Specialized Feed-Additive Blends of Short- and Medium-Chain Fatty Acids Improve Sow and Pig Performance During Nursery and Post-Weaning Phase
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sow Experiment
2.1.1. Animals and Housing
2.1.2. Experimental Design and Dietary Treatments
2.1.3. Experimental Procedures and Sampling
2.2. Pig Experiment
2.2.1. Animals and Housing
2.2.2. Experimental Design and Dietary Treatments
2.2.3. Experimental Procedures and Sampling
2.3. Chemical Analysis
2.4. Microbial Count
2.5. Statistical Analysis
3. Results
3.1. Sow Experiment
3.1.1. Sow Reproductive Performance
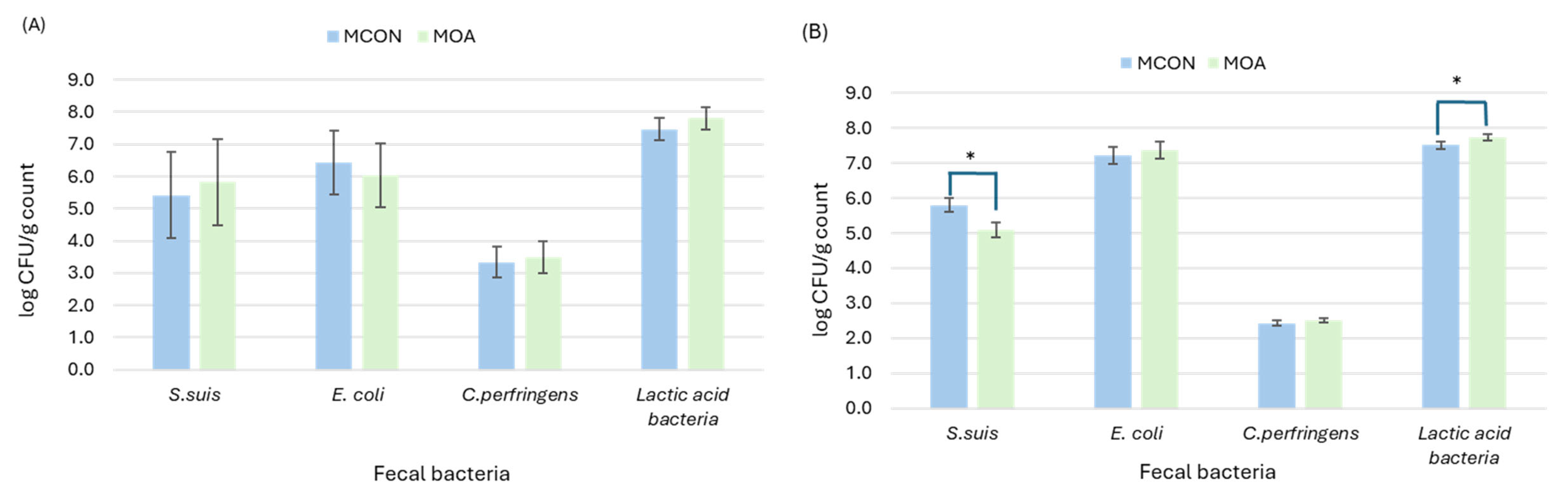
3.1.2. Microbial Counts
3.2. Pig Experiment
3.2.1. Pig Growth Performance
3.2.2. Microbial Counts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonetti, A.; Tugnoli, B.; Piva, A.; Grilli, E. Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhea in Piglets. Animals 2021, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.R.; Segura, M.; Segalés, J.; Gottschalk, M. Review of the Speculative Role of Co-Infections in Streptococcus Suis-Associated Diseases in Pigs. Vet. Res. 2021, 52, 49. [Google Scholar] [CrossRef] [PubMed]
- Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.-J.; Kim, D.W.; Kang, B.N.; Johnson, T.J.; Isaacson, R.E.; Kim, H.B. Piglet Gut Microbial Shifts Early in Life: Causes and Effects. J. Anim. Sci. Biotechnol. 2019, 10, 1. [Google Scholar] [CrossRef]
- Dou, S.; Gadonna-Widehem, P.; Rome, V.; Hamoudi, D.; Rhazi, L.; Lakhal, L.; Larcher, T.; Bahi-Jaber, N.; Pinon-Quintana, A.; Guyonvarch, A.; et al. Characterisation of Early-Life Fecal Microbiota in Susceptible and Healthy Pigs to Post-Weaning Diarrhoea. PLoS ONE 2017, 12, e0169851. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Seok, W.J.; Kim, I.H. Organic Acids Mixture as a Dietary Additive for Pigs—A Review. Animals 2020, 10, 952. [Google Scholar] [CrossRef]
- de Groot, N.; Fariñas, F.; Cabrera-Gómez, C.G.; Pallares, F.J.; Ramis, G. Blend of Organic Acids Improves Gut Morphology and Affects Inflammation Response in Piglets after Weaning. Front. Anim. Sci. 2024, 5, 1308514. [Google Scholar] [CrossRef]
- Gómez-García, M.; Puente, H.; Argüello, H.; Mencía-Ares, Ó.; Rubio, P.; Carvajal, A. In Vitro Assessment of Antiviral Effect of Natural Compounds on Porcine Epidemic Diarrhea Coronavirus. Front. Vet. Sci. 2021, 8, 652000. [Google Scholar] [CrossRef]
- Jackman, J.A.; Boyd, R.D.; Elrod, C.C. Medium-Chain Fatty Acids and Monoglycerides as Feed Additives for Pig Production: Towards Gut Health Improvement and Feed Pathogen Mitigation. J. Anim. Sci. Biotechnol. 2020, 11, 44. [Google Scholar] [CrossRef]
