Prevalence, Risk Factors, and Relationship between Reproductive Performance and the Presence of Antibodies against Coxiellosis in Dairy Farm Milk Tanks in the Northwest of Spain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Milk Sampling
2.2. Sample Analysis for the Diagnosis of Coxiellosis
2.3. Data Collection
2.4. Statistical Analysis
3. Results
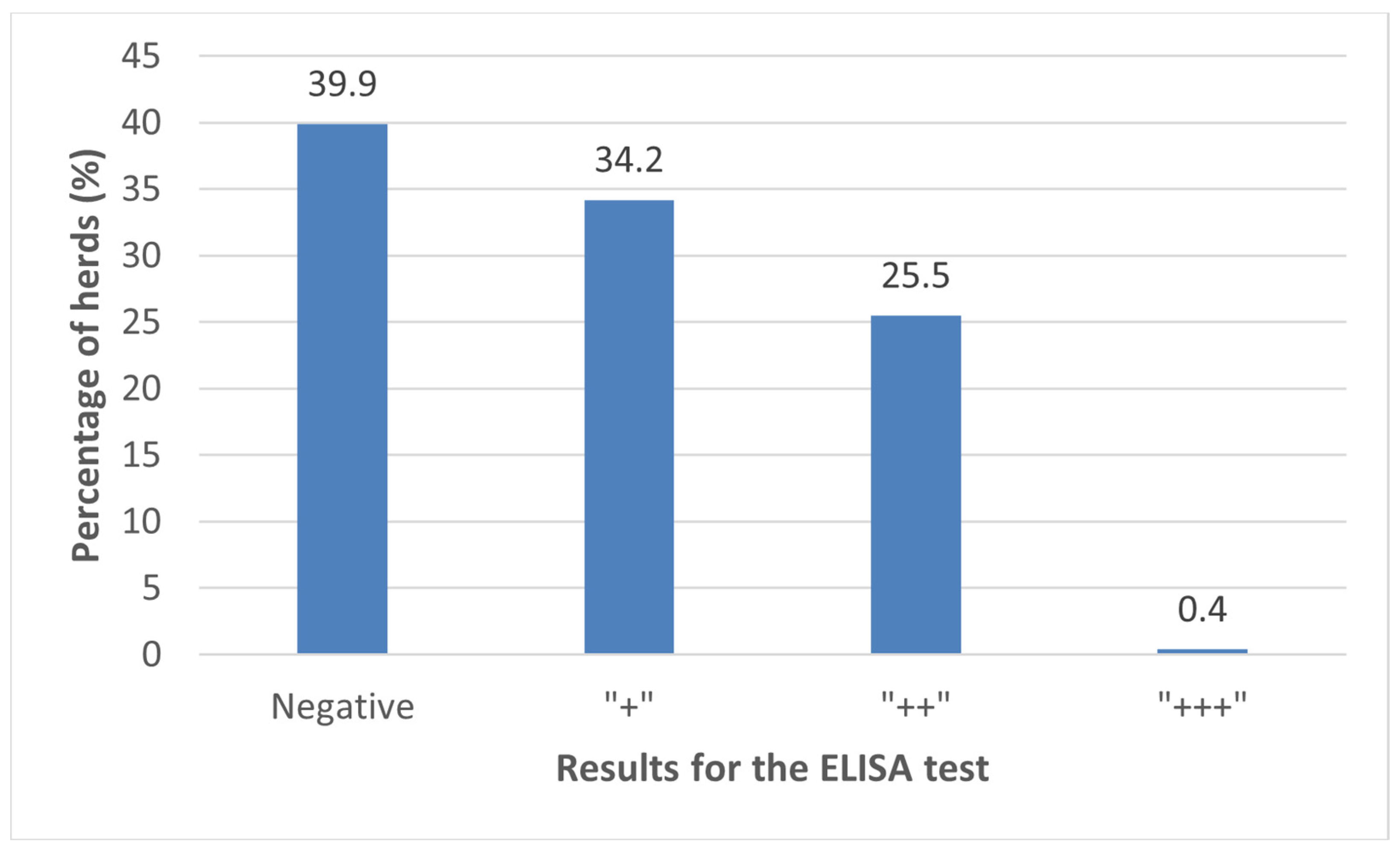
3.1. Prevalence of C. burnetii Antibodies in BTM
3.2. Risk Factors
3.3. Relationship between Reproductive Performance and the Presence of Antibodies against Coxiellosis in BTM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arricau-Bouvery, N.; Rodolakis, A. Is Q Fever an Emerging or Re-Emerging Zoonosis? Vet. Res. 2005, 36, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, C.; Hirsbrunner, G.; Herms, T.L.; Runge, M.; Kiene, F.; Ganter, M.; Zanolari, P.; Bauer, B.U. Two Years after Coxiella burnetii Detection: Pathogen Shedding and Phase-Specific Antibody Response in Three Dairy Goat Herds. Animals 2023, 13, 3048. [Google Scholar] [CrossRef] [PubMed]
- Neupane, K.; Kaswan, D. Coxiella burnetii Infection. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557893/ (accessed on 18 January 2024).
