Isolation and Genomic Characterization of a Chinese Genotype C Bovine Parainfluenza Virus Type 3 from Cattle and Its Pathogenicity in C57BL/6 Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Samples and Etiological Examinations
2.2. Virus Isolation
2.3. Immunofluorescence Test
2.4. Electron Microscopy
2.5. Illumina Sequencing
2.6. Genomic Characterization and Phylogenetic Analysis
2.7. Animal Experiments
3. Results
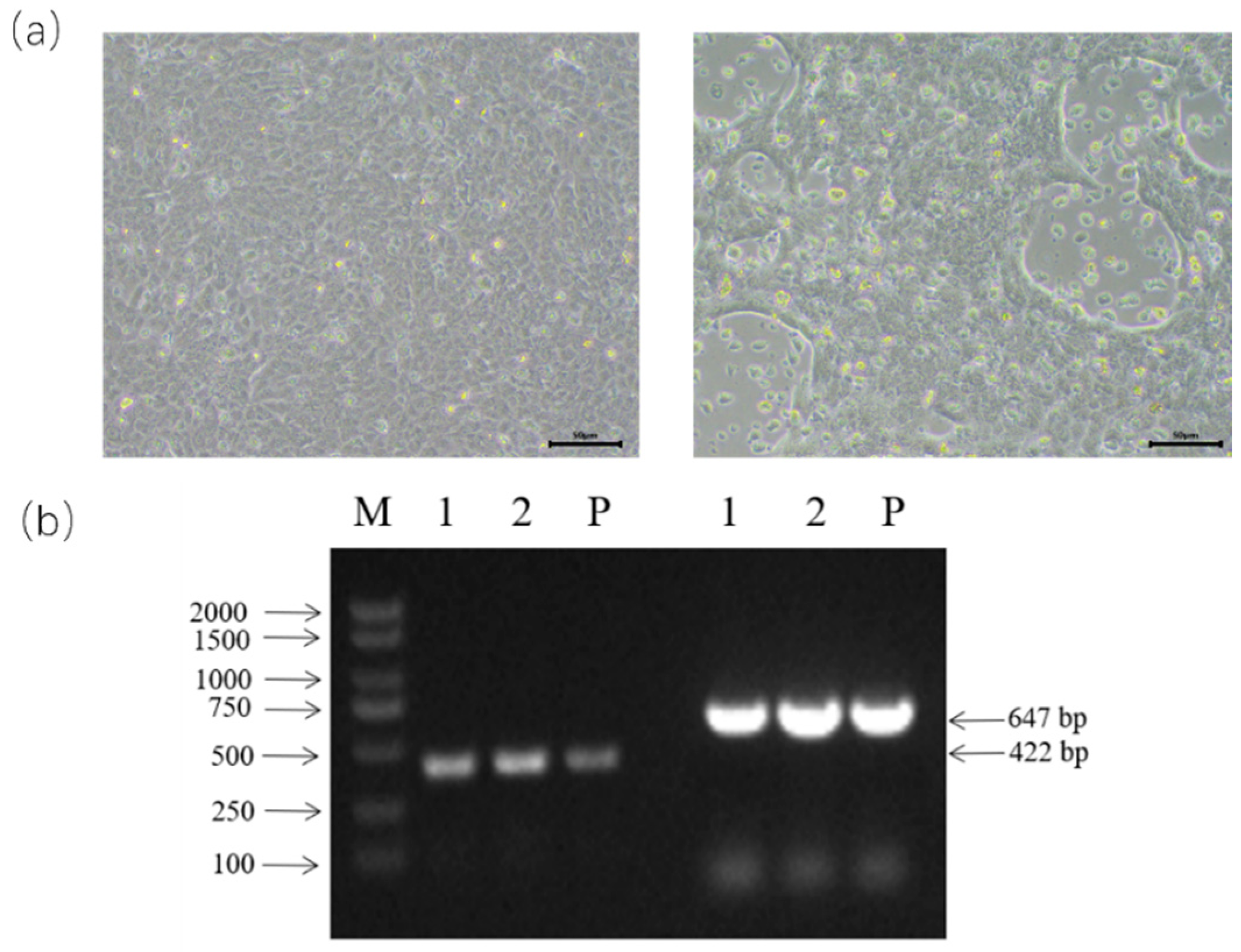
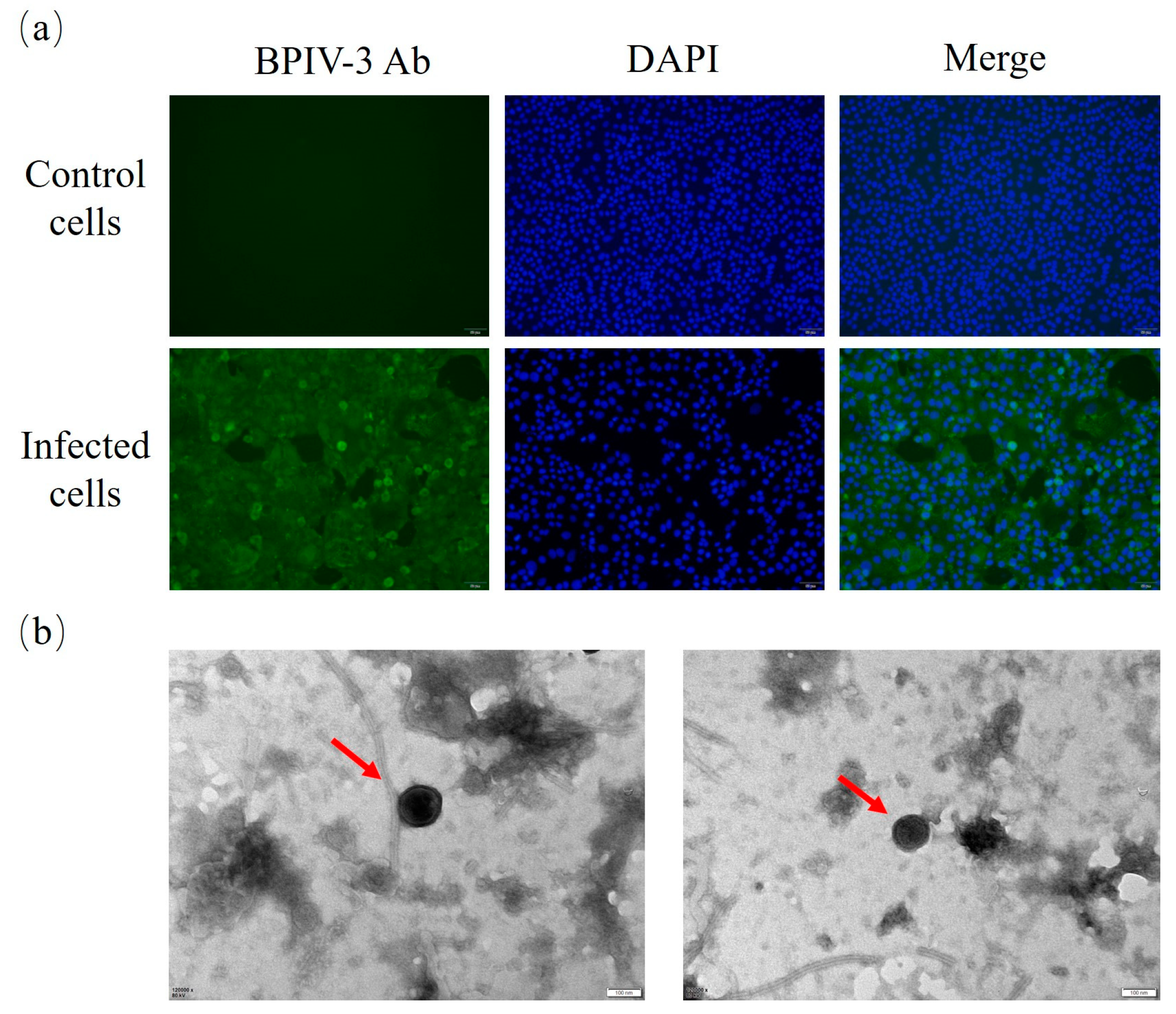
3.1. BPIV-3 Detection, Isolation, and Identification
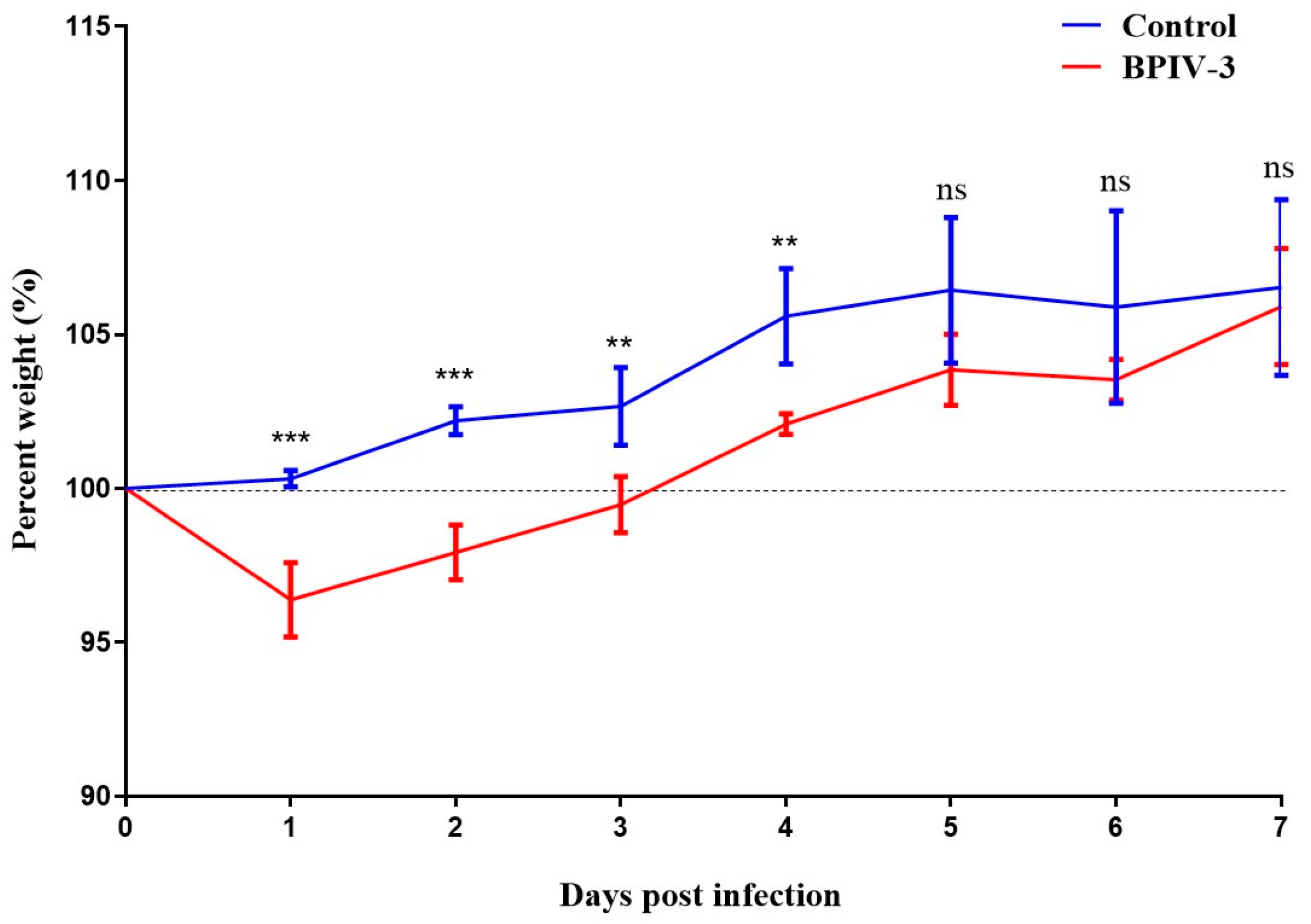
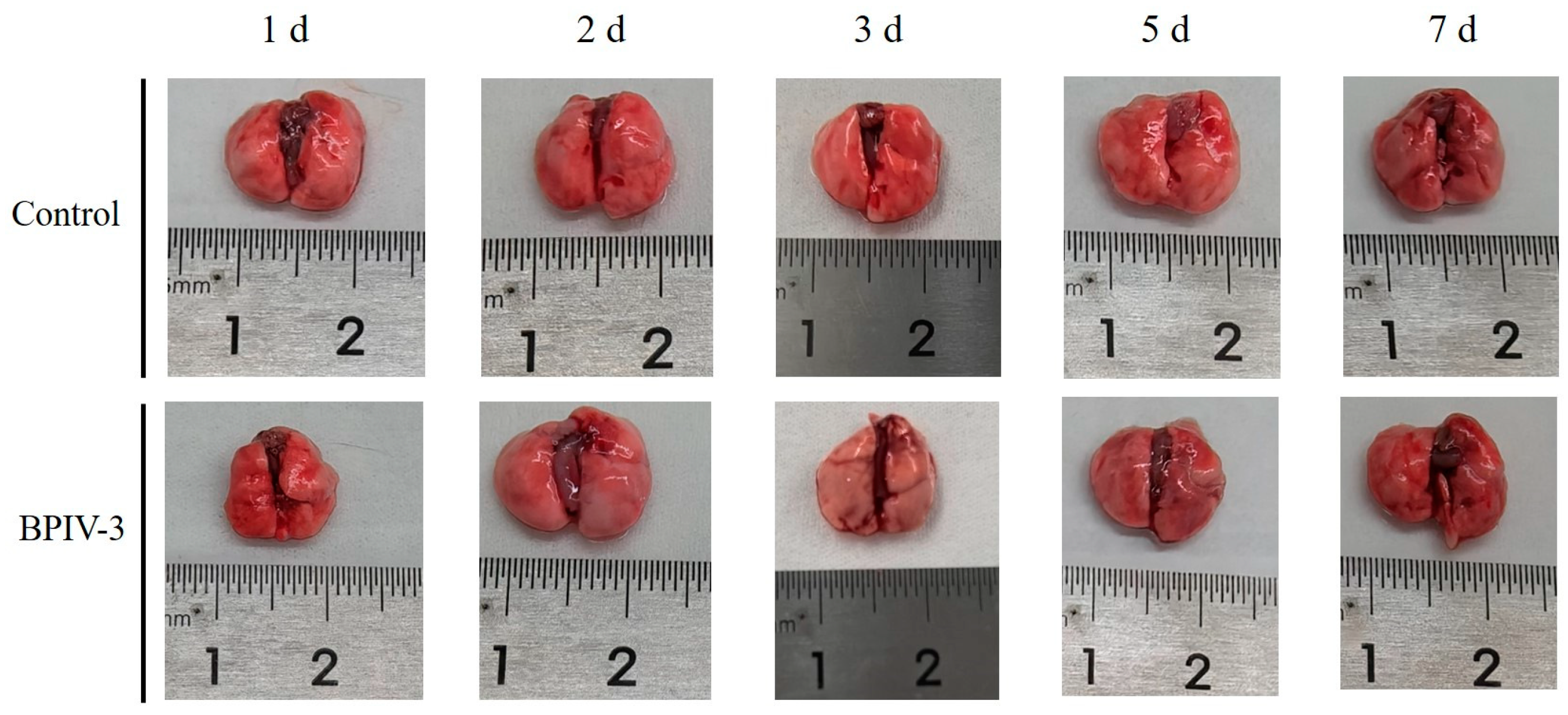
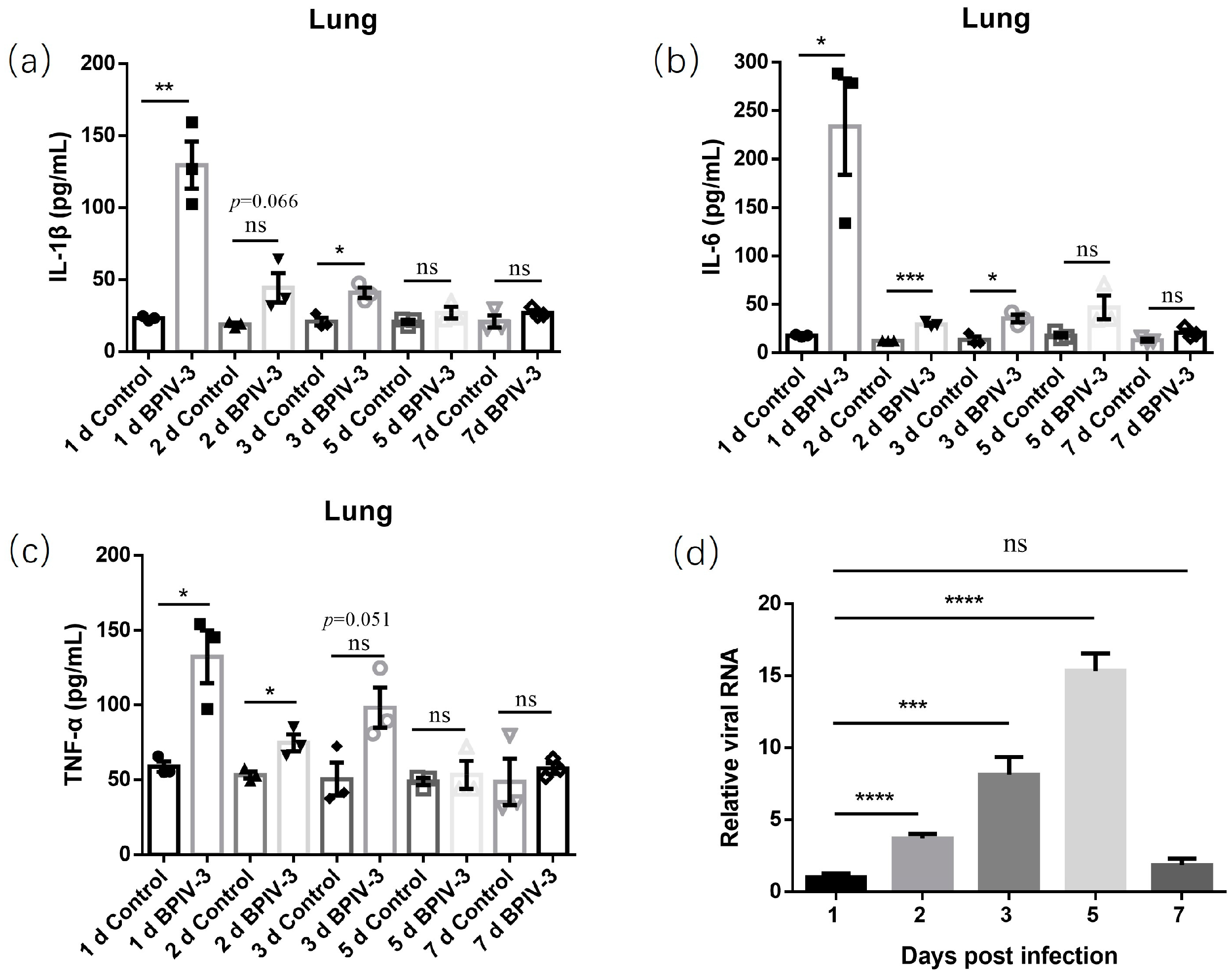
3.2. Pathogenicity of BPIV-3 SC in C57BL/6 Mice
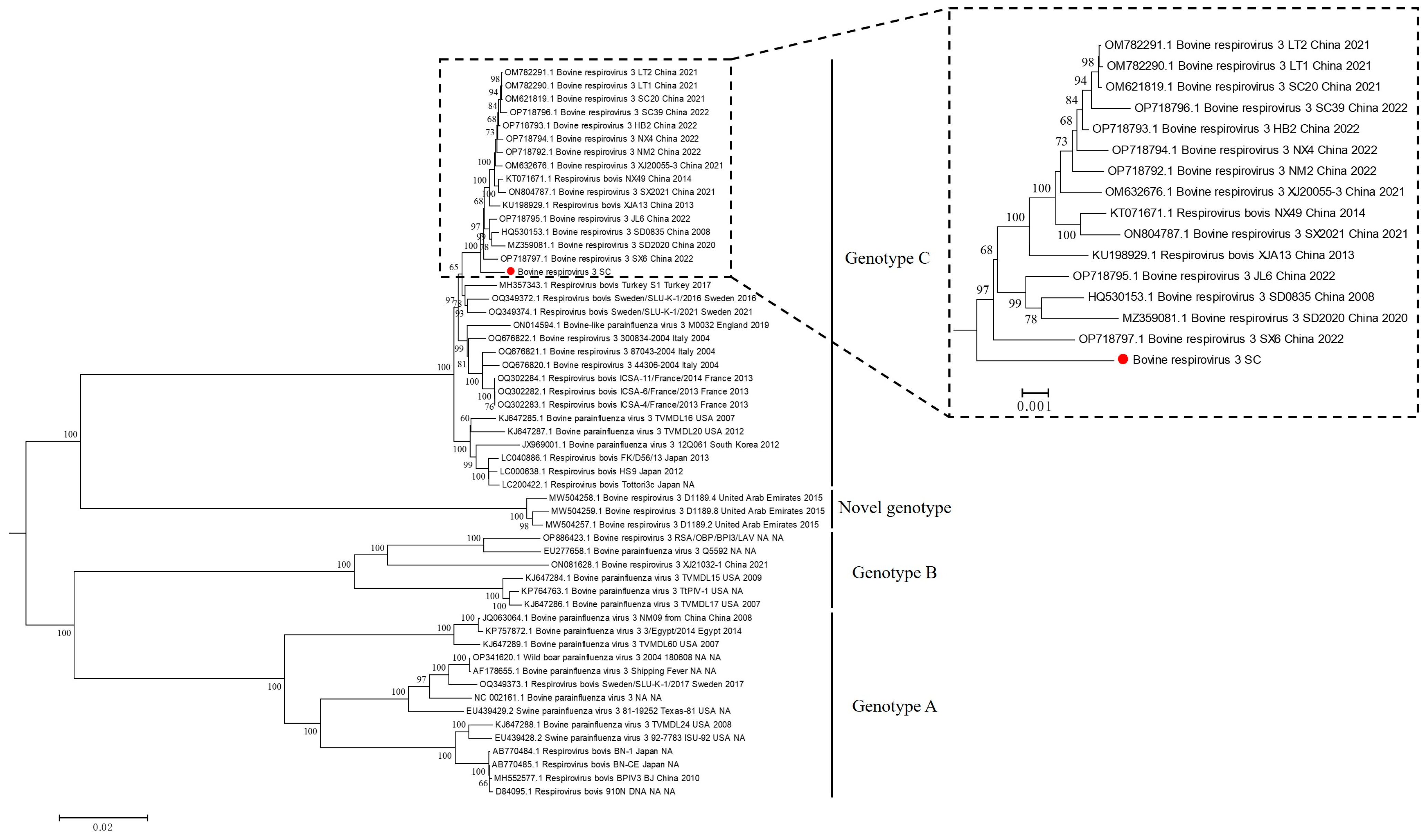
3.3. Whole Genome Sequencing and Phylogenetic Analysis of BPIV-3
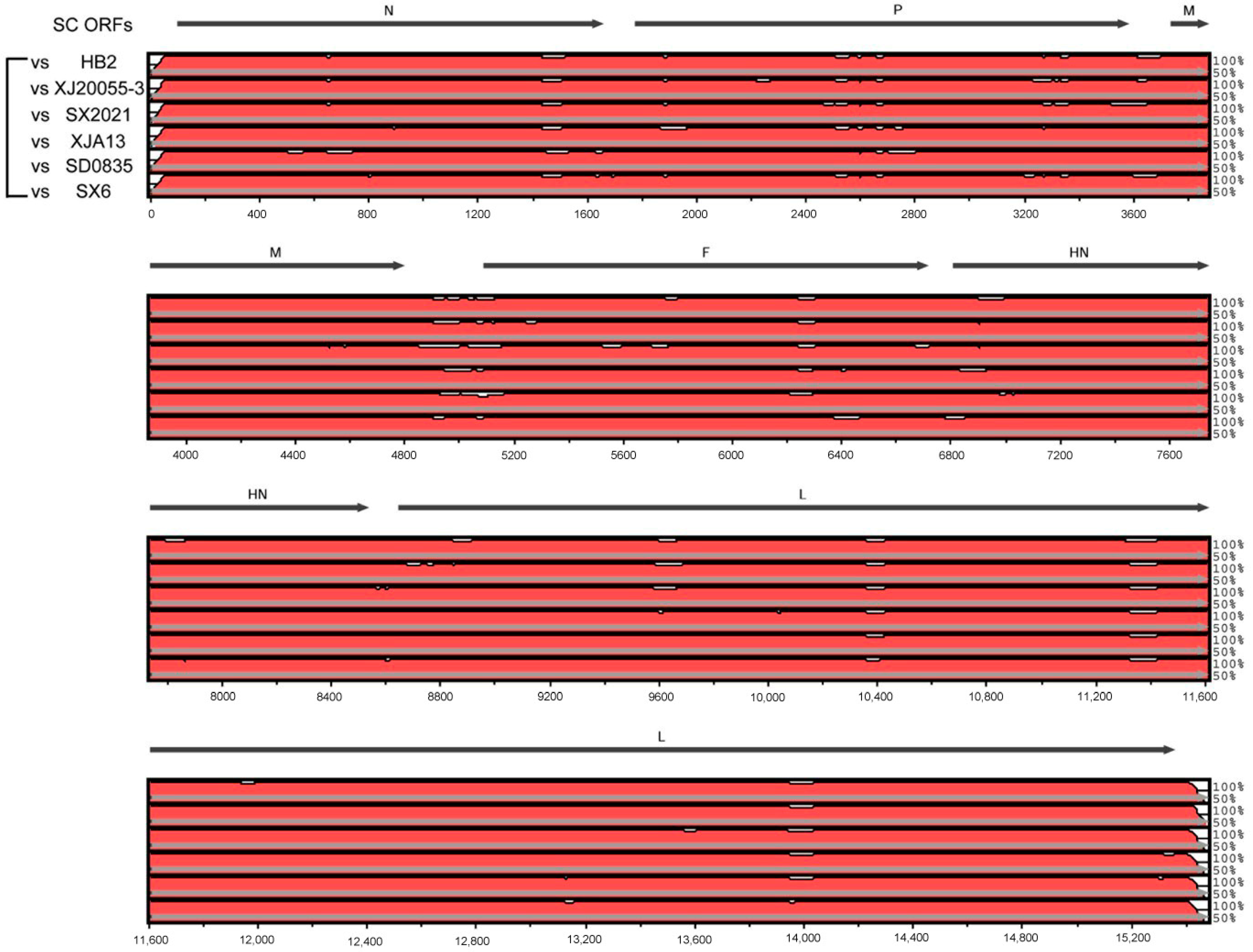
3.4. Genomic Characteristics of BPIV-3 SC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apley, M. The clinical syndrome of BRD: What it is and what it is not. Anim. Health Res. Rev. 2014, 15, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Booker, C.W.; Abutarbush, S.M.; Morley, P.S.; Jim, G.K.; Pittman, T.J.; Schunicht, O.C.; Perrett, T.; Wildman, B.K.; Fenton, R.K.; Guichon, P.T.; et al. Microbiological and histopathological findings in cases of fatal bovine respiratory disease of feedlot cattle in western Canada. Can. Vet. J. 2008, 49, 473–481. [Google Scholar] [PubMed]
