Potential Effects of Garlic (Allium sativum L.) on the Performance, Immunity, Gut Health, Anti-Oxidant Status, Blood Parameters, and Intestinal Microbiota of Poultry: An Updated Comprehensive Review
Abstract
Simple Summary
Abstract
1. Introduction
2. The Different Influences of Dietary Garlic on Poultry Health
2.1. Production Parameters
2.1.1. Performance
2.1.2. Intestinal Architecture
2.2. Immunity
2.3. Gut Health
2.3.1. Antibacterial
2.3.2. Antiparasitic
2.4. Anti-Oxidant Status
2.5. Blood Parameters
2.6. Intestinal Health and Microbiota
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIV | Avian influenza virus |
| BW | Body weight |
| BWG | Body weight gain |
| BA | Brucella abortus |
| CD | Cluster of differentiation |
| ConA | Concanavalin A |
| E. coli | Escherichia coli |
| FCR | Feed conversion ratio |
| FI | Feed intake |
| GOT | Glutamic-oxaloacetic transaminase |
| GPx | Glutathione peroxidase |
| GPT | Glutamic-pyruvic transaminase |
| HDL | High-density lipoprotein |
| Ig | Immunoglobulin |
| IBD | Infectious bursal disease virus |
| INF-γ | Interferon-gamma |
| IL | Interleukin |
| LDL | Low-density lipoprotein |
| MDA | Malondialdehyde |
| NDV | Newcastle disease virus |
| RBCs | Red blood cells |
| SOD | Superoxide dismutase |
| WBCs | White blood cells |
References
- Saleha, A.A.; Myaing, T.T.; Ganapathy, K.K.; Zulkifli, I.; Raha, R.; Arifah, K. Possible effect of antibiotic-supplemented feed and environment on the occurrence of multiple antibiotic resistant Escherichia coli in chickens. Int. J. Poult. Sci. 2009, 8, 28–31. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A. Phytobiotics in poultry industry as growth promoters, antimicrobials and immunomodulators—A review. J. World’s Poult. Res. 2020, 10, 571–579. [Google Scholar] [CrossRef]
- Navidshad, B.; Darabighane, B.; Malecky, M. Garlic: An alternative to antibiotics in poultry production, a review. Iran. J. Appl. Anim. Sci. 2018, 8, 9–17. [Google Scholar]
- Ur Rahman, S.; Khan, S.; Chand, N.; Sadique, U.; Khan, R.U. In vivo effects of Allium cepa L. on the selected gut microflora and intestinal histomorphology in broiler. Acta Histochem. 2017, 119, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Ezeorba, T.P.C.; Chukwudozie, K.I.; Ezema, C.A.; Anaduaka, E.G.; Nweze, E.J.; Okeke, E.S. Potentials for health and therapeutic benefits of garlic essential oils: Recent findings and future prospects. Pharmacol. Res. Mod. Chin. Med. 2022, 3, 100075. [Google Scholar] [CrossRef]
- Najda, A.; Błaszczyk, L.; Winiarczyk, K.; Dyduch, J.; Tchórzewska, D. Comparative studies of nutritional and health-enhancing properties in the “garlic-like” plant Allium ampeloprasum var. ampeloprasum (GHG-L) and A. sativum. Sci. Hortic. 2016, 201, 247–255. [Google Scholar] [CrossRef]
- Ozma, M.A.; Abbasi, A.; Ahangarzadeh Rezaee, M.; Hosseini, H.; Hosseinzadeh, N.; Sabahi, S.; Noori, S.M.A.; Sepordeh, S.; Khodadadi, E.; Lahouty, M.; et al. A critical review on the nutritional and medicinal profiles of garlic’s (A. sativum L.) bioactive compounds. Food Rev. Int. 2022, 39, 6324–6361. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Rybczynska-Tkaczyk, K.; Gawel-Beben, K.; Swieca, M.; Karas, M.; Jakubczyk, A.; Matysiak, M.; Binduga, U.E.; Gminski, J. Characterization of active compounds of different garlic (Allium sativum L.) cultivars. Pol. J. Food Nutr. Sci. 2018, 68, 73–81. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 5, 246. [Google Scholar] [CrossRef]
- Puvača, N.; Kostadinović, L.; Ljubojević, D.; Lukač, D.; Lević, J.; Popović, S.; Novakov, N.; Vidović, B.; Đuragić, O. Effect of garlic, black pepper and hot red pepper on productive performances and blood lipid profile of broiler chickens. Eur. Poult. Sci. 2015, 79, 1–13. [Google Scholar] [CrossRef]
- Saleem, Z.M.; Al-Delaimy, K.S. Inhibition of Bacillus aurous by garlic extracts. J. Food Prot. 1982, 45, 1007–1009. [Google Scholar] [CrossRef]
- Chang, K.J.; Cheong, S.H. Volatile organosulfur and nutrient compounds from garlic by cultivating areas and processing methods. Fed. Am. Soc. Exp. Biol. J. 2008, 22, 1108–1112. [Google Scholar] [CrossRef]
- Bordia, A. Effect of garlic on blood lipids in particles with coronary heart disease. Am. J. Clin. Nutr. 1981, 34, 2100–2103. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.E.; Alagawany, M.; Taha, A.E.; Puvača, N.; Laudadio, V.; Tufarelli, V. Effect of dietary supplementation of garlic powder and phenyl acetic acid on productive performance, blood haematology, immunity and antioxidant status of broiler chickens. Anim. Biosci. 2021, 34, 363. [Google Scholar] [CrossRef] [PubMed]
- Sivam, G.P. Protection against Helicobacter pylori and other bacterial infections by garlic. J. Nutr. 2001, 131, 1106S–1108S. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Lee, H.J.; Yoon, D.K.; Ji, D.S.; Kim, J.H.; Lee, C.H. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci. Biotechnol. 2018, 27, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Morioka, N.; Sze, L.L.; Morton, D.L.; Irie, R.F. A protein fraction from aged garlic extract enhances cytotoxicity and proliferation of human lymphocytes mediated by interleukin-2 and concanavalin A. Cancer Immunol. Immunother. 1993, 37, 316–322. [Google Scholar] [CrossRef]
- Agarwal, K.C. Therapeutic actions of garlic constituents. Med. Res. Rev. 1996, 16, 111–124. [Google Scholar] [CrossRef]
- Ariga, T.; Seki, T. Antithrombotic and anticancer effects of garlic derived sulfur compounds. BioFactors 2006, 26, 93–103. [Google Scholar] [CrossRef]
- Li, W.R.; Shi, Q.S.; Dai, H.Q.; Liang, Q.; Xie, X.B.; Huang, X.M.; Zhao, G.Z.; Zhang, L.X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016, 6, 22805. [Google Scholar] [CrossRef]
- Makwana, R.B.; Raval, A.P.; Chauhan, H.D.; Kulkarni, R.C.; Srivastava, A.K.; Bhagwat, S.R.; Rajgor, B.B. Effects of garlic (Allium sativum) supplementation on growth performance, carcass characteristics and economics of broilers. J. Anim. Res. 2015, 5, 843–848. [Google Scholar] [CrossRef]
