 to 10,000), which was classified into five levels of hazards (I, II, III, IV, and V) [109,110,111]. According to the percentage of PE MPs (60%) and PET MPs (30%) found in this study (not considering the remaining two copolymers), the health risk level of MPs was classified as level III (moderate). Interestingly, Italy and other countries (China, South Africa, New Zealand, South Korea, and the USA) were also classified at this level of risk in the study of Ding et al. [73]. In the latter study, countries classified at level IV (high level risk) presented a high percentage of polymers with high hazard scores, such as PCV (Sn = 10,551), while polymers like PE (Sn = 11) and PET (Sn = 4) presented a lower hazard score.
4. Conclusions
In this study, D. trunculus specimens collected along Tuscan coasts under official monitoring, conducted by the LHA of the Tuscany region, were analyzed with respect to the occurrence of MPs. A low MA of MPs was found when compared to the other studies available for the same species in other areas. This study also found that polymers identified in the analyzed specimens can be classified as moderate risks. Despite the limitations of the study in terms of chemically analyzed items, the outcomes from this study can implement the literature with new data, ensuring reliability guaranteed by the use of strict quality control measures. In particular, this study provides new data on a less-investigated species collected from an area never investigated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani14040618/s1, Table S1: Specimens collected within the five production areas with code, collection date, and collection site (VL: Viareggio Levante; VP: Viareggio Ponente; G-Pi: Gombo Pisa; G-SGT: Gombo San Giuliano Terme; CCT: Centro Coni and Tirrenia); Figure S1: Raman spectrum of a cellulose microfiber (red line) extracted from samples of Donax trunculus. At the time of Raman analysis, microfiber was placed onto scotch tape previously attached to a glass slide and covered with a glass coverslip. The black line represents the spectrum of the background collected before the microfiber analysis, and the reference of the glass can be observed at the same height; Figure S2: Raman spectra of a microfiber (a) and a microfragment (b) and the related reference library spectra, extracted from samples of Donax trunculus. Both microitems were identified as plastic copolymers; in detail, microfibers as Poly(styrene-ethylene-butylene) and microfragments as Poly[(2,3-epoxypropyl methacrylate):Styrene:Ethylene Dimethacrylate)]; Figure S3: Raman spectra of a microfiber (blue line) and the related reference library spectrum (red line) extracted from samples of Donax trunculus. The microfiber was identified as microcrystalline cellulose; Figure S4: Raman spectrum of scotch tape (black line) used to transport microitems extracted from samples of Donax trunculus. The spectrum matched with the Raman fingerprint of polypropylene (red line).
Author Contributions
For conceptualization, A.A. and F.S.; data curation C.M., L.N., M.G. and S.B.; formal analysis, C.M., L.N. and C.C.; funding acquisition, A.A. and F.S.; investigation, S.B., M.G., A.F. and P.G.G.; methodology, A.A., C.M., L.N. and F.G.; project administration, A.A.; supervision, A.A., F.S. and F.G.; writing—original draft, C.M. and L.N.; writing—review and editing A.A., F.S. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed with funds granted partly by the Italian Ministry of Health (grant number IZS LT 12/19 RC), partly by the European Union (NextGeneration EU) through the SAMOTHRACE Project (grant number ECS00000022) of the Ministero dell’Università e della Ricerca (MUR)-Piano Nazionale di Ripresa e Resilienza (PNRR) and partly from the PRIN2022 PLASTACTS project (grant number J53D23007450006).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are included in the manuscript and available in Supplementary Materials attached to the text; further data are also available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment. GESAMP Reports and Studies, 90; UNEP: Paris, France, 2015. [Google Scholar]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2017, 17, 1513–1521. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Song, B.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of micro-and nanoplastic contamination in the food chain. Food Addit. Contam. Part A 2019, 36, 639–673. [Google Scholar] [CrossRef]
