Renal Endocytic Regulation of Vitamin D Metabolism during Maturation and Aging in Laying Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Determination of Calcium and 1,25(OH)2D3 Concentrations in Serum
2.3. Total RNA Extraction, cDNA Synthesis, Quantitative-PCR Analyses
2.4. Protein Extraction and Western Blotting Analyses
2.5. Immunohistochemistry
2.6. Statistical Analyses
3. Results
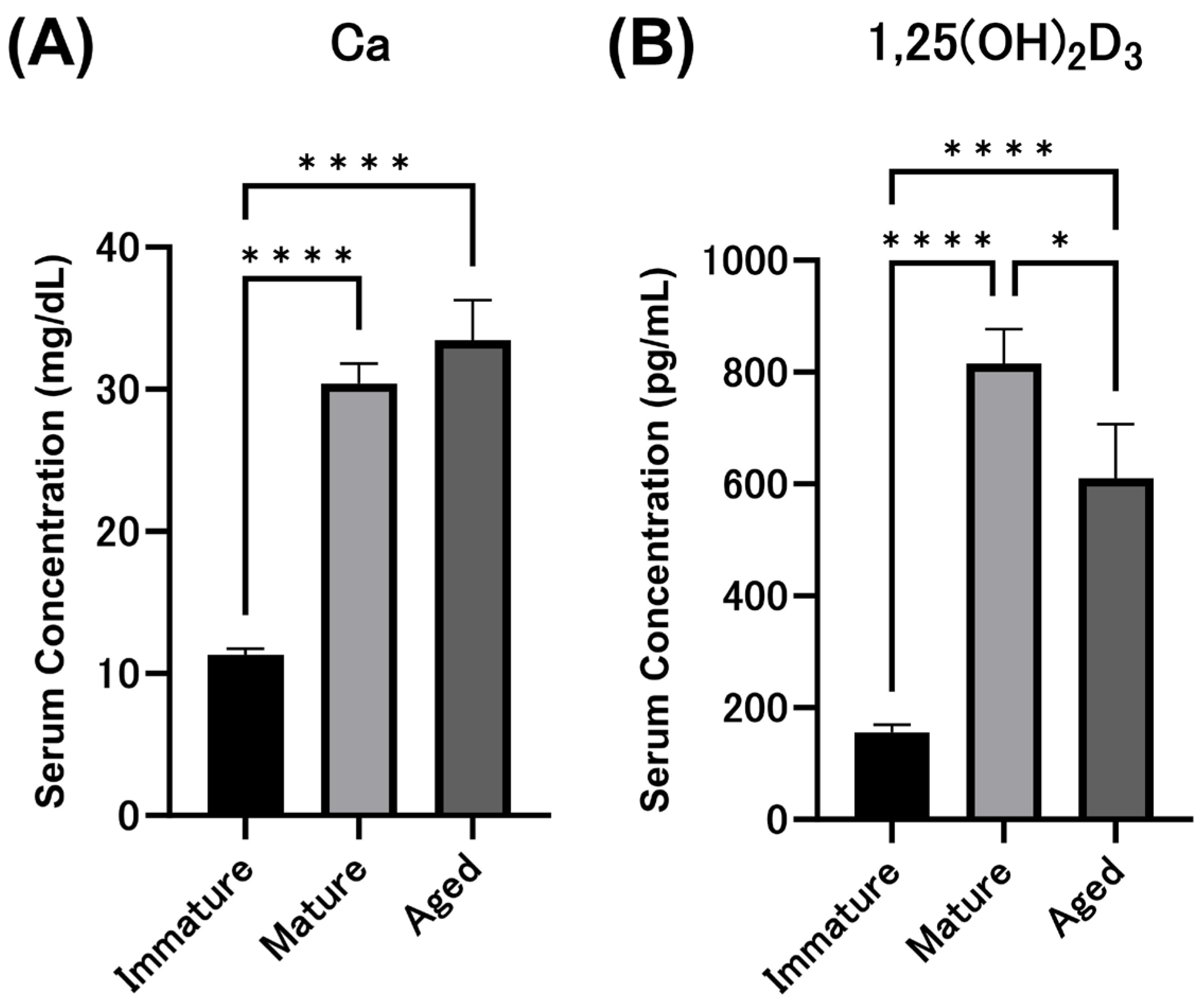
3.1. Serum Calcium and 1,25(OH)2D3 Concentrations
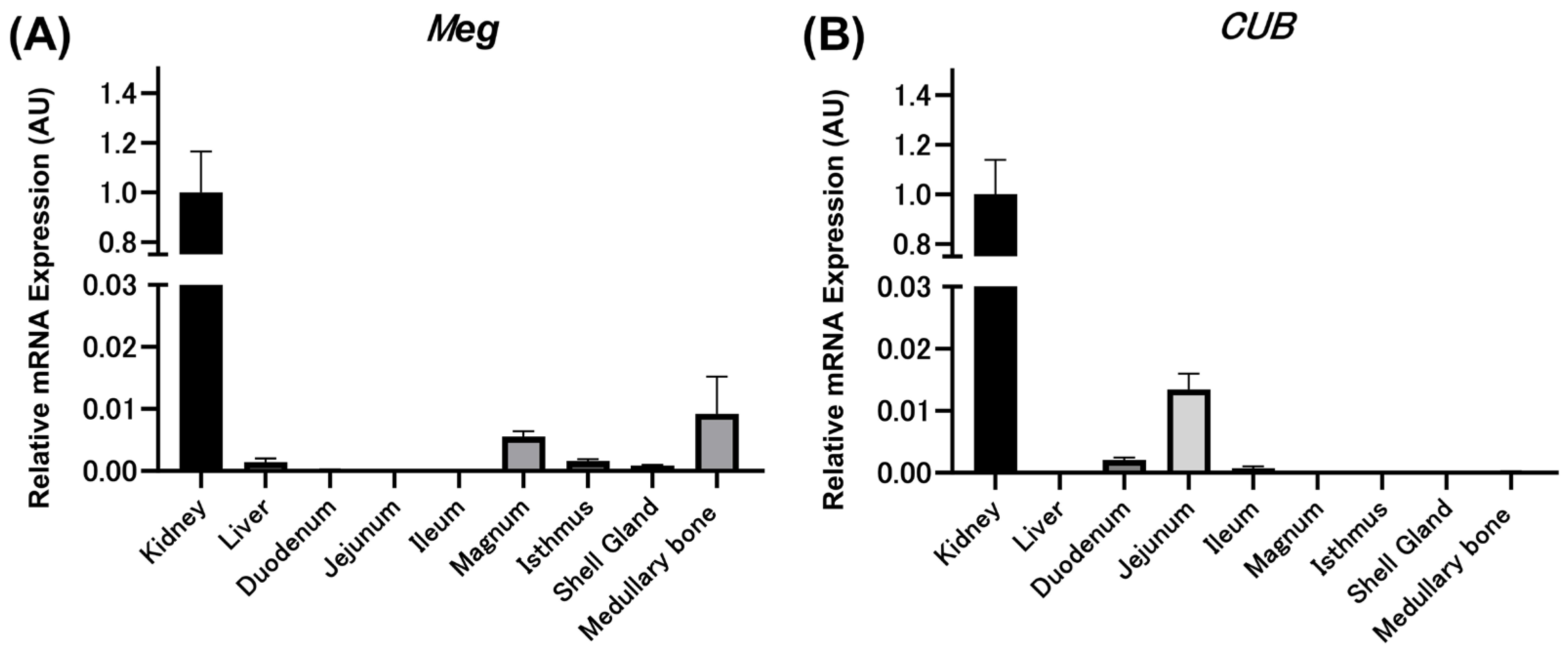
3.2. Relative Expression Levels of Meg and CUB mRNAs in Various Tissues of Mature Laying Hens by qPCR
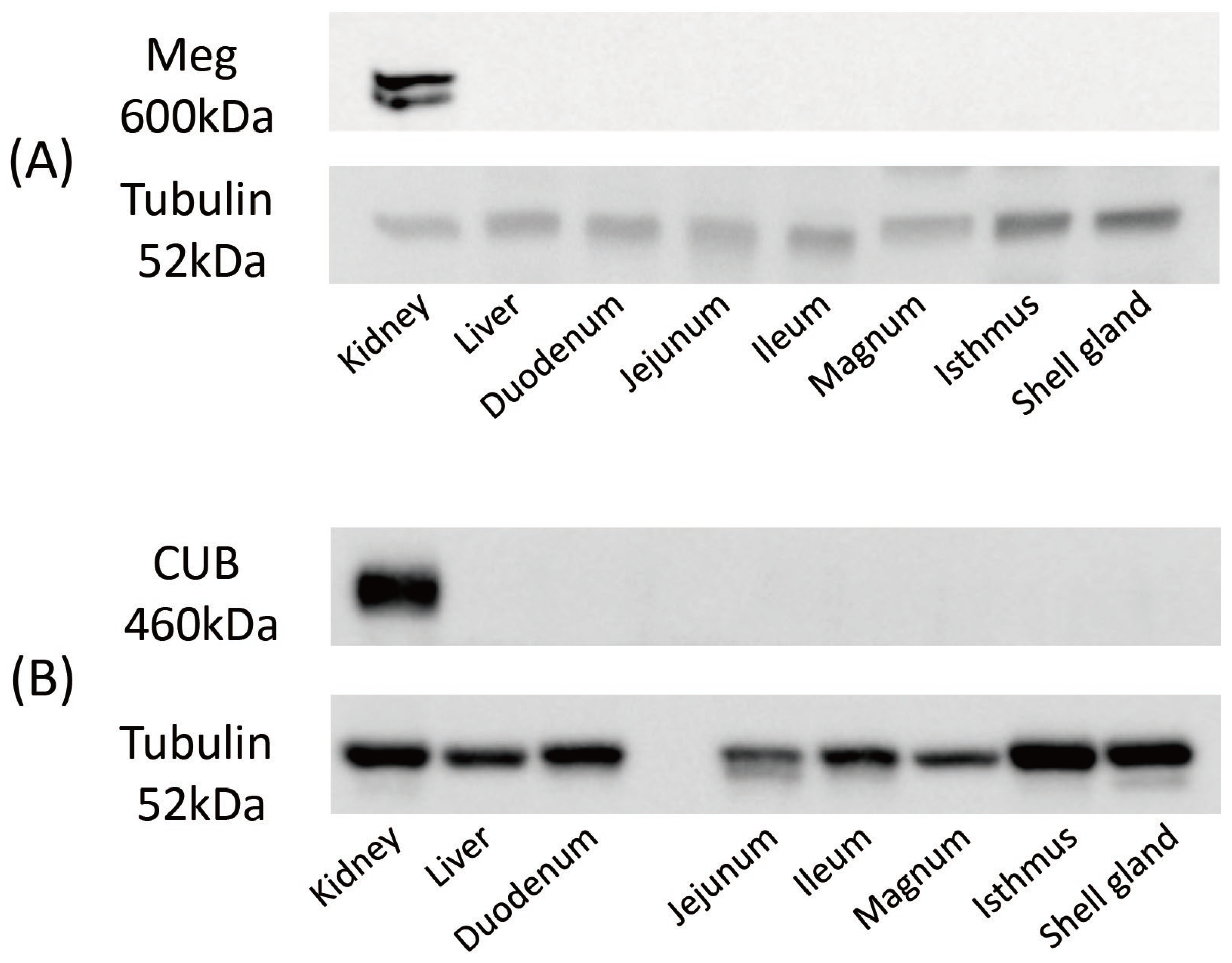
3.3. Expression of Meg and CUB Proteins in Various Tissues of Mature Laying Hens by Western Blotting
3.4. Relative Expression Levels of Meg, CUB and Vitamin D Metabolism-Related Enzyme mRNAs in Immature Chicks and Laying Hens by qPCR
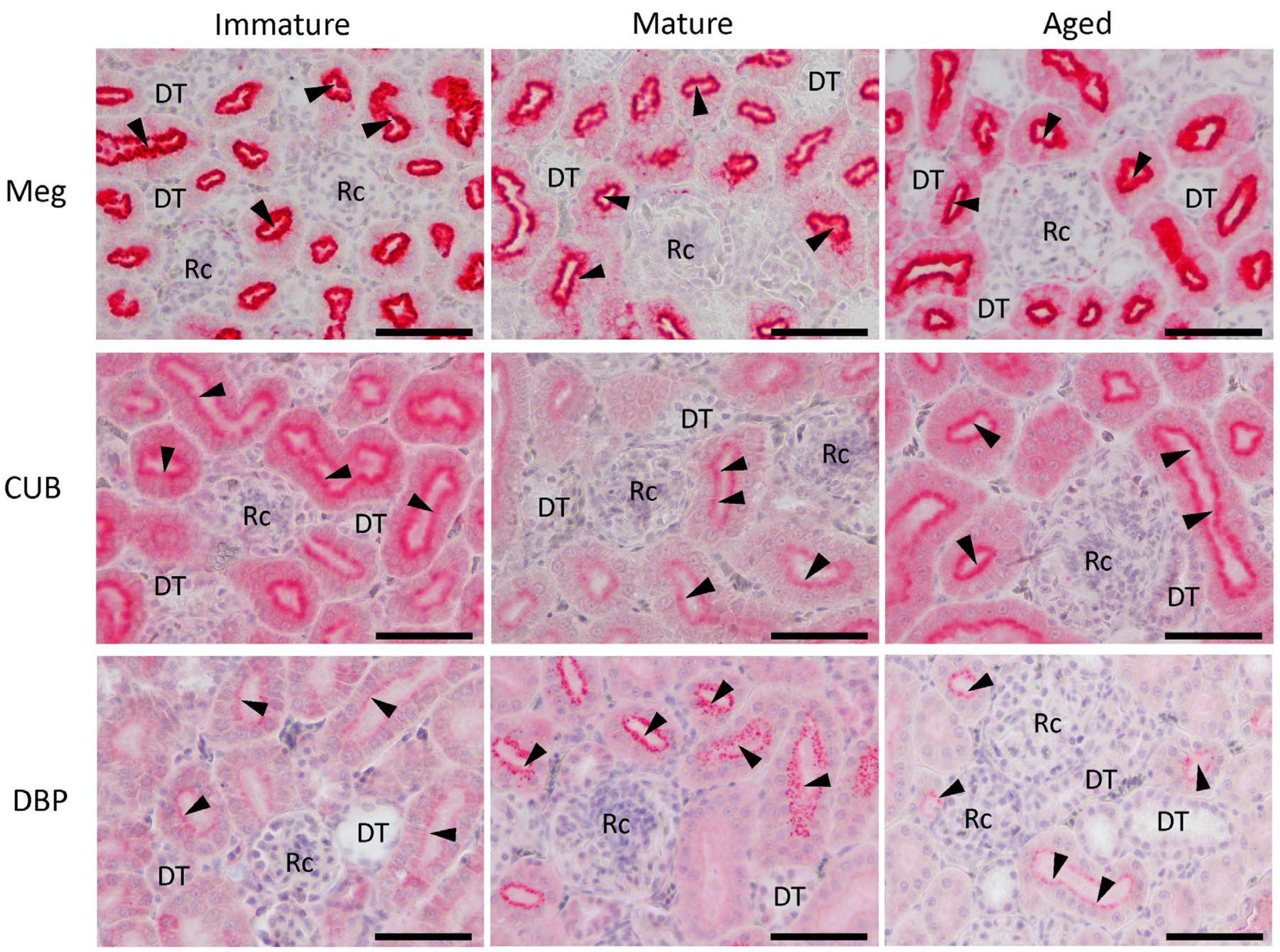
3.5. Localization of Meg, CUB, and DBP Proteins in Laying Hens during Maturation and Aging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, X.; Ning, Z. Research progress on bird eggshell quality defects: A review. Poult. Sci. 2023, 102, 102283. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, K.W. An update on heat stress in laying hens. Worlds Poult. Sci. J. 2023, 79, 689–712. [Google Scholar] [CrossRef]
- Dacke, C.G.; Sugiyama, T.; Gay, C.V. The role of hormones in the regulation of bone turnover and eggshell calcification. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 549–575. [Google Scholar] [CrossRef]
- Nys, Y.; Gautron, J.; Rodriguez-Navarro, A.B.; Hincke, M. Mechanisms and hormonal regulation of shell formation. In Sturkie’s Avian Physiology, 7th ed.; Scanes, C.G., Sami, D., Eds.; Academic Press: San Diego, CA, USA, 2022; pp. 833–879. [Google Scholar] [CrossRef]