- Voss, L.; Huaman, J.; Pacioni, C.; Tolpinrud, A.; Helbig, K.; Carvalho, T.; Firestone, S. Seroprevalence of Coxiella burnetii Antibodies in Wild Deer Populations in Eastern Australia. Aust. Vet. J. 2023, 101, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Agerholm, J.S. Coxiella burnetii Associated Reproductive Disorders in Domestic Animals-a Critical Review. Acta Vet. Scand. 2013, 55, 13. [Google Scholar] [CrossRef] [PubMed]
- Hackstadt, T.; Peacock, M.G.; Hitchcock, P.J.; Cole, R.L. Lipopolysaccharide Variation in Coxiella Burnetti: Intrastrain Heterogeneity in Structure and Antigenicity. Infect. Immun. 1985, 48, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Hackstadt, T. Steric Hindrance of Antibody Binding to Surface Proteins of Coxiella Burnetti by Phase I Lipopolysaccharide. Infect. Immun. 1988, 56, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Maurin, M.; Raoult, D. Q Fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef] [PubMed]
- Pires, H.; Cardoso, L.; Lopes, A.; Fontes, M.; Matos, M.; Pintado, C.; Figueira, L.; Mesquita, J.; Matos, A.; Coelho, A. Seropositivity for Coxiella burnetii in Wild Boar (Sus scrofa) and Red Deer (Cervus elaphus) in Portugal. Pathogens 2023, 12, 421. [Google Scholar] [CrossRef]
- Neare, K.; Tummeleht, L.; Lassen, B.; Viltrop, A. Coxiella burnetii Seroprevalence and Associated Risk Factors in Cattle, Sheep, and Goats in Estonia. Microorganisms 2023, 11, 819. [Google Scholar] [CrossRef]
- Baumann, T.; Studer, E.; Hirsbrunner, G. Examinations of Cattle Births with a Special Focus on Coxiella burnetii. Schweiz. Arch. Tierheilkd. 2023, 165, 127–131. [Google Scholar] [CrossRef]
- Ghanem-Zoubi, N.; Paul, M. Q Fever during Pregnancy: A Narrative Review. Clin. Microbiol. Infect. 2020, 26, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fons, F.; González-Barrio, D.; Aguilar-Ríos, F.; Soler, A.J.; Garde, J.J.; Gortázar, C.; Fernández-Santos, M. del R. Infectious Pathogens Potentially Transmitted by Semen of the Black Variety of the Manchega Sheep Breed: Health Constraints for Conservation Purposes. Anim. Reprod. Sci. 2014, 149, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Ullah, Q.; Jamil, T.; Saqib, M.; Iqbal, M.; Neubauer, H. Q Fever—A Neglected Zoonosis. Microorganisms 2022, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Rabaza, A.; Macías-Rioseco, M.; Fraga, M.; Uzal, F.A.; Eisler, M.C.; Riet-Correa, F.; Giannitti, F. Coxiella burnetii Abortion in a Dairy Farm Selling Artisanal Cheese Directly to Consumers and Review of Q Fever as a Bovine Abortifacient in South America and a Human Milk-Borne Disease. Braz. J. Microbiol. 2021, 52, 2511–2520. [Google Scholar] [CrossRef]
- Thomas, D.R.; Treweek, L.; Salmon, R.L.; Kench, S.M.; Coleman, T.J.; Meadows, D.; Morgan-Capner, P.; Caul, E.O. The Risk of Acquiring Q Fever on Farms: A Seroepidemiological Study. Occup. Environ. Med. 1995, 52, 644–647. [Google Scholar] [CrossRef]
- Trujillo, M.; Conan, A.; Calchi, A.C.; Mertens-Scholtz, K.; Becker, A.A.; Gallagher, C.; Mau, A.; Marchi, S.; Machado, M.; André, M.R.; et al. Coxiella burnetii Shedding and Serological Status in Pregnant and Postpartum Ewes. Acta Trop. 2023, 244, 106962. [Google Scholar] [CrossRef]
