A New Benzo[6,7]oxepino[3,2-b] Pyridine Derivative Induces Apoptosis in Canine Mammary Cancer Cell Lines
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
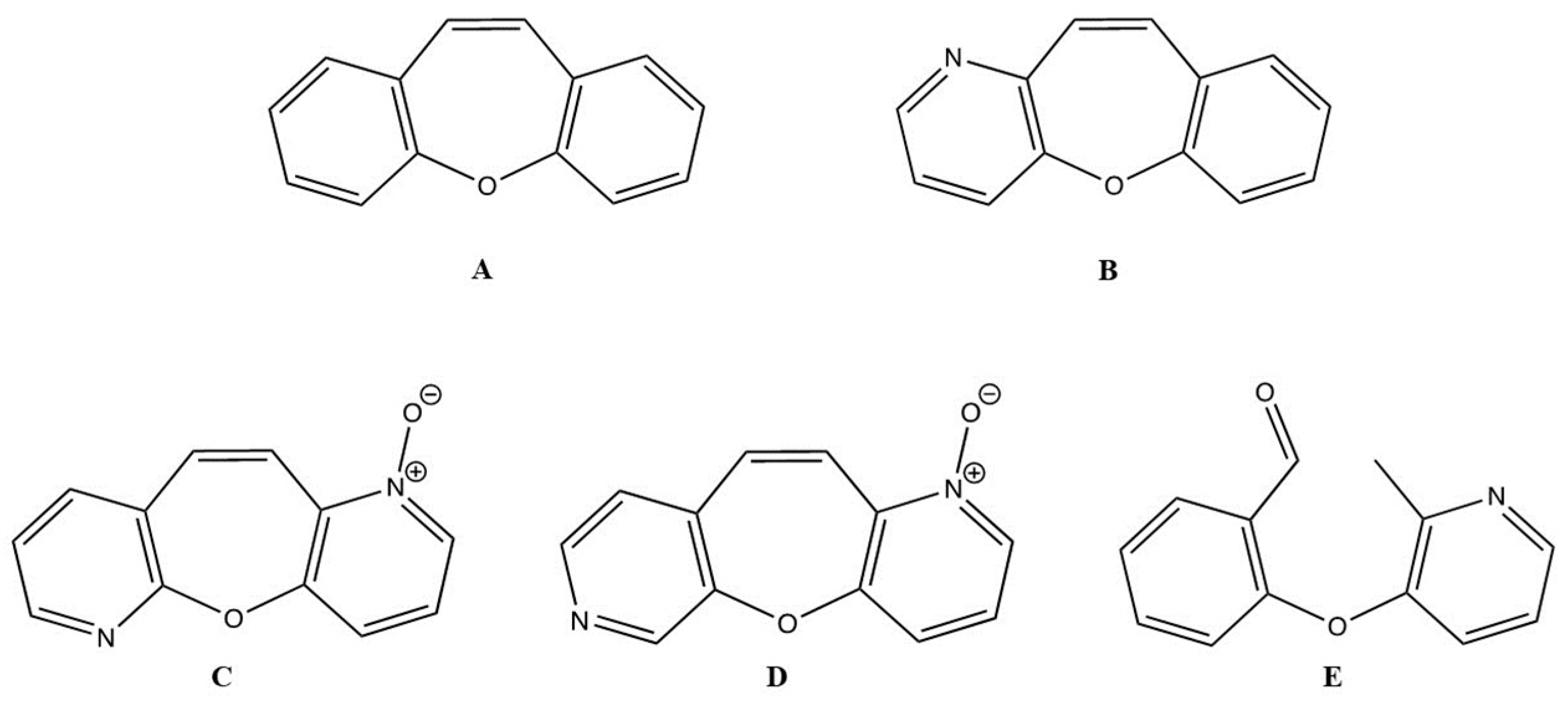
2.1. Synthesis of BZOP Derivatives
2.2. Cell Lines and Cell Cultures
2.3. Cell Proliferation Test
2.4. Morphological Analysis of REM134 and CMGT071020 Cell Lines
2.5. Cell Migration Assay
2.6. Transwell Migration Assay
2.7. Apoptosis Assay
2.8. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
2.9. Immunohistochemistry (IHC)
2.10. Analysis and Interpretation of IHC
2.11. Statistical Analysis
3. Results
3.1. Antiproliferation Effects of BZOP Derivatives on Three Cell Lines
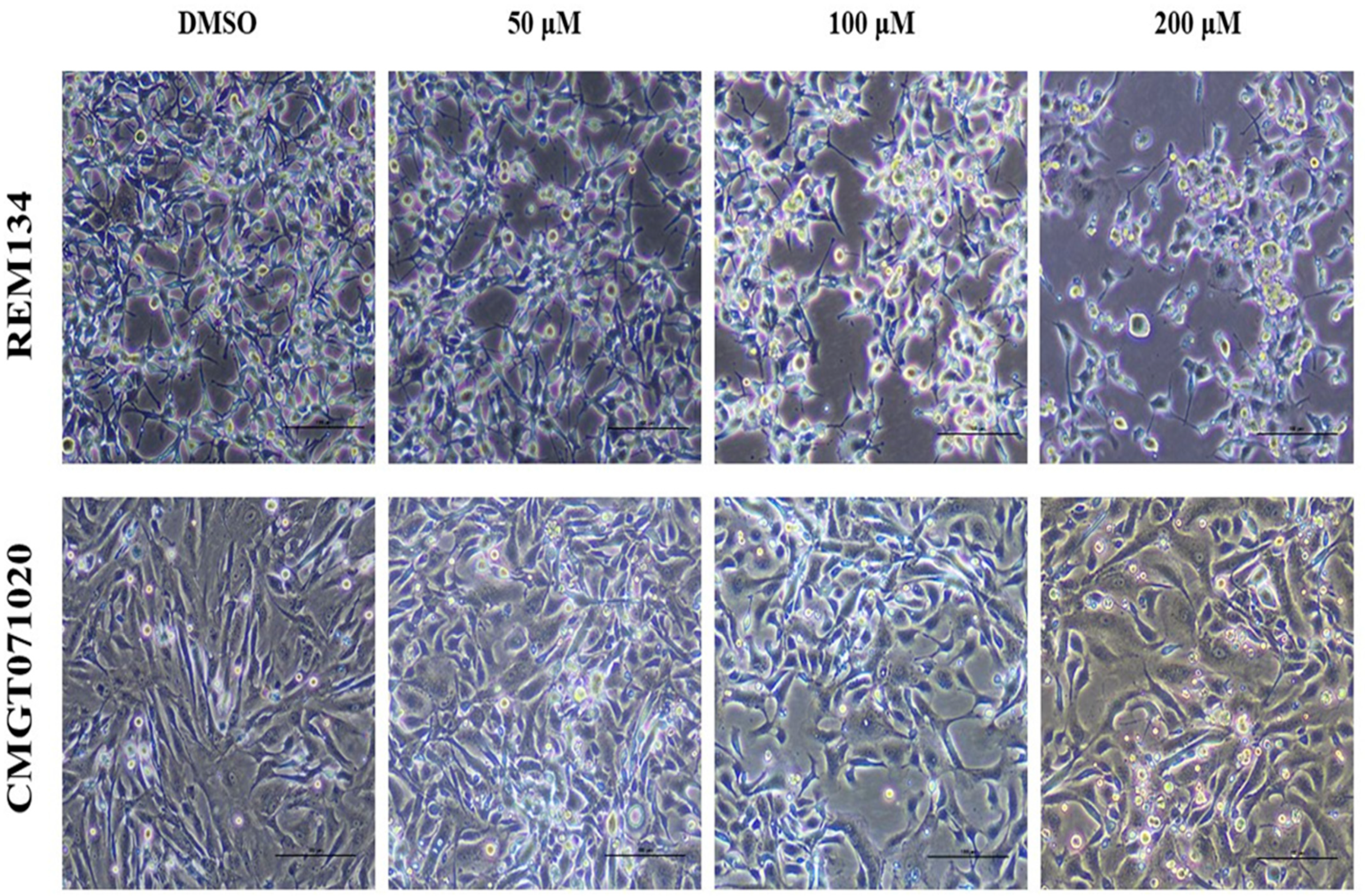
3.2. Effect of MPOBA on Morphology of Both CMC Cell Lines
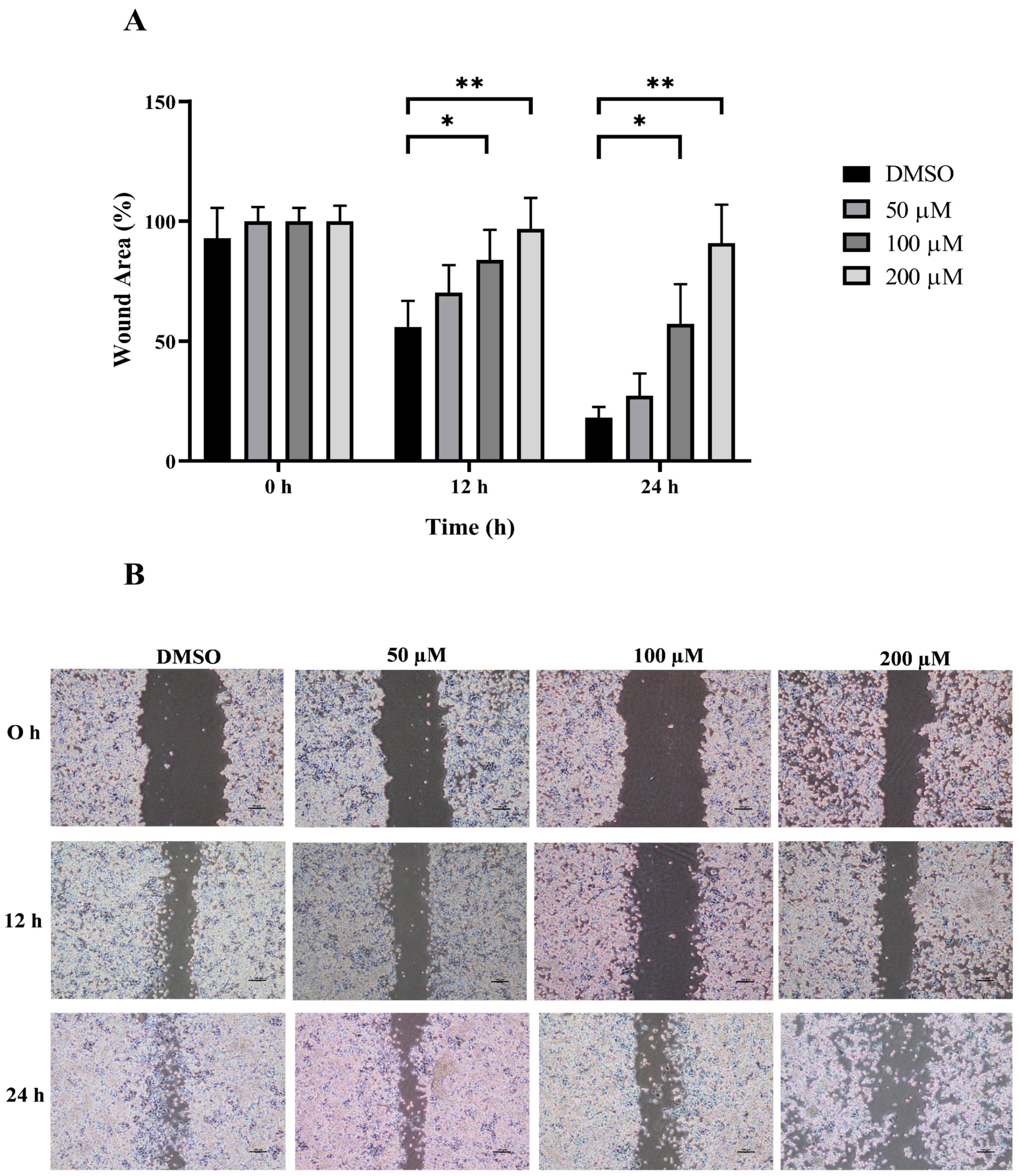
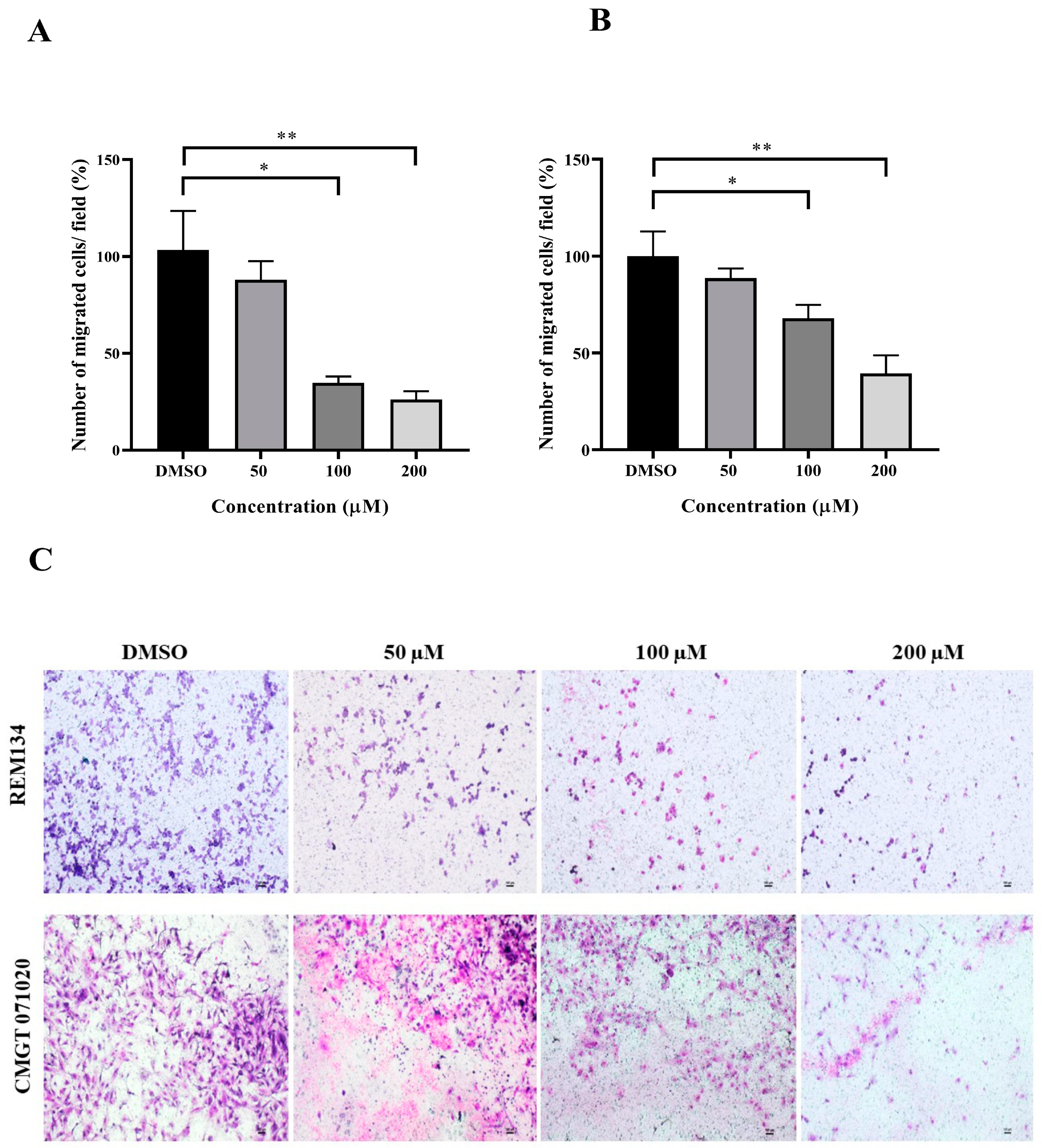
3.3. Anti-Migration Effect of MPOBA on Both CMC Cell Lines
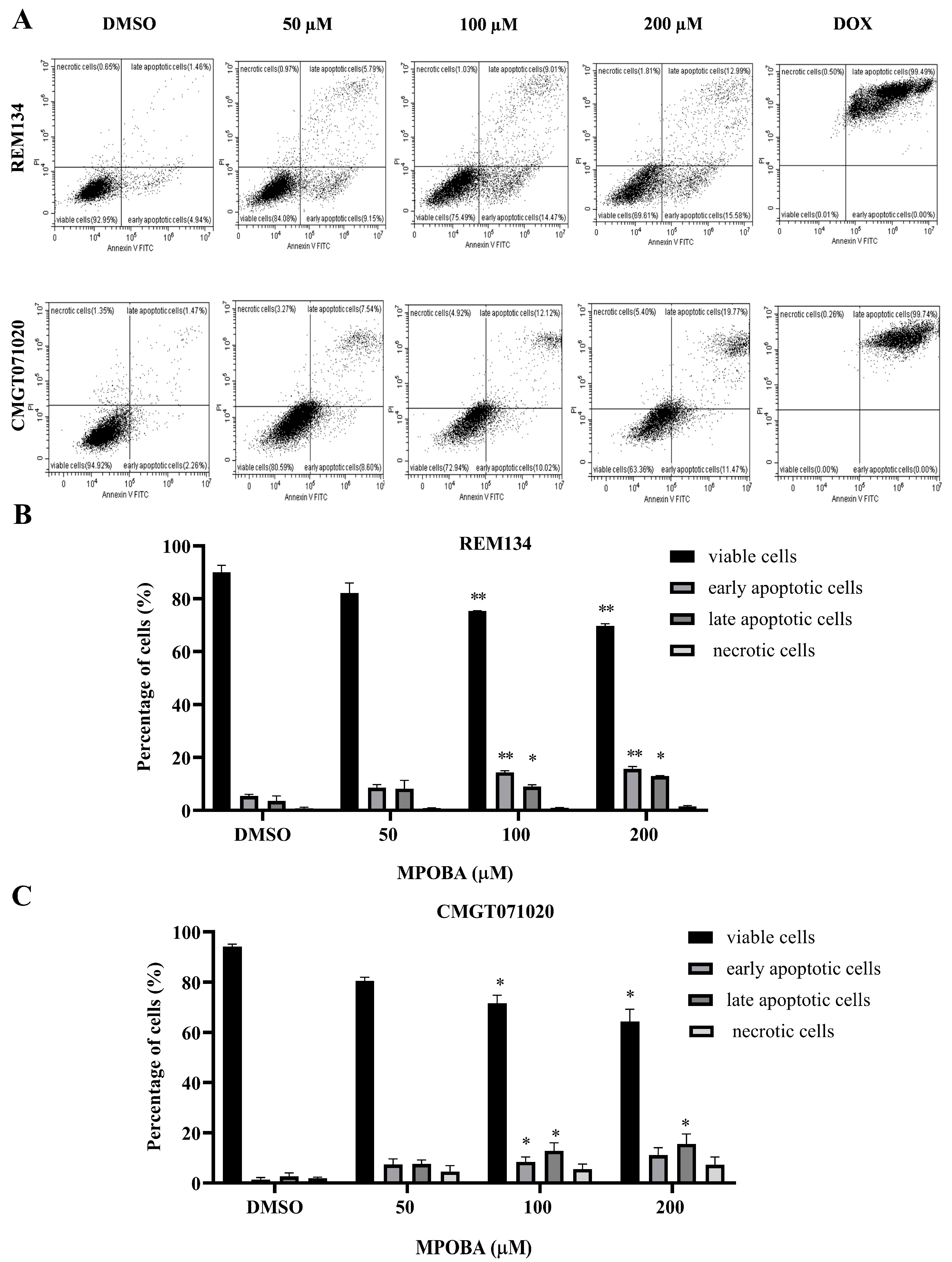
3.4. Effect of MPOBA on Apoptotic Induction in REM134 and CMGT071020 Cells
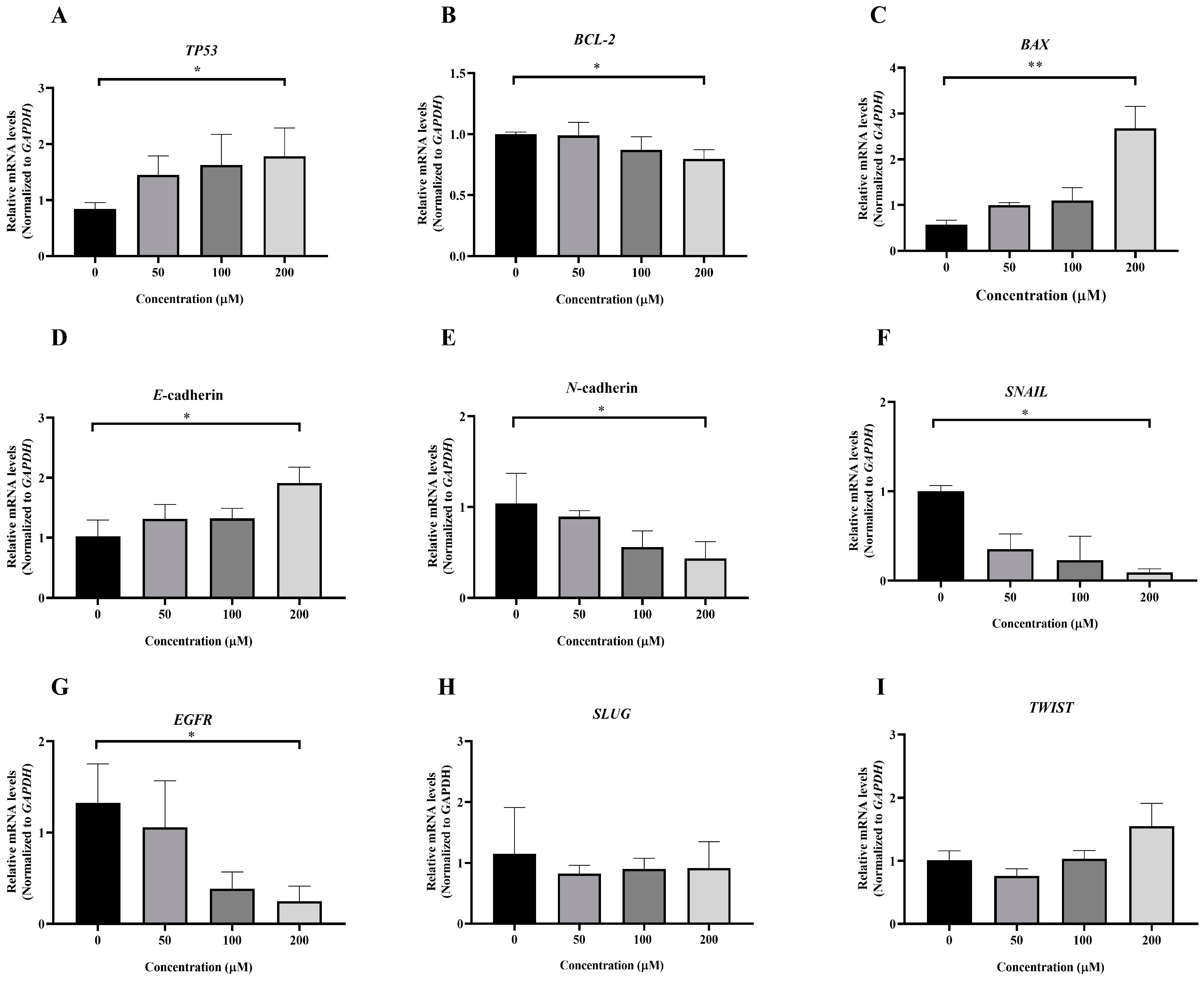
3.5. Effect of MPOBA on Relative mRNA Expression Levels in Both CMC Cell Lines
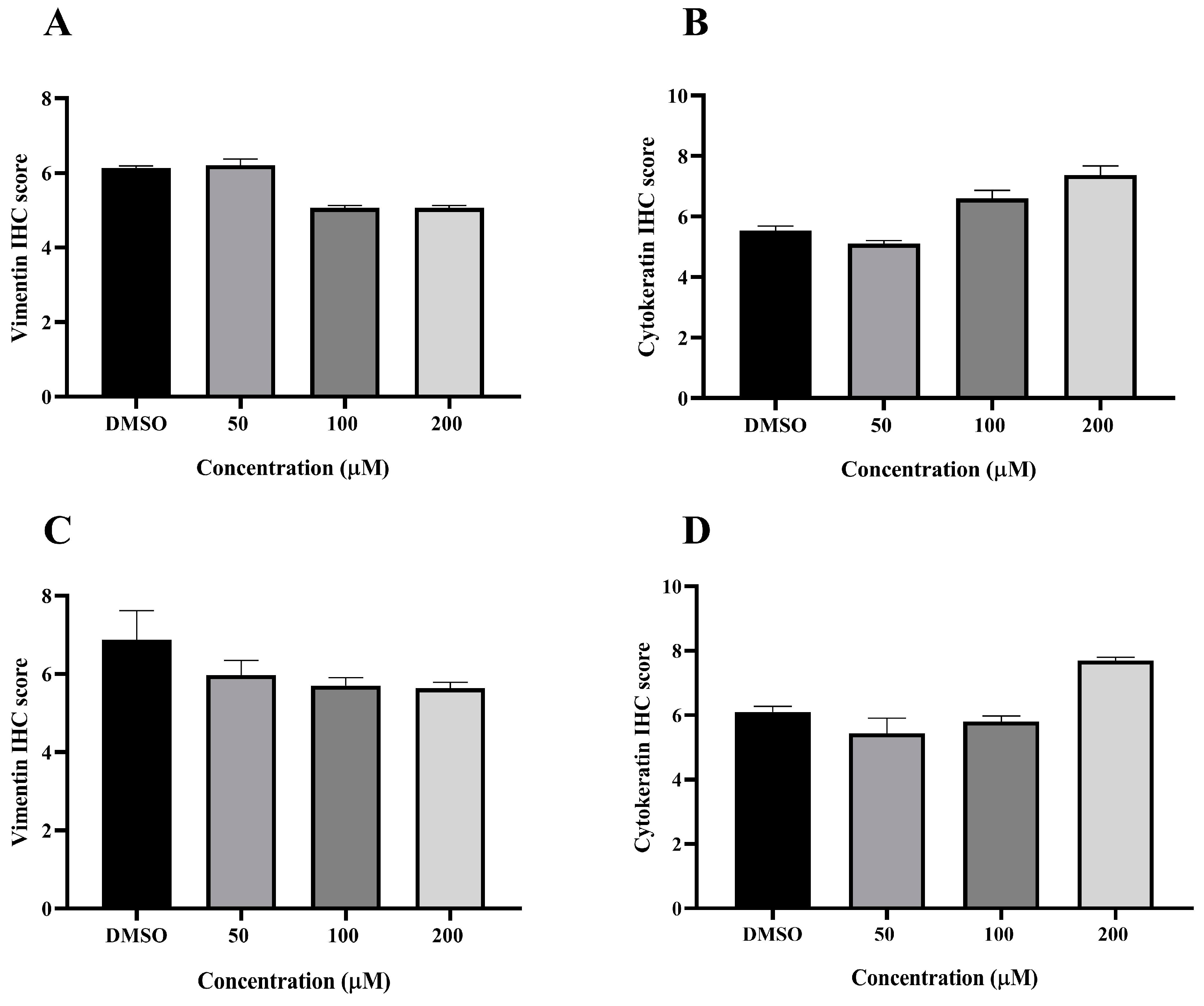
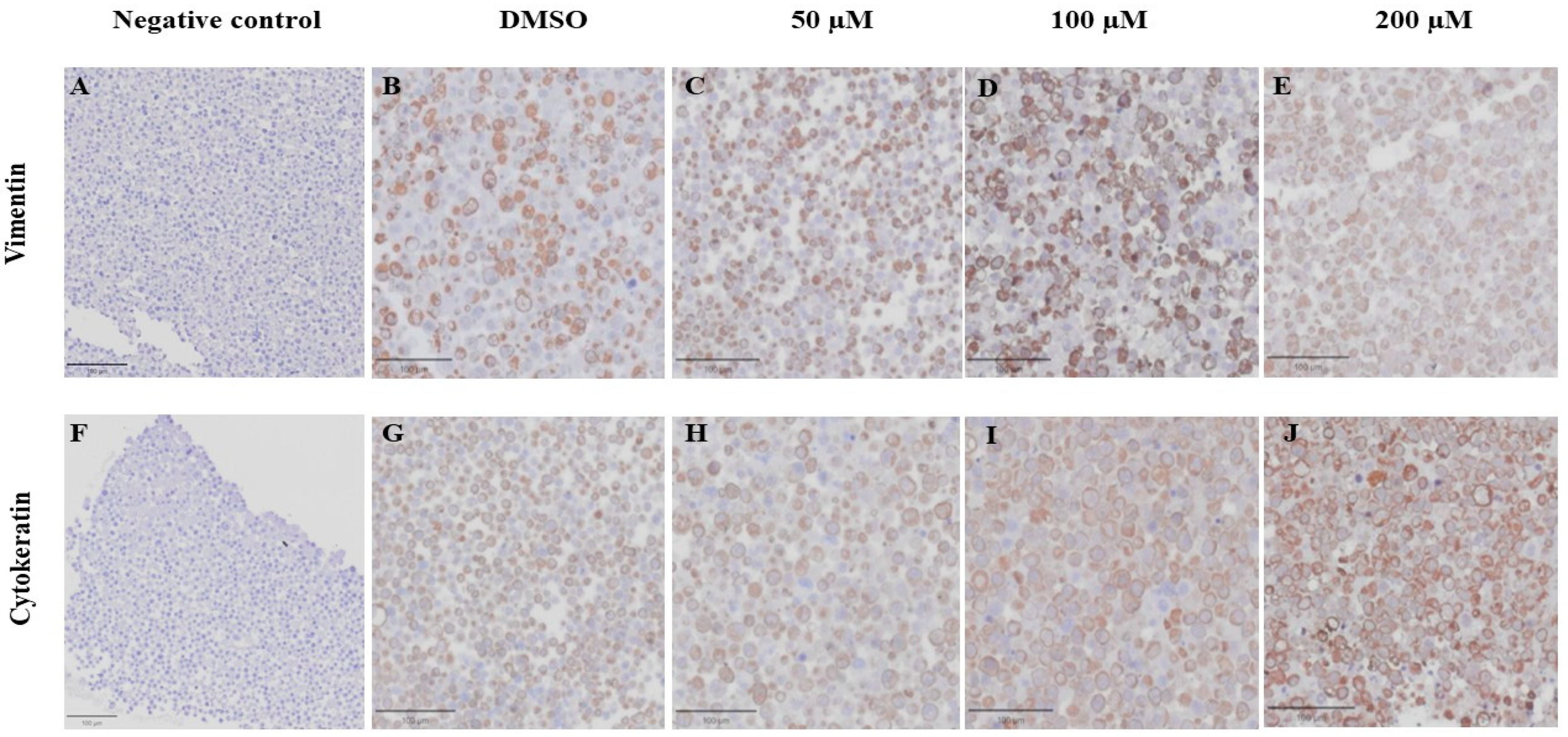
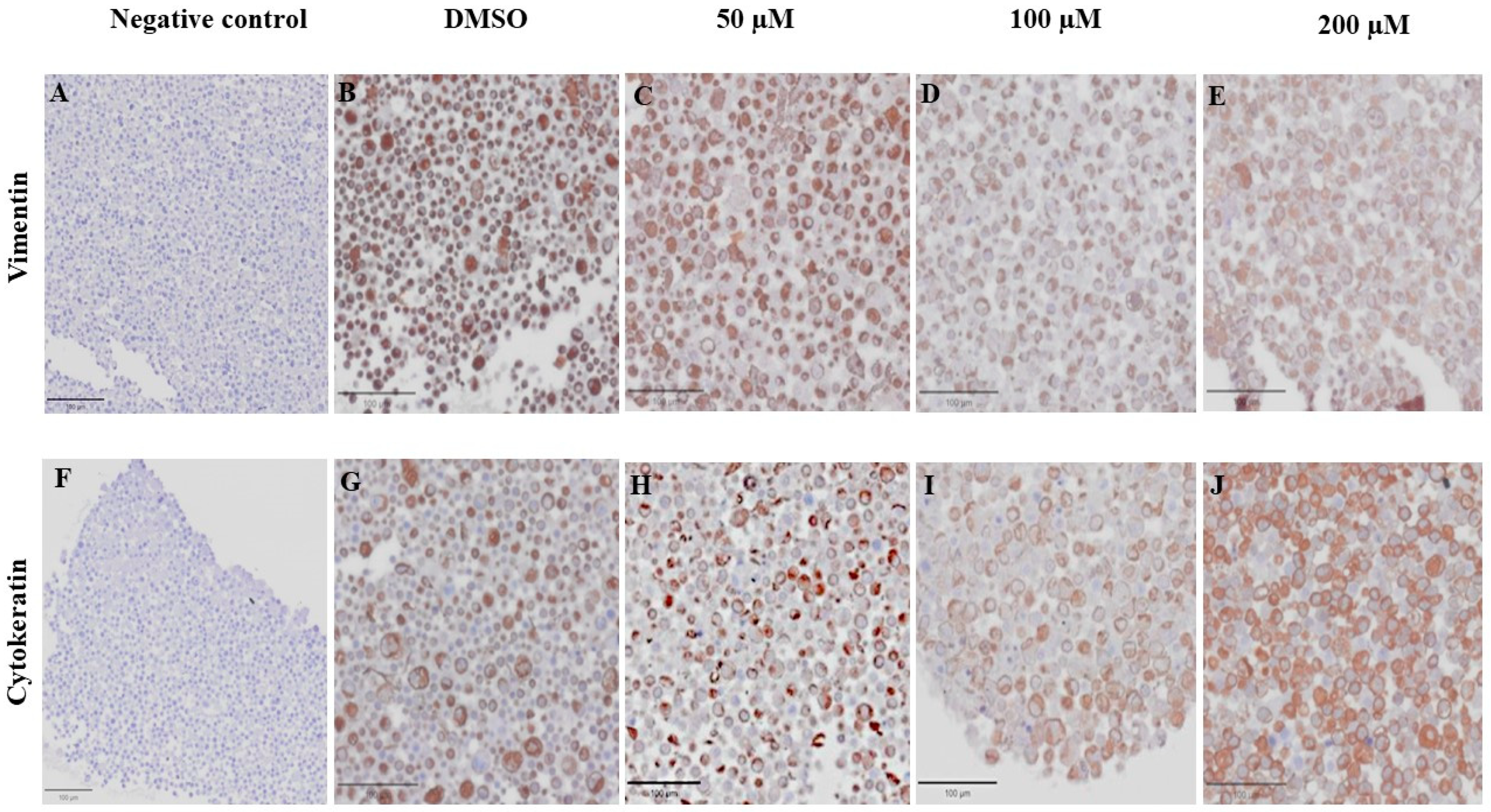
3.6. Effect of MPOBA on Expression of Vimentin (VT) and Cytokeratin (CK) in CMC Cell Lines Based on IHC Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarver, A.L.; Makielski, K.M.; DePauw, T.A.; Schulte, A.J.; Modiano, J.F. Increased risk of cancer in dogs and humans: A consequence of recent extension of lifespan beyond evolutionarily determined limitations? Aging Cancer 2022, 3, 3–19. [Google Scholar] [CrossRef]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological study of mammary tumors in female dogs diagnosed during the period 2002–2012: A growing animal health problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, S.M.; Mohammed, S.I. Canine mammary tumors as a model for human disease. Oncol. Lett. 2018, 15, 8195–8205. [Google Scholar] [CrossRef] [PubMed]
- Sultan, F.; Ganaie, B.A. Comparative oncology: Integrating human and veterinary medicine. Open Vet. J. 2018, 8, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Borges, B.D.N. Epigenetic alterations in canine mammary cancer. Genet. Mol. Biol. 2022, 24, e20220131. [Google Scholar] [CrossRef] [PubMed]
- Sammarco, A.; Gomiero, C.; Beffagna, G.; Cavicchioli, L.; Ferro, S.; Michieletto, S.; Orvieto, E.; Patruno, M.; Zappulli, V. Epithelial-to-mesenchymal transition and phenotypic marker evaluation in human, canine, and feline mammary gland tumors. Animals 2023, 13, 878. [Google Scholar] [CrossRef] [PubMed]
