Mechanisms of Gills Response to Cadmium Exposure in Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis): Oxidative Stress, Immune Response, and Energy Metabolism
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Cultivation of Filefish
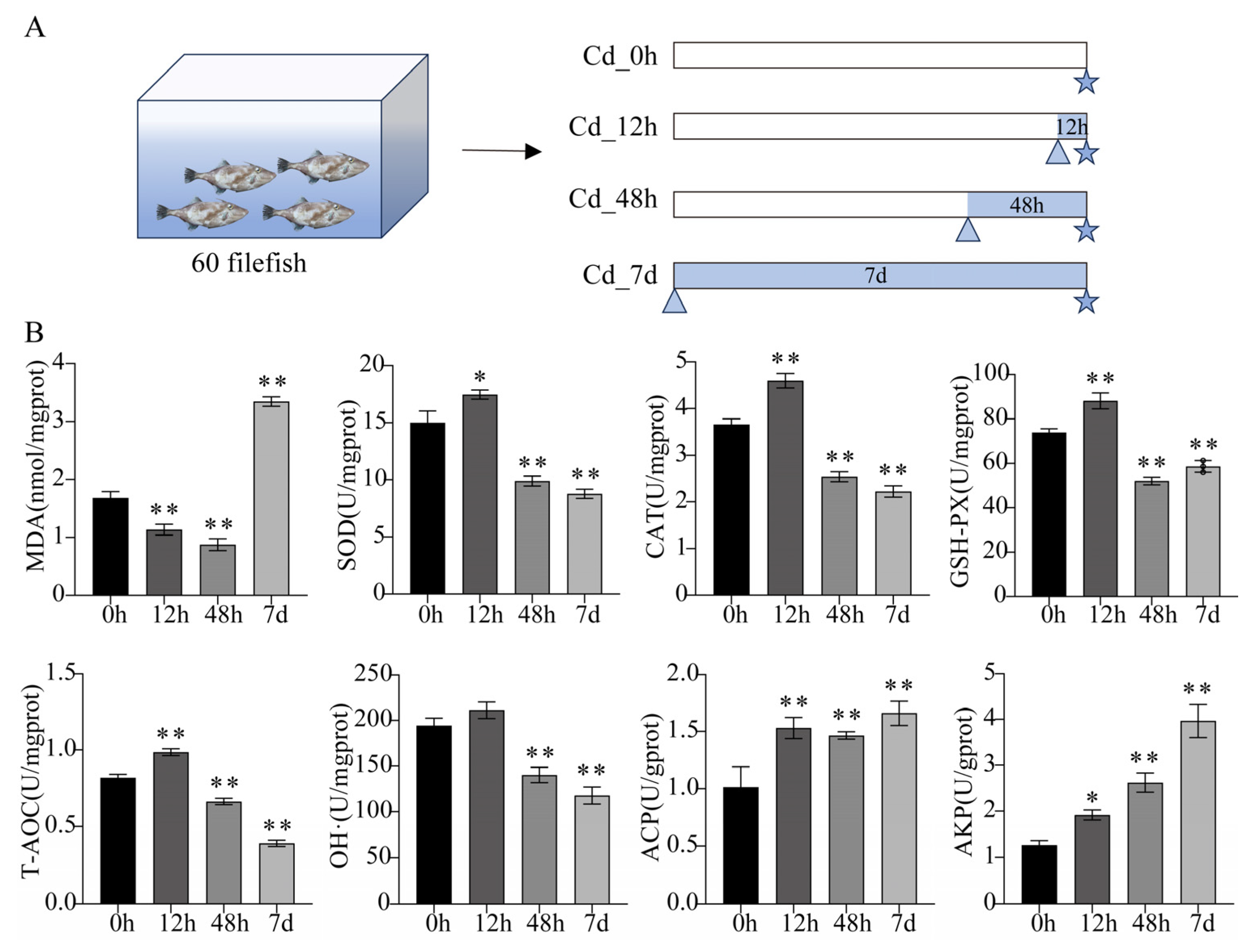
2.2. Adult Filefish Exposure Experiment
2.3. Detection of Biochemical Parameters
2.4. RNA Extraction and Transcriptome Analysis
2.5. Metabolome Analysis
2.6. Quantitative Real-Time RT-PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Biochemical Parameters Analysis of Filefish Gill Tissues after Cd Exposure
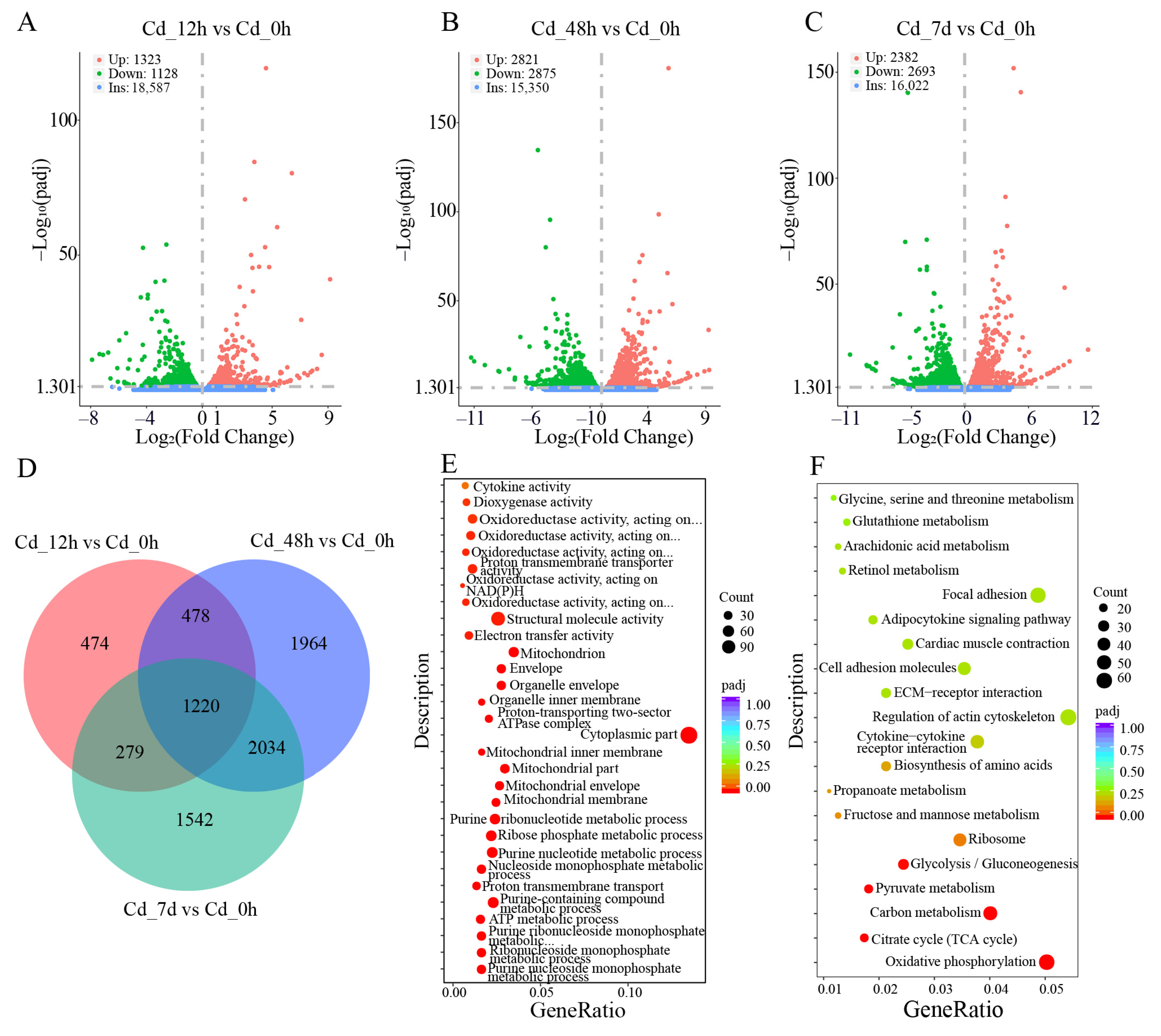
3.2. Transcriptome Analysis of Filefish Gill Tissues after Cd Exposure
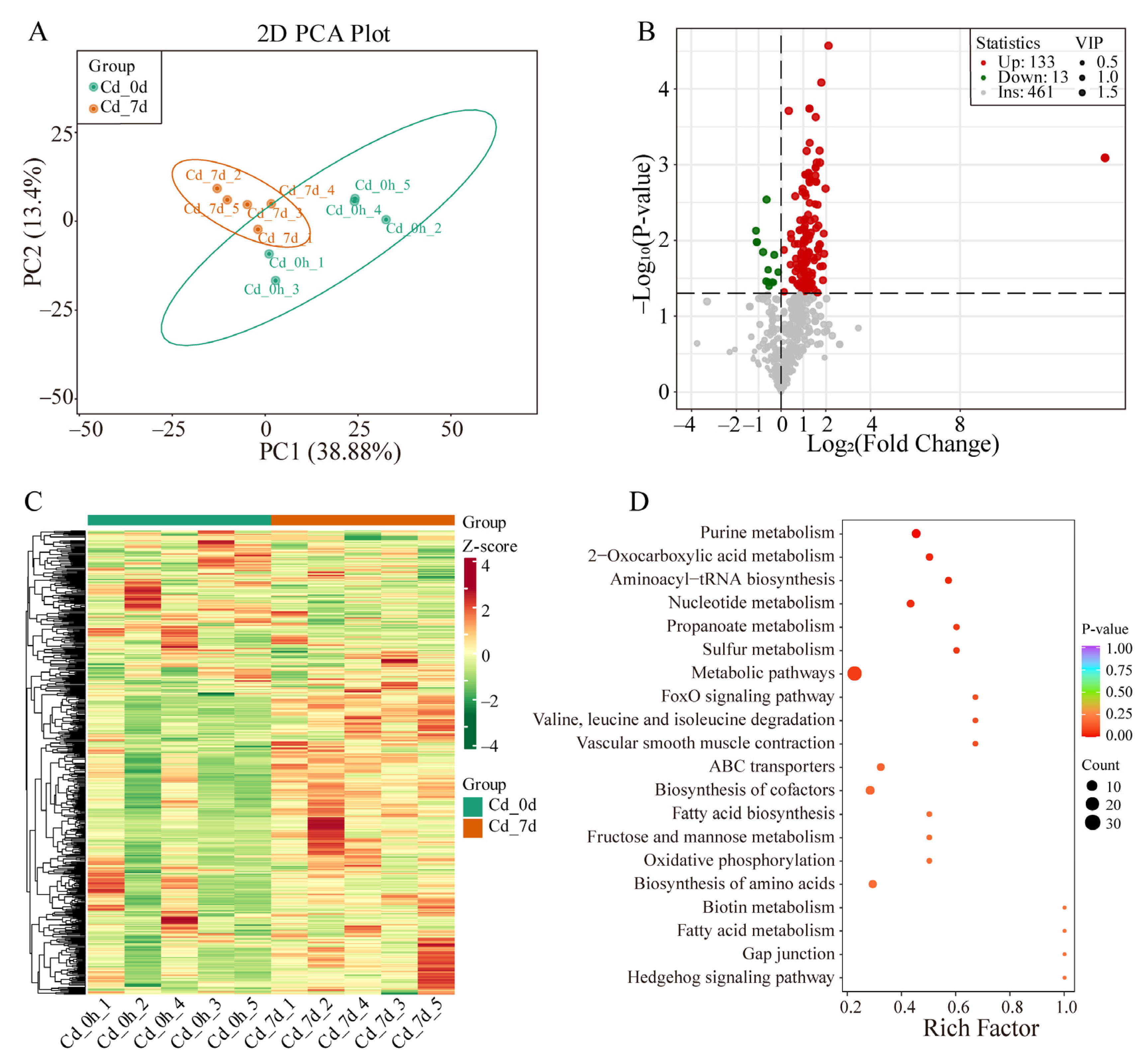
3.3. Metabolomics Analysis of Filefish Gill Tissues after Cd Exposure
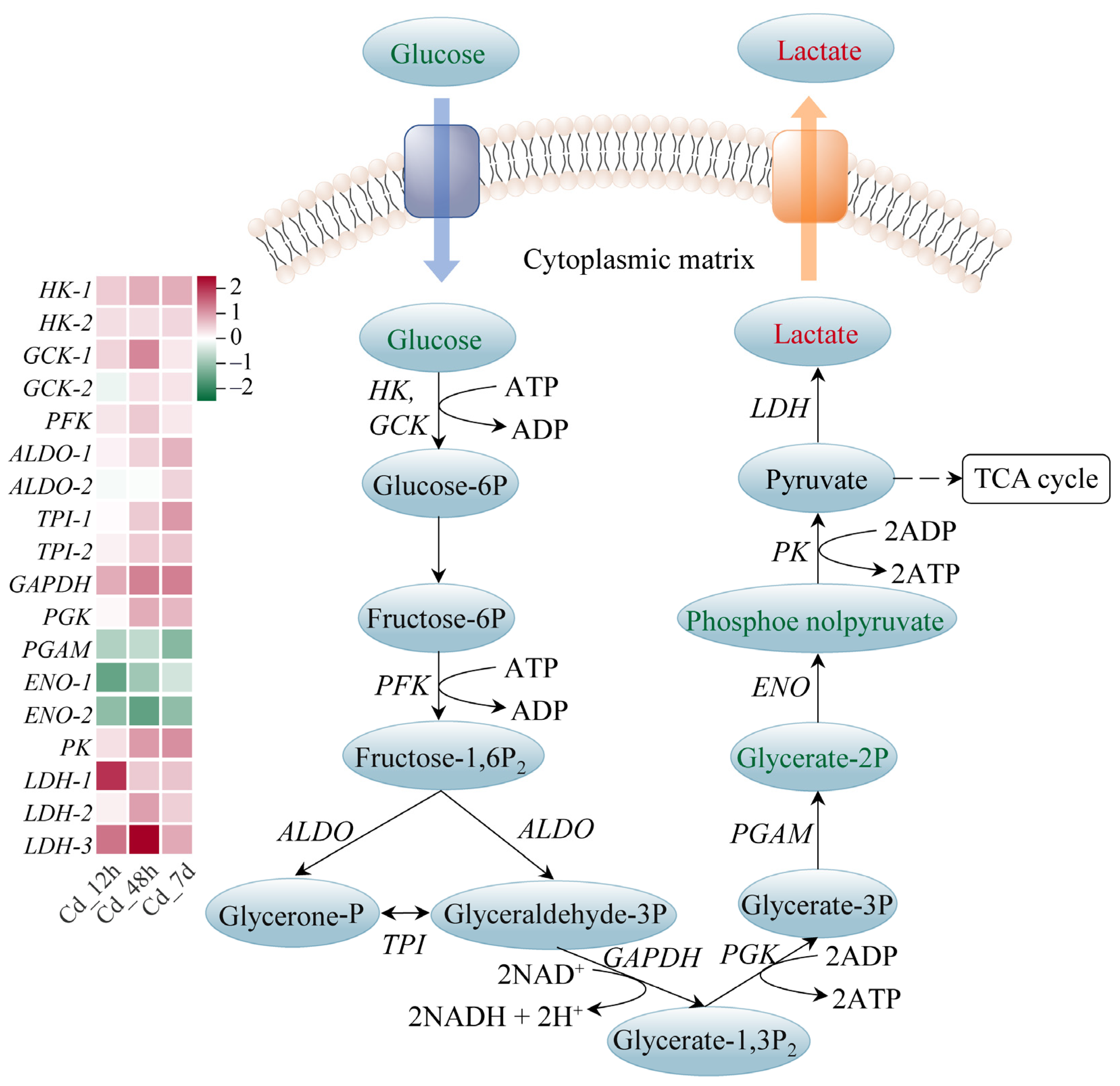
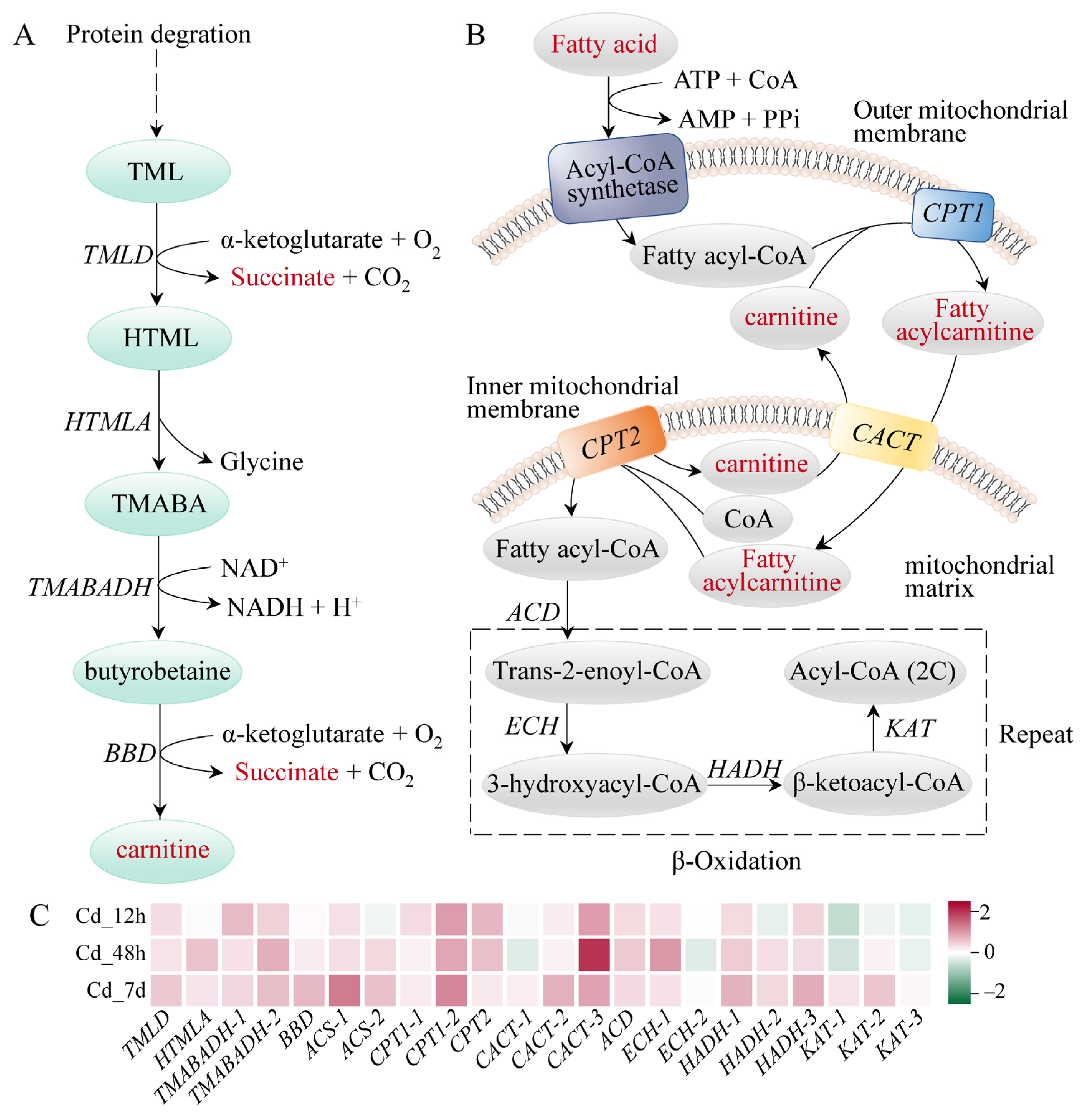
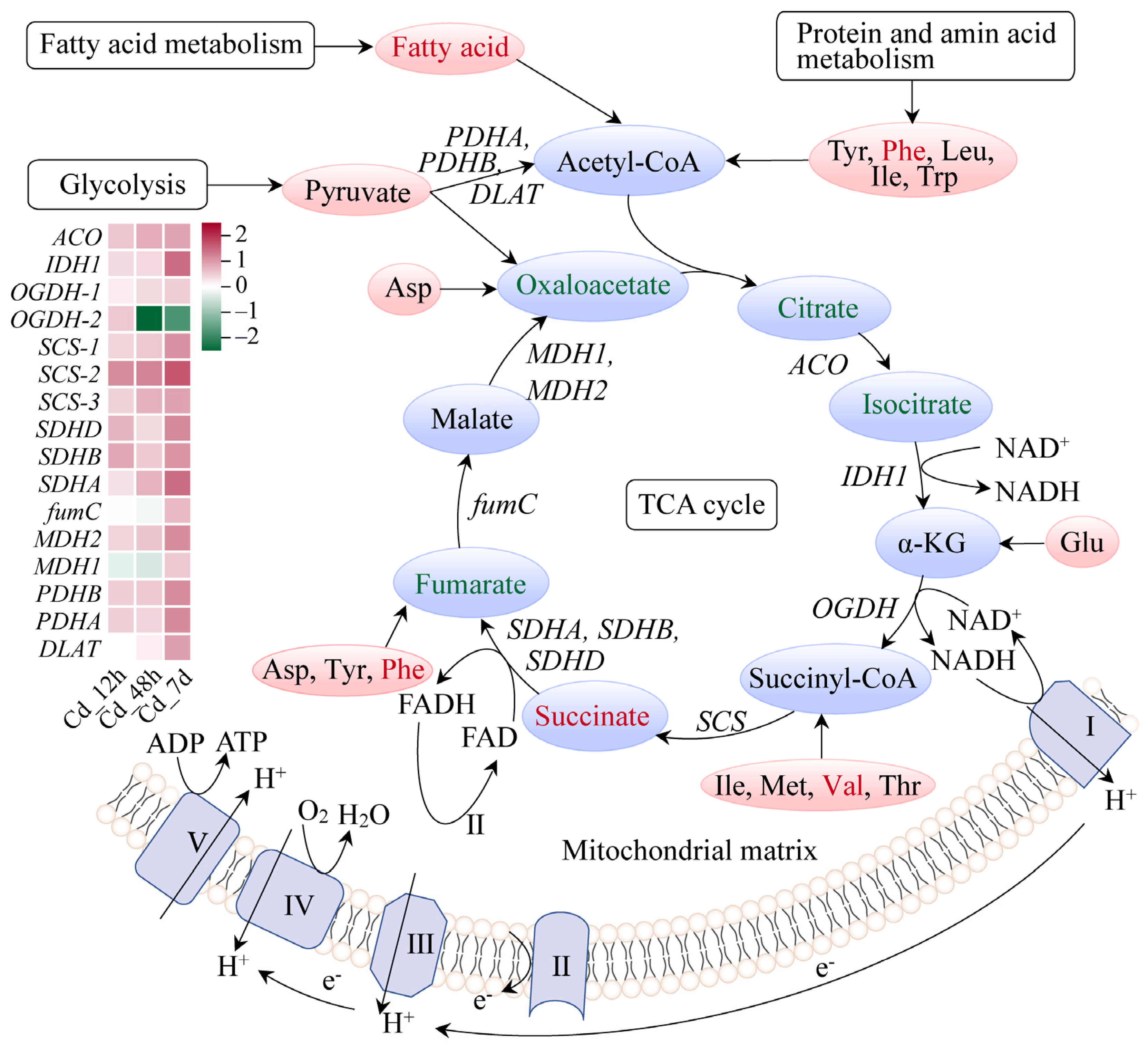
3.4. Response Mechanism Analysis of Filefish Gill Tissues after Cd Exposure
4. Discussion
4.1. Cd Exposure Induced Oxidative Stress and Apoptosis in Gills
4.2. Cd Exposure Caused Energy Metabolism Disorder in Gills
4.3. Cd Exposure Triggered Cellular Inflammation and Activated Immune Response in Gills
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, W.; Yang, Q.; Xiang, D.; Chen, X.; Wen, Z.; Wang, X.; Xu, X.; Peng, C.; Yang, L.; Luo, M.; et al. Combined impacts of microplastics and cadmium on the liver function, immune response, and intestinal microbiota of crucian carp (Carassius carassius). Ecotoxicol. Environ. Saf. 2023, 261, 115104. [Google Scholar] [CrossRef]