- Nakatani, M.; Inoue, R.; Tomonaga, S.; Fukuta, K.; Tsukahara, T. Production, Absorption, and Blood Flow Dynamics of Short-Chain Fatty Acids Produced by Fermentation in Piglet Hindgut during the Suckling–Weaning Period. Nutrients 2018, 10, 1220. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Lee, K.Y.; Serpunja, S.; Song, T.-H.; Kim, I.H. Growth Performance, Nutrient Digestibility, Fecal Microbiota and Fecal Noxious Gas Emission in Weaning Pigs Fed High and Low Density Diet with and without Protected Organic Acid Blends. Anim. Feed Sci. Technol. 2018, 239, 1–8. [Google Scholar] [CrossRef]
- Devi, S.M.; Lee, K.Y.; Kim, I.H. Analysis of the Effect of Dietary Protected Organic Acid Blend on Lactating Sows and Their Piglets. Rev. Bras. Zootec. 2016, 45, 39–47. [Google Scholar] [CrossRef]
- Lan, R.; Kim, I. Effects of Organic Acid and Medium Chain Fatty Acid Blends on the Performance of Sows and Their Piglets. Anim. Sci. J. 2018, 89, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Sampath, V.; Park, J.H.; Pineda, L.; Han, Y.; Kim, I.H. Impact of Synergistic Blend of Organic Acids on the Performance of Late Gestating Sows and Their Offspring. Ital. J. Anim. Sci. 2022, 21, 1334–1342. [Google Scholar] [CrossRef]
- You, C.; Xu, Q.; Chen, J.; Xu, Y.; Pang, J.; Peng, X.; Tang, Z.; Sun, W.; Sun, Z. Effects of Different Combinations of Sodium Butyrate, Medium-Chain Fatty Acids and Omega-3 Polyunsaturated Fatty Acids on the Reproductive Performance of Sows and Biochemical Parameters, Oxidative Status and Intestinal Health of Their Offspring. Animals 2023, 13, 1093. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, Q.; Li, Y.; Tang, Z.; Sun, W.; Zhang, X.; Sun, J.; Sun, Z. Comparative Effects of Dietary Supplementations with Sodium Butyrate, Medium-Chain Fatty Acids, and n-3 Polyunsaturated Fatty Acids in Late Pregnancy and Lactation on the Reproductive Performance of Sows and Growth Performance of Suckling Piglets. J. Anim. Sci. 2019, 97, 4256–4267. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 12th ed.; National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-22423-9. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; Revision 1; Horwitz, W., Latimer, G.W., Eds.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Carlson, A.L.; Xia, K.; Azcarate-Peril, M.A.; Goldman, B.D.; Ahn, M.; Styner, M.A.; Thompson, A.L.; Geng, X.; Gilmore, J.H.; Knickmeyer, R.C. Infant Gut Microbiome Associated With Cognitive Development. Biol. Psychiatry 2018, 83, 148–159. [Google Scholar] [CrossRef]
