Investigating Endoparasites in Captive Birds of Prey in Italy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Laboratory Analysis
2.3. Statistical Analysis
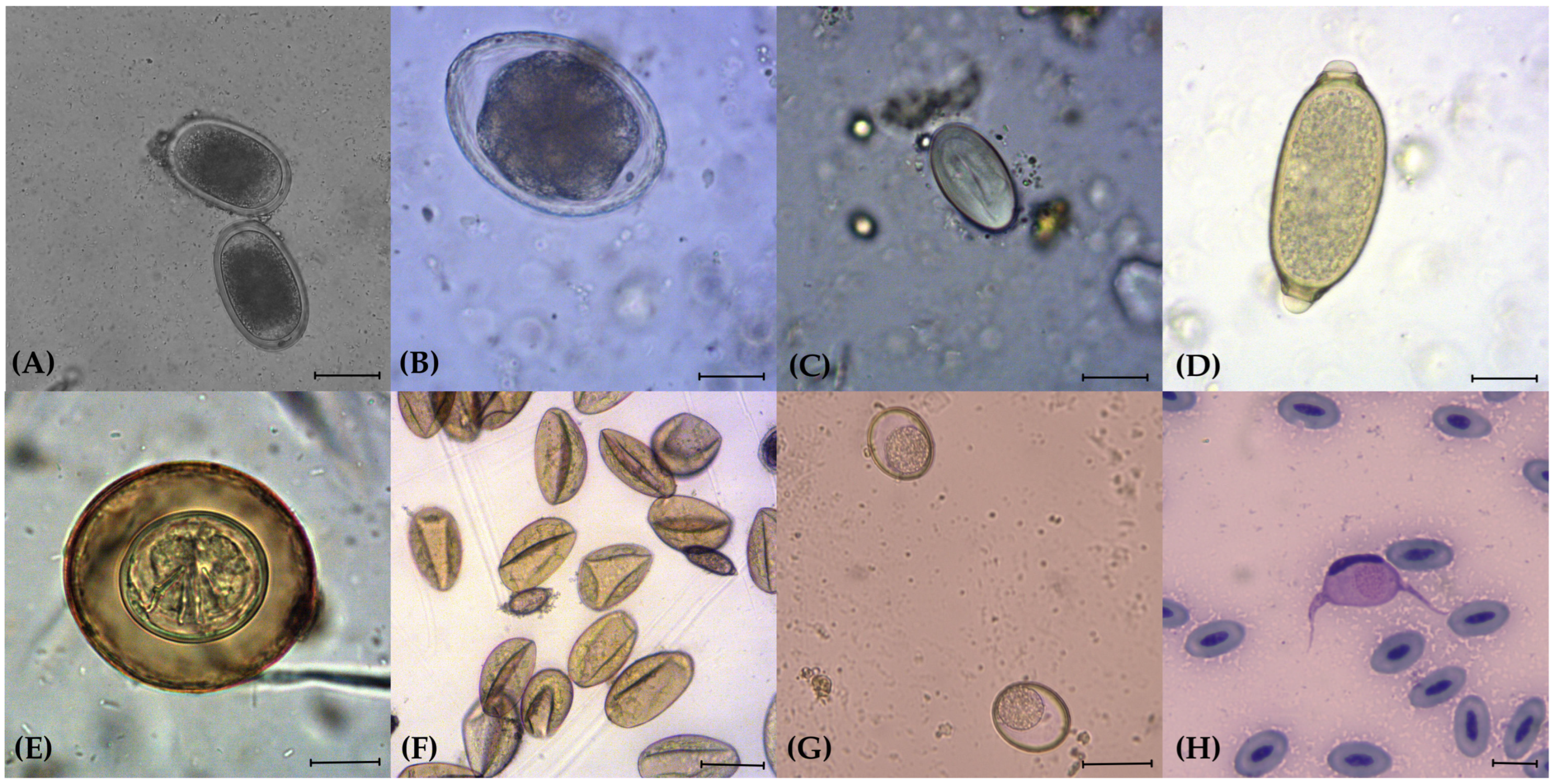
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, F. Behavior and Behavioral Problems of Australian Birds of prey in Captivity. Semin. Avian Exot. Pet Med. 2003, 12, 232–241. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, F.; Huang, W.; Song, J. Occurrence of Nematode Parasites in Raptors in Beijing, China. J. Raptor Res. 2008, 42, 204–209. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Zanzani, S.A.; Santoro, A.; Veronesi, F.; Olivieri, E.; Villa, L.; Lubian, E.; Lovati, S.; Bottura, F.; Epis, S.; et al. Toxoplasma gondii infection in birds of prey from Italy: Seroepidemiology and risk factors analysis. Comp. Immunol. Microbiol. Infect. Dis. 2018, 60, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.G.; Morishita, T.Y.; Bartlett, J.L.; Brooks, D.L. Coprologic survey of internal parasites of Northern California birds of prey. J. Zoo Wildl. Med. 1996, 27, 358–363. [Google Scholar]
- Jones, M.P. Selected Infectious Diseases of Birds of Prey. J. Exot. Pet. Med. 2006, 15, 5–17. [Google Scholar] [CrossRef]
- Ilić, T.; Becskei, Z.; Gajić, B.; Özvegy, J.; Stepanović, P.; Nenadović, K.; Dimitrijević, S. Prevalence of endoparasitic infections of birds in zoo gardens in Serbia. Acta Parasitol. 2018, 63, 134–146. [Google Scholar] [CrossRef]
- Papini, R.; Girivetto, M.; Marangi, M.; Mancianti, M.; Giangaspero, A. Endoparasite Infections in Pet and Zoo Birds in Italy. Sci. World J. 2012, 253127. [Google Scholar] [CrossRef]
- Rossi, G.; Terracciano, G.; Gherardi, R.; Galosi, L.; Perrucci, S. Parasites, Bacteria, and Associated Pathological Changes in the Digestive System of Diurnal and Nocturnal Birds of prey in Central Italy. Pathogens 2021, 10, 1567. [Google Scholar] [CrossRef]
- Borgsteede, F.H.M.; Okulewicz, A.; Zoun, P.E.F.; Okulewicz, J. The helminth fauna of birds of prey (Accipitriformes, Falconiformes and Strigiformes) in the Netherlands. Acta Parasitol. 2003, 48, 200–207. [Google Scholar]
- da Silva, A.S.; Zanette, R.A.; Lara, V.M.; Gressler, L.T.; Carregaro, A.B.; Santurio, J.M.; Monteiro, S.G. Gastrointestinal parasites of Owls (Strigiformes) kept in captivity in the Southern region of Brazil. Parasitol. Res. 2009, 104, 485–487. [Google Scholar] [CrossRef]
