Simple Summary
The pig industry is increasingly seeking natural, safe, and cost-effective feed additives due to the prohibition of antibiotics and growing production demands. Yeast fermentation products have demonstrated antioxidant and anti-inflammatory properties in feed additive studies. However, the mechanism by which yeast fermentation product supplementation improves the performance and health status of weaned piglets needs to be more thoroughly investigated. The results of this study showed that yeast fermentation product supplementation alleviated colon inflammation in weaned piglets challenged with Salmonella typhimurium, and shaped the beneficial microbiota, thereby maintaining gut homeostasis. These findings provide a basis for considering yeast fermentation products as valuable feed additives in the pig industry.
Abstract
Yeast fermentation products (YFPs) are known to contain bioactive compounds, such as nutritional metabolites and cell wall polysaccharides (specifically glucan and mannan), which have been demonstrated to exert positive effects on the growth performance and immunity of livestock and poultry. However, the impact of YFPs on intestinal inflammation and microflora composition in pigs infected with Salmonella typhimurium remains unclear. To investigate this, a total of 18 weaned pigs were divided into three treatment groups: a non-challenged control group (Con), a group challenged with Salmonella typhimurium (ST), and a group challenged with Salmonella typhimurium and supplemented with 0.4% YFP (YFP). The experiment spanned five weeks, encompassing a period of 21 days prior to and 14 days subsequent to the initial Salmonella typhimurium challenge. The findings indicated that the YFP group exhibited an increase in average daily gain (ADG) and a decrease in the feed-gain ratio (F/G) in comparison to the ST group following the Salmonella challenge. Additionally, the YFP group demonstrated a reduction in the levels of inflammatory cytokines in plasma and a decrease in the expression of inflammatory genes in the colon. Treatment with YFP also resulted in improved colon histomorphology, heightened alpha diversity of the gut microbiota, augmented the abundance of butyrate-producing bacteria, and elevated concentrations of short-chain fatty acids (SCFAs). In addition, YFP reprogrammed energy metabolism in colon epithelial cells by blunting glycolysis. Together, dietary YFP supplementation alleviated colon inflammation in weaned pigs challenged with Salmonella typhimurium, and shaped the beneficial microbiota, thereby maintaining gut homeostasis. The results provided evidence supporting the application of yeast fermentation products in livestock production.
1. Introduction
Salmonella enterica serovar typhimurium is a pathogenic bacterium of clinical significance, that causes food-borne zoonotic infections worldwide [1]. Pigs serve as an important infection reservoir for humans due to their ability to carry a broad range of Salmonella serovars [2]. Salmonella infection diminishes gut microbiota diversity, causing poor growth performance [3]. The administration of antibiotics is the primary treatment for Salmonella-related disease, but it also contributes to the spread of drug resistance, a significant global public health issue. Notably, antibiotic treatments increase the risk of Salmonella developing resistance over time [4].
The intestine is regarded as the ecological niche of Salmonella, with the intestinal mucosa performing a crucial role in regulating the immune response to bacterial infection [5]. In the past few years, there has been increasing interest in the role of gut microbiota in the susceptibility to intestinal pathogens. The microbiota contributes to the development and maintenance of the mucosal immune system and acts as a barrier against bacteria invading the epithelial layer, where it competes for nutrients with the host microbiota [6,7]. It has been shown that Salmonella can disrupt the composition of the gut microbiota through its virulence factors, leading to an inflammatory mucosal response [8]. Moreover, evidence also shows that Salmonella can alter the metabolic pattern of colon epithelial cells via its virulence factors [9]. Thus, mitigating the influence of Salmonella digestive tract infections on animals remains a major concern for the livestock industry.
Yeast fermentation products (YFPs) contain bioactive compounds, including nucleotides, nutritional metabolites, and cell wall polysaccharides (specifically β-glucan and mannan). Previous studies have investigated the impact of yeast cell wall components on the immune function of weaning pigs [10,11,12]. It has been reported that yeast β-glucans alleviate the elevation of pro-inflammatory cytokine levels in LPS or Escherichia coli-challenged pigs [13,14]. Additionally, dietary supplementation with yeast-derived mannans could improve weight gain and enhance the percentage of immune cells in pigs [15]. However, the effects of yeast fermentation products on digestive tract inflammation and microbiota composition in pigs challenged with Salmonella typhimurium have not been reported.
Therefore, this study employed weaned pigs challenged with Salmonella typhimurium and supplemented with yeast fermentation products. The results indicate how yeast fermentation products influence colon epithelial cell metabolism and microbiota composition of pigs challenged with Salmonella typhimurium. Our study provides a potentially effective way to prevent the adverse effects of Salmonella infection in livestock production.
2. Materials and Methods
2.1. Ethics Approval
All procedures involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing Agricultural University (IACUC approval number: PT2019015). The sampling procedures followed the “Guidelines on Ethical Treatment of Experimental Animals” (2006) No. 398, set by the Ministry of Science and Technology, China. In this study, all experimental methods were performed in accordance with the Nanjing Agricultural University Health Guide for the Care and Use of Laboratory Animals.