- Ma, C.; Gao, Q.; Zhang, W.; Zhu, Q.; Tang, W.; Blachier, F.; Ding, H.; Kong, X. Supplementing Synbiotic in Sows’ Diets Modifies Beneficially Blood Parameters and Colonic Microbiota Composition and Metabolic Activity in Suckling Piglets. Front. Vet. Sci. 2020, 7, 575685. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, X.; Cui, Y.; Wang, W.; Liu, H.; Li, Z.; Guo, Z.; Ma, S.; Li, D.; Wang, C.; et al. Consumption of Dietary Fiber from Different Sources during Pregnancy Alters Sow Gut Microbiota and Improves Performance and Reduces Inflammation in Sows and Piglets. mSystems 2021, 6. [Google Scholar] [CrossRef]
- Nowland, T.L.; Kirkwood, R.N.; Pluske, J.R. Review: Can Early-Life Establishment of the Piglet Intestinal Microbiota Influence Production Outcomes? Animal 2022, 16, 100368. [Google Scholar] [CrossRef]
- Nowland, T.L.; Kirkwood, R.N.; Plush, K.J.; Barton, M.D.; Torok, V.A. Exposure to Maternal Feces in Lactation Influences Piglet Enteric Microbiota, Growth, and Survival Preweaning. J. Anim. Sci. 2021, 99, 145–164. [Google Scholar] [CrossRef]
- Aviles-Rosa, E.O.; Rakhshandeh, A.; McGlone, J.J. Depriving Piglets of Maternal Feces for the First Seven Days Post-Partum Changes Piglet Physiology and Performance before and after Weaning. Animals 2019, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.S.F.; Pierdon, M.K.; Misic, A.M.; Sullivan, M.C.; O’Brien, K.; Chen, Y.; Murray, S.J.; Ramharack, L.A.; Baldassano, R.N.; Parsons, T.D.; et al. Remodeling of the Maternal Gut Microbiome during Pregnancy Is Shaped by Parity. Microbiome 2021, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Tokach, M.D.; Menegat, M.B.; Gourley, K.M.; Goodband, R.D. Review: Nutrient Requirements of the Modern High-Producing Lactating Sow, with an Emphasis on Amino Acid Requirements. Animal 2019, 13, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Papatsiros, V.G. The Use of Organic Acids in Monogastric Animals (Swine and Rabbits). J. Cell Anim. Biol. 2012, 6, 154–159. [Google Scholar] [CrossRef]
- Suiryanrayna, M.V.A.N.; Ramana, J.V. A Review of the Effects of Dietary Organic Acids Fed to Swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and Medium-Chain Fatty Acids in Energy Metabolism: The Cellular Perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Partanen, K.H.; Mroz, Z. Organic Acids for Performance Enhancement in Pig Diets. Nutr. Res. Rev. 1999, 12, 117–145. [Google Scholar] [CrossRef]
- Øverland, M.; Bikker, P.; Fledderus, J. Potassium Diformate in the Diet of Reproducing Sows: Effect on Performance of Sows and Litters. Livest. Sci. 2009, 122, 241–247. [Google Scholar] [CrossRef]