- Abe, E.; Horikawa, H.; Masumura, T.; Sugahara, M.; Kubota, M.; Suda, T. Disorders of cholecalciferol metabolism in old egg-laying hens. J. Nutr. 1982, 112, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Vax, E.; Striem, S. Relationships among age, eggshell thickness and vitamin D metabolism and its expression in the laying hen. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1999, 123, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Bar, A. Calcium homeostasis and vitamin D metabolism and expression in strongly calcifying laying birds. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Omara, I.I.; Mou, C.T.; Persia, M.E.; Wong, E.A. Effects of available phosphorus source and concentration on performance and expression of sodium phosphate type IIb cotransporter, vitamin D-1α-hydroxylase, and vitamin D-24-hydroxylase mRNA in broiler chicks. Poult. Sci. 2020, 99, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Gloux, A.; Le Roy, N.; Ezagal, J.; Même, N.; Hennequet-Antier, C.; Piketty, M.L.; Prié, D.; Benzoni, G.; Gautron, J.; Nys, Y.; et al. Possible roles of parathyroid hormone, 1,25(OH)2D3, and fibroblast growth factor 23 on genes controlling calcium metabolism across different tissues of the laying hen. Domest. Anim. Endocrinol. 2020, 72, 106407. [Google Scholar] [CrossRef]
- Hrabia, A.; Kamińska, K.; Socha, M.; Grzesiak, M. Vitamin D3 receptors and metabolic enzymes in hen reproductive tissues. Int. J. Mol. Sci. 2023, 24, 17074. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Haussler, C.A.; Hsieh, J.; Thompson, P.D.; Selznick, S.H.; Dominguez, C.E.; Jurutka, P.W. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998, 13, 325–349. [Google Scholar] [CrossRef]