- Al-Massad, M.; Al-Ramamneh, D.; AL-Sharafat, A.; Abdelqader, A.; Hussain, N. Effect of using garlic on the economical and physiological characteristics of broiler chickens. Russ. Agric. Sci. 2018, 44, 276–281. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Ibrahim, N.S.; Shehata, A.M.; Mohamed, N.G.; Abdel-Moneim, A.M. Impact of multi-strain probiotic, citric acid, garlic powder or their combinations on performance, ileal histomorphometry, microbial enumeration and humoral immunity of broiler chickens. Trop. Anim. Health Prod. 2021, 53, 115. [Google Scholar] [CrossRef] [PubMed]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef] [PubMed]
- Abd-ELrahman, S.M.; Mohamed, S.A.-A.; Mohamed, S.E.; El-Khadragy, M.F.; Dyab, A.K.; Hamad, N.; Safwat, M.M.; Nasr, A.A.E.; Alkhaldi, A.A.M.; Gareh, A.; et al. Comparative effect of allicin and alcoholic garlic extract on the morphology and infectivity of Eimeria tenella oocysts in chickens. Animals 2022, 12, 3185. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.M.; Ashmawy, E.S.; Salama, A.A.; Abdel-Moneim, A.E.; Badri, F.B.; Thabet, H.A. Effects of garlic and lemon essential oils on performance, digestibility, plasma metabolite, and intestinal health in broilers under environmental heat stress. BMC Vet. Res. 2022, 18, 430. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Din, Z.Z.; Abuirmeileh, N.; Burger, W.C.; Ahmad, Y.; Elson, C.E. Suppression of avian hepatic lipid metabolism by solvent extracts of garlic: Impact on serum lipids. J. Nutr. 1983, 113, 1746–1755. [Google Scholar] [CrossRef]
- Horton, G.M.J.; Fennell, M.J.; Prasad, B.M. Effects of dietary garlic (Allium sativum) on performance, carcass composition and blood chemistry changes in broiler chickens. Can. J. Anim. Sci. 1991, 71, 939–942. [Google Scholar] [CrossRef]
- Tollba, A.A.H.; Hassan, M.S.H. Using some natural additives to improve physiological and productive performance of broiler chicks under high temperature conditions. 2. Black cumin (Niglla sativa) or garlic (Allium sativum). Egypt. Poult. Sci. J. 2003, 23, 327–340. [Google Scholar]
- Ashayerizadeh, O.; Dastar, B.; Shargh, M.S.; Ashayerizadeh, A.; Rahmatnejad, E.; Hossaini, S.M.R. Use of garlic (Allium sativum), black cumin seeds (Nigella sativa L.) and wild mint (Mentha longifolia) in broiler chickens diets. J. Anim. Vet. Adv. 2009, 8, 1860–1863. [Google Scholar]
- Choi, H.; Park, W.Y.; Kim, Y.J. Effects of dietary garlic powder and α-tocopherol supplementation on performance, serum cholesterol levels, and meat quality of chicken. Poult. Sci. 2010, 89, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Raeesi, M.; Hoseini-Aliabad, S.A.; Roofchaee, A.; Zare Shahneh, A.; Pirali, S. Effect of periodically use of garlic (Allium sativum) powder on performance and carcass characteristics in broiler chickens. World Acad. Sci. Eng. Technol. 2010, 4, 1388–1394. [Google Scholar]
- Suriya, R.; Zulkifli, I.; Alimon, A.R. The effect of dietary inclusion of herbs as growth promoter in broiler chickens. J. Anim. Vet. Adv. 2012, 11, 346–350. [Google Scholar] [CrossRef]
- Elagib, H.A.A.; El-Amin, W.I.A.; Malik, H.E.E. Effect of dietary garlic (Allium sativum) supplementation as a feed additive on broiler performance and blood profile. J. Anim. Sci. Adv. 2013, 3, 58–64. [Google Scholar]
- Oleforuh-Okoleh, V.U.; Harrie, M.; Solomon, N.F.; Olorunleke, O.; Joesph, U. Evaluation of growth performance, hematological and serum biochemical response of broiler chickens to aqueous extract of ginger and garlic. J. Agric. Sci. 2015, 7, 167–173. [Google Scholar] [CrossRef][Green Version]
- Varmaghany, S.; Torshizi, M.A.K.; Rahimi, S.; Lotfollahian, H.; Hassanzadeh, M. The effects of increasing levels of dietary garlic bulb on growth performance, systolic blood pressure, hematology, and ascites syndrome in broiler chickens. Poult. Sci. 2015, 94, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Kirubakaran, A.; Moorthy, M.; Chitra, R.; Prabakar, G. Influence of combinations of fenugreek, garlic, and black pepper powder on production traits of the broilers. Vet. World 2016, 9, 470. [Google Scholar] [CrossRef]
- Gautam, G.; Nabaraj Shishir, B. Effect of Allium sativum on immune status against Newcastle disease virus and the productive performance of broiler chickens. J. Poult. Sci. 2017, 16, 515–521. [Google Scholar] [CrossRef]
- Ratika, K.; Singh, R.J.; Singh, R.K.; Singh, M. Weekly assessment of growth performance of broilers fed a diet supplemented with garlic and turmeric powder and their combination. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1373–1381. [Google Scholar] [CrossRef]
- Sangilimadan, K.; Churchil, R.R.; Premavalli, K.; Omprakash, A.V. Effect of garlic (Allium sativum) on production performances and carcass traits of Nandanam broiler-2. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2531–2538. [Google Scholar] [CrossRef]
- Aydogan, I.; Yildirim, E.; Kurum, A.; Bolat, D.; Cinar, M.; Basalan, M.; Yigit, A. The effect of dietary garlic (Allium Sativum), black cumin (Nigella sativa) and their combination on performance, intestine morphometry, serum biochemistry and antioxidant status of broiler chickens. Braz. J. Poult. Sci. 2020, 22, eRBCA-2020-1317. [Google Scholar] [CrossRef]
- Tanti, A.; Retnani, Y.; Soesanto, I.R.H. Effect of supplementation garlic (Allium sativum) by various processing on performances of broiler. IOP Conf. Ser. Earth Environ. Sci. 2022, 1020, 012015. [Google Scholar] [CrossRef]
- Sharma, R.K.; Singh, R.A.; Pal, R.N.; Aggarwal, C.K. Cholesterol contents of chicken eggs as affected by feeding garlic, sarpagandha and nicotinic acid. Haryana Agric. Univ. J. Res. 1979, 9, 263–265. [Google Scholar]
- Reddy, R.V.; Lightest, S.F.; Maurice, D.V. Effect of feeding garlic oil on performance and egg yolk cholesterol concentration. Poult Sci. 1991, 70, 2006–2009. [Google Scholar] [CrossRef]
- Birrenkott, G.; Brockenfelt, G.E.; Owens, M.; Halpin, E. Yolk and blood cholesterol levels and organoleptic assessment of eggs from hens fed a garlic-supplemented diet. Poult. Sci. 2000, 79, 75–82. [Google Scholar]