- Gamba, C.; Rodrigues, M.; Gomes, D.; Estrela-Lima, A.; Ferreira, E.; Cassali, G. The relationship between E-cadherin and its transcriptional repressors in spontaneously arising canine invasive micropapillary mammary carcinoma. J. Comp. Pathol. 2015, 153, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Mitra, N.; Verma, R.; Deka, D.; Pawar, H.N.; Sood, N.K.; Gupta, K.; Shashi, K.M.; Jitender, M. Differential expression of apoptosis-associated genes in canine mammary tumors. Biologia 2015, 70, 846–852. [Google Scholar] [CrossRef]
- Klopfleisch, R.; von Euler, H.; Sarli, G.; Pinho, S.S.; Gärtner, F.; Gruber, A.D. Molecular carcinogenesis of canine mammary tumors: News from an old disease. Vet. Pathol. 2011, 48, 98–116. [Google Scholar] [CrossRef]
- Beffagna, G.; Sammarco, A.; Bedin, C.; Romualdi, C.; Mainenti, M.; Mollo, A.; Laura, C.; Silvia, F.; Davide, T.; Raffaella, D.M.; et al. Circulating cell-free DNA in dogs with mammary tumors: Short and long fragments and integrity index. PLoS ONE 2017, 12, e0169454. [Google Scholar] [CrossRef]
- Serrano-Gomez, S.J.; Maziveyi, M.; Alahari, S.K. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol. Cancer 2016, 15, 18. [Google Scholar] [CrossRef]
- Jayachandran, J.; Srinivasan, H.; Mani, K.P. Molecular mechanism involved in epithelial to mesenchymal transition. Arch. Biochem. Biophys. 2021, 710, 108984. [Google Scholar] [CrossRef]
- Huat Siar, C.; Ng, K.H. Differential expression of transcription factors Snail, Slug, SIP 1, and Twist. in ameloblastoma. J. Oral Pathol. Med. 2014, 43, 45–52. [Google Scholar] [CrossRef]
- Guimaraes, M.; Carvalho, M.; Pires, I.; Prada, J.; Gil, A.G.; Lopes, C.; Queiroga, F.L. Concurrent expression of cyclo-oxygenase-2 and epidermal growth factor receptor in canine malignant mammary tumours. J. Comp. Pathol. 2014, 150, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Fesseha, H. Mammary tumours in dogs and its treatment option—A review. J. Sci. Tech. Res. 2020, 30, 23552–23561. [Google Scholar] [CrossRef]
- Divya, M.; Maiti, S.K.; Sangeetha, P.; Shivaraju, S.; Kumar, N.; Tiwari, A.K.; Hescheler, J. Evaluation of chemotherapy with nanosomal paclitaxel and gene therapy expressing apoptosis-inducing proteins in the management of spontaneous canine mammary neoplasm. J. Stem Cells Regen. Med. 2019, 15, 24–34. [Google Scholar]
- Valdivia, G.; Alonso-Diez, Á.; Pérez-Alenza, D.; Peña, L. From conventional to precision therapy in canine mammary cancer: A comprehensive review. Front. Vet. Sci. 2021, 8, 623800. [Google Scholar] [CrossRef] [PubMed]
- Cassali, G.D.; Jark, P.C.; Gamba, C.; Damasceno, K.A.; Lima, A.E.; Nardi, A.B.D.; Ferreira, E.; Horta, R.S.; Firmo, B.F.; Sueiro, F.A.; et al. Consensus regarding the diagnosis, prognosis and treatment of canine and feline mammary tumors-2019. Braz. J. Vet. Pathol. 2020, 13, 555–574. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Krawczyk, H. Dibenzo[b,f]oxepine molecules used in biological systems and medicine. Int. J. Mol. Sci. 2023, 24, 12066. [Google Scholar] [CrossRef]