2.2. Animals and Experimental Design
A total of 18 weaned male pigs (Duroc × Yorkshire × Landrace) were housed at the Laboratory Animal Center of Nanjing Agricultural University. The pigs used did not receive Salmonella typhimurium vaccines, antibiotic injections, or antibiotics in feed. All pigs used in this study were susceptible to Salmonella typhimurium. They were allowed free access to feed and water. The pigs were housed in pens (pen size: 1.2 m × 1 m) in an environmentally controlled nursery building. The pens were equipped with a self-feeder, a nipple drinker, and plastic-covered expanded metal floors in an environmentally controlled building (temperature maintained at 25–28 °C with a 16 h light and 8 h dark cycle).
All pigs were randomly allotted to three dietary treatments (n = 6) based on the initial body weight: (1) negative control (Con): control diet, without Salmonella typhimurium challenge; (2) positive control (ST): control diet, with Salmonella typhimurium challenge; (3) YFP: control diet plus 4 g YFP/kg feed with Salmonella typhimurium challenge. The yeast fermentation products were derived from the fermentation of Saccharomyces cerevisiae and were provided by the Cargill Company (XPC, Diamond V, Cedar Rapids, IA, USA). The main components of YFPs include mannan-oligosaccharides, beta-glucan, proteins, peptides, amino acids, organic acids, vitamins, mineral salts, and nucleotides. After 21 days on their experimental diets, the pigs in the ST and YFP groups were orally inoculated with a 5 mL suspension containing 109 CFU of Salmonella typhimurium. A week later, the second challenge was performed the same as the first. The body weight of each piglet was recorded on days 0, 7, 14, 21, and 35, and the feed consumption per pen was recorded every day of the experiment to calculate the average daily gain (ADG), average daily feed intake (ADFI), and feed efficiency (F/G) from day 1 to 21 (pre-challenge) and day 21 to 35 (post-challenge). The experimental diet is described in detail in Table 1.

Table 1.
Ingredient composition of experimental diets (%, as-fed basis).
2.3. Sample Collections
All pigs were weighed and then sacrificed via exsanguination at the end of the study. Blood samples were collected into heparinized tubes, and plasma was separated through centrifugation at 3000× g for 15 min and stored at −80 °C until analysis. Fresh colon content was collected and immediately frozen in liquid nitrogen to isolate bacterial genomic DNA and analyze SCFAs. Colon mucosa was obtained as described previously [16], immediately frozen in liquid nitrogen, and stored at −80 °C for further analysis.
2.4. Biochemical Analysis
The concentrations of interleukin (IL), including IL-1β, IL-6, IL-18, and tumor necrosis factor-α (TNFα) in plasma and IL-1β, IL-6, and TNFα in the colonic mucosa, were measured using porcine-specific ELISA kits (Jiangsu Meimian Industrial Co., Ltd., Yancheng, China). Total protein (TP), globulins (GLOB), albumin (ALB), and lactate dehydrogenase (LDH) were measured with a biochemical automatic analyzer (Hitachi 7020, HITACHI, Tokyo, Japan) using commercial assay kits (Wako Pure Chemical Industries, Ltd., Wako, Japan).
2.5. Histomorphology Analysis
The paraformaldehyde-fixed colon was dehydrated in graded alcohol and embedded in paraffin wax. Then, hematoxylin and eosin (H&E)-stained paraffin sections were viewed under a bright field using the Pannoramic SCAN II, and images were captured with 3DHISTECH software V1.0 (3DHISTECH Ltd., Budapest, Hungary). The degree of intestinal tissue damage was scored as described previously [17,18], evaluating the extent of epithelial loss on intestinal villi and inflammatory infiltration, which were included in the histopathological examination.
2.6. Colon Microbiota Analysis
The extraction of total genomic DNA from the colonic content was performed following the standard protocol of the QIAamp DNA stool Mini Kit (QIAGEN, Hilden, Germany). The integrity of DNA isolation was measured using 1% agarose gel electrophoresis. The V3–V4 region of the 16S rRNA gene was amplified using a specific primer. Amplicon library sequencing was performed on an Illumine Hiseq 2500 platform (Illumina, San Diego, CA, USA) using standard protocols. All raw sequence reads involved in our study were deposited in the NCBI Sequence read archive (SRA). The alpha diversity (Ace, Chao1, Shannon, and Simpson) was measured using the MOTHUR program (version v.1.30; http://www.mothur.org).
2.7. Metabolite Analysis
Gas chromatography was used to determine the concentrations of SCFAs in the colonic content according to the method outlined in Reference [19]. Briefly, 0.30 g of each sample was added to sterile tubes containing 1.5 mL of distilled water. Each mixture was shaken strongly for 5 min and then centrifuged at 12,000× g for 10 min at 4 °C and filtered with 0.22 μm mesh. Finally, 1 μL of supernatant was injected into the Agilent 7890B Gas Chromatograph (Agilent Technologies, Santa Clara, CA, USA). The concentrations of lactate in colon mucosa were measured using an assay kit, following the protocol provided by Nanjing Jiancheng Biological Engineering Institute, Nanjing, China.