- Chowdhury, S.R.; Chowdhury, S.D.; Smith, T.K. Effects of dietary garlic on cholesterol metabolism in laying hens. Poult. Sci. 2002, 81, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Mottaghitalab, M.; Taraz, Z. Effects of garlic (Allium sativum) on egg yolk and blood serum cholesterol in Aryan breed laying hens. Br. Poult. Sci. 2002, 43, S42–S43. [Google Scholar]
- Yalcin, S.; Onbasilar, E.E.; Reisli, Z.; Yalcin, S. Effects of garlic powder on the performance, egg traits and blood parameters of laying hens. J. Sci. Food Agric. 2006, 86, 1336–1339. [Google Scholar] [CrossRef]
- Khan, S.H.; Sardar, R.; Anjum, M.A. Effects of dietary garlic on performance and serum and egg cholesterol concentration in laying hens. Asian Aust. J. Anim. Sci. 2007, 21, 22–27. [Google Scholar] [CrossRef]
- Saffa, H.M. Effect of dietary garlic or fenugreek on cholesterol metabolism in lying hens. Egypt. J. Poult. Sci. 2007, 27, 1207–1221. [Google Scholar]
- Khan, Q.S.H.; Hassan, S.; Sardar, R.; Anjum, M.A. Effects of dietary garlic on cholesterol concentration in native Desi laying hens. Am. J. Food Technol. 2008, 3, 207–213. [Google Scholar]
- Canogullari, S.; Baylan, M.; Erdogan, Z.; Duzguner, V.; Kucukgul, A. The effects of dietary garlic powder on performance, egg yolk and serum cholesterol concentrations in laying quails. Czech. J. Anim. Sci. 2010, 55, 286–293. [Google Scholar] [CrossRef]
- Mahmoud, K.Z.; Saad, M.; Gharaibeh, H.; Zakaria, A.; Amer, M. Garlic (Allium sativum) supplementation: Influence on egg production, quality and yolk cholesterol level in laying hens. Asian Australs. J. Anim. Sci. 2010, 23, 1503–1509. [Google Scholar] [CrossRef]
- Motamedi, S.M.; Taklimi, S.M. Investigating the effect of fenugreek seed powder and garlic powder in the diet on immune response of commercial laying hens’ egg. Indian J. Sci. Res. 2014, 3, 277–283. [Google Scholar]
- Asrat, M.; Zeryehun, T.; Amha, N.; Urge, M. Effects of supplementation of different levels of garlic (Allium sativum) on egg production, egg quality and hatchability of White Leghorn chicken. Livest. Res. Rural Dev. 2018, 30, 37. [Google Scholar]
- Omer, H.A.; El-Mallah, G.M.; Abdel-Magid, S.S.; Bassuony, N.I.; Ahmed, S.M.; El-Ghamry, A.K.A. Impact of adding natural bioactive mixture composed of lemon, onion, and garlic juice at different levels on productive performance, egg quality, and some blood parameters of commercial laying hens. Bull. Natl. Res. Cent. 2019, 43, 137. [Google Scholar] [CrossRef]
- Khan, R.U.; Nikousefat, Z.; Tufarelli, V.; Naz, S.; Javdani, M.; Laudadio, V. Garlic (Allium sativum) supplementation in poultry diets: Effect on production and physiology. Worlds Poult. Sci. J. 2012, 68, 417–424. [Google Scholar] [CrossRef]
- Ziarlarimi, A.; Irani, M.; Gharahveysi, S.; Rahmani, Z. Investigation of antibacterial effects of garlic (Allium sativum), mint (Menthe spp.) and onion (Allium cepa) herbal extracts on Escherichia coli isolated from broiler chickens. Afr. J. Biotechnol. 2011, 10, 10320–10322. [Google Scholar] [CrossRef][Green Version]
- Ross, Z.M.; O’Gara, E.A.; Hill, D.J.; Sleightholme, H.V.; Maslin, D.J. Antimicrobial properties of garlic oil against human enteric bacteria: Evaluation of methodologies and comparisons with garlic oil sulfides and garlic powder. Appl. Environ. Microbiol. 2001, 67, 475–480. [Google Scholar] [CrossRef]
- Olukosi, O.A.; Dono, N.D. Modification of digesta pH and intestinal morphology with the use of benzoic acid or phytobiotics and the Effects on broiler chicken growth performance and energy and nutrient utilization. J. Anim. Sci. 2014, 92, 3945–3953. [Google Scholar] [CrossRef]
- Nusairat, B.M. Dietary Supplementation of Garlic (Allium sativum): Influence on Performance Parameters, Meat Quality and Humoral Immune Response in Broiler Chicks. Master’s Thesis, Jordan University of Science and Technology, Irbi, Jordan, 2007. [Google Scholar]
- Kumar, A.; Kheravii, S.K.; Ionescu, C.; Blanchard, A.; Barekatain, R.; Bajagai, Y.S.; Wu, S.B. A microencapsulated mixture of eugenol and garlic tincture supplementation mitigates the effect of necrotic enteritis on intestinal integrity and increases goblet cells in broilers. Microorganisms 2021, 9, 1451. [Google Scholar] [CrossRef]
- Adibmoradi, M.; Navidshad, B.; Seifdavati, J.; Royan, M. Effect of dietary garlic meal on histological structure of small intestine in broiler chickens. J. Poult. Sci. 2006, 43, 378–383. [Google Scholar] [CrossRef]
- Gotep, J.G.; Tanko, J.T.; Forcados, G.E.; Muraina, I.A.; Ozele, N.; Dogonyaro, B.B.; Oladipo, O.O.; Makoshi, M.S.; Akanbi, O.B.; Kinjir, H.; et al. Therapeutic and safety evaluation of combined aqueous extracts of Azadirachta indica and Khaya senegalensis in chickens experimentally infected with Eimeria oocysts. J. Parasitol. Res. 2016, 2016, 4692424. [Google Scholar] [CrossRef]
- Halliwell, B.; Zhao, K.; Whiteman, M. The gastrointestinal tract: A major site of antioxidant action? Free Radic. Res. 2000, 33, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Iji, P.A.; Choct, M. Dietary modulation of gut microflora in broiler chickens: A review of the role of six kinds of alternatives to in-feed antibiotics. Worlds Poult. Sci. J. 2009, 65, 97–114. [Google Scholar] [CrossRef]
- Grajek, W.; Olejnik, A.; Sip, A. Probiotics, prebiotics and antioxidants as functional foods. Acta Biochim. Pol. 2005, 52, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, S.; Onbaşilar, Ĺ.; Şehu, A.; Yalcin, S. The effects of dietary garlic powder on the performance, egg traits and blood serum cholesterol of laying quails. Asian-Australas. J. Anim. Sci. 2007, 20, 944–947. [Google Scholar] [CrossRef]
- Jafari, R.; Razi, M.; Ghorbanpoor, M.; Marashian, S. Effect of dietary garlic on immune response of broiler chicks to live Newcastle disease vaccine. Pak. J. Biol. Sci. 2008, 11, 1848–1851. [Google Scholar] [CrossRef]
- Hanieh, H.; Narabara, K.; Piao, M.; Gerile, C.; Abe, A.; Kondo, Y. Modulatory effects of two levels of dietary Alliums on immune response and certain immunological variables, following immunization, in White Leghorn chickens. Anim. Sci. J. 2010, 81, 673–680. [Google Scholar] [CrossRef]