- Ng, T.F.; Kondov, N.O.; Deng, X.; Van Eenennaam, A.; Neibergs, H.L.; Delwart, E. A metagenomics and case-control study to identify viruses associated with bovine respiratory disease. J. Virol. 2015, 89, 5340–5349. [Google Scholar] [CrossRef]
- Kirchhoff, J.; Uhlenbruck, S.; Goris, K.; Keil, G.M.; Herrler, G. Three viruses of the bovine respiratory disease complex apply different strategies to initiate infection. Vet. Res. 2014, 45, 20. [Google Scholar] [CrossRef]
- Love, W.J.; Lehenbauer, T.W.; Kass, P.H.; Van Eenennaam, A.L.; Aly, S.S. Development of a novel clinical scoring system for on-farm diagnosis of bovine respiratory disease in pre-weaned dairy calves. PeerJ 2014, 2, e238. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Valarcher, J.F.; Hägglund, S.; Näslund, K.; Zohari, S.; Ducatez, M.F.; Meyer, G. Molecular and genetic characterization of bovine parainfluenza type 3 European field and vaccine strains. Infect. Genet. Evol. 2023, 113, 105483. [Google Scholar] [CrossRef]
- Ellis, J.A. Bovine parainfluenza-3 virus. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 575–593. [Google Scholar] [CrossRef]