- Ugwu, D.; Eze, F.; Ezugwu, J.; Attah, S.; Ugwu, M.; Ekoh, O.; Nyoyoko, I.F. Chemotherapeutic importance of oxepines. Int. J. Chem. Sci. 2021, 19, 401. [Google Scholar]
- Agnew, M.N.; Rizwaniuk, A.; Ong, H.H.; Wichmann, J. Nuclear hydroxylated metabolite of fluradoline (2-fluoro-11-[(β-methylamino) ethylthio] dibenz [b, f] oxepin hydrochloride). Identification and synthesis. J. Heterocycl. Chem. 1986, 23, 265–269. [Google Scholar] [CrossRef]
- Jinno, S.; Okita, T. Synthesis of an antioxidant having a dibenz [b, f] oxepine skeleton. Heterocycles 1999, 51, 303–314. [Google Scholar]
- Kittakoop, P.; Nopichai, S.; Thongon, N.; Charoenchai, P.; Thebtaranonth, Y. Bauhinoxepins A and B: New antimycobacterial dibenzo [b, f] oxepins from Bauhinia saccocalyx. Helv. Chim. Acta 2004, 87, 175–179. [Google Scholar] [CrossRef]
- Lu, Y.H.; Lin, C.N.; Ko, H.H.; Yang, S.Z.; Tsao, L.T.; Wang, J.P. Novel anti-inflammatory constituents of Artocarpus rigida. Helv. Chim. Acta 2003, 86, 2566–2572. [Google Scholar] [CrossRef]
- Acton, D.; Hill, G.; Tait, B.S. Tricyclic triarylethylene antiestrogens: Dibenz [b, f] oxepins, dibenzo [b, f] thiepins, dibenzo [a, e] cyclooctenes, and dibenzo [b, f] thiocins. J. Med. Chem. 1983, 26, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.; Roggo, S.; Kragten, E.; Fürst, P.; Waldmeier, P. Synthesis of tools for target identification of the anti-apoptotic compound CGP 3466; Part I. Bioorg. Med. Chem. Lett. 1998, 8, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Numata, A.; Iwamoto, C.; Usami, Y.; Yamada, T.; Ohishi, H.; Gordon, M.C. Antineoplastic agents. 551. Isolation and structures of bauhiniastatins 1–4 from Bauhinia purpurea. J. Nat. Prod. 2006, 69, 323–327. [Google Scholar] [CrossRef]
- Garbicz, D.; Mielecki, D.; Wrzesinski, M.; Pilzys, T.; Marcinkowski, M.; Piwowarski, J.; Debski, J.; Palak, E.; Szczecinski, P.; Krawczyk, H.; et al. Evaluation of anti-cancer activity of stilbene and methoxydibenzo [b, f] oxepin derivatives. Curr. Cancer Drug Targets 2018, 18, 706–717. [Google Scholar] [CrossRef]
- Apisantiyakom, S.; Kittakoop, P.; Manyum, T.; Kirtikara, K.; Bremner, J.B.; Thebtaranonth, Y. Novel biologically active bibenzyls from Bauhinia saccocalyx Pierre. Chem. Biodivers. 2004, 1, 1694–1701. [Google Scholar] [CrossRef]
- Souza, S.M.; Souza, L.S.; Silva, V.R.; Soares, M.B.P.; Bezerra, D.P.; Gois, R.; da Silva, H.C.; Gilvandete, M.P.S.; Gardenia, C.G.M. Natural dibenzo[b,f]oxepines, pacharin and bauhiniastatin-1, isolated from Bauhinia acuruana induce apoptosis on breast cancer cells via MCL-1 protein reduction. Planta Med. 2023, 89, 286–294. [Google Scholar] [CrossRef]
- Ansari, M.I.; Hussain, M.K.; Arun, A.; Chakravarti, B.; Konwar, R.; Hajela, K. Synthesis of targeted dibenzo[b,f]thiepines and dibenzo[b,f]oxepines as potential lead molecules with promising anti-breast cancer activity. Eur. J. Med. Chem. 2015, 99, 113–124. [Google Scholar] [CrossRef]