2.8. Total RNA Isolation and Real-Time Quantitative PCR
Total RNA was isolated from 30 mg of frozen colonic mucosa with 1 mL of TRIzol (Sango Biotech, Shanghai, China) and reverse-transcribed according to the manufacturer’s protocol (Vazyme Biotech, Nanjing, China). Diluted cDNA (2 μL, 1:25) was used as a template for real-time PCR, which was performed on a real-time PCR system (Mx3000P, Agilent Technologies, Santa Clara, CA, USA). Moreover, β-actin was chosen as a reference gene to normalize the mRNA abundance of target genes. The primer sequences of the target genes are listed in Table 2. The 2−ΔΔCT method was used to analyze real-time PCR data.

Table 2.
The primer sequences of the target genes for RT-PCR.
2.9. Western Blot Analysis
Total protein was extracted from 50 mg of frozen colonic mucosa as previously described [20]. The protein concentration was measured using a Pierce BCA Protein Assay kit (No. 23225, Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Western blot analysis of Occludin (DF7504, Affinity, Liyang, China, diluted 1:1000) was carried out. β-actin (AC026, ABclonal, Wuhan, China, diluted 1:50,000) was used as the internal control.
2.10. Statistical Analysis
All data were checked for normality using exploratory analysis. Data were analyzed using SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA). All data were analyzed using one-way ANOVA, with the YFP diet as a fixed factor, and Duncan’s test was used to determine the difference among groups. The pigs were recognized as a statistical unit. The values were presented as the means ± SEM with significance at p < 0.05, and 0.05 < p < 0.10 considered a tendency. The correlation between differential gut microbiota and plasma parameters or SCFAs and gene expression was analyzed using Pearson correlation analysis with the Pheatmap package in R (version 4.1.2).
3. Results
3.1. Growth Performance
Salmonella typhimurium administration tended (p = 0.09) to decrease the average daily gain (ADG) of pigs compared with the Con group, yet YFP increased (p < 0.05) the ADG of pigs compared with the ST group. The feed/gain ratio (F/G) was observed to significantly increase (p < 0.05) in Salmonella typhimurium-challenged piglets compared with those in the Con group, a finding that was reversed by the YFP (p < 0.05). No significant changes were observed in ADG or F/G among the three groups before Salmonella typhimurium treatment (Table 3).

Table 3.
The effects of YFPs on growth performance.
3.2. Biochemical Index Analysis of Plasma
Salmonella typhimurium challenge markedly increased (p < 0.05) the concentrations of total protein (TP), globulin (GLOB), albumin (ALB), and lactic dehydrogenase (LDH) (Figure 1A–D) in plasma compared with the Con group. Dietary YFP supplementation did not protect the pigs from these changes except LDH. Additionally, the concentrations of inflammatory cytokines interleukin (IL-1β, IL-6, and IL-18) (Figure 1E–G) and TNFα (Figure 1H) in plasma were higher (p < 0.05) in the ST pigs compared to the Con pigs. Compared with ST pigs, the concentrations of IL-1β, IL-6, IL-18, and TNFα were lower (p < 0.05) in YFP pigs (Figure 1E,H).
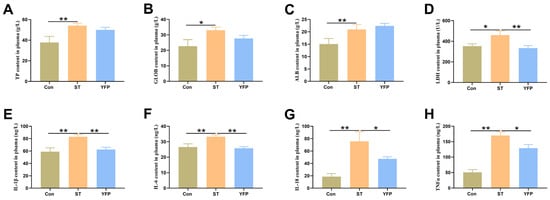
Figure 1.
The effects of YFPs on the concentrations of plasma biochemical index and the contents of inflammatory cytokines in plasma of pigs. (A) TP content in plasma; (B) GLOB content in plasma; (C) ALB content in plasma; (D) LDH content in plasma; (E) IL-Iβ content in plasma; (F) IL-6 content in plasma; (G) IL-I8 content in plasma; (H) TNFα content in plasma. The values are presented as mean ± SEM, n = 6. * p < 0.05, ** p < 0.01.
3.3. Colon Morphology
Salmonella typhimurium-infected pigs showed increased (p < 0.05) infiltration of inflammatory cells and higher histological scores in the colon mucosa compared to the Con pigs, which could be reversed by dietary YFP supplementation (Figure 2A,B). The ST group showed reduced (p < 0.05) Occludin and tight junction ZO1 expression levels in the colon compared with the Con group. In contrast, pigs fed with a YFP had higher (p < 0.05) ZO1 and Occludin expression compared with the ST group (Figure 2C).