- Rima, B.; Balkema-Buschmann, A.; Dundon, W.G.; Duprex, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.; Lee, B.; Rota, P.; et al. ICTV Virus Taxonomy Profile: Paramyxoviridae. J. Gen. Virol. 2019, 100, 1593–1594. [Google Scholar] [CrossRef]
- Neill, J.D.; Ridpath, J.F.; Valayudhan, B.T. Identification and genome characterization of genotype B and genotype C bovine parainfluenza type 3 viruses isolated in the United States. BMC Vet. Res. 2015, 11, 112. [Google Scholar] [CrossRef]
- Bailly, J.E.; McAuliffe, J.M.; Skiadopoulos, M.H.; Collins, P.L.; Murphy, B.R. Sequence determination and molecular analysis of two strains of bovine parainfluenza virus type 3 that are attenuated for primates. Virus Genes 2000, 20, 173–182. [Google Scholar] [CrossRef]
- Horwood, P.F.; Gravel, J.L.; Mahony, T.J. Identification of two distinct bovine parainfluenza virus type 3 genotypes. J. Gen. Virol. 2008, 89, 1643–1648. [Google Scholar] [CrossRef]
- Maidana, S.S.; Lomonaco, P.M.; Combessies, G.; Craig, M.I.; Diodati, J.; Rodriguez, D.; Parreño, V.; Zabal, O.; Konrad, J.L.; Crudelli, G.; et al. Isolation and characterization of bovine parainfluenza virus type 3 from water buffaloes (Bubalus bubalis) in Argentina. BMC Vet. Res. 2012, 8, 83. [Google Scholar] [CrossRef]
- Ohkura, T.; Kokuho, T.; Konishi, M.; Kameyama, K.; Takeuchi, K. Complete Genome Sequences of Bovine Parainfluenza Virus Type 3 Strain BN-1 and Vaccine Strain BN-CE. Genome Announc. 2013, 1, e00247-12. [Google Scholar] [CrossRef]