- Wei, Y.; Ding, D.; Qu, K.; Sun, J.; Cui, Z. Ecological risk assessment of heavy metal pollutants and total petroleum hydrocarbons in sediments of the Bohai Sea, China. Mar. Pollut. Bull. 2022, 184, 114218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, M.; Zhong, Z.; Chen, H.; Wang, M.; Lian, C.; Wang, H.; Zhang, H.; Cao, L.; Li, C. Toxicological effects of cadmium on deep-sea mussel Gigantidas platifrons revealed by a combined proteomic and metabolomic approach. Front. Mar. Sci. 2023, 10, 1087411. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, H.; Yan, Y.; Xie, X. Acute Cd toxicity, metal accumulation, and ion loss in southern catfish (Silurus meridionalis Chen). Toxics 2021, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Ma, H.L.; Liu, G.X.; Fan, S.G.; Deng, Y.Q.; Jiang, J.J.; Feng, J.; Guo, Z.X. Toxic effects of cadmium exposure on intestinal histology, oxidative stress, microbial community, and transcriptome change in the mud crab (Scylla paramamosain). Chemosphere 2023, 326, 138464. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhu, Q.L.; Zheng, J.L.; Wen, Z.Y. Cadmium induced oxidative stress, endoplasmic reticulum (ER) stress and apoptosis with compensative responses towards the up-regulation of ribosome, protein processing in the ER, and protein export pathways in the liver of zebrafish. Aquat. Toxicol. 2022, 242, 106023. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, Y.; Han, Q.; Yao, Y.; Xing, H.; Teng, X. Immunosuppression, oxidative stress, and glycometabolism disorder caused by cadmium in common carp (Cyprinus carpio L.): Application of transcriptome analysis in risk assessment of environmental contaminant cadmium. J. Hazard. Mater. 2019, 366, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, N.; Konig, I.; English, C.; Ivantsova, E.; Souders, C.L.; Hashmi, I.; Martyniuk, C.J. Sulfamethoxazole (SMX) alters immune and apoptotic endpoints in developing zebrafish (Danio rerio). Toxics 2023, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, K.; Wang, P.; Chang, Q.; Chen, S.; Bian, L.; Liu, C.; Ge, J. Analysis of nutritional composition in the muscle of Thamnaconus septentrionalis. Mar. Sci. 2018, 42, 122–129. [Google Scholar]
