Ultrasonographic Measurement of Muscle and Subcutaneous Fat Thickness for the Objective Assessment of the Nutritional Status of Alpacas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Body Condition Score
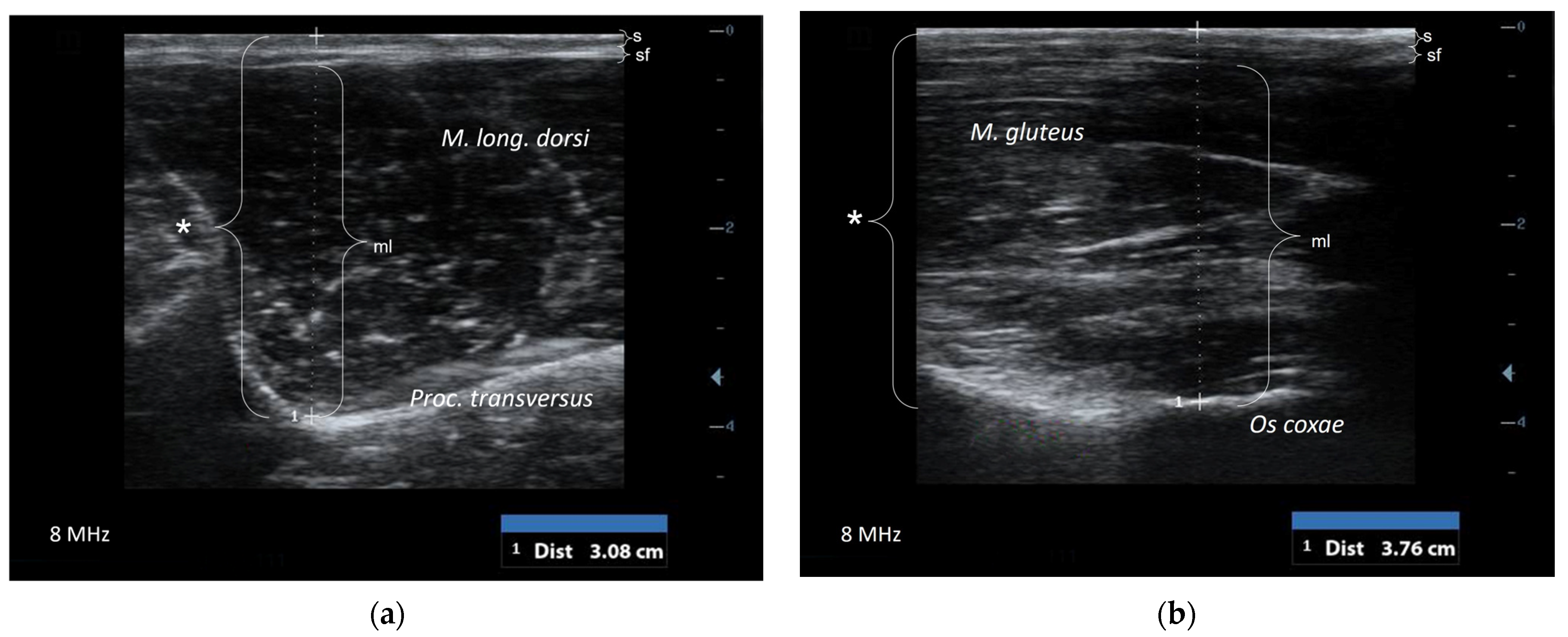
2.3. Ultrasonographic Examination
2.4. Body Weight
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Arithmetic mean |
| BCS | Body condition score |
| BW | Body weight |
| CV | Coefficient of variation |
| MAX | Maximum |
| MIN | Minimum |
| US-G | Ultrasonography soft-tissue thickness gluteal region |
| US-L | Ultrasonography soft-tissue thickness lumbar region |
| RFT | Rump fat thickness |
| SACs | South American camelids |
| SD | Standard deviation |
| SFT | Sternal fat thickness |
References
- Bauerstatter, S.; Lambacher, B.; Stanitznig, A.; Franz, S.; Wittek, T. South American camelids in Austria–Survey on population, husbandry, herd management and preventive measures. Wien. Tierärztl. Mschr. 2018, 105, 191–199. [Google Scholar]
- Wagner, H.; Ulrich, L.; Leisen, A.; Wehrend, A. Populationsstruktur und Haltungsweisen von Neuweltkameliden in Deutschland sowie Fachkunde der Tierhalter (Population structure of South American camelids in Germany). Tierarztl. Prax. Ausg. G Großtiere/Nutztiere 2022, 50, 237–249. [Google Scholar] [PubMed]
- Van Saun, R.J. Nutritional diseases of South American camelids. Small Rumin. Res. 2006, 61, 153–164. [Google Scholar] [CrossRef]
- Van Saun, R.J. Nutritional Requirements and Assessing Nutritional Status in Camelids. Vet. Clin. N. Am. Food Anim. Pract. 2009, 25, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Wittek, T.; Franz, S. Praxishandbuch Neuweltkamele, 2nd ed.; Schlütersche: Hannover, Germany, 2023. [Google Scholar]
- Wagener, M.G.; Ganter, M. Body condition scoring in South American camelids. Prakt. Tierarzt 2020, 101, 684–696. [Google Scholar]
- Wagener, M.G.; Ganter, M.; Leonard-Marek, S. Body condition scoring in alpacas (Vicugna pacos) and llamas (Lama glama)—A scoping review. Vet. Res. Commun. 2024, 48, 665–684. [Google Scholar] [CrossRef]
- Franz, S.; Wittek, T.; Joachim, A.; Hinney, B.; Dadak, A.M. Llamas and alpacas in Europe: Endoparasites of the digestive tract and their Pharmacotherapeutic control. Vet. J. 2015, 204, 255–262. [Google Scholar] [CrossRef]
- San Martín, F.; Van Saun, R.J. Feeding Management Systems. In Llama and Alpaca Care: Medicine, Surgery, Reproduction, Nutrition, and Herd Health, 1st ed.; Cebra, C., Anderson, D.E., Tibary, A., Van Saun, R.J., Johnson, L.W., Eds.; Elsevier: St. Louis, MO, USA, 2014; pp. 91–100. [Google Scholar]
- Bustinza, A.V.; Burfening, P.J.; Blackwell, R.L. Factors affecting survival in young alpacas (Lama pacos). Anim. Sci. J. 1988, 66, 1139–1143. [Google Scholar] [CrossRef]