- Leal, É.; Liu, C.; Zhao, Z.; Deng, Y.; Villanova, F.; Liang, L.; Li, J.; Cui, S. Isolation of a Divergent Strain of Bovine Parainfluenza Virus Type 3 (BPIV3) Infecting Cattle in China. Viruses 2019, 11, 489. [Google Scholar] [CrossRef]
- Sobhy, N.M.; Mor, S.K.; Bastawecy, I.M.; Fakhry, H.M.; Youssef, C.R.B.; Goyal, S.M. Surveillance, isolation and complete genome sequence of bovine parainfluenza virus type 3 in Egyptian cattle. Int. J. Vet. Sci. Med. 2017, 5, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, J.; Meng, F.; Cao, Y.; Wang, Z.; Zhang, Q.; Zhang, Q.; Zhang, X.; Han, M.; Wu, T.; et al. Isolation, identification, and genetic phylogenetic analysis of two different genotypes of bovine parainfluenza 3 virus in China. Viruses 2022, 14, 2221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.M.; Shi, H.F.; Gao, Y.R.; Xin, J.Q.; Liu, N.H.; Xiang, W.H.; Ren, X.G.; Feng, J.K.; Zhao, L.P.; Xue, F. Isolation and genetic characterization of bovine parainfluenza virus type 3 from cattle in China. Vet. Microbiol. 2011, 149, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Oem, J.K.; Lee, E.Y.; Lee, K.K.; Kim, S.H.; Lee, M.H.; Hyun, B.H. Molecular characterization of a Korean bovine parainfluenza virus type 3 isolate. Vet. Microbiol. 