- Chow, E.C.Y.; Quach, H.P.; Vieth, R.; Pang, K.S. Temporal changes in tissue 1,25-dihydroxyvitamin D3, vitamin D receptor target genes, and calcium and PTH levels after 1,25(OH)2D3 treatment in mice. Am. J. Physiol. Endocrinol. Metab. 2013, 304, 977–989. [Google Scholar] [CrossRef]
- Nykjaer, A.; Fyfe, J.C.; Kozyraki, R.; Leheste, J.-R.; Jacobsen, C.; Nielsen, M.S.; Verroust, P.J.; Aminoff, M.; de la Chapelle, A.; Moestrup, S.K.; et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D3. Proc. Natl. Acad. Sci. USA 2001, 98, 13895–13900. [Google Scholar] [CrossRef] [PubMed]
- Kaseda, R.; Hosojima, M.; Sato, H.; Saito, A. Role of megalin and cubilin in the metabolism of vitamin D3. Ther. Apheresis Dial. 2011, 15, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Marzolo, M.P.; Farfán, P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol. Res. 2011, 44, 89–105. [Google Scholar] [CrossRef]
- Elsakka, E.G.E.; Mokhtar, M.M.; Hegazy, M.; Ismail, A.; Doghish, A.S. Megalin, a multi-ligand endocytic receptor, and its participation in renal function and diseases: A review. Life Sci. 2022, 308, 120923. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.C.; Madsen, M.; Højrup, P.; Christensen, E.I.; Tanner, S.M.; de la Chapelle, A.; He, Q.; Moestrup, S.K. The functional cobalamin (vitamin B12)–intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood 2004, 103, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Birn, H.; Storm, T.; Weyer, K.; Nielsen, R. Endocytic receptors in the renal proximal tubule. Physiology 2012, 27, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Plieschnig, J.A.; Gensberger, E.T.; Bajari, T.M.; Schneider, W.J.; Hermann, M. Renal LRP2 expression in man and chicken is estrogen-responsive. Gene 2012, 508, 49–59. [Google Scholar] [CrossRef][Green Version]