- Kapustka, J.; Budzyńska, M. Reproductive Losses and Their Causes in Alpacas—A Survey-Based Study. Animals 2022, 12, 3030. [Google Scholar] [CrossRef]
- Van Saun, R.J. Nutrient requirements of South American camelids: A factorial approach. Small Rumin. Res. 2006, 61, 165–186. [Google Scholar] [CrossRef]
- Cruz, A.; Morante, R.; Cervantes, I.; Burgos, A.; Gutiérrez, J.P. Effect of the gestation and lactation on fiber diameter and its variability in Peruvian alpacas. Livest. Sci. 2017, 198, 31–36. [Google Scholar] [CrossRef]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A Body Condition Scoring Chart for Holstein Dairy Cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Buchalik-Schregel, J.; Kiene, F.; Buchalik, J.; Marahrens, H.; Ossowsky, N.; Schumacher, C.V.; Gerstel, B.; Reimers, U.; Ganter, M.; Wagener, M.G. Relationship between body condition score, body weight and body measurements in alpacas. Ir. Vet. J. 2024, 77, 11. [Google Scholar] [CrossRef] [PubMed]
- Russel, A. Body Condition Scoring of Sheep; CABI: Wallingford, UK, 1984; Volume 6, pp. 91–93. [Google Scholar]
- Shands, C.G.; Mcleod, B.; Lollback, M.L.; Duddy, G.; Hatcher, S.; O’Halloran, W.J. Comparison of manual assessments of ewe fat reserves for on-farm use. Anim. Prod. Sci. 2009, 49, 630–636. [Google Scholar] [CrossRef]
- Yilmaz, M.; Altin, T.; Karaca, O.; Cemal, I.; Bardakcioglu, H.E.; Yilmaz, O.; Taskin, T. Effect of body condition score at mating on the reproductive performance of Kivircik sheep under an extensive production system. Trop. Anim. Health Prod. 2011, 43, 1555–1560. [Google Scholar] [CrossRef]
- Vatankhah, M.; Talebi, M.A.; Zamani, F. Relationship between ewe body condition score (BCS) at mating and reproductive and productive traits in Lori-Bakhtiari sheep. Small Rumin. Res. 2012, 106, 105–109. [Google Scholar] [CrossRef]
- Keinprecht, H.; Pichler, M.; Pothmann, H.; Huber, J.; Iwersen, M.; Drillich, M. 2016. Short term repeatability of body fat thickness measurement and body condition scoring in sheep as assessed by a relatively small number of assessors. Small Rumin. Res. 2012, 139, 30–38. [Google Scholar] [CrossRef]
- Johnson, L.W. Update Llama nutrition. Vet. Clin. N. Am. Food Anim. Pract. 1994, 10, 187–201. [Google Scholar] [CrossRef]
- Hilton, C.D.; Pugh, D.G.; Heath, A.M. How to determine and when to use body weight and condition in llamas. Vet. Med. 1998, 93, 1015–1018. [Google Scholar]
- Fowler, M.E. Medicine and Surgery of Camelids, 3rd ed.; Wiley-Blackwell: Ames, IA, USA, 2010. [Google Scholar]
- Wagner, H.; Hümmelchen, H.; Bartl, E.M.; Ulrich, L. Tiergesundheitsmanagementplan. Available online: https://nwk-projekt.de/tiergesundheitsmanagementplan-und-checklisten (accessed on 27 November 2024).