- Rahimi, S.; Teymouri Zadeh, Z.; Karimi Torshizi, M.A.; Omidbaigi, R.; Rokni, H. Effect of the three herbal extracts on growth performance, immune system, blood factors and intestinal selected bacterial population in broiler chickens. J. Agric. Sci. Technol. 2011, 13, 527–539. [Google Scholar]
- El-katcha, M.I.; Soltan, M.A.; Sharaf, M.M.; Hasen, A. Growth performance, immune response, blood serum parameters, nutrient digestibility and carcass traits of broiler chicken as affected by dietary supplementation of garlic extract (allicin). Alex. J. Vet. Sci. 2016, 49, 50–54. [Google Scholar] [CrossRef]
- Oladele, O.; Esan, O.; Akpan, I.; Enibe, F. Garlic feed inclusion and susceptibility of broiler chickens to infectious bursal disease. J. Adv. Vet. Anim. Res. 2018, 5, 275–281. [Google Scholar] [CrossRef]
- Chang, L.Y.; Di, K.Q.; Xu, J.; Chen, Y.F.; Xi, J.Z.; Wang, D.H.; Hao, E.Y.; Xu, L.J.; Chen, H.; Zhou, R.Y. Effect of natural garlic essential oil on chickens with artificially infected E. tenella. Veter. Parasitol. 2021, 300, 109614. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.S. Aged garlic extract modifies human immunity. J. Nutr. 2016, 146, 433S–436S. [Google Scholar] [CrossRef] [PubMed]
- Dorhoi, A.; Dobrean, V.; Zahan, M.; Virag, P. Modulatory effects of several herbal extracts on avian peripheral blood cell immune responses. Phytother. Res. 2006, 20, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, T.; Hassan, Z.; Ebtekar, M.; Ahamdiani, A.; Naderi, G.; Azar, A. Garlic induces a shift in cytokine pattern in Leishmania major-infected Balb/c mice. Scand. J. Immunol. 2000, 52, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Hanieh, H.; Narabara, K.; Tanaka, Y.; Gu, Z.; Abe, A.; Kondo, Y. Immunomodulatory effects of Alliums and Ipomoea batata extracts on lymphocytes and macrophages functions in White Leghorn chickens: In vitro study. Anim. Sci. J. 2012, 83, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kyo, E.; Uda, N.; Ushijima, M.; Kasuga, S.; Itakura, Y. Prevention of psychological stress-induced immune suppression by aged garlic extract. Phytomedicine 1999, 6, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhang, G.; Hao, T.; Zhou, B.; Zhang, H.; Jiang, Z. Effect of diallyl trisulfide on the activation of T cell and macrophage-mediated cytotoxicity. J. Tongii Med. Univ. 1994, 14, 142–147. [Google Scholar] [CrossRef]
- Nantz, M.P.; Rowe, C.A.; Muller, C.E.; Creasy, R.A.; Stanika, J.M.; Percival, S.S. Supplementation with aged garlic improves both NK and –T cell function and reduces the severity of cold and flu symptoms: A randomized, double-blind placebo-controlled nutrition intervention. Clin. Nutr. 2012, 31, 337–344. [Google Scholar] [CrossRef]
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L. Ortuño-Sahagún, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef]
- Costantini, D.; Møller, A. Does immune response cause oxidative stress in birds? A meta-analysis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 153, 339–344. [Google Scholar] [CrossRef]
- Karangiya, V.; Savsani, H.; Patil, S.S.; Garg, D.; Murthy, K.; Ribadiya, N.; Vekariya, S. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Veter. World. 2016, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Pirgozliev, V.; Rose, S.; Catherine, I.; Blanchard, A. Phytogenic Feed Additives Can Alleviate the Negative Impact of Necrotic Enteritis in Broilers. In Proceedings of the 6th International Conference Poultry Intestinal Health, Rome, Italy, 3–5 April 2019. [Google Scholar]
- Rees, L.P.; Minney, S.F.; Plummer, N.T.; Slater, J.H.; Skyrme, D.A. A quantitative assessment of the antimicrobial activity of garlic (Allium sativum). World J. Microbiol. Biotechnol. 1993, 9, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Skyrme, D.A. The Antimicrobial Activity of Allium sativum. Ph.D. Thesis, Cardiff University, Cardiff, UK, 1997. [Google Scholar]
- Elmahallawy, E.K.; Fehaid, A.; El-Shewehy, D.M.; Ramez, A.M.; Alkhaldi, A.A.; Mady, R.; Nasr, N.E.; Arafat, N.; Hassanen, E.A.; Alsharif, K.F.; et al. S-methylcysteine ameliorates the intestinal damage induced by Eimeria tenella infection via targeting oxidative stress and inflammatory modulators. Front. Veter. Sci. 2022, 8, 754991. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Eller, J.; Albrecht, F.; Prochnow, P.; Kuhlmann, K.; Bandow, J.E.; Slusarenko, A.J.; Leichert, L.I.O. Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. J. Biol. Chem. 2016, 291, 11477–11490. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Torres, S.; Soria Fregozo, C.; Jaramillo-Juarez, F.; Moreno-Hernandez-Duque, J. Allium sativum aqueous extract prevents potassium dichromateinduced nephrotoxicity and lipid oxidation in rats. J. Pharm. Pharmac. Res. 2014, 2, 45–52. [Google Scholar] [CrossRef]
- Abou-Elkhair, R.; Gaafar, K.M.; Elbahy, N.M.; Helal, M.A.; Mahboub, H.D.; Sameh, G. Bioactive effect of dietary supplementation with essential oils blend of oregano, thyme and garlic oils on performance of broilers infected with Eimeria species. Global Veter. 2014, 13, 977–985. [Google Scholar] [CrossRef]
- El-Khtam, A.; Shata, A.; El-Hewaity, M.H. Efficacy of turmeric (Curcuma longa) and garlic (Allium sativum) on Eimeria spp. in broilers. Int. J. Bas. Appl. Sci. 2014, 3, 349–356. [Google Scholar] [CrossRef][Green Version]
- El Dakroury, M.F.; Reda, S.F.; Baz, G.M. A study of the anticoccidial effects of clopidol and garlic powder on Eimeria-infected broilers. Assiut. Veter. Med. J. 2016, 62, 84–89. [Google Scholar]
- Kefyalew, D.; Sibhat, B.; Cheru, H. Anticoccidial effect of garlic on leghorn chickens. Biomed. Nurs. 2018, 4, 70–74. [Google Scholar]
- Udo, E.J.; Abba, A.M. Comparative study of in-vitro anticoccidial efficacy of Allium sativum and Carica papaya. J. Zool. Res. 2018, 2, 10–14. [Google Scholar] [CrossRef]
- Al-Shaibani, I.R.M.; Al-Khadher, A.M.A.; AlHibah, A.Z.H. Anticoccidial activity of Allium sativum and Punica granatum against experimentally induced Eimeria tenella infection in broiler chickens. Asian J. Res. Anim. Vet. Sci. 2020, 5, 20–29. [Google Scholar] [CrossRef]