- Bauer, R.; Plieschnig, J.A.; Finkes, T.; Riegler, B.; Hermann, M.; Schneider, W.J. The developing chicken yolk sac acquires nutrient transport competence by an orchestrated differentiation process of its endodermal epithelial cells. J. Biol. Chem. 2013, 288, 1088–1098. [Google Scholar] [CrossRef]
- Kerschnitzki, M.; Zander, T.; Zaslansky, P.; Fratzl, P.; Shahar, R.; Wagermaier, W. Rapid alterations of avian medullary bone material during the daily egg-laying cycle. Bone 2014, 69, 109–117. [Google Scholar] [CrossRef]
- Christensen, E.I.; Willnow, T.E. Essential role of megalin in renal proximal tubule for vitamin homeostasis. J. Am. Soc. Nephrol. 1999, 10, 2224–2236. [Google Scholar] [CrossRef]
- Christensen, E.I.; Birn, H. Megalin and Cubilin: Multifunctional endocytic receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 258–268. [Google Scholar] [CrossRef]
- Cui, S.; Verroust, P.J.; Moestrup, S.K.; Christensen, E.I. Megalin/gp330 mediates uptake of albumin in renal proximal tubule. Am. J. Physiol. 1996, 271, F900–F907. [Google Scholar] [CrossRef]
- Nykjaer, A.; Dragun, D.; Walther, D.; Vorum, H.; Jacobsen, C.; Herz, J.; Melsen, F.; Christensen, E.I.; Willnow, T.E. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999, 96, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Kozyraki, R.; Fyfe, J.; Verroust, P.J.; Jacobsen, C.; Dautry-Varsat, A.; Gburek, J.; Willnow, T.E.; Christensen, E.I.; Moestrup, S.K. Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc. Natl. Acad. Sci. USA 2001, 98, 12491–12496. [Google Scholar] [CrossRef] [PubMed]