- Pearlin, B.V.; Muthuvel, S.; Govidasamy, P.; Villavan, M.; Alagawany, M.; Ragab Farag, M.; Dhama, K.; Gopi, M. Role of Acidifiers in Livestock Nutrition and Health: A Review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 558–569. [Google Scholar] [CrossRef]
- Patterson, R.; Connor, M.L.; Krause, D.O.; Nyachoti, C.M. Response of Piglets Weaned from Sows Fed Diets Supplemented with Conjugated Linoleic Acid (CLA) to an Escherichia Coli K88+ Oral Challenge. Animal 2008, 2, 1303–1311. [Google Scholar] [CrossRef]
- Corino, C.; Pastorelli, G.; Rosi, F.; Bontempo, V.; Rossi, R. Effect of Dietary Conjugated Linoleic Acid Supplementation in Sows on Performance and Immunoglobulin Concentration in Piglets1. J. Anim. Sci. 2009, 87, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.G.; Sweeney, T.; Pierce, K.M.; Bahar, B.; Lynch, B.P.; O’Doherty, J.V. The Effects of Supplementing the Diet of the Sow with Seaweed Extracts and Fish Oil on Aspects of Gastrointestinal Health and Performance of the Weaned Piglet. Livest. Sci. 2010, 134, 135–138. [Google Scholar] [CrossRef]
- De Bettio, S.; Maiorka, A.; Barrilli, L.N.E.; Bergsma, R.; Silva, B.A.N. Impact of Feed Restriction on the Performance of Highly Prolific Lactating Sows and Its Effect on the Subsequent Lactation. Animal 2016, 10, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal Health and Function in Weaned Pigs: A Review of Feeding Strategies to Control Post-weaning Diarrhoea without Using In-feed Antimicrobial Compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Tugnoli, B.; Giovagnoni, G.; Piva, A.; Grilli, E. From Acidifiers to Intestinal Health Enhancers: How Organic Acids Can Improve Growth Efficiency of Pigs. Animals 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C. Effects of Dietary Fatty Acids on Gut Health and Function of Pigs Pre- and Post-Weaning. J. Anim. Sci. 2020, 98, skaa086. [Google Scholar] [CrossRef]
- Hanczakowska, E. The Use of Medium-Chain Fatty Acids in Piglet Feeding—A Review. Ann. Anim. Sci. 2017, 17, 967–977. [Google Scholar] [CrossRef]
- Zentek, J.; Buchheit-Renko, S.; Ferrara, F.; Vahjen, W.; Van Kessel, A.G.; Pieper, R. Nutritional and Physiological Role of Medium-Chain Triglycerides and Medium-Chain Fatty Acids in Piglets. Anim. Health Res. Rev. 2011, 12, 83–93. [Google Scholar] [CrossRef]
- Lei, X.J.; Park, J.W.; Baek, D.H.; Kim, J.K.; Kim, I.H. Feeding the Blend of Organic Acids and Medium Chain Fatty Acids Reduces the Diarrhea in Piglets Orally Challenged with Enterotoxigenic Escherichia Coli K88. Anim. Feed Sci. Technol. 2017, 224, 46–51. [Google Scholar] [CrossRef]
- Dibner, J.J.; Buttin, P. Use of Organic Acids as a Model to Study the Impact of Gut Microflora on Nutrition and Metabolism. J. Appl. Poult. Res. 2002, 11, 453–463. [Google Scholar] [CrossRef]