- Espí, A.; del Cerro, A.; Oleaga, Á.; Rodríguez-Pérez, M.; López, C.M.; Hurtado, A.; Rodríguez-Martínez, L.D.; Barandika, J.F.; García-Pérez, A.L. One Health Approach: An Overview of Q Fever in Livestock, Wildlife and Humans in Asturias (Northwestern Spain). Animals 2021, 11, 1395. [Google Scholar] [CrossRef]
- Kidder, I.; Kobayashi, T.; Ford, B.; Sekar, P. Hip Periprosthetic Joint Infection Due to Coxiella burnetii in an Adult Male. IDCases 2023, 31, e01661. [Google Scholar] [CrossRef]
- Christodoulou, M.; Malli, F.; Tsaras, K.; Billinis, C.; Papagiannis, D. A Narrative Review of Q Fever in Europe. Cureus 2023, 15, e38031. [Google Scholar] [CrossRef]
- Hemsley, C.M.; Essex-Lopresti, A.; Chisnall, T.; Millar, M.; Neale, S.; Reichel, R.; Norville, I.H.; Titball, R.W. MLVA and Com1 Genotyping of Coxiella burnetii in Farmed Ruminants in Great Britain. Vet. Microbiol. 2023, 277, 109629. [Google Scholar] [CrossRef]
- Ahmadinezhad, M.; Mounesan, L.; Doosti-Irani, A.; Behzadi, M.Y. The Prevalence of Q Fever in the Eastern Mediterranean Region: A Systematic Review and Meta-Analysis. Epidemiol. Health 2022, 44, e2022097. [Google Scholar] [CrossRef] [PubMed]
- Dobos, A.; Fodor, I.; Tekin, T.; Ðuričić, D.; Samardzija, M. Presence of Coxiella burnetii in Dairy Cattle and Farms in the Czech Republic. Pol. J. Vet. Sci. 2022, 25, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Pexara, A.; Solomakos, N.; Govaris, A. Q Fever and Prevalence of Coxiella burnetii in Milk. Trends Food Sci. Technol. 2018, 71, 65–72. [Google Scholar] [CrossRef]
- Nobrega, D.B.; French, J.E.; Kelton, D.F. A Scoping Review of the Testing of Bulk Milk to Detect Infectious Diseases of Dairy Cattle: Diseases Caused by Bacteria. J. Dairy. Sci. 2023, 106, 1986–2006. [Google Scholar] [CrossRef] [PubMed]
- Brito, B.; Hick, P. Milk as a Diagnostic Fluid to Monitor Viral Diseases in Dairy Cattle. Aust. Vet. J. 2023, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nokhodian, Z.; Feizi, A.; Moradi, A.; Yaran, M.; Hoseini, S.G.; Ataei, B.; Hosseini, M. Detection and Risk Factors of Coxiella burnetii Infection in Dairy Cattle Based on Bulk Tank Milk Samples in Center of Iran. Prev. Vet. Med. 2016, 134, 139–144. [Google Scholar] [CrossRef]
- Lindberg, A.L.E. Regional Eradication of Bovine Viral Diarrhoea in Sweden—An Approach Complementary to the Current Control Scheme. In Proceedings of the Society of Veterinary Epidemiology and Preventive Medicine, Reading, UK, 29–31 March 1995; pp. 146–156. [Google Scholar]
- Niskanen, R.; Alenius, S.; Larsson, B.; Jacobsson, S.-O. Determination of Level of Antibodies to Bovine Virus Diarrhoea Virus (BVDV) in Bulk Tank Milk as a Tool in the Diagnosis and Prophylaxis of BVDV Infections in Dairy Herds. In Ruminant Pestivirus Infections; Liess, B., Moenning, V., Pohlenz, J., Trautwein, G., Eds.; Springer: Vienna, Austria, 1991; pp. 245–251. [Google Scholar]
- Joly, A.; Fourichon, C.; Beaudeau, F. Description and First Results of a BVDV Control Scheme in Brittany (Western France). Prev. Vet. Med. 2005, 72, 209–213. [Google Scholar] [CrossRef]