2013, 162, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Ohkura, T.; Shimizu, M.; Akiyama, M.; Kameyama, K.; Takeuchi, K. Complete genome sequence of the first isolate of genotype C bovine parainfluenza virus type 3 in Japan. Genome Announc. 2014, 2, e01215-14. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Tong, Q.; Wang, W.; Wu, H. Serological survey of antibodies against bovine parainfluenza virus type 3 in 12 provinces. Chin. J. Prev. Vet. Med. 2014, 36, 154–156. (In Chinese) [Google Scholar]
- Guo, L.; Wang, W.; Zhang, S.Q.; Sun, N.; Wang, F.X.; Wu, H. A multiplex PCR to Detect the Viruses of BVDV, BPIV3, IBRV and BRSV Associated with the Bovine Respiratory Disease Complex in Clinical. Spec. Wild Econ. Anim. Plant Res. 2014, 36, 12–16. (In Chinese) [Google Scholar]
- Sun, F.Y.; Li, Z.J.; Wei, Y.; Sun, L.; Wang, X.W.; Wang, N.; Ye, J.F.; Feng, E.K.; Guo, L. Establishment and preliminary application of a nano-PCR method for detection of bovine coronavirus. Chin. Vet. Sci. 2021, 51, 1221–1226. (In Chinese) [Google Scholar]
- Jin, J.; Yin, Z.; Zhang, Q.; Xu, X.G. Laboratory diagnosis of coinfection with coronavirus, rotavirus and Escherichia coli in calves. Prog. Vet. Med. 2021, 42, 124–127. (In Chinese) [Google Scholar]