- Tlili, S.; Mouneyrac, C. The wedge clam donax trunculus as sentinel organism for Mediterranean coastal monitoring in a global change context. Reg. Environ. Chang. 2019, 19, 995–1007. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Sendra, M.; Sparaventi, E.; Novoa, B.; Figueras, A. An overview of the internalization and effects of microplastics and nanoplastics as pollutants of emerging concern in bivalves. Sci. Total Environ. 2021, 753, 142024. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). EFSA Panel on Contaminants in the Food Chain (CONTAM) (2016). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- GESAMP. Guidelines or the Monitoring and Assessment of Plastic Litter and Microplastics in the Ocean; GESAMP Reports and Studies, 90; UNEP: Nairobi, Kenya, 2019; p. 130. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. EST 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Colloquium 25—A coordinated approach to assess the human health risks of micro- and nanoplastics in food. EFSA J. 2021, 18, EN-6815. [Google Scholar] [CrossRef]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: A scoping review. Sci. Total Env. 2021, 757, 143872. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.S.L.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of microplastics and nanoplastics on human health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Wijsman, J.W.M.; Troost, K.; Fang, J.; Roncarati, A. Global production of marine bivalves. Trends and challenges. In Goods and Services of Marine Bivalves; Springer: Cham, Switzerland, 2019; pp. 7–26. [Google Scholar]
- Suffredini, E.; Lanni, L.; Arcangeli, G.; Pepe, T.; Mazzette, R.; Ciccaglioni, G.; Croci, L. Qualitative and quantitative assessment of viral contamination in bivalve molluscs harvested in Italy. Int. J. Food Microbiol. 2014, 184, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Cunha, A.; Castilho, F.; Romalde, J.L.; Pereira, M.J. Microbial contamination and purification of bivalve shellfish: Crucial aspects in monitoring and future perspectives–A mini-review. Food Control 2011, 22, 805–816. [Google Scholar] [CrossRef]
- Vitali, C.; Peters, R.J.; Janssen, H.G.; Nielen, M.W. Microplastics and nanoplastics in food, water, and beverages; part I. Occurrence. TrAC 2023, 159, 116670. [Google Scholar] [CrossRef]
- Ward, J.E.; Rosa, M.; Shumway, S.E. Capture, ingestion, and egestion of microplastics by suspension-feeding bivalves: A 40-year history. Anthr. Coasts 2019, 2, 39–49. [Google Scholar] [CrossRef]
- Venugopal, V.; Gopakumar, K. Shellfish: Nutritive value, health benefits, and consumer safety. CRFSFS 2017, 16, 1219–1242. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; Li, J.; Shi, H.; Xu, X.; Ju, P.; Jiang, F.; Li, F. Microplastics in global bivalve mollusks: A call for protocol standardization. J. Hazard. Mater. 2022, 438, 129490. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; McLachlan, A. The Ecology of Sandy Shores; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Fernández-Pérez, J.; Nantón, A.; Méndez, J. An alternative method for rapid and specific authentication of four European Donax species, including D. trunculus a commercially-important bivalve. Eur. Food Res. Technol. 2018, 244, 1815–1820. [Google Scholar] [CrossRef]
- Salas-Casanova, C. Ecología de los Donacidae (Mollusca Bivalvia) de la bahia de Malaga (SE de España). Investig. Pesq. 1987, 51, 67–77. [Google Scholar]