- Patil, Y.; Gooneratne, R.; Ju, X.-H. Interactions between Host and Gut Microbiota in Domestic Pigs: A Review. Gut Microbes 2020, 11, 310–334. [Google Scholar] [CrossRef] [PubMed]
- McCormack, U.M.; Curião, T.; Buzoianu, S.G.; Prieto, M.L.; Ryan, T.; Varley, P.; Crispie, F.; Magowan, E.; Metzler-Zebeli, B.U.; Berry, D.; et al. Exploring a Possible Link between the Intestinal Microbiota and Feed Efficiency in Pigs. Appl. Environ. Microbiol. 2017, 83, e00380-17. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yang, T.; Wang, Y.; Xing, K.; Zhang, F.; Zhao, X.; Ao, H.; Chen, S.; Liu, J.; Wang, C. Metagenomic Analysis of Cecal Microbiome Identified Microbiota and Functional Capacities Associated with Feed Efficiency in Landrace Finishing Pigs. Front. Microbiol. 2017, 8, 1546. [Google Scholar] [CrossRef] [PubMed]
- McCormack, U.M.; Curião, T.; Metzler-Zebeli, B.U.; Magowan, E.; Berry, D.P.; Reyer, H.; Prieto, M.L.; Buzoianu, S.G.; Harrison, M.; Rebeiz, N.; et al. Porcine Feed Efficiency-Associated Intestinal Microbiota and Physiological Traits: Finding Consistent Cross-Locational Biomarkers for Residual Feed Intake. mSystems 2019, 4, 10–1128. [Google Scholar] [CrossRef]

| Ingredients, % | Lactation |
|---|---|
| Corn | 21.51 |
| Wheat | 15.00 |
| Wheat bran | 15.00 |
| Field pea | 12.11 |
| Soybean | 11.75 |
| Rice bran | 10.00 |
| Sorghum | 5.00 |
| Sugarbeet pulp | 5.00 |
| Calcium carbonate | 1.66 |
| Palm oil | 1.06 |
| Lysine 50 | 0.30 |
| L-Threonine | 0.10 |
| DL-Methionine | 0.06 |
| Salt | 0.60 |
| Mono calcium phosphate | 0.43 |
| Vit-Min Premix 1 | 0.40 |
| Phytase | 0.02 |
| Calculated composition | |
| NE, kcal/kg | 2230 |
| CP, % | 15.94 |
| Lysine, % | 0.94 |
| Ether extract, % | 4.50 |
| Crude fiber, % | 5.08 |
| Ca, % | 0.85 |
| Total P, % | 0.62 |
| Dig P, % | 0.30 |
| Analyzed nutrient composition, % | |
| CP | 15.90 |
| Ether Extract | 4.00 |
| NDF | 16.49 |
| Ash | 5.94 |
| Ingredients, % | Pre-Starter | Starter |
|---|---|---|
| Wheat | 33.40 | 30.27 |
| Barley Extruded | 30.00 | 10.00 |
| Soybean meal, 47 | 7.79 | 10.91 |
| Corn | - | 20.00 |
| Sorghum | - | 15.00 |
| Dextrose | 7.50 | - |
| Whey powder acid | 5.00 | - |
| Fish meal, 70 | 5.00 | 2.50 |
| Mucous hydrolyzed, 50 | - | 2.50 |
| Animal plasma, 80 | 2.50 | - |
| Soybean concentrate | 2.00 | 2.50 |
| Rapeseed oil | 1.20 | - |
| Bi-calcium phosphate | 1.00 | - |
| Lard 1.5 | - | 1.68 |
| Lysine 50 | - | 0.95 |
| Calcium carbonate | - | 0.90 |
| Monocalcium phosphate | - | 0.70 |
| Vitamin Premix 1 | 2.50 | 1.00 |
| Lysine sulphate 70 | 0.85 | - |