- Delalay, G.; Di Labio, E.; Glauser, D.L.; Schwermer, H. BVD Situation on Farms with High Serological Values in the Tank Milk—Individual Animals Have a Greater Influence than Previously Known. Schweiz. Arch. Tierheilkd. 2021, 164, 635–649. [Google Scholar] [CrossRef]
- Muskens, J.; van Engelen, E.; van Maanen, C.; Bartels, C.; Lam, T.J.G.M. Prevalence of Coxiella burnetii Infection in Dutch Dairy Herds Based on Testing Bulk Tank Milk and Individual Samples by PCR and ELISA. Vet. Rec. 2011, 168, 79. [Google Scholar] [CrossRef]
- Guatteo, R.; Seegers, H.; Taurel, A.-F.; Joly, A.; Beaudeau, F. Prevalence of Coxiella burnetii Infection in Domestic Ruminants: A Critical Review. Vet. Microbiol. 2011, 149, 1–16. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining Postpartum Uterine Disease in Cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- More, S.J.; Stegeman, J.A.; Rodolakis, A.; Roest, H.J.; Vellema, P.; Thiéry, R.; Neubauer, H.; Van Der Hoek, W.; Staerk, K.D.C.; Needham, H.; et al. Scientific Opinion on Q Fever. EFSA J. 2010, 8, 1595. [Google Scholar] [CrossRef]
- Van den Brom, R.; Schimmer, B.; Schneeberger, P.M.; Swart, W.A.; van der Hoek, W.; Vellema, P. Seroepidemiological Survey for Coxiella burnetii Antibodies and Associated Risk Factors in Dutch Livestock Veterinarians. PLoS ONE 2013, 8, e54021. [Google Scholar] [CrossRef] [PubMed]
- Sidi-Boumedine, K.; Adam, G.; Angen, Ø.; Aspán, A.; Bossers, A.; Roest, H.-J.; Prigent, M.; Thiéry, R.; Rousset, E. Whole Genome PCR Scanning (WGPS) of Coxiella burnetii Strains from Ruminants. Microbes Infect. 2015, 17, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Pablos-Tanarro, A.; Eiras, C.; Calavia, P.; San Miguel-Ayanz, J.M.; Ortega-Mora, L.M.; Ferre, I. Presencia de Anticuerpos Específicos Frente a Coxiella burnetii En Explotaciones Bovinas Lecheras de Galicia y Extensivas de Carne En Sistemas de Dehesa. In Proceedings of the XVII Congreso Internacional ANEMBE de Medicina Bovina, Santander, Spain, 18 April 2012; pp. 216–218. [Google Scholar]
- Piñeiro, A.; Astobiza, I.; Barandika, F.K.; Hurtado, A.; Atxaerandio, R.; García-Pérez, A.I. Evolución de La Prevalencia de Coxiella burnetii En Explotaciones de Bovino Lechero En Un Periodo de 2 Años. In Proceedings of the XVII Congreso Internacional ANEMBE de Medicina Bovina, Santander, Spain, 18 April 2012; pp. 218–220. [Google Scholar]
- Ruiz-Fons, F.; Rodríguez, Ó.; Torina, A.; Naranjo, V.; Gortázar, C.; de la Fuente, J. Prevalence of Coxiella Burnetti Infection in Wild and Farmed Ungulates. Vet. Microbiol. 2008, 126, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.; Perez, A.; Mardones, F.O.; Pérez-Sancho, M.; García-Seco, T.; Pagés, E.; Mirat, F.; Díaz, R.; Carpintero, J.; Domínguez, L. Epidemiological Factors Associated with the Exposure of Cattle to Coxiella burnetii in the Madrid Region of Spain. Vet. J. 2012, 194, 102–107. [Google Scholar] [CrossRef]
- Ruiz-Fons, F.; Astobiza, I.; Barandika, J.F.; Hurtado, A.; Atxaerandio, R.; Juste, R.A.; García-Pérez, A.L. Seroepidemiological Study of Q Fever in Domestic Ruminants in Semi-Extensive Grazing Systems. BMC Vet. Res. 2010, 6, 3. [Google Scholar] [CrossRef]