- Gburek, J.; Verroust, P.J.; Willnow, T.E.; Fyfe, J.C.; Nowacki, W.; Jacobsen, C.; Moestrup, S.K.; Christensen, E.I. Megalin and cubilin are endocytic receptors involved in renal clearance of hemoglobin. J. Am. Soc. Nephrol. 2002, 13, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.J.; Anderson, P.H.; Findlay, D.M.; Welldon, K.J.; Vincent, C.; Zannettino, A.C.W.; O’Loughlin, P.D.; Morris, H.A. Metabolism of vitamin D3 in human osteoblasts: Evidence for autocrine and paracrine activities of 1α,25-dihydroxyvitamin D3. Bone 2007, 40, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Choi, M.Y.; Longtine, M.S.; Nelson, D.M. Vitamin D effects on pregnancy and the placenta. Placenta 2010, 31, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, R.; Selvaraj, R.K. Vitamin D-1α-hydroxylase and vitamin D-24-hydroxylase mRNA studies in chickens. Poult. Sci. 2012, 91, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.; Tanaka, Y.; Wineland, M.J.; Jowsey, J.O.; Deluca, H.F. Production of 1,25-dihydroxyvitamin D3 and formation of medullary bone in the egg-laying hen. Endocrinology 1979, 104, 1598–1601. [Google Scholar] [CrossRef]
- Sedrani, S.H. Changes in serum levels of 1,25-dihydroxyvitamin D3, calcium and phosphorus with age and vitamin D status in chickens. Br. J. Nutr. 1984, 52, 329–334. [Google Scholar] [CrossRef]
- Karras, S.N.; Wagner, C.L.; Castracane, V.D. Understanding vitamin D metabolism in pregnancy: From physiology to pathophysiology and clinical outcomes. Metabolism 2018, 86, 112–123. [Google Scholar] [CrossRef]