- Chirinos-Peinado, D.; Castro-Bedriñana, J.; Ríos-Ríos, E.; Mamani-Gamarra, G.; Quijada-Caro, E.; Huacho-Jurado, A.; Nuñez-Rojas, W. Lead and cadmium bioaccumulation in fresh cow’s milk in an intermediate area of the Central Andes of Peru and risk to human health. Toxics 2022, 10, 317. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.; Wang, A.; Miao, Y. Next-generation sequencing of the mitochondrial genome of Thamnaconus septentrionalis Gunther, 1877 (Aluteridae: Thamnaconus) specimen collected in China. Mitochondrial DNA Part B 2021, 6, 2198–2199. [Google Scholar] [CrossRef]
- Wang, X.; Bian, L.; Hu, Q.; Qin, B.; Chang, Q.; Ying, N.; Wu, Y.; Yang, L.; Chen, S. Acute effects of cadmium on the antioxidant enzyme activities and histological structure of the gills and liver of juvenile Thamnaconus septentrionalis. Prog. Fish. Sci. 2023, 44, 74–84. [Google Scholar]
- Figueroa, J.A.; Stiner, C.A.; Radzyukevich, T.L.; Heiny, J.A. Metal ion transport quantified by ICP-MS in intact cells. Sci. Rep. 2016, 6, 20551. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, C.; Li, L.; Mohsen, M.; Wang, T.; Wang, X.; Zhang, L.; Huang, W. Single and combined effects of microplastics and cadmium on the sea cucumber Apostichopus japonicus. Mar. Environ. Res. 2023, 186, 105927. [Google Scholar] [CrossRef] [PubMed]
- Nigro, M.; Bernardeschi, M.; Costagliola, D.; Della Torre, C.; Frenzilli, G.; Guidi, P.; Lucchesi, P.; Mottola, F.; Santonastaso, M.; Scarcelli, V.; et al. n-TiO2 and CdCl2 co-exposure to titanium dioxide nanoparticles and cadmium: Genomic, DNA and chromosomal damage evaluation in the marine fish European sea bass (Dicentrarchus labrax). Aquat. Toxicol. 2015, 168, 72–77. [Google Scholar] [CrossRef]