- Paiba, G.A.; Green, L.E.; Lloyd, G.; Patel, D.; Morgan, K.L. Prevalence of Antibodies to Coxiella Burneti (Q Fever) in Bulk Tank Milk in England and Wales. Vet. Rec. 1999, 144, 519–522. [Google Scholar] [CrossRef]
- Fanelli, A.; Trotta, A.; Bono, F.; Corrente, M.; Buonavoglia, D. Seroprevalence of Coxiella burnetii in Dairy Cattle and Buffalo from Southern Italy: C.Burnetii in Southern Italy. Vet. Ital. 2020, 56, 193–197. [Google Scholar]
- Anastácio, S.; Carolino, N.; Sidi-Boumedine, K.; da Silva, G.J. Q Fever Dairy Herd Status Determination Based on Serological and Molecular Analysis of Bulk Tank Milk. Transbound. Emerg. Dis. 2016, 63, e293–e300. [Google Scholar] [CrossRef]
- Menadi, S.E.; Chisu, V.; Santucciu, C.; Di Domenico, M.; Curini, V.; Masala, G. Serological, Molecular Prevalence and Genotyping of Coxiella burnetii in Dairy Cattle Herds in Northeastern Algeria. Vet. Sci. 2022, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Kim, E.H.; Lafferty, C.J.; Dubovi, E. Coxiella burnetii in Bulk Tank Milk Samples, United States. Emerg. Infect. Dis. 2005, 11, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Dobos, A.; Fodor, I. Prevalence of Coxiella burnetii in Bovine Placentas in Hungary and Slovakia: Detection of a Novel Sequence Type—Short Communication. Acta Vet. Hung. 2021, 69, 303–307. [Google Scholar] [CrossRef]
- Agger, J.F.; Christoffersen, A.-B.; Rattenborg, E.; Nielsen, J.; Agerholm, J.S. Prevalence of Coxiella burnetii Antibodies in Danish Dairy Herds. Acta Vet. Scand. 2010, 52, 5. [Google Scholar] [CrossRef]
- Saegerman, C.; Speybroeck, N.; Dal Pozzo, F.; Czaplicki, G. Clinical Indicators of Exposure to Coxiella burnetii in Dairy Herds. Transbound. Emerg. Dis. 2015, 62, 46–54. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, A.; Aziz, M.U.; Song, B.; Zeb, J.; Hasib, F.M.Y.; Li, J.; Rehman, A.; George, D.; Cabezas-Cruz, A.; et al. First Serological Evidence of Q Fever in Large Ruminants and Its Associated Risk Factors in Punjab, Pakistan. Sci. Rep. 2022, 12, 17278. [Google Scholar] [CrossRef]
- Lurier, T.; Rousset, E.; Gasqui, P.; Sala, C.; Claustre, C.; Abrial, D.; Dufour, P.; de Crémoux, R.; Gache, K.; Delignette-Muller, M.L.; et al. Evaluation Using Latent Class Models of the Diagnostic Performances of Three ELISA Tests Commercialized for the Serological Diagnosis of Coxiella burnetii Infection in Domestic Ruminants. Vet. Res. 2021, 52, 56. [Google Scholar] [CrossRef]
- Sadiki, V.; Gcebe, N.; Mangena, M.L.; Ngoshe, Y.B.; Adesiyun, A.A. Prevalence and Risk Factors of Q Fever (Coxiella burnetii) in Cattle on Farms of Limpopo Province, South Africa. Front. Vet. Sci. 2023, 10, 1101988. [Google Scholar] [CrossRef]
- Taurel, A.-F.; Guatteo, R.; Joly, A.; Seegers, H.; Beaudeau, F. Seroprevalence of Q Fever in Naturally Infected Dairy Cattle Herds. Prev. Vet. Med. 2011, 101, 51–57. [Google Scholar] [CrossRef]
- McCaughey, C.; Murray, L.J.; McKenna, J.P.; Menzies, F.D.; McCullough, S.J.; O’Neill, H.J.; Wyatt, D.E.; Cardwell, C.R.; Coyle, P.V. Coxiella burnetii (Q Fever) Seroprevalence in Cattle. Epidemiol. Infect. 2010, 138, 21–27. [Google Scholar] [CrossRef]