- Sidiropoulou, E.; Skoufos, I.; Marugan-Hernandez, V.; Giannenas, I.; Bonos, E.; Aguiar-Martins, K.; Lazari, D.; Blake, D.P.; Tzora, A. In vitro anticoccidial study of oregano and garlic essential oils and effects on growth performance, fecal oocyst output, and intestinal microbiota in vivo. Front. Veter. Sci. 2020, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.H.; Soad, M.; Mervat, A.A.; Gehan, N.A.; Rania, A.A. Comparative biochemical and pathological studies between toltrazuril and garlic supplementation in chickens experimentally infected with coccidiosis. Egypt. J. Anim. Health 2021, 1, 65–80. [Google Scholar] [CrossRef]
- Adjei-Mensah, B.; Atuahene, C.C. Avian coccidiosis and anticoccidial potential of garlic (Allium sativum L.) in broiler production: A review. J. Appl. Poult. Res. 2023, 32, 100314. [Google Scholar] [CrossRef]
- Ali, M.; Chand, N.; Khan, R.U.; Naz, S.; Gul, S. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 2019, 47, 79–84. [Google Scholar] [CrossRef]
- Asghar, M.; Durrani, U.F.; Hussain, R.; Matloob, K.; Mahmood, A.K.; Anees, M.; Oneeb, M. Comparative efficacy of amprolium, garlic oil (A. sativum) and ginger oil (Zingiber officinale) against coccidiosis in common quail (Coturnix coturnix). J. Hellen. Veter. Med. Soc. 2020, 71, 2273–2278. [Google Scholar] [CrossRef]
- Allen, P.C.; Danforth, H.D.; Augustine, P.C. Diet modulation of avian coccidiosis. Int. J. Parasitol. 1998, 28, 1131–1140. [Google Scholar] [CrossRef]
- Pourali, M.; Kermanshahi, H.; Golian, A.; Razmi, G.R.; Soukhtanloo, M. Antioxidant and anticoccidial effects of garlic powder and sulfur amino acids on Eimeria infected and uninfected broiler chickens. Iran. J. Veter. Res. 2014, 15, 227–232. [Google Scholar] [CrossRef]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Lillehoj, E.P.; Bravo, D. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br. J. Nutr. 2013, 109, 76–88. [Google Scholar] [CrossRef]
- Christaki, E.; Florou-Paneri, P.; Giannenas, I.; Papazahariadou, M.; Botsoglou, N.A.; Spais, A.B. Effect of a mixture of herbal extracts on broiler chickens infected with Eimeria tenella. Anim. Res. 2004, 53, 137–144. [Google Scholar] [CrossRef]
- Jamil, M.; Aleem, M.T.; Shaukat, A.; Khan, A.; Mohsin, M.; Rehman, T.U.; Abbas, R.Z.; Saleemi, M.K.; Khatoon, A.; Babar, W.; et al. Medicinal plants as an alternative to control poultry parasitic diseases. Life 2022, 12, 449. [Google Scholar] [CrossRef] [PubMed]
- Tanweer, A.J.; Saddique, U.; Bailey, C.A.; Khan, R.U. Antiparasitic effect of wild rue (Peganum harmala L.) against experimentally induced coccidiosis in broiler chicks. Parasitol. Res. 2014, 113, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.; Al-Sagan, A.A.; Abd El-Hack, M.E.; Taha, A.E.; M. AbdElhakim, Y.; Prasad Devkota, H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, E.; Turel, I.; Gul, A.; Yilmaz, O. Evaluation of the anthelmentic activity of garlic (Allium sativum) in mice naturally infected with Aspiculuris tetraptera. Rec. Pat. Antiinfect. Drug Discov. 2008, 3, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Abdel-Baki, A.S.; Wunderlich, F.; Sies, H.; AlQuraishy, S. Anticoccidial and antiinflammatory activity of garlic in murine Eimeria papillata infections. Veter. Parasitol. 2011, 175, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Sadek, H.A.; Abdel-Rahman, S.M.; Bakir, H.Y.; Arafa, M.I.; Ahmed, A.A.; Gaber, M.M. The potential convention of garlic and black seed different extracts as an effective treatment of Cryptosporidium spp.: An experimental study. J. Egypt. Soc. Parasitol. 2020, 50, 613–621. [Google Scholar] [CrossRef]
- Sklan, D.; Berner, Y.N.; Rabinowitch, H.D. The effect of dietary onion and garlic on hepatic lipid concentrations and activity of antioxidative enzymes in chicks. J. Nutr. Biochem. 1992, 3, 322–325. [Google Scholar] [CrossRef]
- Konjufca, V.H.; Pesti, G.M.; Bakalli, R.I. Modulation of cholesterol levels in broiler meat by dietary garlic and copper. Poult. Sci. 1997, 76, 1264–1271. [Google Scholar] [CrossRef]
- Lim, K.S.; You, S.J.; An, H.K.; Kang, C.W. Effects of dietary garlic powder and copper on cholesterol content and quality characteristics of chicken eggs. Asian-Australas. J. Anim. Sci. 2006, 19, 582–586. [Google Scholar] [CrossRef]
- Azeke, M.; Ekpo, K.E. Egg yolk cholesterol lowering effects of garlic and tea. J. Biol. Sci. 2008, 8, 456–460. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.J.; Jin, S.K.; Yang, H.S. Effect of dietary garlic bulb and husk on the physicochemical properties of chicken meat. Poult. Sci. 2009, 88, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Yoo, J.S.; Lee, J.H.; Jang, H.D.; Wang, J.P.; Zhou, T.X.; Kim, I.H. Effects of fermented garlic powder on production performance, egg quality, blood profiles and fatty acids composition of egg yolk in laying hens. Asian Australs. J. Anim. Sci. 2010, 23, 786–791. [Google Scholar] [CrossRef]
- Ghasemi, R.; Zarei, M.; Torki, M. Adding medicinal herbs including garlic (Allium sativum) and thyme (Thymus vulgaris) to diet of laying hens and evaluating productive performance and egg quality characteristics. Am. J. Anim. Veter. Sci. 2010, 5, 151–154. [Google Scholar] [CrossRef]
- Issa, K.J.; Omar, J.M.A. Effect of garlic powder on performance and lipid profile of broilers. Open J. Anim. Sci. 2012, 2, 62–68. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, S.O.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Chang, Y.C.; Kim, W.J.; Kim, C.M.; Yoo, Y.H.; Choi, Y.H. An exploration of the antioxidant effects of garlic saponins in mouse-derived C2C12 myoblasts. Int. J. Mol. Med. 2016, 37, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.O.; Kabutu, F.R.; Bello, M.; Babayo, U. Inhibition of paracetamol-induced oxidative stress in rats by extracts of lemongrass (Cymbropogon citratus) and green tea (Camellia sinensis) in rats. Afr. J. Biotechnol. 2006, 5, 1227–1232. [Google Scholar]