| DL-Methionine | 0.32 | 0.21 |
| L-Threonine | 0.32 | 0.26 |
| Salt | 0.20 | 0.30 |
| L-Tryptophan | 0.12 | 0.06 |
| L-Valine | 0.12 | 0.08 |
| Enzymes | 0.10 | 0.10 |
| Phytase | 0.08 | 0.08 |
| Calculated composition | ||
| NE, kcal/kg | 2430 | 2380 |
| CP, % | 17.62 | 17.47 |
| Lysine, % | 1.39 | 1.29 |
| Ether extract, % | 4.50 | 3.91 |
| Crude fiber, % | 2.86 | 2.58 |
| Ca, % | 0.52 | 0.6 |
| Total P, % | 0.62 | 0.54 |
| Dig P, % | 0.73 | 0.67 |
| Analyzed nutrient composition, % | ||
| CP | 18.54 | 17.28 |
| Ether Extract | 4.58 | 4.20 |
| NDF | 10.53 | 12.37 |
| Ash | 5.77 | 5.56 |
| Item | MCON | MOA | SEM | p-Value |
|---|---|---|---|---|
| Nº of sows | 36 | 36 | - | - |
| Parity, n | 4.6 | 4.9 | 0.18 | 0.251 |
| Sow BW, kg | ||||
| Gestation (day 110) | 266.2 | 267.8 | 16.64 | 0.783 |
| Weaning | 223.5 | 226.5 | 18.81 | 0.551 |
| Sow backfat, mm | ||||
| Gestation (day 110) | 17.2 | 17.1 | 0.79 | 0.942 |
| Weaning | 13.4 | 14.2 | 0.65 | 0.215 |
| Loss backfat, mm | −3.73 | −2.88 | 0.28 | 0.023 |
| Feed intake, kg | ||||
| Total Feed intake | 117.7 | 124.0 | 6.96 | 0.268 |
| ADFI | 4.6 | 4.9 | 0.26 | 0.268 |
| Weaning-estrus interval, d | 4.9 | 4.8 | 0.30 | 0.943 |
| Item | MCON | MOA | SEM | p-Value |
|---|---|---|---|---|
| Litter birth weight, kg | 19.18 | 18.85 | 0.601 | 0.673 |
| Born alive pig weight, kg | 1.40 | 1.38 | 0.042 | 0.695 |
| Total born pigs 1, n | 14.36 | 14.66 | 0.482 | 0.574 |
| Pigs born alive, n | 13.20 | 12.91 | 0.527 | 0.523 |
| Pigs stillborn, n | 1.03 | 1.35 | 0.227 | 0.307 |
| Suckling litter performance 2 | ||||
| Litter size at cross-fostering (CF), n | 12.62 | 12.24 | 0.165 | 0.109 |
| Pig CF weight, kg | 1.71 | 1.74 | 0.048 | 0.612 |
| Litter performance day 20 lactation 3 | ||||
| Litter weight, kg | 65.30 | 65.78 | 1.839 | 0.844 |
| Pig weight, kg | 5.65 | 5.97 | 0.101 | 0.027 |
| Total weaned pigs, n | 11.58 | 11.08 | 0.245 | 0.140 |
| CV, % | 18.56 | 15.88 | 0.833 | 0.024 |
| Sow Diet | Pig Diet | BW, kg | ADFI, g/d | ADG, g/d | G:F | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d 0 | d 14 | d 35 | d 0–14 | d 14–35 | d 0–35 | d 0–14 | d 14–35 | d 0–35 | d 0–14 | d 14–35 | d 0–35 | ||
| MCON | 6.20 | 8.10 | 15.29 | 151.0 | 409.9 | 306.4 | 141.2 | 342.0 | 261.9 | 0.92 | 0.84 | 0.86 | |
| MOA | 6.19 | 8.10 | 15.20 | 152.8 | 403.8 | 301.5 | 136.5 | 338.0 | 257.6 | 0.89 | 0.84 | 0.86 | |
| SEM | 0.632 | 0.725 | 1.116 | 6.641 | 25.558 | 16.667 | 6.410 | 21.663 | 13.979 | 0.023 | 0.053 | 0.036 | |
| CON | 6.19 | 8.02 | 15.07 | 150.5 | 406.4 | 303.6 | 135.4 | 335.9 | 255.9 | 0.89 | 0.83 | 0.85 | |
| OA | 6.19 | 8.19 | 15.42 | 153.3 | 407.3 | 304.3 | 142.3 | 344.1 | 263.6 | 0.93 | 0.85 | 0.87 | |