- Turcotte, M.-È.; Buczinski, S.; Leboeuf, A.; Harel, J.; Bélanger, D.; Tremblay, D.; Gagnon, C.A.; Arsenault, J. Epidemiological Study of Coxiella burnetii in Dairy Cattle and Small Ruminants in Québec, Canada. Prev. Vet. Med. 2021, 191, 105365. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, M.; Lakošeljac, D.; Knežević, L.; Bubonja-Šonje, M.; Abram, M.; Špičić, S.; Zdelar-Tuk, M.; Duvnjak, S.; Reil, I.; Valjin, O.; et al. Coxiella burnetii (Q-fever) Outbreak Associated with Non-occupational Exposure in a Semi-urban Area of Western Croatia in 2022. Zoonoses Public Health 2023, 70, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Czaplicki, G.; Houtain, J.-Y.; Mullender, C.; Porter, S.R.; Humblet, M.-F.; Manteca, C.; Saegerman, C. Apparent Prevalence of Antibodies to Coxiella burnetii (Q Fever) in Bulk Tank Milk from Dairy Herds in Southern Belgium. Vet. J. 2012, 192, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Marawan, M.A.; Abdelhady, A.; Alshammari, F.A.; Alqhtani, A.H.; Ba-Awadh, H.A.; Olarinre, I.O.; Swelum, A.A. Coxiella burnetii and Its Risk Factors in Cattle in Egypt: A Seroepidemiological Survey. BMC Vet. Res. 2023, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Robi, D.T.; Demissie, W.; Temteme, S. Coxiellosis in Livestock: Epidemiology, Public Health Significance, and Prevalence of Coxiella burnetii Infection in Ethiopia. Vet. Med. 2023, 14, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, P.; Malik, S.V.S.; Yadav, J.P.; Kumar, M.; Barbuddhe, S.B.; Rawool, D.B. Apparent Prevalence and Risk Factors of Coxiellosis (Q Fever) among Dairy Herds in India. PLoS ONE 2020, 15, e0239260. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Ali, S.; Javid, A.; Imran, M.; Rashid, M.I.; Mertens-Scholz, K.; Neubauer, H. Sero-Epidemiology of Coxiella burnetii Infection in Small Ruminants in the Eastern Region of Punjab, Pakistan. Pathogens 2022, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, M.-È.; Denis-Robichaud, J.; Dubuc, J.; Harel, J.; Tremblay, D.; Gagnon, C.A.; Arsenault, J. Prevalence of Shedding and Antibody to Coxiella burnetii in Post-Partum Dairy Cows and Its Association with Reproductive Tract Diseases and Performance: A Pilot Study. Prev. Vet. Med. 2021, 186, 105231. [Google Scholar] [CrossRef]
- Ferrara, G.; Colitti, B.; Pagnini, U.; D’Angelo, D.; Iovane, G.; Rosati, S.; Montagnaro, S. Serological Evidence of Q Fever among Dairy Cattle and Buffalo Populations in the Campania Region, Italy. Pathogens 2022, 11, 901. [Google Scholar] [CrossRef]
- Bwatota, S.F.; Shirima, G.M.; Hernandez-Castro, L.E.; Bronsvoort, B.M.d.C.; Wheelhouse, N.; Mengele, I.J.; Motto, S.K.; Komwihangilo, D.M.; Lyatuu, E.; Cook, E.A.J. Seroprevalence and Risk Factors for Q Fever (Coxiella burnetii) Exposure in Smallholder Dairy Cattle in Tanzania. Vet. Sci. 2022, 9, 662. [Google Scholar] [CrossRef]
- Freick, M.; Enbergs, H.; Walraph, J.; Diller, R.; Weber, J.; Konrath, A. Coxiella burnetii: Serological Reactions and Bacterial Shedding in Primiparous Dairy Cows in an Endemically Infected Herd—Impact on Milk Yield and Fertility. Reprod. Domest. Anim. 2017, 52, 160–169. [Google Scholar] [CrossRef] [PubMed]