- Qureshi, A.A.; Abuirmeileh, N.; Din, Z.Z.; Elson, C.E.; Burger, W.C. Inhibition of cholesterol and fatty acid biosynthesis in liver enzymes and chicken. Hepatocytes by polar fractions of garlic. Lipids 1983, 18, 343–348. [Google Scholar] [CrossRef]
- Horie, T.; Awazu, S.; Itakura, Y.; Fuwa, T. Identified diallyl polysulfides from an aged garlic extract which protects the membranes from lipid peroxidation. Planta Med. 1992, 58, 468–469. [Google Scholar] [CrossRef]
- Chen, C.H.; Chan, H.C.; Chu, Y.T.; Ho, H.Y.; Chen, P.Y.; Lee, T.H.; Lee, C.K. Antioxidant activity of some plant extracts towards xanthine oxidase, lipoxygenase and tyrosinase. Molecules 2009, 14, 2947–2958. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Lee, M.T.; Chang, S.C.; Chang, Y.L.; Shih, C.H.; Yu, B.; Lee, T.T. Effects of mulberry leaves on production performance and the potential modulation of antioxidative status in laying hens. Poult. Sci. 2017, 96, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Pourakbari, M.; Seidavi, A.; Asadpour, L.; Martínez, A. Probiotic level effects on growth performance, carcass traits, blood parameters, cecal microbiota, and immune response of broilers. An. Acad. Bras. Ciênc. 2016, 88, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Cavallito, C.J.; Bailey, J.H. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Ko, J.W.; Park, S.H.; Lee, I.C.; Lee, S.M.; Shin, I.S.; Kang, S.S.; Moon, C.; Kim, S.H.; Heo, J.D.; Kim, J.C. Protective effects of garlic oil against 1,3-dichloro-2-propanol-induced hepatotoxicity: Role of CYP2E1 and MAPKs. Mol. Cell. Toxicol. 2016, 12, 185–195. [Google Scholar] [CrossRef]
- Guan, M.J.; Zhao, N.; Xie, K.Q.; Zeng, T. Hepatoprotective effects of garlic against ethanol-induced liver injury: A mini-review. Food Chem. Toxicol. 2018, 111, 467–473. [Google Scholar] [CrossRef]
- Lai, Y.S.; Chen, W.C.; Ho, C.T.; Lu, K.H.; Lin, S.H.; Tseng, H.C.; Lin, S.Y.; Sheen, L.Y. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J. Agric. Food Chem. 2014, 62, 5897–5906. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Z.U.; Munir, M.T. Effect of garlic on the health and performance of broilers. Veterinaria 2015, 3, 32–39. [Google Scholar]
- Peinado, M.J.; Ruiz, R.; Echávarri, A.; Rubio, L.A. Garlic derivative propyl propane thiosulfonate is effective against broiler enteropathogens in vivo. Poult. Sci. 2012, 91, 2148–2157. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, M.; Singh, N.; Kaur, P.; Sethi, A.; Sikka, S. Effect of sun dried whole bulb garlic powder on nutrient utilization, blood parameters, duodenum morphology and faecal microbial load in broiler chickens. Indian J. Anim. Sci. 2017, 87, 195–198. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, J.; Liu, Y.; Wu, Y.; Xu, Y.; Feng, J. Dietary garlic powder alleviates lipopolysaccharide-induced inflammatory response and oxidative stress through regulating the immunity and intestinal barrier function in broilers. Animals 2022, 12, 2281. [Google Scholar] [CrossRef]
- Bjerrum, L.; Engberg, R.M.; Leser, T.D.; Jensen, B.B.; Finster, K.; Pedersen, K. Microbial community composition of the ileum and cecum of broiler chickens as revealed by molecular and culture-based techniques. Poult. Sci. 2006, 85, 1151–1164. [Google Scholar] [CrossRef]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955–962. [Google Scholar] [CrossRef]
- Peinado, M.J.; Ruiz, R.; Echávarri, A.; Aranda-Olmedo, I.; Rubio, L.A. Garlic derivative PTS-O modulates intestinal microbiota composition and improves digestibility in growing broiler chickens. Anim. Feed Sci. Technol. 2013, 181, 87–92. [Google Scholar] [CrossRef]
- Lee, S.H.; Bang, S.; Jang, H.H.; Lee, E.B.; Kim, B.S.; Kim, S.H.; Kang, S.H.; Lee, K.W.; Kim, D.W.; Kim, J.B.; et al. Effects of Allium hookeri on gut microbiome related to growth performance in young broiler chickens. PLoS ONE 2020, 15, e0226833. [Google Scholar] [CrossRef] [PubMed]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gutmorphology in broiler chicks. Poult Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Havlik, J.; Cong, W.; Mullen, W.; Preston, T.; Morrison, D.J.; Combet, E. Polyphenols and health: Interactions between fibre, plant polyphenols and the gut microbiota. Nutr. Bull. 2017, 42, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Carbonero, F.; Benefiel, A.C.; Alizadeh-Ghamsari, A.H.; Gaskins, H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012, 3, 448. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Levina, N.; van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (allicin), a volatile antimicrobial from garlic (Allium sativum), kills human lung pathogenic bacteria, including MDR strains, as a vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef] [PubMed]
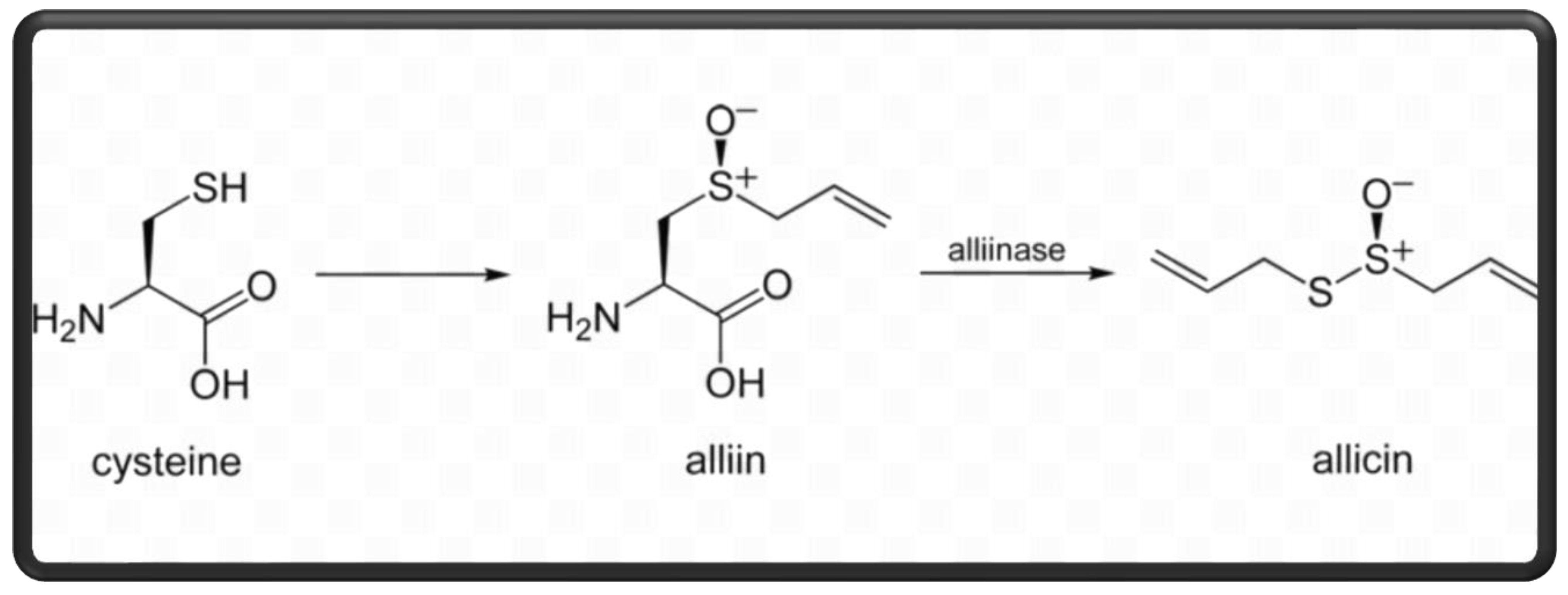
| Component | Importance |
|---|---|
| Carbohydrates (33.06 g) | Important for energy, immunity, disease prevention, and blood clotting |
| Protein (6.36 g) | Development of body tissues |
| Fiber (2.1 g) | Shortens the stagnant time in the gut |
| Sugar (1 g) | Important for energy, immunity, disease prevention, and blood clotting |
| Fats (0.5 g) | Formation of cell membranes |
| Vitamin B3 (0.7 g) | Formation of coenzyme NAD |
| Vitamin B5 (0.6 g) | Formation of coenzymes of amino acid metabolism |
| Vitamin B2 (0.1 g) | Formation of coenzyme FAD |
| Vitamin B1 (0.2 mg) | Carbohydrate metabolism and synthesis of acetylcholine |
| Vitamin B6 (1.2 mg) | Formation of coenzymes in different reactions |
| Vitamin C (31.2 mg) | Protein synthesis |
| Vitamin B9 (3 µg) | Synthesis of DNA |
| Calcium (181 mg) | Formation of bone and coagulation process |
| Phosphorus (153 mg) | Formation of lipids, proteins, sugars, and nucleic acid |
| Magnesium (25 mg) | Cofactor for kinase and decarboxylase |
| Sodium (17 mg) | Formation of membrane |
| Zinc (1.16 mg) | Cofactor for some enzymes |
| Selenium (14.2 µg) | Cofactor for glutathione coagulase |
| Sulfur (16%) | Antimicrobial |
| Dose/Route | Effects | Reference |
|---|---|---|
| Garlic paste (3.8%), solvent fractions, or garlic oil equal to this quantity in feed | No effect on FI | [27] |
| Garlic 0, 0.01, 0.1 or 1% in meal | No improvement in the performance | [28] |