| SEM | 0.632 | 0.725 | 1.116 | 6.641 | 25.558 | 16.667 | 6.410 | 21.639 | 13.979 | 0.023 | 0.053 | 0.040 | |
| p-value | |||||||||||||
| Sow diet | 0.309 | 0.993 | 0.647 | 0.579 | 0.532 | 0.372 | 0.305 | 0.557 | 0.367 | 0.194 | 0.919 | 0.977 | |
| Pig diet | 0.926 | 0.099 | 0.068 | 0.373 | 0.927 | 0.900 | 0.136 | 0.236 | 0.113 | 0.050 | 0.373 | 0.233 | |
| Sow × pig diet | 0.602 | 0.310 | 0.315 | 0.779 | 0.857 | 0.603 | 0.878 | 0.571 | 0.530 | 0.176 | 0.589 | 0.421 | |
| Sow Diet | Pig Diet | 7 d Post-Weaning | 35 d Post-Weaning | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S. suis | E. coli | C. perfringens | Lactic acid bacteria | S. suis | E. coli | C. perfringens | Lactic acid bacteria | ||
| MCON | CON | 3.42 | 7.97 | 3.65 a | 8.05 a | 5.36 | 7.21 a | 3.14 | 7.85 |
| OA | 2.97 | 7.58 | 3.62 a | 7.82 b | 5.22 | 6.66 b | 3.23 | 7.34 | |
| MOA | CON | 3.21 | 7.80 | 3.34 b | 8.02 a | 5.24 | 6.86 ab | 3.22 | 7.43 |
| OA | 2.98 | 7.37 | 3.60 a | 8.02 a | 5.30 | 6.96 ab | 3.43 | 7.22 | |
| SEM | 0.112 | 0.215 | 0.060 | 0.060 | 0.138 | 0.144 | 0.104 | 0.274 | |
| p-value | |||||||||
| Sow diet | 0.372 | 0.216 | 0.0001 | 0.035 | 0.820 | 0.837 | 0.067 | 0.161 | |
| Pig diet | 0.003 | 0.008 | 0.009 | 0.007 | 0.675 | 0.028 | 0.038 | 0.065 | |
| Sow × pig diet | 0.344 | 0.910 | 0.001 | 0.008 | 0.321 | 0.002 | 0.448 | 0.435 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villagómez-Estrada, S.; Melo-Durán, D.; van Kuijk, S.; Pérez, J.F.; Solà-Oriol, D. Specialized Feed-Additive Blends of Short- and Medium-Chain Fatty Acids Improve Sow and Pig Performance During Nursery and Post-Weaning Phase. Animals 2024, 14, 3692. https://doi.org/10.3390/ani14243692
Villagómez-Estrada S, Melo-Durán D, van Kuijk S, Pérez JF, Solà-Oriol D. Specialized Feed-Additive Blends of Short- and Medium-Chain Fatty Acids Improve Sow and Pig Performance During Nursery and Post-Weaning Phase. Animals. 2024; 14(24):3692. https://doi.org/10.3390/ani14243692
Chicago/Turabian StyleVillagómez-Estrada, Sandra, Diego Melo-Durán, Sandra van Kuijk, José F. Pérez, and David Solà-Oriol. 2024. "Specialized Feed-Additive Blends of Short- and Medium-Chain Fatty Acids Improve Sow and Pig Performance During Nursery and Post-Weaning Phase" Animals 14, no. 24: 3692. https://doi.org/10.3390/ani14243692
APA StyleVillagómez-Estrada, S., Melo-Durán, D., van Kuijk, S., Pérez, J. F., & Solà-Oriol, D. (2024). Specialized Feed-Additive Blends of Short- and Medium-Chain Fatty Acids Improve Sow and Pig Performance During Nursery and Post-Weaning Phase. Animals, 14(24), 3692. https://doi.org/10.3390/ani14243692