- Santos, T.; de Oliveira, J.B.; Vaughan, C.; Santiago, H. Health of an ex-situ population of birds of prey (Falconiformes and Strigiformes) in Mexico: Diagnosis of internal parasites. Rev. Biol. Trop. 2011, 59, 1265–1274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cardozo, S.V.; Berto, B.P.; Caetano, I.; Maniero, V.C.; da Fonseca, I.P.; Lopes, C.W.G. Caryospora peneireiroi n. sp. (Apicomplexa: Eimeriidae) in the Common Kestrel, Falco tinnunculus (Falconiformes: Falconidae), in mainland Portugal. Rev. Bras. Parasitol. Vet. 2016, 25, 202–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crespo-Ginés, R.; López, D.S.; Berriatua, E.; Blanco, G.; Candela, M.G.; Pérez-Garciá, J.M. Coccidian prevalence and intensity in free-ranging and rehabilitating wild birds of prey. Ardeola 2019, 66, 65–76. [Google Scholar] [CrossRef]
- Sehgal, R.N.M.; Hull, A.C.; Anderson, N.L.; Valkiunas, G.; Markovets, M.J.; Kawamura, S.; Tell, L.A. Evidence for cryptic speciation of Leucocytozoon spp. (Haemosporida, Leucocytozoidae) in diurnal birds of prey. J. Parasitol. 2006, 92, 375–379. [Google Scholar] [CrossRef]
- Santos, N.G.; Pereira, M.C.; Melo, P.M.; Madeira de Carvalho, L.M. Study of haemoprotozoa in birds of prey (orders Falconiformes and Strigiformes) in wildlife rehabilitation facilities in Portugal. Rev. Port. Ciências Vet. 2008, 103, 195–200. [Google Scholar]
- Svobodová, M.; Weidinger, K.; Peške, L.; Volf, P.; Votýpka, J.; Voříšek, P. Trypanosomes and haemosporidia in the Buzzard (Buteo buteo) and Sparrowhawk (Accipiter nisus): Factors affecting the prevalence of parasites. Parasitol. Res. 2015, 114, 551–560. [Google Scholar] [CrossRef]
- Ferrer, D.; Molina, R.; Castellà, J.; Kinsella, J.M. Parasitic helminths in the digestive tract of six species of Owls (Strigiformes) in Spain. Vet. J. 2004, 167, 181–185. [Google Scholar] [CrossRef]
- Sanmartín, M.L.; Alvarez, F.; Barreiro, G.; Leiro, J. Helminth fauna of Falconiform and Strigiform birds of prey in Galicia, Northwest Spain. Parasitol. Res. 2004, 92, 255–263. [Google Scholar] [CrossRef]
- Santoro, M.; Tripepi, M.; Kinsella, J.M.; Panebianco, A.; Mattiucci, S. Helminth infestation in birds of prey (Accipitriformes and Falconiformes) in Southern Italy. Vet. J. 2010, 186, 119–122. [Google Scholar] [CrossRef]
- Smith, O.M.; Snyder, W.E.; Owen, J.P. Are we overestimating risk of enteric pathogen spillover from wild birds to humans? Biol. Rev. Camb. Philos. Soc. 2020, 95, 652–679. [Google Scholar] [CrossRef]
- Cringoli, G.; Rinaldi, L.; Maurelli, M.P.; Utzinger, J. FLOTAC: New multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010, 5, 503–515. [Google Scholar] [CrossRef] [PubMed]