- Arun, A.; Ansari, M.I.; Popli, P.; Jaiswal, S.; Mishra, A.K.; Dwivedi, A.; Hajela, K.; Konwar, R. New piperidine derivative DTPEP acts as dual-acting anti-breast cancer agent by targeting ERα and downregulating PI3K/Akt-PKCα leading to caspase-dependent apoptosis. Cell Prolif. 2018, 51, e12501. [Google Scholar] [CrossRef]
- Bharath, Y.; Thirupathi, B.; Ranjit, G.; Mohapatra, D.K. An efficient synthesis of dibenzo [b, f] oxepins by ring-closing metathesis. Asian J. Org. Chem. 2013, 2, 848–851. [Google Scholar] [CrossRef]
- Rossington, S.B.; Hadfield, J.A.; Shnyder, S.D.; Wallace, T.W.; Williams, K.J. Tubulin-binding dibenz [c, e] oxepines: Part 2. Structural variation and biological evaluation as tumour vasculature disrupting agents. Bioorg. Med. Chem. 2017, 25, 1630–1642. [Google Scholar] [CrossRef][Green Version]
- Chiacchio, M.A.; Iannazzo, D.; Romeo, R.; Giofrè, S.V.; Legnani, L. Pyridine and pyrimidine derivatives as privileged scaffolds in biologically active agents. Curr. Med. Chem. 2019, 26, 7166–7195. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Pingaew, R.; Worachartcheewan, A.; Sinthupoom, N.; Prachayasittikul, V.; Ruchirawat, S.; Prachayasittikul, V. Roles of pyridine and pyrimidine derivatives as privileged scaffolds in anticancer agents. Mini Rev. Med. Chem. 2017, 17, 869–901. [Google Scholar] [CrossRef]
- Rahnamay, M.; Mahdavi, M.; Shekarchi, A.; Zare, P.; Hosseinpour Feizi, M. Cytotoxic and apoptosis inducing effect of some pyrano [3, 2-c] pyridine derivatives against MCF-7 breast cancer cells. Acta Biochim. Pol. 2018, 65, 397–402. [Google Scholar] [CrossRef]
- Mohammadi-Motlagh, H.-R.; Yarani, R.; Sadeghalvad, M.; Adham, E.; Rasouli, H.; Mostafaie, A. 2-Methylpyridine-1-ium-1-sulfonate as an inducer of apoptosis and cell cycle arrest: A comparative in vitro and computational study. Nutr. Cancer 2019, 71, 643–656. [Google Scholar] [CrossRef]
- Thongaram, P.; Borwornpinyo, S.; Kanjanasirirat, P.; Jearawuttanakul, K.; Kongsema, M.; Chuanopparat, N.; Ngernmeesri, P. Synthesis and anticancer activity evaluation of benzo[6,7]oxepino[3,2-b] pyridine derivatives. Tetrahedron 2020, 76, 131473. [Google Scholar] [CrossRef]
- Inkol, J.M.; Hocker, S.E.; Mutsaers, A.J. Combination therapy with cannabidiol and chemotherapeutics in canine urothelial carcinoma cells. PLoS ONE 2021, 16, e0255591. [Google Scholar] [CrossRef]
- Komatsu, T.; Iwano, H.; Ebisawa, M.; Watabe, A.; Endo, Y.; Hirayama, K.; Taniyama, H.; Kadosawa, T. Pathological classification of canine mammary tumor based on quantifying mRNA levels of hormonal receptors, SATB1, and Snail in tissue and fine needle biopsy samples. J. Vet. Med. Sci. 2012, 74, 719–726. [Google Scholar] [CrossRef]
- Panja, K.; Buranapraditkun, S.; Roytrakul, S.; Kovitvadhi, A.; Lertwatcharasarakul, P.; Nakagawa, T.; Chunsumon, L.; Jaroensong, T. Scorpion venom peptide effects on inhibiting proliferation and inducing apoptosis in canine mammary gland tumor cell lines. Animals 2021, 11, 2119. [Google Scholar] [CrossRef]