| Garlic powder 0.2% and 0.4% of feed | No effects on BWG, FI, FCR, carcass cuts, and visceral organs | [29] |
| Garlic at 1 kg/ton feed | Enhanced carcass yield | [30] |
| Garlic 1, 3, and 5% and 3% garlic powder + 200 IU of α-tocopherol/kg of feed | No influence on performance Increased crude protein Decreased crude fat contents of carcass, the pH, and thiobarbituric acid reactive substances of meat | [31] |
| Garlic 0.5%, 1.0%, and 3% | Decreased heart weight | [32] |
| Garlic powder 0.5% of feed | Increased live BWG | [33] |
| Garlic powder 3% and 5% of diet | Increased breast weight (3%) Low BW (5%) | [34] |
| A mixture of ginger and garlic (1:1 ratio) 50 mL/L of the drinking water | Improved BW, BWG, FI, and FCR | [35] |
| Garlic bulb 5, 10, or 15 g/kg meal | Decreased BW (high dose and standard temperature) No effect on the FCR | [36] |
| The 5 g/kg garlic powder + 1 g/kg black pepper powder and 10 g/kg garlic powder + 2 g/kg black pepper powder | Improved WG and FCR | [37] |
| Fresh garlic paste 0.2, 0.4, 0.6, and 0.8%/L of drinking water | No effect on BWG or FCR Decreased mortality | [38] |
| Garlic powder 3% in diet and a mixture of garlic powder 1.5% plus turmeric powder 0.25% | Improved BWG, FI, FCR, performance index, and protein efficiency ratio | [39] |
| Garlic paste 0.25% and 0.50% with basal diet | Improved BWG, FCR, and livability No influence on carcass attributes | [40] |
| Garlic 5 g/kg feed, black cumin 5 g/kg, or their combination | No difference in BWG, FI, FCR, and relative organ weights | [41] |
| A basal diet plus 0.25, 0.50, and 0.75 g garlic powder/kg diet | Increased BW and BWG at 21 and 42 days of age High length and average width of small intestine | [14] |
| Garlic essential oil (200 mg/kg diet) alone/or in combination with lemon essential oil (200 mg/kg diet) under heat stress | Enhancement in BW, FCR, carcass dressing, and increasing the digestive enzymes Decreasing mortality rate and abdominal fat content | [26] |
| Garlic powder 3% of feed | Improved BWG and final BW | [42] |
| Dose/Route | Effects | Reference |
|---|---|---|
| 1 or 3% garlic meal | Decreased egg yolk cholesterol | [43] |
| Garlic paste (3.8%), solvent fractions, or garlic oil equal to this quantity in feed | No effect on daily FI | [27] |
| Garlic oil 0.02% in meal | No effect on egg production, egg mass, body weight, feed consumption, and feed efficiency | [44] |
| Garlic powder 3% in diet | No differences in the color and flavor of eggs No change in yolk cholesterol concentrations | [45] |
| Sun-dried garlic paste 0, 2, 4, 6, 8, or 10% of diet | No effect on egg weight, egg mass, feed consumption, and feed efficiency among diets or birds’ strain Increased Yolk weight with increasing levels of dietary garlic Decreased yolk cholesterol concentrations | [46] |
| Garlic powder 0, 5, 10, and 15 g/kg feed | Decreased yolk weight | [47] |
| Garlic powder 0.5 and 10 g/kg feed | Increased egg weight Decreased egg yolk cholesterol triglyceride No effect on performance or egg albumin index, eggshell index, and egg Haugh unit | [48] |
| Garlic powder 0, 2, 6, or 8% in feed | Increased egg production | [49] |
| Garlic 2% and fenugreek 2% | No effect on FI, FCR, BW, BWG, egg rate, egg weight, and egg mass Increased yolk weight and color and Haugh units Decreased albumen weight | [50] |
| Garlic powder 8% in feed | Better egg production No effect on egg mass and egg weight | [51] |
| Garlic powder 1, 2, and 4% in feed | Increased egg production No effect on egg weight, yolk index, shell weight, shell thickness, yolk weight (1% garlic) Decreased eggshell index and Haugh unit (4% garlic) | [52] |
| Garlic juice at 0.25, 0.50, and 1% | Improved egg albumin, yolk and shell weight, albumin height, and Haugh unit | [53] |
| Garlic powder 1%, fenugreek 1%, and garlic powder 1% + fenugreek 0.5% | No effect on laying hens’ performance | [54] |
| Garlic 1, 2, and 3% of ration | No effect on BWG, FCR, egg production, egg mass, albumen weight, albumen height, Haugh unit, yolk index, yolk height, egg weight, fertility, hatchability, embryonic mortality, chick weight, and chick visual score, shell thickness, and shell weight An improvement in yolk diameter, yolk weight, chick length, and yolk color | [55] |
| A mixture of lemon, onion, and garlic juice in portions 1.00, 1.00, and 0.125/L of the drinking water, respectively | Improved FCR Increased number of eggs/hen, percentage of egg production, and egg mass/hen Enhanced yolk color and yolk percentage | [56] |
| Dose/Route | Type of Production | Effects | Reference |
|---|---|---|---|
| Garlic powder 1% or 3% garlic | Broiler chickens | Enhanced antibody production against NDV and leukocyte count | [69] |
| Garlic 10 and 30 g/kg diet | White Leghorn chickens | Enhanced antibodies against NDV, SRBCs, and BA Augmented splenocyte and thymocyte proliferations Reduced CD4+, but increasing CD4: CD8- lymphocyte ratios and WBCs count Increased relative weights of immune organs (spleen, thymus glands, and bursa of Fabricius) | [70] |
| Garlic 0.5%, 1.0%, and 3% | Broiler chickens | Lower weights of bursa of Fabricius and spleen | [32] |
| Garlic powder 0.1% | Broiler chickens | Improved relative weight of bursa of Fabricius without effect on the spleen weight No effect on NDV vaccine (LaSota) antibody response | [71] |
| Garlic powder 3% and 5% of diet | Broiler chickens | No influence on bursa of Fabricius and thymus weights Decrease spleen weight | [34] |
| A mixture of ginger and garlic (1:1 ratio) 50 mL/L of the drinking water | Marshal broiler chickens | Increased total protein, albumin, and globulin | [35] |
| Garlic extract (allicin) 25, 50, 75, or 100 mg/kg diet | Broiler chickens | Increased total protein and albumin concentrations by about 4.7 and 5.9%, respectively (50 mg/kg) No effect on total protein, albumin, or globulin concentrations (25, 75, or 100 mg/kg) | [72] |
| Fresh garlic paste 0.2, 0.4, 0.6, and 0.8%/L of drinking water | Broiler chickens | Increased antibody titer against NDV | [38] |