- Manca-Zeichen, M.; Agnesi, S.; Mariani, A.; Maccaroni, A.; Ardizzone, G.D. Biology and population dynamics of Donax trunculus L. (Bivalvia: Donacidae) in the South Adriatic Coast (Italy). Estuar. Coast. Shelf Sci. 2002, 54, 971–982. [Google Scholar] [CrossRef]
- La Valle, P.; Nicoletti, L.; Finoia, M.G.; Ardizzone, G.D. Donax trunculus (Bivalvia: Donacidae) as a potential biological indicator of grain-size variations in beach sediment. Ecol. Indic. 2011, 11, 1426–1436. [Google Scholar] [CrossRef]
- Ribeiro, F.; O’Brien, J.W.; Galloway, T.; Thomas, K.V. Accumulation and fate of nano- and micro-plastics and associated contaminants in organisms. TrAC 2019, 111, 139–147. [Google Scholar] [CrossRef]
- Ben-Haddad, M.; Abelouah, M.R.; Hajji, S.; De-la-Torre, G.E.; Abou Oualid, H.; Rangel-Buitrago, N.; Alla, A.A. The wedge clam Donax trunculus L., 1758 as a bioindicator of microplastic pollution. Mar. Pollut. Bull. 2022, 178, 113607. [Google Scholar] [CrossRef]
- Olivieri, Z.; Cesarini, G.; Orsini, M.; De Santis, S.; Scalici, M. Uptake of Microplastics in the Wedge Clam Donax trunculus: First Evidence from the Mediterranean Sea. Water 2022, 14, 4095. [Google Scholar] [CrossRef]
- Şentürk, Y.; Esensoy, F.B.; Öztekin, A.; Aytan, Ü. Microplastics in bivalves in the southern Black Sea. In Marine Litter in the Black Sea; Turkish Marine Research Foundation (TUDAV) Publication: Istanbul, Turkey, 2020; pp. 303–313. [Google Scholar]
- Alexandrova, A.V.; Ignatova-Ivanova, T.V.; Bachvarova, D.G.; Toschkova, S.G.; Doichinov, A.H.; Ibryamova, S.F.; Chipev, N.H. Pilot Screening and Assessment of Microplastic Bioaccumulation in Wedge Clams Donax trunculus Linnaeus, 1758 (Bivalvia) from the Bulgarian Black Sea Coast. Acta Zool 2022, 74, 568–578. [Google Scholar]
- Expósito, N.; Rovira, J.; Sierra, J.; Gimenez, G.; Domingo, J.L.; Schuhmacher, M. Levels of microplastics and their characteristics in molluscs from North-West Mediterranean Sea: Human intake. Mar. Pollut. Bull. 2022, 181, 113843. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Maximenko, N.; Thiel, M.; Cummins, A.; Lattin, G.; Wilson, S.; Hafner, J.; Zellers, A.; Rifman, S. Plastic pollution in the South Pacific subtropical gyre. Mar. Pollut. Bull. 2013, 68, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Llorca, M.; Álvarez-Muñoz, D.; Ábalos, M.; Rodríguez-Mozaz, S.; Santos, L.H.; León, V.M.; Campillo, J.A.; Martínez-Gómez, C.; Abad, E.; Farré, M. Microplastics in Mediterranean coastal area: Toxicity and impact for the environment and human health. Trends Environ. Anal. Chem. 2020, 27, e00090. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation) Text with EEA relevance. Off. J. Eur. Union 2017, L95, 1–142. [Google Scholar]
- European Union. Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as regards official controls (Text with EEA relevance.) C/2019/13 OJ L 131, 17.5. 2019, p. 51–100. Off. J. Eur. Union 2019, L131, 51–100. [Google Scholar]
- European Union. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union 2004, L139, 55–205. [Google Scholar]
- Community Guide to the Principles of Good Practice for the Microbiological Classification and Monitoring of Bivalve Mollusc Production and Relaying Areas with regard to Implementing Regulation 2019/627. Available online: https://www.aesan.gob.es/en/CRLMB/web/public_documents/seccion/EURLMB_Guides.htm (accessed on 15 December 2023).
- European Union. Regional Government resolution no. 1401 of 11 12 2017 Guidelines for the classification of marine waters lagoons or estuaries, for the production, harvesting and relaying of live bivalve molluscs live echinoderms and live tunicates for human consumption. Revocation of DGRT n. 899/2012. Off. J. Eur. Union L273, 1–75.
- FAO. Ministerial Decree (DM) of 22 December 2000. Amendments to the Ministerial Decree of 21 July 1998 concerning the regulation of bivalve mollusc fishing; Mipaf; OJ General Series no. 102 of 04-05-2001; FAO: Rome, Italy.