- Jermnak, U.; Supsavhad, W.; Kunakornsawat, S.; Jaroensong, T.; Watcharasit, P.; Visitnonthachai, D.; Pairor, S.; Phaochoosak, N. Anti-cancer potentials of Gynura procumbens leaves extract against two canine mammary cancer cell lines. Vet. Med. Sci. 2022, 8, 69–84. [Google Scholar] [CrossRef]
- Yu, C.; Zheng, H.; Liu, X.; Xie, G. The analysis of E-Cadherin, N-Cadherin, Vimentin, HER-2, CEA, CA15-3 and SF Expression in the diagnosis of canine mammary tumors. Animals 2022, 12, 3050. [Google Scholar] [CrossRef]
- Choudhury, K.R.; Yagle, K.J.; Swanson, P.E.; Krohn, K.A.; Rajendran, J.G. A robust automated measure of average antibody staining in immunohistochemistry images. J. Histochem. Cytochem. 2010, 58, 95–107. [Google Scholar] [CrossRef]
- Allred, D.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Amaral, C.B.; Leite, J.D.S.; Fonseca, A.B.M.; Ferreira, A.M.R. Vimentin, osteocalcin and osteonectin expression in canine primary bone tumors: Diagnostic and prognostic implications. Mol. Biol. Rep. 2018, 45, 1289–1296. [Google Scholar] [CrossRef]
- Fortier, A.M.; Asselin, E.; Cadrin, M. Keratin 8 and 18 loss in epithelial cancer cells increases collective cell migration and cisplatin sensitivity through claudin1 up-regulation. J. Biol. Chem. 2013, 288, 11555–11571. [Google Scholar] [CrossRef]
- Rasotto, R.; Berlato, D.; Goldschmidt, M.H.; Zappulli, V. Prognostic significance of canine mammary tumor histologic subtypes: An observational cohort study of 229 cases. Vet. Pathol. 2017, 54, 571–578. [Google Scholar] [CrossRef]
- Mandal, B.; Karmakar, I.; Brahmachari, G. An updated review on biologically promising natural oxepines. Chem. Biodivers. 2022, 19, e202200484. [Google Scholar] [CrossRef]
- Borys, F.; Tobiasz, P.; Sobel, J.; Krawczyk, H. Synthesis and study of dibenzo[b, f]oxepine combined with fluoroazobenzenes-new photoswitches for application in biological systems. Molecules 2022, 27, 5836. [Google Scholar] [CrossRef]
- Kishbaugh, T.L.S. Six-membered ring systems: Pyridine and benzo derivatives. In Progress in Heterocyclic Chemistry, 1st ed.; Gribble, G.W., Joule, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 343–391. [Google Scholar]
- Marinescu, M.; Popa, C.V. Pyridine Compounds with antimicrobial and antiviral activities. Int. J. Mol. Sci. 2022, 23, 5659. [Google Scholar] [CrossRef]
- Islam, M.B.; Islam, M.I.; Nath, N.; Emran, T.B.; Rahman, M.R.; Sharma, R.; Matin, M.M. Recent advances in pyridine scaffold: Focus on chemistry, synthesis, and antibacterial activities. BioMed Res. Int. 2023, 2023, 9967591. [Google Scholar] [CrossRef]
- Fayed, E.A.; Sabour, R.; Harras, M.F.; Mehany, A.B. Design, synthesis, biological evaluation, and molecular modeling of new coumarin derivatives as potent anticancer agents. Med. Chem. Res. 2019, 28, 1284–1297. [Google Scholar] [CrossRef]