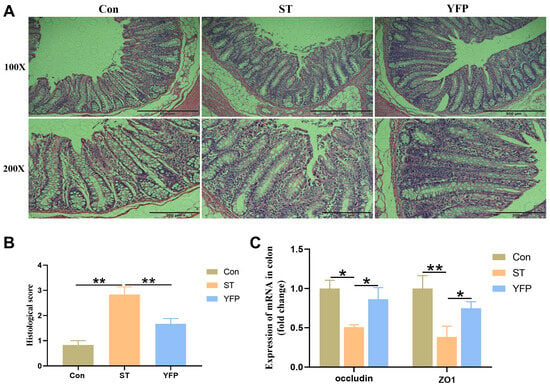
Figure 2.
The effects of YFPs on colon injury. (A) Morphology of the colon; (B) Histological score of colon tissue; (C) The mRNA expression of Occludin and ZO1 in the colon. The values are presented as mean ± SEM, n = 6. * p < 0.05, ** p < 0.01.
3.4. Genes Expression and Metabolite Analysis of Colonic Mucosa Related to Glycolysis, and Colonic Inflammation Cytokine Expression
To examine whether YFP influences glycolysis, we measured glycolysis-related genes and lactate levels in the colonic mucosa. Salmonella typhimurium challenge significantly increased (p < 0.05) the glycolysis-related mRNA expression of glucose transporter 1 (Glut1), pyruvate kinase (PK), phosphofructokinase (PFK), hexokinase1 (HK1), hexokinase3 (HK3), and lactate dehydrogenase A (LDHA) (Figure 3A); meanwhile, the concentrations of lactate in the colon mucosa were higher (p < 0.05) compared to those in the Con group (Figure 3B). However, dietary YFP supplementation decreased (p < 0.05) the expression of these genes as well as the concentration of lactate in the colonic mucosa (Figure 3A,B). From these results, it could be deduced that YFPs could alleviate colonic mucosa metabolic disorders induced by Salmonella typhimurium.
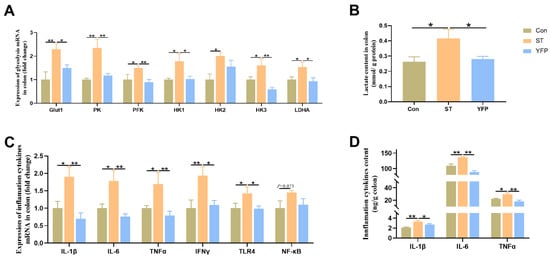
Figure 3.
The effects of YFP on glycolysis and inflammation levels. (A) Glycolysis-related mRNA expression; (B) The concentration of lactate in the colon; (C) Inflammatory cytokines mRNA expression; (D) Inflammatory cytokine content in the colon. The values are presented as mean ± SEM, n = 6. * p < 0.05, ** p < 0.01.
Salmonella typhimurium challenge significantly increased (p < 0.05) the mRNA expression of IL-1β, IL-6, TNFα, IFNγ, and TLR4 (Figure 3C) and the concentrations of IL-1β, IL-6, and TNFα in the colonic mucosa (Figure 3D). However, YFP supplementation could decrease the mRNA expression and the concentrations of inflammatory cytokines.
3.5. Metabolite Analysis of Colonic Content
To evaluate whether alterations to the gut microbial composition influenced the fermentative capacity, the concentrations of colonic-content SCFAs were determined. Salmonella typhimurium challenge significantly decreased (p < 0.05) the concentrations of acetate, butyrate, isobutyrate, valerate, and total SCFA compared to the Con group. Dietary YFP supplementation significantly increased (p < 0.05) the concentrations of acetate, butyrate, and total SCFA. However, the Salmonella typhimurium challenge had no influence on the propionate content in the colon content (Figure 4A–F).
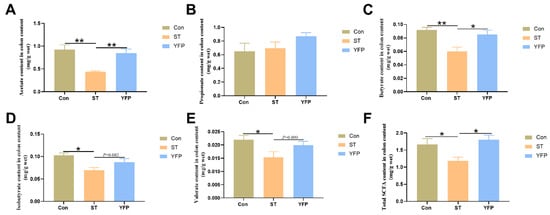
Figure 4.
The effects of YFP on concentrations of SCFAs in colon content. (A) Acetate content in colon content; (B) Propionate content in colon content; (C) Butyrate content in colon content; (D) Isobutyrate content in colon content; (E) Valerate content in colon content; (F) Total SCFA content in colon content. The values are presented as mean ± SEM, n = 6. * p < 0.05, ** p < 0.01.
3.6. Microbiota Composition of Colonic Content
The effects of dietary YFPs on the gut microbiota of pigs under the Salmonella typhimurium challenge have rarely been reported. To confirm this issue, we analyzed colonic microbiota compositions. Alpha diversity analysis showed a significant difference (p < 0.05) in community diversity based on the Simpson and Shannon indices (Figure 5A,B); however, no significant differences were observed in Chao1 and ACE community richness (Figure 5C,D).
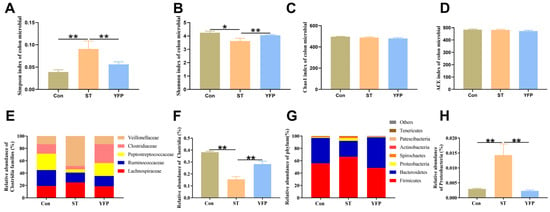
Figure 5.