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Nalbone, L.; Cincotta, F.; Giarratana, F.; Ziino, G.; Panebianco, A. Microplastics in fresh and processed mussels sampled from fish shops and large retail chains in Italy. Food Control 2021, 125, 108003. [Google Scholar] [CrossRef]
- Hermsen, E.; Mintenig, S.M.; Besseling, E.; Koelmans, A.A. Quality criteria for the analysis of microplastic in biota samples: A critical review. Environ. Sci. Technol. 2018, 52, 10230–10240. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Eo, S.; Han, G.M.; Shim, W.J. Rapid production of micro- and nanoplastics by fragmentation of expanded polystyrene exposed to sunlight. Environ. Sci. Technol. 2020, 54, 11191–11200. [Google Scholar] [CrossRef]
- Ziino, G.; Nalbone, L.; Giarratana, F.; Romano, B.; Cincotta, F.; Panebianco, A. Microplastics in vacuum packages of frozen and glazed icefish (Neosalanx spp.): A freshwater fish intended for human consumption. IJFS 2021, 10, 9974. [Google Scholar] [CrossRef]
- Prata, J.C.; Reis, V.; da Costa, J.P.; Mouneyrac, C.; Duarte, A.C.; Rocha-Santos, T. Contamination issues as a challenge in quality control and quality assurance in microplastics analytics. J. Hazard. Mater. 2021, 403, 123660. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, E.; Jenner, L.C.; Twiddy, M.; Rotchell, J.M. Microplastic contamination of seafood intended for human consumption: A systematic review and meta-analysis. EHP 2020, 128, 126002. [Google Scholar] [CrossRef] [PubMed]
- EUMOFA. The EU Fish Market. 2022. Available online: https://www.eumofa.eu/the-eu-fish-market-2022-edition-is-now-online (accessed on 15 December 2023).
- World Trade Organization. Microplastics in Drinking-Water. 2019. Available online: https://www.who.int/news/item/20-08-2019-microplastics-in-drinking-water (accessed on 15 December 2023).
- Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Union 2008, L164, 19–40.
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. ESPR 2017, 24, 21530–21547. [Google Scholar] [CrossRef] [PubMed]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E. Evaluation of existing methods to extract microplastics from bivalve tissue: Adapted KOH digestion protocol improves filtration at single-digit pore size. Mar. Pollut. Bull. 2019, 142, 384–393. [Google Scholar] [CrossRef]
- Miller, M.E.; Kroon, F.J.; Motti, C.A. Recovering microplastics from marine samples: A review of current practices. Mar. Pollut. Bull. 2017, 123, 6–18. [Google Scholar] [CrossRef]
- Claessens, M.; De Meester, S.; Van Landuyt, L.; De Clerck, K.; Janssen, C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast, 2011. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Bour, A.; Avio, C.G.; Gorbi, S.; Regoli, F.; Hylland Ketil, H. Presence of microplastics in benthic and epibenthic organisms: Influence of habitat, feeding mode and trophic level. Environ. Pollut. 2018, 243, 1217–1225. [Google Scholar] [CrossRef]
- Frias, J.; Pagter, E.; Nash, R.; O’Connor, I.; Carretero, O.; Filgueiras, A.; Vinas, L.; Gago, J.; Antunes, J.; Bessa, F.; et al. Standardized Protocol for Monitoring Microplastics in Sediments; JPI-Oceans BASEMAN project: Nairobi, Kenya, 2018; 24p. [Google Scholar] [CrossRef]
- Jin-Feng, D.I.N.G.; Jing-Xi, L.I.; Cheng-Jun, S.U.N.; Chang-Fei, H.E.; Jiang, F.H.; Feng-Lei, G.A.O.; Zheng, L. Separation and identification of microplastics in digestive system of bivalves. Chin. J. Anal. Chem. 2018, 46, 690–697. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Hossain, M.S.; Li, Q.; Kolandhasamy, P.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Arneborg, L.; Broström, G.; Almroth, B.C.; Gipperth, L.; Hassellöv, M. The unaccountability case of plastic pellet pollution. Mar. Pollut. Bull. 2018, 129, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Sathish, M.N.; Jeyasanta, K.I.; Patterson, J. Monitoring of microplastics in the clam Donax cuneatus and its habitat in Tuticorin coast of Gulf of Mannar (GoM), India. Environ. Pollut. 2020, 266, 115219. [Google Scholar] [CrossRef]
- Xu, Q.; Deng, T.; LeBlanc, G.A.; An, L. An effective method for evaluation of microplastic contaminant in gastropod from Taihu Lake, China. ESPR 2020, 27, 22878–22887. [Google Scholar] [CrossRef]