- El-Naggar, M.; Almahli, H.; Ibrahim, H.S.; Eldehna, W.M.; Abdel-Aziz, H.A. Pyridine-ureas as potential anticancer agents: Synthesis and in vitro biological evaluation. Molecules 2018, 23, 1459. [Google Scholar] [CrossRef]
- Kurteva, V. Recent progress in metal-free direct synthesis of imidazo[1,2-a]pyridines. ACS Omega 2021, 6, 35173–35185. [Google Scholar] [CrossRef]
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. BioMed Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef]
- Singh, V.; Khurana, A.; Navik, U.; Allawadhi, P.; Bharani, K.K.; Weiskirchen, R. Apoptosis and pharmacological therapies for targeting thereof for cancer therapeutics. Sci 2022, 4, 15. [Google Scholar] [CrossRef]
- Rouzier, R.; Perou, C.M.; Symmans, W.F.; Ibrahim, N.; Cristofanilli, M.; Anderson, K.; Hess, K.R.; Stec, J.; Ayers, M.; Wagner, P.; et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 2005, 11, 5678–5685. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.; Lessene, G.; Strasser, A.; Adams, J. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2013, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Park, M.N.; Rahman, M.H.; Rashid, M.M.; Islam, R.; Uddin, M.J.; Hannan, M.A.; Kim, B. p53 modulation of autophagy signaling in cancer therapies: Perspectives mechanism and therapeutic targets. Front. Cell Dev. Biol. 2022, 10, 761080. [Google Scholar] [CrossRef]
- Almeida, A.; Sánchez-Morán, I.; Rodríguez, C. Mitochondrial-nuclear p53 trafficking controls neuronal susceptibility in stroke. IUBMB Life 2021, 73, 582–591. [Google Scholar] [CrossRef]
- Yao, K.; Xing, H.; Wu, B.; Li, Y.; Liao, A.J.; Yang, W.; Liu, Z.G. Effect of TIEG1 on apoptosis and expression of Bcl-2/Bax and Pten in leukemic cell lines. Genet. Mol. Res. 2015, 14, 1968–1974. [Google Scholar] [CrossRef]
- Raposo-Ferreira, T.M.M.; Brisson, B.K.; Durham, A.C.; Laufer-Amorim, R.; Kristiansen, V.; Puré, E.; Volk, S.W.; Sorenmo, K. Characteristics of the epithelial-mesenchymal transition in primary and paired metastatic canine mammary carcinomas. Vet. Pathol. 2018, 55, 622–633. [Google Scholar] [CrossRef]
- Vu, T.; Datta, P.K. Regulation of EMT in colorectal cancer: A culprit in metastasis. Cancers 2017, 9, 171. [Google Scholar] [CrossRef]
- Gaponova, A.V.; Rodin, S.; Mazina, A.A.; Volchkov, P.V. Epithelial-Mesenchymal Transition: Role in cancer progression and the perspectives of antitumor treatment. Acta Naturae 2020, 12, 4–23. [Google Scholar] [CrossRef]
- Vora, H.H.; Patel, N.A.; Rajvik, K.N.; Mehta, S.V.; Brahmbhatt, B.V.; Shah, M.J.; Shukla, S.N.; Shah, P.M. Cytokeratin and vimentin expression in breast cancer. Int. J. Biol. Markers 2009, 24, 38–46. [Google Scholar] [CrossRef]