The effects of YFP on the microbial content of the colon. (A) Simpson index of microbial in colon content; (B) Shannon index of microbial in colon content; (C) Chao1 index of microbial in colon content; (D) ACE index of microbial in colon content; (E) Families of butyrate-producer content of colon content; (F) Clostridia content of colon content; (G) Abundant phyla of colon content; (H) Proteobacteria content of colon content. The values are presented as mean ± SEM, n = 4. * p < 0.05, ** p < 0.01.
We investigated whether dietary YFP supplementation would influence butyrate-producing bacteria. ST treatment significantly reduced the abundance of Clostridia (phylum Firmicutes) (0.16%) compared to the Con group (0.38%), which are obligate anaerobes that include abundant butyrate-producers, specifically Ruminococcaceae (25.73% in Con, 16.01% in ST and 16.63% in YFP), Peptostreptococcaceae (26.50% in Con, 5.07% in ST and 20.88% in YFP) and Clostridiaceae (15.36% in Con, 5.70% in ST and 30.67% in YFP) (Figure 5E,F).
We found that the predominant phyla in the colon content at the phylum level were Firmicutes (55.79% in Con, 66.50% in ST, and 48.48% in YFP), Bacteroidetes (41.08% in Con, 25.75% in ST, and 49.51% in YFP), and Proteobacteria (1.18% in Con, 4.29% in ST, and 0.92% in YFP) were. The relative abundance of Proteobacteria, a common marker of gut dysbiosis, was higher in the ST group (4.29%) as compared to the Con group (1.18%). YFP supplementation prevented the expansion of Proteobacteria (0.92%) (Figure 5G,H).
3.7. Correlation Analysis of the Gut Microbiota, Plasma Parameters, and SCFAs of the Colon Content
To understand how the differential gut microbiota community of predominant families impacts host metabolism, a Pearson correlation matrix was generated to explore these relationships. We detected the correlations between colon differential microbiota related to SCFAs, glycolysis, and inflammatory cytokines. The relative abundance of Peptostreptococcaceae, Ruminococcaceae, and Lachnospiraceae showed a negative correlation with IL-1β, IL-18, and TNFα levels in plasma. The relative abundance of Lachnospiraceae and Clostridiaceae_1 showed a negative correlation with IL-6 and LDH levels in plasma. The relative abundance of Veillonellaceae showed a positive correlation with IL-1β and IL-6 levels in plasma (Figure 6A) (p < 0.05).
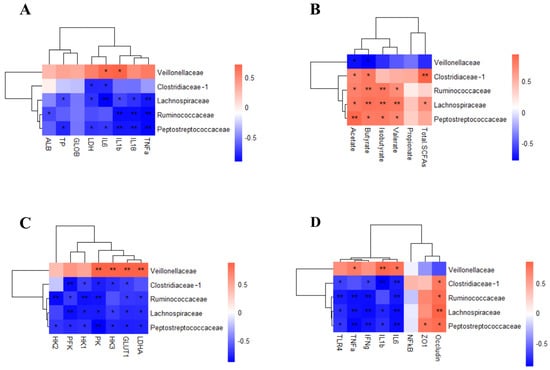
Figure 6.
Pearson correlation analysis between the colonic microbiota and metabolism and inflammation. (A) Relationship between colonic microbiota and inflammation markers; (B) Relationship between colonic microbiota and organic acids; (C) Relationship between colonic microbiota and glycolysis gene expression; (D) Relationship between colonic microbiota and colon inflammatory cytokines. n = 4. * p < 0.05, ** p < 0.01.
Peptostreptococcaceae, Lachnospiraceae, and Ruminococcaceae were positively correlated with acetate, butyrate, isobutyrate, and valerate. Clostridiaceae_1 was positively correlated with acetate, butyrate, and total SCFAs. Veillonellaceae was negatively correlated with acetate and butyrate (Figure 6B) (p < 0.05).
Peptostreptococcaceae, Ruminococcaceae, Lachnospiraceae, and Clostridiaceae_1 were negatively correlated with glycolysis-related genes such as PK, PFK, HK1, HK3, and Glut1. The gene expression of PK, HK3, Glut1, and LDHA was positively correlated with Veillonellaceae (Figure 6C) (p < 0.05).
To determine whether the gut microbiota influences intestinal barrier function, the correlations between intestinal barrier gene expression levels and differential microbiota families were measured. The relative abundance of Peptostreptococcaceae, Lachnospiraceae, and Ruminococcaceae were negatively correlated with IL-6, IFNγ, TNFα, and TLR4. Clostridiaceae_1 was negatively correlated with IL-6, IL-1β, IFNγ, and TLR4. Peptostreptococcaceae and Lachnospiraceae were negatively correlated with IL-1β. The relative abundance of Veillonellaceae was positively correlated with IL-6, IL-1β, and TNFα. The gene expression of tight junction protein Occludin was positively correlated with Peptostreptococcaceae, Lachnospiraceae, Ruminococcaceae, and Clostridiaceae_1. Similarly, the gene expression of tight junction protein ZO1 was positively correlated with Peptostreptococcaceae (Figure 6D) (p < 0.05).