- Shen, Y.; Liu, J.; Zhang, Y.H.; Ma, X.Y.; Yue, H.; Tang, C. Prevalence and characteristics of a novel bovine adenovirus type 3 with a natural deletion fiber gene. Infect. Genet. Evol. 2020, 83, 104348. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, X.; Tang, C.; Yue, H. First Isolation and characteristics of bovine parainfluenza virus type 3 from Yaks. Pathogens 2022, 11, 962. [Google Scholar] [CrossRef]
- Dong, X.M.; Zhu, Y.M.; Cai, H.; Lv, C.; Gao, Y.R.; Yu, Z.; Xue, F. Studies on the pathogenesis of a Chinese strain of bovine parainfluenza virus type 3 infection in Balb/c mice. Vet. Microbiol. 2012, 158, 199–204. [Google Scholar] [CrossRef]
- Qu, Z.; Li, M.; An, R.; Dai, H.; Yu, Y.; Li, C.; Cao, C.; Meng, Y.; Wang, J.; Gao, M. Self-assembled BPIV3 nanoparticles can induce comprehensive immune responses and protection against BPIV3 challenge by inducing dendritic cell maturation in mice. Vet. Microbiol. 2022, 268, 109415. [Google Scholar] [CrossRef]
- Teng, J.L.L.; Wernery, U.; Lee, H.H.; Fung, J.; Joseph, S.; Li, K.S.M.; Elizabeth, S.K.; Fong, J.Y.H.; Chan, K.H.; Chen, H.; et al. Co-circulation of a novel dromedary camel parainfluenza virus 3 and middle east respiratory syndrome coronavirus in a dromedary herd with respiratory tract infections. Front. Microbiol. 2021, 12, 739779. [Google Scholar] [CrossRef]