- López-Gatius, F.; Almeria, S.; Garcia-Ispierto, I. Serological Screening for Coxiella burnetii Infection and Related Reproductive Performance in High Producing Dairy Cows. Res. Vet. Sci. 2012, 93, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ispierto, I.; López-Helguera, I.; Tutusaus, J.; Serrano, B.; Monleón, E.; Badiola, J.; López-Gatius, F. Coxiella burnetii Shedding During the Peripartum Period and Subsequent Fertility in Dairy Cattle. Reprod. Domest. Anim. 2013, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.S.; Rodolakis, A.; Cochonneau, D.; Agger, J.F.; Christoffersen, A.-B.; Jensen, T.K.; Agerholm, J.S. Coxiella burnetii Associated Placental Lesions and Infection Level in Parturient Cows. Vet. J. 2011, 190, e135–e139. [Google Scholar] [CrossRef] [PubMed]
- Bildfell, R.J.; Thomson, G.W.; Haines, D.M.; McEwen, B.J.; Smart, N. Coxiella burnetii Infection Is Associated with Placentitis in Cases of Bovine Abortion. J. Vet. Diagn. Investig. 2000, 12, 419–425. [Google Scholar] [CrossRef]
- De Biase, D.; Costagliola, A.; Del Piero, F.; Di Palo, R.; Coronati, D.; Galiero, G.; Uberti, B.D.; Lucibelli, M.G.; Fabbiano, A.; Davoust, B.; et al. Coxiella burnetii in Infertile Dairy Cattle With Chronic Endometritis. Vet. Pathol. 2018, 55, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.-J. Defining Postpartum Uterine Disease and the Mechanisms of Infection and Immunity in the Female Reproductive Tract in Cattle1. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Giuliodori, M.J.; Magnasco, R.P.; Becu-Villalobos, D.; Lacau-Mengido, I.M.; Risco, C.A.; de la Sota, R.L. Metritis in Dairy Cows: Risk Factors and Reproductive Performance. J. Dairy Sci. 2013, 96, 3621–3631. [Google Scholar] [CrossRef]
- Mahnani, A.; Sadeghi-Sefidmazgi, A.; Cabrera, V.E. Consequences and Economics of Metritis in Iranian Holstein Dairy Farms. J. Dairy Sci. 2015, 98, 6048–6057. [Google Scholar] [CrossRef]
- Gaafar, H.M.A.; Shamiah, S.H.M.; Shitta, A.A.; Ganah, H.A.B. Factors Affecting Retention of Placenta and Its Influence on Postpartum Reproductive Performance and Milk Production in Friesian Cows. Slovak J. Anim. Sci. 2010, 43, 6–12. [Google Scholar]
- Martins, T.M.; Muniz, C.S.; Andrade, V.B.; Paixão, T.A.; Santos, R.L.; Borges, Á.M. Changes in Endometrial Transcription of TLR2, TLR4, and CD14 during the First-Week Postpartum in Dairy Cows with Retained Placenta. Theriogenology 2016, 85, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A.; Burfeind, O.; Heuwieser, W. The Associations between Postpartum Serum Haptoglobin Concentration and Metabolic Status, Calving Difficulties, Retained Fetal Membranes, and Metritis. J. Dairy Sci. 2015, 98, 4544–4551. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, N.; Poudel, S.; Pandey, D.; Khanal, D.R. Sero-detection of Coxiella burnetii Infection in Cattle, Sheep and Goats in Selected Regions of Nepal. Vet. Med. Sci. 2021, 7, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Changoluisa, D.; Rivera-Olivero, I.A.; Echeverria, G.; Garcia-Bereguiain, M.A.; de Waard, J.H. Serology for Neosporosis, Q Fever and Brucellosis to Assess the Cause of Abortion in Two Dairy Cattle Herds in Ecuador. BMC Vet. Res. 2019, 15, 194. [Google Scholar] [CrossRef]