4. Discussion
Yeast fermentation products have been used in the livestock industry, but the mechanism of action is largely unclear, especially in inflammation in animals. In this study, during the pre-challenge period, supplementation with YFPs had no significant effect on the growth performance of pigs compared with the control group. However, during the Salmonella typhimurium challenge period, YFPs significantly enhanced ADG. Moreover, YFPs decreased the colon inflammation levels and improved the intestinal microbial community compared to the ST group. The present study indicates that YFPs provide colon protection against Salmonella typhimurium.
It has been shown that yeast supplementation increases body weight and ADG, and yeast supplementation decreases the total bacteria and lactobacilli in the feces of nursery pigs [21]. Sows fed with Bacillus spp. and yeast extracts derived from S. cerevisiae increased ADG in piglets, but pigs fed yeast tended to demonstrate decreased ADG in the nursery. Sows fed with live yeast (S. cerevisiae strain NCYC Sc 47) and yeast-based prebiotics derived from S. cerevisiae improved pigs’ growth in the nursery period [22]. These studies show that feeding yeast at different growth stages of pigs had different effects on growth performance. In this study, weaned pigs fed with YFPs showed a significant increase in growth performance after a Salmonella typhimurium challenge.
The enteric pathogen Salmonella can overcome niche protection through its virulence factors to induce intestinal inflammation by stimulating the production of cytokines such as IL-1, IL-6, and TNFα [23]. In the present study, increased IL-1β, IL-6, IL-18, and TNFα concentrations in plasma and IL-1β, IL-6, and TNFα concentrations in the colon indicated that the intestine successfully induced inflammation after Salmonella treatment. Pigs receiving YFPs had lower concentrations of IL-1β, IL-6, IL-18, and TNFα in plasma and IL-1β, IL-6, and TNFα in the colonic mucosa compared to the ST group, implying that YFPs decreased the inflammation reaction.
Intestinal morphology can be used as an indicator of intestinal health. In response to inflammation induced by pathogens, deeper crypts exhibit faster cellular turnover, allowing the renewal of villi as needed [24]. In this current study, dietary YFP supplementation improved intestinal histomorphology in infected pigs. Tight junctions between intestinal epithelial cells, which protect the body from intestinal pathogens, influence intestinal mucosal barrier function to a great extent [25]. In this current study, YFP supplementation significantly increased tight junction ZO1 and Occludin expression in the colon, which is expected to improve intestinal mucosal barrier function. Our results demonstrated that YFPs maintain intestinal integrity and barrier function, partly by elevating intestinal tight junction protein expression.
There are a large number of microbial communities in the gastrointestinal tracts of humans and animals, which play a crucial role in the development of intestinal function and the host’s metabolism and immune system [26]. According to our current study, YFPs significantly improved the decreased microbiota diversity and altered the microbiota structure of the colonic microbiota communities. There is increasing interest in the crosstalk between dietary supplementation and gut microbiota to improve the production of SCFAs, mainly from Bacteroidetes and Firmicutes phyla in the colon [27]. SCFAs in the colon, including dominant acetate, propionate, and butyrate, are an energy source for epithelial cells and play an important role in inflammatory and immune signaling [28]. Our study found that YFP could decrease the ratio of Firmicutes and Bacteroidetes at the phylum level, which is related to the metabolism level. Meanwhile, dietary YFPs increased the number of butyrate-producing bacteria, such as Ruminococcaceae, Peptostreptococcaceae, and Clostridiaceae, at the family level, thereby promoting the production of acetate and butyrate. Additionally, butyrate contributes to the anti-inflammation reaction, as the relative abundance of butyrate-produced bacteria was negatively correlated with the production of inflammatory cytokines.
There is an increasing amount of evidence showing that some bacterial pathogens can alter the pattern of energy metabolism to obtain nutrients from host cells and support their own growth and long-term persistence in host tissue [29,30,31]. However, these studies were limited to mice and were not replicated in pigs. In addition, the effect of YFPs on intestinal energy metabolism in Salmonella-infected pigs has not been reported. Therefore, we detected the gene expression and metabolites related to glycolysis in colon epithelial cells. We found that Salmonella typhimurium increased the expression of glycolysis-related genes, including PK, PFK, HK1, HK2, HK3, Glut1, and LDHA, and the concentration of lactate in colon epithelial cells. Dietary YFP supplementation reprogrammed the energy metabolism of colon epithelial cells by blunting the expression of PK, PFK, HK1, HK3, Glut1, and LDHA, and the concentration of lactate. The results showed that Veillonellaceae promoted colonic inflammation and anaerobic glycolysis of colonic epithelial cells and had a destructive effect on the colonic mucosal barrier. The short-chain fatty acid-producing bacteria Ruminococcaceae, Peptostreptococcaceae, Lachnospiraceae, and Clostridiaceae increase the concentration of short-chain fatty acids in the colon, inhibit colon inflammation, and inhibit anaerobic glycolysis of colon epithelial cells.