- Dastjerdi, A.; Floyd, T.; Swinson, V.; Davies, H.; Barber, A.; Wight, A. Parainfluenza and corona viruses in a fallow deer (Dama dama) with fatal respiratory disease. Front. Vet. Sci. 2022, 9, 1059681. [Google Scholar] [CrossRef]
- Qiao, D.; Janke, B.H.; Elankumaran, S. Molecular characterization of glycoprotein genes and phylogenetic analysis of two swine paramyxoviruses isolated from United States. Virus Genes 2009, 39, 53–65. [Google Scholar] [CrossRef]
- Qiao, D.; Janke, B.H.; Elankumaran, S. Complete genome sequence and pathogenicity of two swine parainfluenzavirus 3 isolates from pigs in the United States. J. Virol. 2010, 84, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, E.; Lelli, D.; Barbieri, I.; Chiapponi, C.; Moreno, A.; Trogu, T.; Tosi, G.; Lavazza, A. Isolation and molecular characterisation of respirovirus 3 in wild boar. Animals 2023, 13, 1815. [Google Scholar] [CrossRef] [PubMed]

| Pathogens | Sequence (5′-3′) | Length of Amplicon (bp) | References |
|---|---|---|---|
| BVDV | GGTAGCAACAGTGGTGAGTTC | 130 | [21] |
| CTCAGGTTAAGATGTGCTGTG | |||
| BCoV | ACGTTCTTTTAAAACAGCCGATG | 409 | [22] |
| TGCCAGAACAAGACTAGCAA | |||
| BRSV | TATGCTATGTCCCGATTGG | 600 | [21] |
| ACTGATTTGGCTAGTACACCC | |||
| BRV | GGTAGCGGCGTTATTTCC | 407 | [23] |
| CGCCATCTGAGTGATTACTC | |||
| BPIV-3 | GCATCACAAACTCCGCAATAT | 1048 | [17] |
| TGCTTGATTTTTCCGACTCCT | |||