- Ding, J.; Li, J.; Sun, C.; Jiang, F.; He, C.; Zhang, M.; Ju, P.; Ding, N.X. An examination of the occurrence and potential risks of microplastics across various shellfish. Sci. Total Environ. 2020, 739, 139887. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; He, C.; Li, J.; Ju, P.; Li, F. Microplastics in four bivalve species and basis for using bivalves as bioindicators of microplastic pollution. Sci. Total Environ. 2021, 782, 146830. [Google Scholar] [CrossRef]
- Yozukmaz, A. Investigation of microplastics in edible wild mussels from İzmir Bay (Aegean Sea, Western Turkey): A risk assessment for the consumers. Mar. Pollut. Bull. 2021, 171, 112733. [Google Scholar] [CrossRef]
- Ding, J.; Sun, Y.; He, C.; Li, J.; Li, F. Towards risk assessments of microplastics in bivalve mollusks globally. J. Mar. Sci. Eng. 2022, 10, 288. [Google Scholar] [CrossRef]
- Keisling, C.; Harris, R.D.; Blaze, J.; Coffin, J.; Byers, J.E. Low concentrations and low spatial variability of marine microplastics in oysters (Crassostrea virginica) in arural Georgia estuary. Mar. Pollut. Bull. 2020, 150, 110672. [Google Scholar] [CrossRef] [PubMed]
- Rist, S.; Steensgaard, I.M.; Guven, O.; Nielsen, T.G.; Jensen, L.H.; Møller, L.F.; Hartmann, N.B. The fate of microplastics during uptake and depuration phases in a blue mussel exposure system. Environ. Toxicol. Chem. 2019, 38, 99–105. [Google Scholar] [CrossRef]
- Garrido Gamarro, E.; Ryder, J.; Elvevoll, E.O.; Olsen, R.L. Microplastics in fish and shellfish—A threat to seafood safety? J. Aquat. Food Prod. Technol. 2020, 29, 417–425. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in mussels along the coastal waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef]
- Qu, X.; Su, L.; Li, H.; Liang, M.; Shi, H. Assessing the relationship between the abundance and properties of microplastics in water and in mussels. Sci. Total Environ. 2018, 621, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Cai, H.; Kolandhasamy, P.; Wu, C.; Rochman, C.M.; Shi, H. Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environ. Pollut. 2018, 234, 347–355. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Zhao, Q.; Qu, L.; Ma, D.; Wang, J. Spatio-temporal distribution of plastic and microplastic debris in the surface water of the Bohai Sea, China. Mar. Pollut. Bull. 2020, 158, 111343. [Google Scholar] [CrossRef]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef]
- Huntington, A.; Corcoran, P.L.; Jantunen, L.; Thaysen, C.; Rochman, C.M. A first assessment of microplastics and other anthropogenic particles in Hudson Bay and the surrounding eastern Canadian Arctic waters of Nunavut. Facets 2020, 5, 432–454. [Google Scholar] [CrossRef]
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Kaliszewicz, A.; Panteleeva, N.; Karaban, K.; Runka, T.; Winczek, M.; Beck, E.; Poniatowska, A.; Olejnicza, I.; Boniecki, P.; Golovanova, J.; et al. First Evidence of Microplastic Occurrence in the Marine and Freshwater Environments in a Remote Polar Region of the Kola Peninsula and a Correlation with Human Presence. Biology 2023, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.; Johnson, M.; Nathanail, P.; MacNaughtan, W.; Gomes, R.L. Freshwater and airborne textile fibre populations are dominated by ‘natural’, not microplastic, fibres. Sci. Total Environ. 2019, 666, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, M.; Ru, S.; Liu, X. High levels of microplastic pollution in the sediments and benthic organisms of the South Yellow Sea, China. Sci. Total Environ. 2019, 651, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, S.; Volgare, M.; Cocca, M.; Dorigato, G.; Giaccone, V.; Colavita, G. Impact of Fibrous Microplastic Pollution on Commercial Seafood and Consumer Health: A Review. Animals 2023, 13, 1736. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Nationwide monitoring of microplastics in bivalves from the coastal environment of Korea. Environ. Pollut. 2021, 270, 116175. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.; De Beni, E.; Martellini, T.; Sarti, C.; Randazzo, D.; Ciraolo, R.; Cincinelli, A. Occurrence of Natural and Synthetic Micro-Fibers in the Mediterranean Sea: A Review. Toxics 2022, 10, 391. [Google Scholar] [CrossRef]