5. Conclusions
Collectively, dietary YFP supplementation positively influenced growth performance and intestinal barrier function while reducing intestinal inflammation in weaned pigs challenged with Salmonella typhimurium. Additionally, YFPs could reprogram the energy metabolism of colon epithelial cells and shape the beneficial microbiota, thereby maintaining gut homeostasis.
Author Contributions
G.F. contributed to all data analysis and drafting of the manuscript. Y.Z. and X.S. were responsible for animal care, breeding, and sampling. Y.L. provided technical support. X.Y. contributed to the experimental design, data interpretation, and critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the project of Seed Industry Revitalization in Jiangsu Province (JBGS [2021] 024) and the National Natural Science Foundation of China (32072808).
Institutional Review Board Statement
The experimental procedures and animal management in this study were approved by the Animal Ethics Committee of Nanjing Agricultural University (approval code: NO.PT2019015).
Informed Consent Statement
Not applicable.
Data Availability Statement
Original data are available from the corresponding author upon reasonable request.
Conflicts of Interest
The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results. The authors declare no conflict of interest.
References
- Chirullo, B.; Pesciaroli, M.; Drumo, R.; Ruggeri, J.; Razzuoli, E.; Pistoia, C.; Petrucci, P.; Martinelli, N.; Cucco, L.; Moscati, L.; et al. Salmonella Typhimurium exploits inflammation to its own advantage in piglets. Front. Microbiol. 2015, 6, 985. [Google Scholar] [CrossRef] [PubMed]
- Drumo, R.; Pesciaroli, M.; Ruggeri, J.; Tarantino, M.; Chirullo, B.; Pistoia, C.; Petrucci, P.; Martinelli, N.; Moscati, L.; Manuali, E.; et al. Salmonella enterica Serovar Typhimurium Exploits Inflammation to Modify Swine Intestinal Microbiota. Front. Cell Infect. Microbiol. 2015, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, Y.H.; Yang, G.Y.; Liu, X.; Xia, B.; Hu, X.; Su, J.H.; Wang, J.F. Lactobacillus rhamnosus GG Affects Microbiota and Suppresses Autophagy in the Intestines of Pigs Challenged with Salmonella Infantis. Front. Microbiol. 2017, 8, 2705. [Google Scholar] [CrossRef]
- Xia, B.; Yu, J.; He, T.; Liu, X.; Su, J.; Wang, M.; Wang, J.; Zhu, Y. Lactobacillus johnsonii L531 ameliorates enteritis via elimination of damaged mitochondria and suppression of SQSTM1-dependent mitophagy in a Salmonella infantis model of piglet diarrhea. FASEB J. 2020, 34, 2821–2839. [Google Scholar] [CrossRef] [PubMed]
- Hallstrom, K.; McCormick, B.A. Salmonella Interaction with and Passage through the Intestinal Mucosa: Through the Lens of the Organism. Front. Microbiol. 2011, 2, 88. [Google Scholar] [CrossRef]
- Littman, D.R.; Pamer, E.G. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 2011, 10, 311–323. [Google Scholar] [CrossRef]
- Sassone-Corsi, M.; Raffatellu, M. No vacancy: How beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J. Immunol. 2015, 194, 4081–4087. [Google Scholar] [CrossRef]
- Barman, M.; Unold, D.; Shifley, K.; Amir, E.; Hung, K.; Bos, N.; Salzman, N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect. Immun. 2008, 76, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Litvak, Y.; Byndloss, M.X.; Baumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, eaat9076. [Google Scholar] [CrossRef] [PubMed]
- Seljelid, R.; Rasmussen, L.T.; Larm, O.; Hoffman, J. The protective effect of beta 1-3D-glucan-derivatized plastic beads against Escherichia coli infection in mice. Scand. J. Immunol. 1987, 25, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Muchmore, A.V.; Sathyamoorthy, N.; Decker, J.; Sherblom, A.P. Evidence that specific high-mannose oligosaccharides can directly inhibit antigen-driven T-cell responses. J. Leukoc. Biol. 1990, 48, 457–464. [Google Scholar] [CrossRef]