- McRae, N.K.; Gaw, S.; Glover, C.N. Effects of waterborne cadmium on metabolic rate, oxidative stress, and ion regulation in the freshwater fish, inanga (Galaxias maculatus). Aquat. Toxicol. 2018, 194, 1–9. [Google Scholar] [CrossRef]
- Liu, S.; Li, T.; Fang, S.; Zhang, P.; Yi, D.; Cong, B.; Zhang, Z.; Zhao, L. Metabolic profiling and gene expression analyses provide insights into cold adaptation of an Antarctic moss Pohlia nutans. Front. Plant Sci. 2022, 13, 1006991. [Google Scholar] [CrossRef]
- Bian, L.; Li, F.; Ge, J.; Wang, P.; Chang, Q.; Zhang, S.; Li, J.; Liu, C.; Liu, K.; Liu, X.; et al. Chromosome-level genome assembly of the greenfin horse-faced filefish (Thamnaconus septentrionalis) using Oxford Nanopore PromethION sequencing and Hi-C technology. Mol. Ecol. Resour. 2020, 20, 1069–1079. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Jiang, W.; Fang, J.; Du, M.; Gao, Y.; Fang, J.; Jiang, Z. Integrated transcriptomics and metabolomics analyses reveal benzo[a]pyrene enhances the toxicity of mercury to the Manila clam, Ruditapes philippinarum. Ecotoxicol. Environ. Saf. 2021, 213, 112038. [Google Scholar] [CrossRef]
- Xiang, J.; Wu, H.; Gao, J.; Jiang, W.; Tian, X.; Xie, Z.; Zhang, T.; Feng, J.; Song, R. Niclosamide exposure disrupts antioxidant defense, histology, and the liver and gut transcriptome of Chinese soft-shelled turtle (Pelodiscus sinensis). Ecotoxicol. Environ. Saf. 2023, 260, 115081. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, D.H.; Lee, J.S.; Seo, M.-S.; Kim, M.E.; Lee, J.S. Sargassum horneri (Turner) C. Agardh extract regulates neuroinflammation in vitro and in vivo. Curr. Issues Mol. Biol. 2022, 44, 5416–5426. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Luo, Y.; Huang, S. MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. J. Neurochem. 2007, 105, 251–261. [Google Scholar] [CrossRef]