- Lehner, S.; Lohan, K.; Dieckhoff, H.-J.; Gerdes, U. Erfahrungen von Tierhaltern in Niedersächsischen Milchkuhbetrieben Mit Der Impfung Gegen Q-Fieber. Tierärztliche Prax. Ausg. G Großtiere/Nutztiere 2017, 45, 141–149. [Google Scholar] [CrossRef]


| Variable | Groups | Seropositivity (%) (Seropositive/Total) |
|---|---|---|
| Geographical area | C a | 80.0 (28/35) |
| L b | 56.8 (126/222) | |
| P a,b | 80.0 (4/5) | |
| Herd size (number of cows) | ≤36 a | 44.4 (55/124) |
| 37–60 b | 66.7 (58/87) | |
| >60 c | 82.2 (45/51) | |
| Average herd age (years) | ≤5 a | 64.1 (109/170) † |
| >5 a | 52.7 (48/91) | |
| Purchase of livestock | No a | 53.7 (79/147) |
| Yes b | 68.7 (79/115) | |
| Housing type | Free stalls a | 72.3 (73/101) |
| Free stalls + pasture a,b | 58.6 (17/29) | |
| Free stalls + exercise area a,b | 63.6 (14/22) | |
| Tie stalls/Stanchion barns b | 51.0 (26/51) | |
| Tie stalls/Stanchion barns + pasture b | 47.5 (28/59) | |
| Heifer raising | At the farm a | 59.4 (152/256) |
| Custom raised b | 100 (6/6) | |
| Use of bulls | No a | 56.0 (117/209) |
| Yes b | 77.4 (41/53) |
| Variable | Positive Farms (±SD) | Negative Farms (±SD) |
|---|---|---|
| Abortions (%) | 9.79 (±9.36) | 8.91 (±8.12) |
| Metritis (%) | 11.52 (±9.37) | 10.22 (±8.26) |
| Endometritis (%) * | 13.73 (±9.25) | 11.23 (±9.34) |
| Culling rate (%) | 26.55 (±11.95) | 27.87 (±16.7) |
| FSCR (%) * | 32.90 (±11.80) | 36.13 (±13.16) |
| CR (%) * | 37.07 (±10.28) | 39.78 (±11.72) |
| Calving to first AI interval (days) | 78.50 (±14.06) | 84.99 (±16.03) |
| Days open | 153.11 (±32.36) | 154.03 (±36.36) |
| SCC (×103 cells/mL) | 277.49 (±142.03) | 316.92 (±137.89) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yáñez, U.; Álvarez, J.; Pisón, C.; Acción, A.; Becerra, J.J.; Jiménez, A.; Gisbert, P.; Herradón, P.G.; Peña, A.I.; Prieto, A.; et al. Prevalence, Risk Factors, and Relationship between Reproductive Performance and the Presence of Antibodies against Coxiellosis in Dairy Farm Milk Tanks in the Northwest of Spain. Animals 2024, 14, 367. https://doi.org/10.3390/ani14030367
Yáñez U, Álvarez J, Pisón C, Acción A, Becerra JJ, Jiménez A, Gisbert P, Herradón PG, Peña AI, Prieto A, et al. Prevalence, Risk Factors, and Relationship between Reproductive Performance and the Presence of Antibodies against Coxiellosis in Dairy Farm Milk Tanks in the Northwest of Spain. Animals. 2024; 14(3):367. https://doi.org/10.3390/ani14030367
Chicago/Turabian StyleYáñez, Uxía, Jacobo Álvarez, Cristina Pisón, Antía Acción, Juan J. Becerra, Antonio Jiménez, Philippe Gisbert, Pedro G. Herradón, Ana I. Peña, Alberto Prieto, and et al. 2024. "Prevalence, Risk Factors, and Relationship between Reproductive Performance and the Presence of Antibodies against Coxiellosis in Dairy Farm Milk Tanks in the Northwest of Spain" Animals 14, no. 3: 367. https://doi.org/10.3390/ani14030367
APA StyleYáñez, U., Álvarez, J., Pisón, C., Acción, A., Becerra, J. J., Jiménez, A., Gisbert, P., Herradón, P. G., Peña, A. I., Prieto, A., Díaz-Cao, J. M., & Quintela, L. A. (2024). Prevalence, Risk Factors, and Relationship between Reproductive Performance and the Presence of Antibodies against Coxiellosis in Dairy Farm Milk Tanks in the Northwest of Spain. Animals, 14(3), 367. https://doi.org/10.3390/ani14030367