- International Union for Conservation of Nature (IUCN). Available online: https://www.iucn.org (accessed on 3 April 2024).
- Cringoli, G. FLOTAC®, a novel apparatus for a multivalent faecal egg count technique. Parassitologia 2006, 48, 381–384. [Google Scholar] [PubMed]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Tristan, T. The aging raptor. Vet. Clin. N. Am. Exot. Anim. Pract. 2010, 13, 51–84. [Google Scholar] [CrossRef] [PubMed]
- Perrucci, S.; Rinaldi, L.; Tampieri, M.P. Parassiti degli uccelli domestici e selvatici. In Parassitologia e Malattie Parassitarie degli Animali; Garippa, G., Manfredi, M.T., Otranto, D., Eds.; EMSI: Roma, Italy, 2010; pp. 473–490. [Google Scholar]
- Lloyd, C. Control of nematode infections in captive birds. Practice 2003, 25, 198–206. [Google Scholar] [CrossRef]
- Willette, M.; Ponder, J.; Cruz-Martinez, L.; Arent, L.; Bueno Padilla, I.; de Francisco, O.N.; Redig, P. Management of Select Bacterial and Parasitic Conditions of Birds of prey. Vet. Clin. N. Am. Exot. Anim. Pract. 2009, 12, 491–517. [Google Scholar] [CrossRef]
- Komorová, P.; Sitko, J.; Špakulová, M.; Hurníková, Z.; Sałamatin, R.; Chovancová, G. New data on helminth fauna of birds of prey (Falconiformes, Accipitriformes, Strigiformes) in the Slovak Republic. Helminthologia 2017, 54, 314–321. [Google Scholar] [CrossRef]
- Globokar, M.; Fischer, D.; Pantchev, N. Occurrence of endoparasites in captive birds between 2005 to 2011 as determined by faecal flotation and review of literature. Berl. Münch. Tierärztl. Wochenschr. 2017, 130, 461–473. [Google Scholar] [CrossRef]
- Krone, O.; Priemer, J.; Streich, J.; Sömmer, P.; Langgemach, T.; Lessow, O. Haemosporida of birds of prey and Owls from Germany. Acta Protozool. 2001, 40, 281–289. [Google Scholar]
- Santana-Sánchez, G.; Flores-Valle, I.T.; González-Gómez, M.; Vega-Sánchez, V.; Salgado-Miranda, C.; Soriano-Vargas, E. Caryospora neofalconis and other enteroparasites in birds of prey from Mexico. Int. J. Parasitol. Parasites Wildl. 2015, 4, 351–355. [Google Scholar] [CrossRef]
- De Freitas, M.F.L.; De Oliveira, J.B.; De Brito Cavalcanti, M.D.; Soares Leite, A.; Santiago Magalhaes, V.; Alves De Oliveira, R.; Sobrino, A.E. Gastrointestinal parasites of captive wild birds in Pernambuco state, Brazil. Parasitol. Latinoam. 2002, 57, 50–54. [Google Scholar] [CrossRef]
- Borgsteede, F.H.M.; Okulewicz, A. Justification of the species Cyathostoma (Hovorkonema) americana (Chapin, 1925) (Syngamidae–Nematoda). Helminthologia 2001, 38, 151–154. [Google Scholar]