- Queiroga, F.L.; Perez-Alenza, M.D.; González-Gil, A.; Silván, G.; Peña, L.; Illera, J.C. Quantification of epidermal growth factor receptor (EGFR) in canine mammary tumours by ELISA assay: Clinical and prognostic implications. Vet. Comp. Oncol. 2017, 15, 383–390. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Guimarães, M.J.; Pires, I.; Prada, J.; Silva-Carvalho, R.; Lopes, C.; Queiroga, F.L. EGFR and microvessel density in canine malignant mammary tumours. Res. Vet. Sci. 2013, 95, 1094–1099. [Google Scholar]
- Manupati, K.; Dhoke, N.R.; Debnath, T.; Yeeravalli, R.; Guguloth, K.; Saeidpour, S.; De, U.C.; Debnath, S.; Das, A. Inhibiting epidermal growth factor receptor signalling potentiates mesenchymal–epithelial transition of breast cancer stem cells and their responsiveness to anticancer drugs. FEBS J. 2017, 284, 1830–1854. [Google Scholar] [CrossRef]
- Yang, W.-N.; Ai, Z.-H.; Wang, J.; Xu, Y.-L.; Teng, Y.-C. Correlation between the overexpression of epidermal growth factor receptor and mesenchymal makers in endometrial carcinoma. J. Gynecol. Oncol. 2014, 25, 36–42. [Google Scholar] [CrossRef]
- Takeda, T.; Tsubaki, M.; Matsuda, T.; Kimura, A.; Jinushi, M.; Obana, T.; Takegami, M.; Nishida, S. EGFR inhibition reverses epithelial-mesenchymal transition and decreases tamoxifen resistance via Snail and Twist downregulation in breast cancer cells. Oncol. Rep. 2022, 47, 109. [Google Scholar] [CrossRef]
- Antar, S.; Al-Karmalawy, A.A.; Mourad, A.; Mourad, M.; Elbadry, M.; Saber, S.; Khodir, A. Protective effects of mirazid on gentamicin-induced nephrotoxicity in rats through antioxidant, anti-inflammatory, JNK1/iNOS, and apoptotic pathways; novel mechanistic insights. Pharm. Sci. 2022, 28, 525–540. [Google Scholar] [CrossRef]
- Abdelsalam, E.A.; Abd El-Hafeez, A.A.; Eldehna, W.M.; El Hassab, M.A.; Marzouk, H.M.M.; Elaasser, M.M.; Abou Taleb, N.A.; Amin, K.M.; Abdel-Aziz, H.A.; Ghosh, P.; et al. Discovery of novel thiazolyl-pyrazolines as dual EGFR and VEGFR-2 inhibitors endowed with in vitro antitumor activity towards non-small lung cancer. J. Enzym. Inhib. Med. Chem. 2022, 37, 2265–2282. [Google Scholar] [CrossRef] [PubMed]
- Raslan, R.R.; Ammar, Y.A.; Fouad, S.A.; Hessein, S.A.; Shmiess, N.A.M.; Ragab, A. Evaluation of the anti-proliferative activity of 2-oxo-pyridine and 1’H-spiro-pyridine derivatives as a new class of EGFRWt and VEGFR-2 inhibitors with apoptotic inducers. RSC Adv. 2023, 13, 10440–10458. [Google Scholar] [CrossRef] [PubMed]
- Kaori, A.-K.; Ken, H.; Osamu, A.; Jun, S.; Mutsuyuki, K.; Hiroshi, S. Tumor-specific cytotoxicity and type of cell death induced by benzaldehyde. Anticancer Res. 2010, 30, 5069. [Google Scholar]
- Liu, Y.; Sakagami, H.; Hashimoto, K.; Kikuchi, H.; Amano, O.; Ishihara, M.; Kanda, Y.; Kunii, S.; Kochi, M.; Zhang, W.; et al. Tumor-specific cytotoxicity and type of cell death induced by beta-cyclodextrin benzaldehyde inclusion compound. Anticancer Res. 2008, 28, 229–236. [Google Scholar]