- Kleiböhmer, C.; Heuwieser, W.; Bergmann, J.; Ochsmann, A. Untersuchungen zur Erlernbarkeit und Genauigkeit der Körperkonditionsbeurteilung (BCS) beim Rind. Prakt. Tierarzt 1998, 79, 50–61. [Google Scholar]
- Schröder, U.; Staufenbiel, R. Ultrasonographic assessment of body condition in dairy herd management. Part 4: Practical application. Tierarztl. Prax. Ausg. G Grosstiere/Nutztiere 2004, 32, 1–6. [Google Scholar]
- Schröder, U.J.; Staufenbiel, R. Methods to Determine Body Fat Reserves in the Dairy Cow with Special Regard to Ultrasonographic Measurement of Backfat Thickness. J. Dairy Sci. 2006, 89, 1–14. [Google Scholar] [CrossRef] [PubMed]
- De Kruif, A.; Mansfeld, R.; Hoedemaker, M. Tierärztliche Bestandsbetreuung beim Milchrind, 2nd ed.; Enke: Stuttgart, Germany, 2007. [Google Scholar]
- Pothmann, H.; Erlen, A.; Pichler, M.; Huber, J.; Drillich, M. Relationship and repeatability of body condition scoring and backfat thickness measurement in dairy cows by different investigators. Berl. Münch. Tierärztl. Wschr. 2015, 128, 319–325. [Google Scholar]
- Boge, S.; Tichy, A.; Michael, S.; Drillich, M.; Pothmann, H. The body condition of Simmental dairy cows–An update to the reference curve for backfat thickness, with special consideration to the growth phase of younger cows. Wien. Tierärztl. Mschr. 2020, 108, 96–103. [Google Scholar]
- Emenheiser, J.C.; Greiner, S.P.; Lewis, R.M.; Notter, D.R. Validation of live animal ultrasonic measurements of body composition in market lambs. J. Anim. Sci. 2010, 88, 2932–2939. [Google Scholar] [CrossRef]
- Van Burgel, A.J.; Oldham, C.M.; Behrendt, R.; Curnow, M.; Gordon, D.J.; Thompson, A.N. The merit of condition score and fat score as alternatives to liveweight for managing the nutrition of ewes. Anim. Prod. Sci. 2011, 51, 834–841. [Google Scholar] [CrossRef]
- Navarre, C.B.; Heath, A.M.; Wenzel, J.; Simpkins, A.; Blair, E.; Belknap, E.; Pugh, D.G. A comparison of physical examination and clinicopathologic parameters between sheared and nonsheared alpacas (Lama pacos). Small Rumin. Res. 2001, 39, 11–17. [Google Scholar] [CrossRef]
- Heath, A.M.; Navarre, C.B.; Simpkins, A.; Purohit, R.C.; Pugh, D.G. A comparison of surface and rectal temperatures between sheared and non-sheared alpacas (Lama pacos). Small Rumin. Res. 2001, 39, 19–23. [Google Scholar] [CrossRef]
- Smith, C.L.; Peter, A.T.; Pugh, D.G. Reproduction in llamas and alpacas: A review. Theriogenology 1994, 41, 573–592. [Google Scholar] [CrossRef]
- Van Saun, R.J. Body Condition Scoring of Llamas and Alpacas. Available online: https://extension.psu.edu/body-condition-scoring-of-llamas-and-alpacas (accessed on 28 June 2024).