- Joyner, C.J.; Peddie, M.J.; Taylor, T.G. The effect of age on egg production in the domestic hen. Gen. Comp. Endocrinol. 1987, 65, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Mata-Greenwood, E.; Huber, H.F.; Li, C.; Nathanielsz, P.W. Role of pregnancy and obesity on vitamin D status, transport, and metabolism in baboons. Am. J. Physiol. Endocrinol. Metab. 2019, 316, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Odera, K.; Goto, S.; Takahashi, R. Age-related change of endocytic receptors megalin and cubilin in the kidney in rats. Biogerontology 2007, 8, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.; White, D.; House, J.D.; Kim, W.K. Effects of additional dosage of vitamin D3, vitamin D2, and 25-hydroxyvitamin D3 on calcium and phosphorus utilization, egg quality and bone mineralization in laying hens. Poult. Sci. 2020, 99, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Turner, B.; Applegate, T.J.; Litta, G.; Kim, W.K. Role of long-term supplementation of 25-hydroxy vitamin D3 on egg production and egg quality of laying hen. Poult. Sci. 2020, 99, 6899–6906. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ding, X.; Bai, S.; Wang, J.; Zeng, Q.; Peng, H.; Xuan, Y.; Zhang, K. Effects of supplementation of 25-hydroxy vitamin D3 as a vitamin D3 substitute on performance, bone traits, and egg quality of laying hens from 1 day to 72 weeks of age. Agriculture 2023, 13, 383. [Google Scholar] [CrossRef]

| Target Genes NCBI Gene Bank No. | Primer Sequence | Product Size | |
|---|---|---|---|
| Megalin (Meg) XM_040676662.2 | Forward Reverse | 5′-TGCTCTGGGCATCCTTACCAC-3′ 5′-ACATTATGGGAACAACATTCTGCAC-3′ | 151 bp |
| Cubilin (CUB) XM_040663409.2 | Forward Reverse | 5′-ACAGCTGGAGGAGAGCAATG-3′ 5′-GGACAGACGAGGCTGTTCAT-3′ | 149 bp |