| BADV-3 | CTCCTGGGTCCTGGCCTTAGTT | 1182 | [24] |
| AGTGTTTGTGGGTAAAGGGCAATAG | |||
| BHV-1 | GCTCGCCAACTTCTTTCAGGG | 306 | [21] |
| GCGTCAAACTCCTCCTCTTCCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Qiu, Y.; Xiong, P.; Wang, Z.; Li, N.; Ye, C.; Peng, Y. Isolation and Genomic Characterization of a Chinese Genotype C Bovine Parainfluenza Virus Type 3 from Cattle and Its Pathogenicity in C57BL/6 Mice. Animals 2024, 14, 463. https://doi.org/10.3390/ani14030463
Chen J, Qiu Y, Xiong P, Wang Z, Li N, Ye C, Peng Y. Isolation and Genomic Characterization of a Chinese Genotype C Bovine Parainfluenza Virus Type 3 from Cattle and Its Pathogenicity in C57BL/6 Mice. Animals. 2024; 14(3):463. https://doi.org/10.3390/ani14030463
Chicago/Turabian StyleChen, Jing, Yangyang Qiu, Pan Xiong, Zhijie Wang, Nengzhang Li, Chao Ye, and Yuanyi Peng. 2024. "Isolation and Genomic Characterization of a Chinese Genotype C Bovine Parainfluenza Virus Type 3 from Cattle and Its Pathogenicity in C57BL/6 Mice" Animals 14, no. 3: 463. https://doi.org/10.3390/ani14030463
APA StyleChen, J., Qiu, Y., Xiong, P., Wang, Z., Li, N., Ye, C., & Peng, Y. (2024). Isolation and Genomic Characterization of a Chinese Genotype C Bovine Parainfluenza Virus Type 3 from Cattle and Its Pathogenicity in C57BL/6 Mice. Animals, 14(3), 463. https://doi.org/10.3390/ani14030463