- Grund, S.; Vogel, M.; Mülling, C.K.W. Morphometric evaluation of the growth of Alpacas (Vicugna pacos) from birth to 36 months of age. Small Rumin. Res. 2018, 166, 61–65. [Google Scholar] [CrossRef]
- Junkuszew, A.; Ringdorfer, F. Computer tomography and ultrasound measurement as methods for the prediction of the body composition of lambs. Small Rumin. Res. 2005, 56, 121–125. [Google Scholar] [CrossRef]

| Male | Female | |||
|---|---|---|---|---|
| Alpacas | Intact | Castrated | Pregnant > 6 Months | Non-Pregnant or Pregnant < 6 Months |
| n | 56 | 23 | 44 | 37 |
| % | 35.00 | 14.38 | 27.50 | 23.12 |
| Measured Parameter | N * | AM | SD | MAX | MIN |
|---|---|---|---|---|---|
| BW (kg) | 157 | 65.60 | 14.26 | 106.60 | 24.50 |
| BCS | 160 | 2.84 | 0.70 | 4.50 | 1.25 |
| US-L (cm) | 160 | 3.90 | 0.66 | 5.75 | 2.10 |
| US-G (cm) | 157 | 4.09 | 0.65 | 5.86 | 2.04 |
| Measured Parameter | Gender | N * | AM | SD |
|---|---|---|---|---|
| BW (kg) | Male | 78 | 67.63 a | 14.98 |
| Female | 79 | 63.57 b | 13.30 | |
| BCS | Male | 79 | 3.01 a | 0.69 |
| Female | 81 | 2.68 b | 0.68 | |
| US-L (cm) | Male | 76 | 4.04 a | 0.72 |
| Female | 78 | 3.75 b | 0.58 | |
| US-G (cm) | Male | 75 | 4.23 a | 0.68 |
| Female | 75 | 3.96 b | 0.60 |
| Alpacas | Correlation Coefficients (p-Value) Between Measured Parameters | |||||
|---|---|---|---|---|---|---|
| BW:BCS | BW:US-L | BW:US-G | BCS:US-L | BCS:US-G | US-L:US-G | |
| All alpacas (n = 160) | 0.46 (<0.05) | 0.63 (<0.001) | 0.71 (<0.001) | 0.77 (<0.001) | 0.58 (<0.01) | 0.77 (<0.001) |
| Males (n = 79) | 0.59 (<0.001) | 0.76 (<0.001) | 0.84 (<0.001) | 0.77 (<0.001) | 0.72 (<0.001) | 0.86 (<0.001) |
| Females pregnant > 6 months (n = 44) | 0.12 (0.43) | 0.39 (<0.01) | 0.40 (<0.01) | 0.70 (<0.001) | 0.25 (0.12) | 0.53 (<0.001) |
| Females non-pregnant or pregnant < 6 months (n = 37) | 0.43 (<0.001) | 0.75 (<0.001) | 0,73 (<0.001) | 0.85 (<0.001) | 0.78 (<0.001) | 0.89 (<0.001) |
| BCS | Gender | N * | US-L (cm): AM ± SD | US-G (cm): AM ± SD |
|---|---|---|---|---|
| 1 to <2 | Male | 5 | 2.50 ± 0.34 (a) | 2.86 ± 0.58 (a) |
| Females non-pregnant or pregnant < 6 months | 7 | 2.80 ± 0.49 (1) | 3.13 ± 0.46 (1) | |
| 2 to <3 | Male | 30 | 3.80 ± 0.57 (b) | 3.99 ± 0.49 (b) |
| Females non-pregnant or pregnant < 6 months | 17 | 3.57 ± 0.32 (2) | 3.72 ± 0.41 (2) | |
| 3 to <4 | Male | 38 | 4.28 ± 0.54 (c) | 4.44 ± 0.48 (c) |
| Females non-pregnant or pregnant < 6 months | 12 | 4.24 ± 0.49 (3) | 4.34 ±0.52 (3) | |
| 4 to 5 | Male | 6 | 4.89 ± 0.49 (c) | 5.22 ± 0.62 (d) |
| Females non-pregnant or pregnant < 6 months | 1 | 4.84 | 5.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franz, S.; Andrich, M.; Wittek, T. Ultrasonographic Measurement of Muscle and Subcutaneous Fat Thickness for the Objective Assessment of the Nutritional Status of Alpacas. Animals 2024, 14, 3695. https://doi.org/10.3390/ani14243695
Franz S, Andrich M, Wittek T. Ultrasonographic Measurement of Muscle and Subcutaneous Fat Thickness for the Objective Assessment of the Nutritional Status of Alpacas. Animals. 2024; 14(24):3695. https://doi.org/10.3390/ani14243695
Chicago/Turabian StyleFranz, Sonja, Melanie Andrich, and Thomas Wittek. 2024. "Ultrasonographic Measurement of Muscle and Subcutaneous Fat Thickness for the Objective Assessment of the Nutritional Status of Alpacas" Animals 14, no. 24: 3695. https://doi.org/10.3390/ani14243695
APA StyleFranz, S., Andrich, M., & Wittek, T. (2024). Ultrasonographic Measurement of Muscle and Subcutaneous Fat Thickness for the Objective Assessment of the Nutritional Status of Alpacas. Animals, 14(24), 3695. https://doi.org/10.3390/ani14243695