- Abidli, S.; Lahbib, Y.; El Menif, N.T. Microplastics in commercial molluscs from the lagoon of Bizerte (Northern Tunisia). Mar. Pollut. Bull. 2019, 142, 243–252. [Google Scholar] [CrossRef]
- Renzi, M.; Guerranti, C.; Blašković, A. Microplastic contents from maricultured and natural mussels. Mar. Pollut. Bull. 2019, 131, 248–251. [Google Scholar] [CrossRef]
- Jang, M.; Shim, W.J.; Cho, Y.; Han, G.M.; Song, Y.K.; Hong, S.H. A close relationship between microplastic contamination and coastal area use pattern. Water Res. 2020, 171, 115400. [Google Scholar] [CrossRef]
- Thushari, G.G.N.; Senevirathna, J.D.M.; Yakupitiyage, A.; Chavanich, S. Effects of microplastics on sessile invertebrates in the eastern coast of Thailand: An approach to coastal zone conservation. Mar. Pollut. Bull. 2017, 124, 349–355. [Google Scholar] [CrossRef]
- Sparks, C. Microplastics in mussels along the coast of Cape Town, South Africa. Bull. Environ. Contam. Toxicol. 2020, 104, 423–431. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Paul-Pont, I.; Cassone, A.L.; Himber, C.; Receveur, J.; Jezequel, R.; El Rakwe, M.; Rinnert, E.; Rivière, G.; Lambert, C.; et al. Microplastic contamination and pollutant levels in mussels and cockles collected along the channel coasts. Environ. Pollut. 2019, 250, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Ríos, M.F.; Hernández-Moresino, R.D.; Galván, D.E. Assessing urban microplastic pollution in a benthic habitat of Patagonia Argentina. Mar. Pollut. Bull. 2020, 159, 111491. [Google Scholar] [CrossRef] [PubMed]
- Tirkey, A.; Upadhyay, L.S.B. Microplastics: An overview on separation, identification and characterization of microplastics. Mar. Pollut. Bull. 2021, 170, 112604. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical analysis of microplastics and nanoplastics: Challenges, advanced methods, and perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Castro, J.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.; Cerqueira, M. The importance of contamination control in airborne fibers and microplastic sampling: Experiences from indoor and outdoor air sampling in Aveiro, Portugal. Mar. Pollut. Bull. 2021, 159, 111522. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Cavalca Bom, F.C.; Sá, F. Concentration of microplastics in bivalves of the environment: A systematic review. Environ. Monit. Assess. 2021, 193, 846. [Google Scholar] [CrossRef]
- Laskar, N.; Kumar, U. Plastics and microplastics: A threat to environment. Environ. Technol. Innov. 2019, 14, 100352. [Google Scholar] [CrossRef]
- Dalla Fontana, G.; Mossotti, R.; Montarsolo, A. Assessment of microplastics release from polyester fabrics: The impact of different washing conditions. Environ. Pollut. 2020, 264, 113960. [Google Scholar] [CrossRef] [PubMed]
- Suaria, G.; Achtypi, A.; Perold, V.; Lee, J.R.; Pierucci, A.; Bornman, T.G.; Aliani, S.; Ryan, P.G. Microfibers in oceanic surface waters: A global characterization. Sci. Adv. 2020, 6, eaay8493. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics–the Facts 2022. An analysis of European plastics production, demand, conversion and end-of-life management. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 15 December 2023).
- Gomiero, A.; Strafella, P.; Fabi, G. From macroplastic to microplastic litter: Occurrence, composition, source identification and interaction with aquatic organisms. Experiences from the Adriatic Sea. In Plastics in the Environment; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Birnstiel, S.; Soares-Gomes, A.; da Gama, B.A.P. Depuration reduces microplastic content in wild and farmed mussels. Mar. Pollut. Bull. 2019, 140, 241–247. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Xu, P.; Peng, G.; Su, L.; Gao, Y.; Gao, L.; Li, D. Microplastic risk assessment in surface waters: A case study in the Changjiang Estuary, China. Mar. Pollut. Bull. 2018, 133, 647–654. [Google Scholar] [CrossRef]
- Fang, C.; Zheng, R.; Chen, H.; Hong, F.; Lin, L.; Lin, H.; Guo, H.; Bailey, H.; Mu, J.; Bo, J. Comparison of microplastic contamination in fish and bivalves from two major cities in Fujian province, China and the implications for human health. Aquaculture 2019, 512, 734322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).