- Ryabinina, O.P.; Subbian, E.; Iordanov, M.S. D-MEKK1, the Drosophila orthologue of mammalian MEKK4/MTK1, and Hemipterous/D-MKK7 mediate the activation of D-JNK by cadmium and arsenite in Schneider cells. BMC Cell Biol. 2006, 7, 7. [Google Scholar] [CrossRef]
- Strader, M.E.; Wong, J.M.; Hofmann, G.E. Ocean acidification promotes broad transcriptomic responses in marine metazoans: A literature survey. Front. Zool. 2020, 17, 7. [Google Scholar] [CrossRef]
- Shen, H.-M.; Liu, Z.-g. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2006, 40, 928–939. [Google Scholar] [CrossRef]
- Dong, X.; Tang, Y. Ntrk1 promotes mesangial cell proliferation and inflammation in rat glomerulonephritis model by activating the STAT3 and p38/ERK MAPK signaling pathways. BMC Nephrol. 2022, 23, 413. [Google Scholar] [CrossRef]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 2011, 711, 193–201. [Google Scholar] [CrossRef]
- Cheng, C.; Ma, H.; Liu, G.; Fan, S.; Guo, Z. Mechanism of cadmium exposure induced hepatotoxicity in the mud crab (Scylla paramamosain): Activation of oxidative stress and nrf2 signaling pathway. Antioxidants 2022, 11, 978. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Zheng, J.; Zhang, X.; Shao, J.; Zhao, L.; Hao, J. Anti-inflammatory and antioxidant effects of acetyl-l-carnitine on Atherosclerotic Rats. Med. Sci. Monit. 2020, 26, e920250. [Google Scholar] [CrossRef]
- Wei, X.; Qi, Y.; Zhang, X.; Gu, X.; Cai, H.; Yang, J.; Zhang, Y. ROS act as an upstream signal to mediate cadmium-induced mitophagy in mouse brain. NeuroToxicology 2015, 46, 19–24. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Nazhand, A.; Souto, S.B.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A. The nutraceutical value of carnitine and its use in dietary supplements. Molecules 2020, 25, 2127. [Google Scholar] [CrossRef]