- Krone, O.; Friedrich, D.; Honisch, M. Specific status and pathogenicity of syngamid nematodes in bird species (Ciconiformes, Falconiformes, Gruiformes) from Germany. J. Helminthol. 2007, 81, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Forbes, N.A. Birds of prey: Parasitic disease. In BSAVA Manual of Birds of Prey, Pigeons and Passerine Birds; Chitty, J., Lierz, M.M., Eds.; BSAVA: Gloucester, UK, 2008; pp. 202–211. [Google Scholar]
- Pérez Cordón, G.; Hitos Prados, A.; Romero, D.; Sánchez Moreno, M.; Pontes, A.; Osuna, A.; Rosales, M.J. Intestinal and haematic parasitism in the birds of the Almuñecar (Granada, Spain) ornithological garden. Vet. Parasitol. 2009, 165, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.E.; Cannizzo, F.T.; Chiappino, L.; Sereno, A.; Ripepi, M.; Salamida, S.; Manuali, E.; Bollo, E. Plasmodium spp. in a captive raptor collection of a safari park in northwest Italy. Res. Vet. Sci. 2018, 104, 123–125. [Google Scholar] [CrossRef]
- Basto, N.; Rodriguez, O.A.; Marinkelle, C.J.; Gutierrez, R.; Matta, N.E. Haematozoa in birds from la Macarena National Park (Colombia). Caldasia 2006, 28, 371–377. [Google Scholar]
- Giorgiadis, M.; Guillot, J.; Duval, L.; Landau, I.; Quintard, B. Haemosporidian parasites from captive Strigiformes in France. Parasitol. Res. 2020, 119, 2975–2981. [Google Scholar] [CrossRef]
- Ziman, M.; Colagross-Schouten, A.; Griffey, S.; Stedman, B. Haemoproteus spp. and Leukocytozoon spp. in a captive raptor population. J. Wildl. Dis. 2004, 40, 137–140. [Google Scholar] [CrossRef]
- Valkunias, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005; pp. 1–932. [Google Scholar]
- Krone, O.; Waldenström, J.; Valkiunas, G.; Lessow, O.; Müller, K.; Iezhova, T.A.; Fickel, J.; Bensch, S. Haemosporidian blood parasites in European birds of prey and owls. J. Parasitol. 2008, 94, 709–715. [Google Scholar] [CrossRef]
| Birds of Prey Common Name | Birds of Prey Scientific Name | N° Birds of Prey | N° Faecal Samples | N° Blood Smears | N° Positive Samples/Total | Detected Parasites |
|---|---|---|---|---|---|---|
| Accipitriformes | ||||||
| Cinereous vulture | Aegypius monachus | 1 | 1 | 0 | 1/1 | Spiruridae |
| Golden eagle | Aquila chrysaetos | 1 | 1 | 0 | 1/1 | Eimeria spp. |
| Bonelli’s eagle | Aquila fasciata | 1 | 1 | 0 | 0/1 | - |
| Steppe eagle | Aquila nipalensis | 2 | 2 | 0 | 2/2 | Eimeria spp., Porrocaecum spp., Strongylidae |
| African hawk-eagle | Aquila spilogaster | 1 | 0 | 1 | 0/1 | - |
| Eurasian buzzard | Buteo buteo | 3 | 3 | 3 | 2/3 | Ascarididae, Capillariidae, Porrocaecum spp., Haemoproteus/Plasmodium spp., Leucocytozoon spp., Trematoda |