- Lin, C.-F.; Yang, J.-S.; Chang, C.-Y.; Kuo, S.-C.; Lee, M.-R.; Huang, L.-J. Synthesis and anticancer activity of benzyloxybenzaldehyde derivatives against HL-60 cells. Bioorg. Med. Chem. 2005, 13, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Tamang, N.; Andrews, C.; Mavileti, S.K.; Nanduri, S.; Golakoti, N.R.; Karanam, B. Anti-cancer activity of heteroaromatic acetals of andrographolide and its isomers. New J. Chem. 2022, 46, 9745–9754. [Google Scholar] [CrossRef] [PubMed]
- Rabanal, R.M.; Else, R.W. Immunohistochemical localisation of cytokeratin and vimentin intermediate filament proteins in canine mammary tumours. Res. Vet. Sci. 1994, 56, 225–233. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer (5′ to 3′) |
|---|---|
| SNAIL | F: GACTCCCAGACTCGCAAGG R: GACATGCGGGAGAAGGTTCG |
| SLUG | F: GGCAAGGCGTTTTCCAGACCCT R: GGGCAAGAAAAAGGCTTCTCCCCAG |
| TWIST | F: GGCAGGGCCGGAGACCTAGATG R: TCCACGGGCCTGTCTCGCTT |
| E-cadherin | F: TCCTGGGCAGGGTGAGTT R: GAGGCCGCTTGACTGTAATC |
| N-cadherin | F: AGCACCCTCCTCAGTCAACG R: TGTCAACATGGTCCCAGCA |
| TP53 | F: GCGGCCCAT CCTCACTATC R: CACAAACGCGTACCTCAAAGC |
| EGFR | F: CGAGCACAAGGACAACATCG R: CTCCACACATCGCTTTGGTG |
| BAX | F: GGTTGTTGCCCTCCTCTACT R: GTAAGCACTCCAGCCACAAA |
| BCL-2 | F: TGGATGACTGAGTACCTGAA R: GGCCTACTGACTTCACTTAT |
| GAPDH | F: CCCACTCTTCCACCTTCGAC R: AGCCAAATTCATTGTCATACCAGG |
| Compounds | Half-Maximal Inhibitory Concentration (IC50) (µM) | |||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | |||||
| REM134 | CMGT071020 | MDCK | REM134 | CMGT071020 | MDCK | |
| OPDOb | >300 | >300 | >300 | 172 | >300 | >300 |
| OPDOc | >300 | >300 | >300 | >300 | >300 | >300 |
| MPOBA | 265 | >300 | >300 | 87 | 104 | 267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jianpraphat, N.; Supsavhad, W.; Ngernmeesri, P.; Siripattarapravat, K.; Soontararak, S.; Akrimajirachoote, N.; Phaochoosak, N.; Jermnak, U. A New Benzo[6,7]oxepino[3,2-b] Pyridine Derivative Induces Apoptosis in Canine Mammary Cancer Cell Lines. Animals 2024, 14, 386. https://doi.org/10.3390/ani14030386
Jianpraphat N, Supsavhad W, Ngernmeesri P, Siripattarapravat K, Soontararak S, Akrimajirachoote N, Phaochoosak N, Jermnak U. A New Benzo[6,7]oxepino[3,2-b] Pyridine Derivative Induces Apoptosis in Canine Mammary Cancer Cell Lines. Animals. 2024; 14(3):386. https://doi.org/10.3390/ani14030386
Chicago/Turabian StyleJianpraphat, Natamon, Wachiraphan Supsavhad, Paiboon Ngernmeesri, Kannika Siripattarapravat, Sirikul Soontararak, Nattaphong Akrimajirachoote, Napasorn Phaochoosak, and Usuma Jermnak. 2024. "A New Benzo[6,7]oxepino[3,2-b] Pyridine Derivative Induces Apoptosis in Canine Mammary Cancer Cell Lines" Animals 14, no. 3: 386. https://doi.org/10.3390/ani14030386
APA StyleJianpraphat, N., Supsavhad, W., Ngernmeesri, P., Siripattarapravat, K., Soontararak, S., Akrimajirachoote, N., Phaochoosak, N., & Jermnak, U. (2024). A New Benzo[6,7]oxepino[3,2-b] Pyridine Derivative Induces Apoptosis in Canine Mammary Cancer Cell Lines. Animals, 14(3), 386. https://doi.org/10.3390/ani14030386