- Podzorski, R.P.; Gray, G.R.; Nelson, R.D. Different effects of native Candida albicans mannan and mannan-derived oligosaccharides on antigen-stimulated lymphoproliferation in vitro. J. Immunol. 1990, 144, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, J.; Li, D.; Wang, X.; Zhao, L.; Lv, S.; Huang, D. Effects of beta-glucan extracted from Saccharomyces cerevisiae on humoral and cellular immunity in weaned piglets. Arch. Anim. Nutr. 2005, 59, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, D.F.; Xing, J.J.; Cheng, Z.B.; Lai, C.H. Effects of beta-glucan extracted from Saccharomyces cerevisiae on growth performance, and immunological and somatotropic responses of pigs challenged with Escherichia coli lipopolysaccharide. J. Anim. Sci. 2006, 84, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Maxwell, C.V.; Erf, G.F.; Brown, D.C.; Wistuba, T.J. Dietary supplementation with phosphorylated mannans improves growth response and modulates immune function of weanling pigs. J. Anim. Sci. 2004, 82, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Li, D.; Yin, Y.; Wang, X.; Li, P.; Dangott, L.J.; Hu, W.; Wu, G. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J. Nutr. 2008, 138, 60–66. [Google Scholar] [CrossRef]
- Huang, S.; Wu, Z.; Liu, C.; Han, D.; Feng, C.; Wang, S.; Wang, J. Milk Fat Globule Membrane Supplementation Promotes Neonatal Growth and Alleviates Inflammation in Low-Birth-Weight Mice Treated with Lipopolysaccharide. BioMed Res. Int. 2019, 2019, 4876078. [Google Scholar] [CrossRef]
- Ji, Y.; Dai, Z.; Sun, S.; Ma, X.; Yang, Y.; Tso, P.; Wu, G.; Wu, Z. Hydroxyproline Attenuates Dextran Sulfate Sodium-Induced Colitis in Mice: Involvment of the NF-kappaB Signaling and Oxidative Stress. Mol. Nutr. Food Res. 2018, 62, e1800494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, K.; Chen, H.; Su, Y.; Zhu, W. Caecal infusion of the short-chain fatty acid propionate affects the microbiota and expression of inflammatory cytokines in the colon in a fistula pig model. Microb. Biotechnol. 2018, 11, 859–868. [Google Scholar] [CrossRef]
- Lee, T.I.; Kao, Y.H.; Tsai, W.C.; Chung, C.C.; Chen, Y.C.; Chen, Y.J. HDAC Inhibition Modulates Cardiac PPARs and Fatty Acid Metabolism in Diabetic Cardiomyopathy. PPAR Res. 2016, 2016, 5938740. [Google Scholar] [CrossRef] [PubMed]
- van Heugten, E.; Funderburke, D.W.; Dorton, K.L. Growth performance, nutrient digestibility, and fecal microflora in weanling pigs fed live yeast. J. Anim. Sci. 2003, 81, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Chance, J.A.; DeRouchey, J.M.; Amachawadi, R.G.; Ishengoma, V.; Nagaraja, T.G.; Goodband, R.D.; Woodworth, J.C.; Tokach, M.D.; Kang, Q.; Loughmiller, J.A.; et al. Influence of yeast-based pre- and probiotics in lactation and nursery diets on nursery pig performance and antimicrobial resistance of fecal Escherichia coli. J. Anim. Sci. 2022, 100, skac166. [Google Scholar] [CrossRef]
- Stecher, B.; Robbiani, R.; Walker, A.W.; Westendorf, A.M.; Barthel, M.; Kremer, M.; Chaffron, S.; Macpherson, A.J.; Buer, J.; Parkhill, J.; et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007, 5, 2177–2189. [Google Scholar] [CrossRef] [PubMed]
- Paiva, D.; Walk, C.; McElroy, A. Dietary calcium, phosphorus, and phytase effects on bird performance, intestinal morphology, mineral digestibility, and bone ash during a natural necrotic enteritis episode. Poult. Sci. 2014, 93, 2752–2762. [Google Scholar] [CrossRef] [PubMed]
- Ballard, S.T.; Hunter, J.H.; Taylor, A.E. Regulation of tight-junction permeability during nutrient absorption across the intestinal epithelium. Annu. Rev. Nutr. 1995, 15, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Backhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Noriega, B.S.; Sanchez-Gonzalez, M.A.; Salyakina, D.; Coffman, J. Understanding the Impact of Omega-3 Rich Diet on the Gut Microbiota. Case Rep. Med. 2016, 2016, 3089303. [Google Scholar] [CrossRef] [PubMed]
- Young, G.P. Prevention of colon cancer: Role of short chain fatty acids produced by intestinal flora. Asia Pac. J. Clin. Nutr. 1996, 5, 44–47. [Google Scholar]
- Sekirov, I.; Gill, N.; Jogova, M.; Tam, N.; Robertson, M.; de Llanos, R.; Li, Y.; Finlay, B.B. Salmonella SPI-1-mediated neutrophil recruitment during enteric colitis is associated with reduction and alteration in intestinal microbiota. Gut Microbes 2010, 1, 30–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopez, C.A.; Winter, S.E.; Rivera-Chavez, F.; Xavier, M.N.; Poon, V.; Nuccio, S.P.; Tsolis, R.M.; Baumler, A.J. Phage-mediated acquisition of a type III secreted effector protein boosts growth of salmonella by nitrate respiration. mBio 2012, 3, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.A.; Rivera-Chavez, F.; Byndloss, M.X.; Baumler, A.J. The Periplasmic Nitrate Reductase NapABC Supports Luminal Growth of Salmonella enterica Serovar Typhimurium during Colitis. Infect. Immun. 2015, 83, 3470–3478. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).