- Nishio, T.; Kishi, R.; Sato, K.; Sato, K. Blue light exposure enhances oxidative stress, causes DNA damage, and induces apoptosis signaling in B16F1 melanoma cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2022, 883–884, 503562. [Google Scholar] [CrossRef]
- Pirinccioglu, A.G.; Gökalp, D.; Pirinccioglu, M.; Kizil, G.; Kizil, M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin. Biochem. 2010, 43, 1220–1224. [Google Scholar] [CrossRef]
- Yim, W.W.; Mizushima, N. Lysosome biology in autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef]
- Zhou, C.; Song, H.; Feng, J.; Hu, Z.; Yang, M.-J.; Shi, P.; Li, Y.-R.; Guo, Y.-J.; Li, H.-Z.; Zhang, T. Metabolomics and biochemical assays reveal the metabolic responses to hypo-salinity stress and osmoregulatory role of cAMP-PKA pathway in Mercenaria mercenaria. Comput. Struct. Biotechnol. J. 2022, 20, 4110–4121. [Google Scholar] [CrossRef]
- Zhang, A.; Matsushita, M.; Zhang, L.; Wang, H.; Shi, X.; Gu, H.; Xia, Z.; Cui, J.Y. Cadmium exposure modulates the gut-liver axis in an Alzheimer’s disease mouse model. Commun. Biol. 2021, 4, 1398. [Google Scholar] [CrossRef]
- Pi, J.; Li, X.; Zhang, T.; Li, D. Effects of acute exposure to sublethal waterborne cadmium on energy homeostasis in silver carp (Hypophthalmichthys molitrix). Bull. Environ. Contam. Toxicol. 2016, 97, 497–503. [Google Scholar] [CrossRef]
- Dando, P.R. Lactate Metabolism in Fish. J. Mar. Biolog. Assoc. UK 1969, 49, 209–223. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Scaglia, F. Disorders of carnitine biosynthesis and transport. Mol. Genet. Metab. 2015, 116, 107–112. [Google Scholar] [CrossRef]