| Garlic meal 0.125% of feed | Broiler chickens | Reducing scores of IBDV signs Higher mortality rate High antibody response to IBDV | [73] |
| Garlic essential oil 0.06 mL/L drinking water | Broiler chickens | Improved immune organ index, IgM, IgG, and IgA | [74] |
| A basal diet plus 0.25, 0.50, and 0.75 g garlic powder/kg diet | Broiler chickens | Increasing total protein, globulin, IgM, and IgG Improved liver and immune-related organ weight | [14] |
| Garlic essential oil (200 mg/kg diet) alone/or in combination with lemon essential oil (200 mg/kg diet) under heat stress | Broiler chickens | Increasing the relative weight of bursa of Fabricius and the serum antibody titer against NDV No changes in relative weights of spleen and thymus glands, and antibody titer against AIV | [26] |
| Dose/Route | Type of Production | Effects | Reference |
|---|---|---|---|
| Garlic paste (3.8%), solvent fractions, or garlic oil equal to this quantity in feed | Broiler chickens Leghorn laying pullets | Decreasing serum cholesterol by 18 and 23% in broilers and Leghorn pullets, respectively | [27] |
| Garlic oil 0.02% in meal | Babcock B-300 strain of laying hens | No effect on serum cholesterol | [44] |
| Garlic 2% in feed | Broiler chickens | Lowering in hepatic cholesterol concentrations | [112] |
| Garlic 3% in meal | Broiler chickens | Decreased cholesterol in plasma and breast and thigh muscles | [113] |
| Garlic powder 3% in diet | Laying hens | No change in serum cholesterol concentrations | [54] |
| Sun-dried garlic paste 0, 2, 4, 6, 8, or 10% of diet | Hisex Brown, Isa Brown, Lohmann, Starcross, Babcock, and Starcross-579 strains of laying hens | Decreased serum cholesterol concentrations | [46] |
| Garlic 0, 1, 3, or 5% in meal | Laying hens | No change in HDL level | [114] |
| Garlic powder 0.5 and 10 g/kg feed | Laying hens | Decreased serum triglyceride | [48] |
| Garlic 2% and fenugreek 2% | Lohmann Brown laying hens | Increased HDL Reduced serum cholesterol and LDL | [50] |
| Garlic powder 1% or 3% garlic | Broiler chickens | No effect on leukocyte count | [69] |
| Garlic powder 10 and 20 g kg−1 | Laying hens | Reduced total cholesterol, triglyceride, LDL, and HDL | [115] |
| Garlic powder 5–20 g kg−1 | Broiler chickens | Decreased plasma LDL cholesterol No effect on HDL cholesterol | [116] |
| Fermented garlic powder 3% in diet | Laying hens | Decreased serum cholesterol | [117] |
| Garlic powder 1, 2, and 4% in feed | Laying hens | Increased plasma HDL and LDL (1, 2, and 4%). | [52] |
| Garlic 1, 3, and 5% and 3% garlic powder + 200 IU of α-tocopherol/kg of feed | Broiler chickens | Reduced the total and LDL levels Increased HDL levels | [31] |
| A mixture of garlic and thyme powder 0.1 and 0.2 g kg−1 | Laying hens | No effect on cholesterol, triglyceride, HDL, and LDL | [118] |
| Garlic powder 0.1% | Broiler chickens | Decreased triglycerides, total cholesterol, and LDL Increased HDL | [71] |
| Garlic powder at 0.2% and 0.4% of feed | Cobb broiler chickens | Reduced triglycerides, cholesterol, and LDL Increased HDL | [119] |
| Garlic powder 3% and 5% of diet | Broiler chickens | Decrease spleen weight, RBCs, WBCs, and packed cells volume | [34] |
| Garlic powder 1%, fenugreek 1%, and garlic powder 1% + fenugreek 0.5% Garlic and fenugreek 2% | Laying hens | Decreased LDL Beneficial effects on cholesterol metabolism | [54] |
| A mixture of ginger and garlic (1:1 ratio) 50 mL/L of the drinking water | Marshal broiler chickens | Increased hemoglobin, packed cell volume, WBCs, RBCs, total protein, albumin, and globulin Decreased cholesterol | [35] |
| A mixture of lemon, onion, and garlic juice in portions of 1.00, 1.00, and 0.125/liter of drinking water, respectively | Bovan Brown layer chickens | Decreasing total plasma cholesterol content, GPT, GOT, and creatinine | [56] |
| Garlic 5 g/kg feed, black cumin 5 g/kg, or their combination | Ross-308 broiler chickens | Increasing total protein Reduced GOT | [41] |
| Probiotic, citric acid, and garlic supplemented with 0.5 g kg−1 multi-strain probiotic mixture, citric acid, and garlic powder, respectively. Probiotic-citric and probiotic-garlic groups treated with 0.5 g kg−1 multi-strain probiotic mixture and 0.5 g kg−1 citric acid and garlic powder, respectively, while citric-garlic group fed diet with 0.5 g kg−1 of citric acid and garlic powder. | Broiler chickens | Decreased cholesterol, triglycerides, and LDL Elevated HDL | [23] |
| A basal diet plus 0.25, 0.50, and 0.75 g garlic powder/kg diet | Broiler chickens | Increasing RBCs, hemoglobin HDL, SOD, and total anti-oxidant capacity Decreasing total cholesterol, LDL, GOT, and AMD | [14] |
| Garlic essential oil (200 mg/kg diet) alone/or in combination with lemon essential oil (200 mg/kg diet) under heat stress | Broiler chickens | Reducing MDA, triglycerides, cholesterol, and LDL Increasing HDL, SOD, and GPx | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Ghany, W.A. Potential Effects of Garlic (Allium sativum L.) on the Performance, Immunity, Gut Health, Anti-Oxidant Status, Blood Parameters, and Intestinal Microbiota of Poultry: An Updated Comprehensive Review. Animals 2024, 14, 498. https://doi.org/10.3390/ani14030498
Abd El-Ghany WA. Potential Effects of Garlic (Allium sativum L.) on the Performance, Immunity, Gut Health, Anti-Oxidant Status, Blood Parameters, and Intestinal Microbiota of Poultry: An Updated Comprehensive Review. Animals. 2024; 14(3):498. https://doi.org/10.3390/ani14030498
Chicago/Turabian StyleAbd El-Ghany, Wafaa A. 2024. "Potential Effects of Garlic (Allium sativum L.) on the Performance, Immunity, Gut Health, Anti-Oxidant Status, Blood Parameters, and Intestinal Microbiota of Poultry: An Updated Comprehensive Review" Animals 14, no. 3: 498. https://doi.org/10.3390/ani14030498
APA StyleAbd El-Ghany, W. A. (2024). Potential Effects of Garlic (Allium sativum L.) on the Performance, Immunity, Gut Health, Anti-Oxidant Status, Blood Parameters, and Intestinal Microbiota of Poultry: An Updated Comprehensive Review. Animals, 14(3), 498. https://doi.org/10.3390/ani14030498