| β-actin NM_205518.2 | Forward Reverse | 5′-ATTGTCCACCGCAAATGCTTC-3′ 5′-AAATAAAGCCATGCCAATCTCGTC-3′ | 113 bp |
| 1α-hydroxylase (Cyp27C1) XM_040676644.2 | Forward Reverse | 5′-TCGTGGCAGGAATACAGAGA-3′ 5′-ACTGCCACATCTTTGGGTTT-3′ | 125 bp |
| 24-hydroxylase (Cyp24A1) NM_001396287.1 | Forward Reverse | 5′-AGCCAAGCACTCCATTGACA-3′ 5′-AACTGTTGGCCGTCGTTTCA-3′ | 161 bp |
| 25-hydroxylase (Cyp27A1) XM_040676620.2 | Forward Reverse | 5′-TATCCCCAAGATGCCGATGC-3′ 5′-TGGGGAAGAGGTAGTCTCCG-3′ | 125 bp |
| VitaminD binding protein (DBP) NM_204882.2 | Forward Reverse | 5′-TGAGGACCTTTCCCCTCTCG-3′ 5′-GTGTGCTCCGACAACTTCTG-3′ | 102 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuwata, N.; Mukohda, H.; Uchida, H.; Takamatsu, R.; Binici, M.M.; Yamada, T.; Sugiyama, T. Renal Endocytic Regulation of Vitamin D Metabolism during Maturation and Aging in Laying Hens. Animals 2024, 14, 502. https://doi.org/10.3390/ani14030502
Kuwata N, Mukohda H, Uchida H, Takamatsu R, Binici MM, Yamada T, Sugiyama T. Renal Endocytic Regulation of Vitamin D Metabolism during Maturation and Aging in Laying Hens. Animals. 2024; 14(3):502. https://doi.org/10.3390/ani14030502
Chicago/Turabian StyleKuwata, Nami, Hatsune Mukohda, Hiroto Uchida, Ryo Takamatsu, Muhammet Mustafa Binici, Takahisa Yamada, and Toshie Sugiyama. 2024. "Renal Endocytic Regulation of Vitamin D Metabolism during Maturation and Aging in Laying Hens" Animals 14, no. 3: 502. https://doi.org/10.3390/ani14030502
APA StyleKuwata, N., Mukohda, H., Uchida, H., Takamatsu, R., Binici, M. M., Yamada, T., & Sugiyama, T. (2024). Renal Endocytic Regulation of Vitamin D Metabolism during Maturation and Aging in Laying Hens. Animals, 14(3), 502. https://doi.org/10.3390/ani14030502