- Richard, A.J.; Stephens, J.M. Emerging roles of JAK-STAT signaling pathways in adipocytes. Trends Endocrinol. Metab. 2011, 22, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Lin, X.; Xu, Y.; Tong, T.; Zhang, J.; He, H.; Yang, L.; Lu, Y.; Zhou, Z. Cadmium induces ferroptosis mediated inflammation by activating Gpx4/Ager/p65 axis in pancreatic β-cells. Sci. Total Environ. 2022, 849, 157819. [Google Scholar] [CrossRef] [PubMed]
- Selak, M.A.; Armour, S.M.; MacKenzie, E.D.; Boulahbel, H.; Watson, D.G.; Mansfield, K.D.; Pan, Y.; Simon, M.C.; Thompson, C.B.; Gottlieb, E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 2005, 7, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xiao, Z.; Wu, S.; Song, J.; Peng, X. Proteomic and metabolomic analysis on cadmium-induced mitochondrial toxicity in liver tissues of juvenile olive flounder Paralichthys olivaceus. Front. Mar. Sci. 2022, 9, 1041705. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Eilers, S.; Kurochkin, I.O.; Chung, J.S.; Techa, S.; Piontkivska, H.; Sokolov, E.P.; Sokolova, I.M. Effects of cadmium exposure and intermittent anoxia on nitric oxide metabolism in eastern oysters, Crassostrea virginica. J. Exp. Biol. 2010, 213, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, L.; Zhang, J.; Wang, X.; Yang, X.; Xin, Y.; Chen, L.; Li, J.; Niu, P. Multi-omics analysis reveals Mn exposure affects ferroptosis pathway in zebrafish brain. Ecotoxicol. Environ. Saf. 2023, 253, 114616. [Google Scholar] [CrossRef] [PubMed]
- Krzak, G.; Willis, C.M.; Smith, J.A.; Pluchino, S.; Peruzzotti-Jametti, L. Succinate receptor 1: An emerging regulator of myeloid cell function in inflammation. Trends Immunol. 2021, 42, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Wootton, E.C.; Dyrynda, E.A.; Pipe, R.K.; Ratcliffe, N.A. Comparisons of PAH-induced immunomodulation in three bivalve molluscs. Aquat. Toxicol. 2003, 65, 13–25. [Google Scholar] [CrossRef]
- Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Méndez, A.A.E.; Gallego, S.M. Oxidative post translational modifications of proteins related to cell cycle are involved in cadmium toxicity in wheat seedlings. Plant Sci. 2012, 196, 1–7. [Google Scholar] [CrossRef]
- Zhou, Y.; Dahms, H.-U.; Dong, F.; Jing, W.; Wang, L. Immune-associated parameters and antioxidative responses to cadmium in the freshwater crab Sinopotamon henanense. Ecotoxicol. Environ. Saf. 2016, 129, 235–241. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, W.; Zhao, L.; Zheng, L.; Wang, B.; Song, C.; Liu, S. Mechanisms of Gills Response to Cadmium Exposure in Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis): Oxidative Stress, Immune Response, and Energy Metabolism. Animals 2024, 14, 561. https://doi.org/10.3390/ani14040561
Zhang X, Zhang W, Zhao L, Zheng L, Wang B, Song C, Liu S. Mechanisms of Gills Response to Cadmium Exposure in Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis): Oxidative Stress, Immune Response, and Energy Metabolism. Animals. 2024; 14(4):561. https://doi.org/10.3390/ani14040561
Chicago/Turabian StyleZhang, Xuanxuan, Wenquan Zhang, Linlin Zhao, Li Zheng, Bingshu Wang, Chengbing Song, and Shenghao Liu. 2024. "Mechanisms of Gills Response to Cadmium Exposure in Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis): Oxidative Stress, Immune Response, and Energy Metabolism" Animals 14, no. 4: 561. https://doi.org/10.3390/ani14040561
APA StyleZhang, X., Zhang, W., Zhao, L., Zheng, L., Wang, B., Song, C., & Liu, S. (2024). Mechanisms of Gills Response to Cadmium Exposure in Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis): Oxidative Stress, Immune Response, and Energy Metabolism. Animals, 14(4), 561. https://doi.org/10.3390/ani14040561