| Red-tailed hawk | Buteo jamaicensis | 2 | 2 | 1 | 1/2 | Strongylidae |
| Ferruginous hawk | Buteo regalis | 1 | 0 | 1 | 0/1 | - |
| Turkey vulture | Cathartes aura | 1 | 1 | 0 | 1/1 | Spiruridae |
| Black-chested buzzard-eagle | Geranoaetus melanoleucus | 1 | 1 | 0 | 1/1 | Strongylidae |
| White-backed vulture | Gyps africanus | 1 | 1 | 0 | 0/1 | - |
| Bald eagle | Haliaeetus leucocephalus | 3 | 3 | 1 | 2/3 | Strongylidae, Trematoda |
| Harris’s hawk | Parabuteo unicinctus | 11 | 11 | 6 | 3/11 | Strongylidae |
| White-headed vulture | Trigonoceps occipitalis | 1 | 1 | 0 | 0/1 | - |
| Falconiformes | ||||||
| Crested caracara | Caracara cheriway | 3 | 2 | 1 | 0/3 | - |
| Lanner falcon | Falco biarmicus | 2 | 2 | 0 | 0/2 | - |
| Barbary falcon | Falco pelegrinoides | 1 | 1 | 0 | 0/1 | - |
| Peregrine falcon | Falco peregrinus | 1 | 1 | 0 | 0/1 | - |
| Eurasian hobby | Falco subbuteo | 2 | 2 | 2 | 2/2 | Haemoproteus/Plasmodium spp., Spiruridae |
| Common kestrel | Falco tinnunculus | 2 | 1 | 2 | 2/2 | Spiruridae, Strongylidae, Trematoda |
| Gyrfalcon/saker falcon (hybrid) | Falco rusticolus/Falco cherrug | 3 | 3 | 0 | 3/3 | Caryospora spp. |
| Gyrfalcon/lanner falcon (hybrid) | Falco rusticolus/Falco biarmicus | 3 | 2 | 2 | 1/3 | Caryospora spp., Porrocaecum spp. |
| Strigiformes | ||||||
| Northern long-eared owl | Asio otus | 1 | 1 | 1 | 0/1 | - |
| Little owl | Athene noctua | 8 | 8 | 6 | 2/8 | Haemoproteus/Plasmodium spp. |
| Rock eagle-owl | Bubo bengalensis | 1 | 1 | 1 | 1/1 | Eimeria spp. |
| Eurasian eagle-owl | Bubo bubo | 12 | 8 | 1 | 2/12 | Eimeria spp., Strongylidae |
| Snowy owl | Bubo scandiacus | 4 | 4 | 0 | 3/4 | Capillariidae, Eimeria spp., Spiruridae, Strongylidae |
| Great horned owl | Bubo virginianus | 1 | 1 | 0 | 1/1 | Capillariidae, Cestoda, Eimeria spp., Strongylidae |
| Great horned owl subarcticus | Bubo virginianus subarcticus | 1 | 1 | 0 | 1/1 | Capillariidae, Eimeria spp., Strongylidae |
| Eurasian scops owl | Otus scops | 2 | 2 | 1 | 2/2 | Capillariidae, Cestoda |
| Common barn owl | Tyto alba | 4 | 4 | 3 | 0/4 | - |
| Phylum | Family | Genus | N° Positive Samples/Total | Prevalence (95% CI a) | EPG/OPG b (SD c) | Min–Max |
| Nematoda | Ascarididae | Porrocaecum spp. | 4/72 | 5.6 (1.5–13.6) | 39.7 (281.9) | 0–2368 |
| Other genera | 1/72 | 1.4 (0.03–7.5) | 84.2 (714.6) | 0–6064 | ||
| Capillariidae | Nd d | 6/72 | 8.3 (3.1–17.3) | 86.6 (519.3) | 0–3888 | |
| Spiruridae | Nd | 6/72 | 8.3 (3.1–17.3) | 9.6 (58.7) | 0–440 | |
| Strongylidae | Nd | 14/72 | 19.4 (11.1–30.5) | 15.63 (71.1) | 0–512 | |
| Phylum | Class | N° Positive Samples/Total | Prevalence (95% CI a) | EPG/OPG b (SD c) | Min–Max | |
| Platyhelminthes | Cestoda | 2/72 | 2.8 (0.3–9.7) | Nd | Nd | |
| Trematoda | 4/72 | 5.6 (1.5–13.6) | 967.3 (796.5) | 0–67,584 | ||
| Phylum | Subclass | Genus | N° Positive Samples/Total | Prevalence (95% CI a) | EPG/OPG b (SD c) | Min–Max |
| Apicomplexa | Coccidia | Caryspora spp. | 4/72 | 5.5 (2.2–13.4) | 48.9 (403.5) | 0–3424 |
| Eimeria spp. | 8/72 | 11.1 (5.7–20.4) | 247.6 (2100.9) | 0–17,952 | ||
| Total | 30/72 | 41.7 (30.1–53.9) | ||||
| Risk Factor | Category | p-Value | OR a | 95% CI b |
|---|---|---|---|---|
| Age | Adult | <0.05 * | 15.20 | 1.54–150.4 |
| Young | 1 | |||
| Diet | Also fresh meat | <0.05 * | 35.4 | 1.6–771.2 |
| Only thawed meat | 1 | |||
| Family | Accipitridae | >0.05 | 1.4 × 1010 | ∞ |
| Cathartidae | >0.05 | 1.1 × 1021 | ∞ | |
| Falconidae | >0.05 | 3.7 × 109 | ∞ | |
| Strigidae | >0.05 | 1 × 1010 | 0 | |
| Tytonidae | 1 | |||
| Housing | Free in the aviary | >0.05 | 11 | 0.54–226.3 |
| Tied to a perch | 1 | |||
| Origin | Permanently in captivity | >0.05 | 7.2 | 0.35–150.4 |
| Wild temporarily in captivity | 1 | |||
| Placement in aviary | In groups | >0.05 | 2.21 | 0.23–20.9 |
| In pairs | >0.05 | 0.075 | 0.002–2.7 | |
| Individually | 1 | |||
| Order of Birds of Prey | N° Positive Samples/Total (Prevalence, 95% CI a) | Species of Bird of Prey | Haemoproteus/Plasmodium spp. | Leucocytozoon spp. |
|---|---|---|---|---|
| Accipitriformes | 2/13 | Buteo buteo | 2 | 1 |
| Falconiformes | 4/7 | Falco subbuteo | 2 | 0 |
| Falco tinnunculus | 2 | 0 | ||
| Strigiformes | 1/13 | Athene noctua | 1 | 0 |
| Total | 7/33 (21.2%, 9–38.9) | 7/33 | 1/33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allievi, C.; Zanzani, S.A.; Bottura, F.; Manfredi, M.T. Investigating Endoparasites in Captive Birds of Prey in Italy. Animals 2024, 14, 3579. https://doi.org/10.3390/ani14243579
Allievi C, Zanzani SA, Bottura F, Manfredi MT. Investigating Endoparasites in Captive Birds of Prey in Italy. Animals. 2024; 14(24):3579. https://doi.org/10.3390/ani14243579
Chicago/Turabian StyleAllievi, Carolina, Sergio A. Zanzani, Fulvio Bottura, and Maria Teresa Manfredi. 2024. "Investigating Endoparasites in Captive Birds of Prey in Italy" Animals 14, no. 24: 3579. https://doi.org/10.3390/ani14243579
APA StyleAllievi, C., Zanzani, S. A., Bottura, F., & Manfredi, M. T. (2024). Investigating Endoparasites in Captive Birds of Prey in Italy. Animals, 14(24), 3579. https://doi.org/10.3390/ani14243579





